Abstract
Introduction:
Cystic echinococcosis (CE) is a chronic zoonosis which presents with variable clinical manifestations. Currently the diagnosis of this disease is based on radiological findings and serological tests which lack specificity. Although antigen detection from the cyst fluid is the most specific, it is seldom done due to the complications involved. Detecting the presence of Echinococcus granulosus specific deoxyribonucleic acid (DNA) by the polymerase chain reaction (PCR) could provide a definitive diagnosis of CE.
Materials and Methods:
An in-house PCR assay was devised to detect E. granulosus specific DNA in serum, urine and hydatid cyst fluid. The ability of the PCR to detect E. granulosus in the above mentioned samples were observed in comparison with other antigen and antibody detection tests.
Results:
Serum samples from surgically confirmed patients of CE with ruptured cysts contained the corresponding DNA while the in the majority of cases who had an intact cyst had no DNA of E. granulosus in their serum. DNA of E. granulosus was not found to be excreted in urine. PCR performed equal to antigen detection ELISA while testing hydatid cyst fluid samples.
Conclusions:
Serum and urine might not serve as useful samples for the molecular diagnosis of cystic echinococcosis. However, PCR can be useful on serum samples to detect ruptured hydatid cysts and on hydatid cyst fluid to confirm the parasitic diagnosis.
Keywords: Cystic echinococcosis, hydatid disease, polymerase chain reaction, serum
INTRODUCTION
Cystic echinococcosis (CE) also known as hydatid disease, is a chronic zoonosis of humans, caused by the dog tapeworm Echinococcus granulosus. The disease usually remains asymptomatic throughout the life but in symptomatic cases, the clinical manifestations are highly variable.[1] Among the species of Echinococcus causing human infections, E. granulosus is the most common and occurs chiefly in South America, Middle East, Australia, Russia, and China. In India, large number of cases has been reported from Andhra Pradesh, Gujarat, Tamil Nadu, West Bengal, and Pondicherry.[2]
As the clinical diagnosis of CE is difficult due to the variable presentation, it is always supported by imaging and immunological methods.[3] The immunodiagnostic methods are based on antibody detection in the serum and include tests such as indirect hemagglutination (IHA), counter-current immunoelectrophoresis (CIEP), and enzyme-linked immunosorbent assay (ELISA) and enzyme immunotransfer blot (EITB).[4] However, the antibody-based serological tests have the disadvantage of the inability to differentiate between recent and past infections.[3]
In this context, molecular methods such as polymerase chain reaction (PCR) with a superior sensitivity and specificity and with the ability to provide a definite parasitic diagnosis, have an edge over the conventional serological tests.[5] In the present study, an attempt is made to diagnose CE by detecting E. granulosus specific deoxyribonucleic acid (DNA) in the serum by PCR. The efficacy of this PCR, using serum as a sample has been compared with ELISA and EITB. Furthermore, the possibility of hydatid cyst fluid and urine as a sample for molecular diagnosis of CE has been tried.
MATERIALS AND METHODS
The study was initiated following the ethical clearance sanctioned by the Institutional Ethics Committee and carried over a period of 2 years. A total of 50 subjects comprised of consecutive patients and healthy volunteers were included in the study. The study subjects were divided into the following groups: Group I: “Surgically confirmed CE” which included 10 surgically proved cases of CE. Group II: “Ultrasound proven CE” which included 15 cases of un-operated CE but proved by ultrasonography. Group III: “Controls with other parasitic diseases” which included 15 patients with various parasitic diseases other than CE. Group IV: “Healthy controls” which included 10 healthy adults (blood donors and students) who had not suffered from CE.
Serum and urine samples were collected from all subjects and hydatid cyst fluid samples were collected from Group I. All serum samples were tested for the presence of anti-echinococcal antibodies using ELISA and EITB. All hydatid cyst fluid samples were tested for the presence of hydatid antigens using an in-house sandwich ELISA. All procedures involving sample collection and processing, serological tests and the antigen detection ELISA were performed as mentioned earlier by Chaya and Parija.[6]
Conventional PCR targeting the E. granulosus specific DNA sequences of the NADH1 gene was performed on all serum, urine and hydatid cyst fluid samples. DNA extraction was carried out by the phenol-chloroform-isoamyl alcohol (25:24:1) method as described elsewhere.[7] The PCR reaction was performed in a total reaction mixture of 25 μL (1-5 ng of template DNA, 40 nM dNTP mix (10 nM each), 0.5 nmol each primer, 2 mM MgCl2 , 2.5 μL Taq Buffer, 2 units Taq Polymerase) using forward (5'GTCGTAACAAGGTTTCCGTA3') and reverse (5'TCTAGATGCGTTCGAA (G/A) TGTCGATG3') oligonucleotide primers.[1,8] The amplification was carried out at one cycle of 96°C for 4 min, 30 cycles of 94°C for 1 min, 40°C for 1 min, 72°C for 2 min and 1 cycle of final extension of 72°C for 7 min. The resultant 450 bp amplification products were observed on a 1.2% agarose gel following electrophoresis.
The performance of PCR in detecting E. granulosus from serum samples were compared with antibody detecting tests, ELISA and EITB. The performance of PCR in detecting E. granulosus from hydatid fluid was compared with sandwich ELISA detecting antigen in hydatid cyst fluid. The PCR was also performed on all urine samples to check whether the DNA of the organism is excreted in urine and if urine could be used as an alternate sample for the diagnosis of CE.
RESULTS
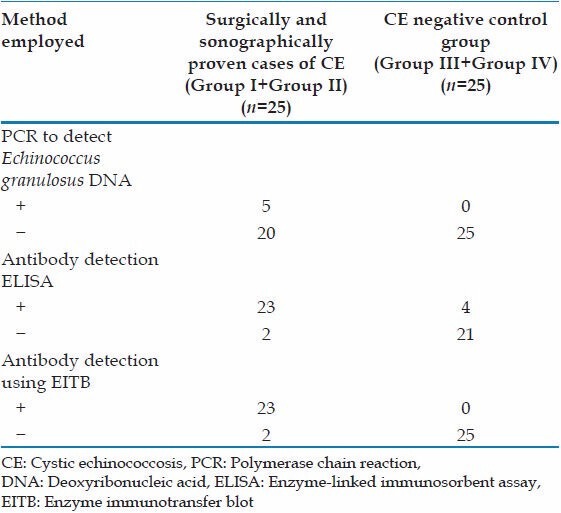
PCR identified E. granulosus specific DNA in the serum of 5 out of 10 surgically confirmed CE (Group I) and none in sera of ultrasound proven cases and controls (Groups II-IV) [Figure 1]. The results of the comparative evaluation of ELISA for hydatid antibody and antigen detection and PCR for E. granulosus specific DNA in serum is summarized in Table 1. The comparative evaluation of the tests employed on serum samples showed that the EITB for detection of hydatid antibody was found to be highly sensitive (92%) and specific (100%). ELISA for hydatid antibody detection also had a high sensitivity (92%), but the sensitivity of the PCR was found to be very low for testing serum samples.
Figure 1.
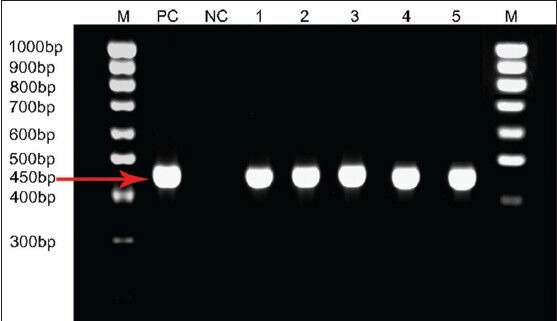
Polymerase chain reaction amplification of the 450 bp Echinococcus granulosus specific NADH1 gene from five serum samples of surgically confirmed cases with a ruptured cyst. M: Molecular ladder, PC: Positive control, NC: Negative control, 1-5 depicts the number of cases
Table 1.
Performance of the different tests when tested with serum sample
Both the PCR and the antigen detection ELISA detected E. granulosus specific DNA and hydatid antigens respectively in all of the 10 hydatid cyst fluid samples. E. granulosus specific DNA was not detected in any of the urine samples.
DISCUSSION
The laboratory methods for the diagnosis of parasitic diseases essentially consist of parasitic, immunodiagnosis and molecular methods. Parasitic diagnosis is the simplest method to establish the specific diagnosis of the CE, which includes microscopy of the aspirated hydatid fluid to demonstrate brood capsules and protoscolices. Although it confirms the diagnosis of the CE, the greatest disadvantage of diagnostic aspiration is the risk of anaphylactic reaction from leakage of cyst fluid.[4]
Hence, a wide number of serological tests have been developed for the detection of either hydatid antibodies or antigens in sera.[9] The antibody-based serological tests include IHA, CIEP, ELISA, EITB, and many other tests.[4] But the antibody-based tests have the disadvantages of low specificity and low sensitivity. These tests cannot differentiate between recent and past infections. The mere demonstration of hydatid antibody does not confirm clinical diagnosis because of non-specific reactions and persistence of anti-hydatid antibodies for several years after surgical removal of hydatid cyst.
Diagnostic methods based on the detection of parasite antigens are more useful as it helps in more direct measure of parasite burden, giving an indication of the activity and intensity of infection and also in assessing medical and chemotherapeutic treatment of CE.[10] Detection of hydatid antigen in the serum or other body fluid is suggested to be more efficacious in the diagnosis of CE than antibody detection.[11] Antigen-based tests such as CIEP, co-agglutination and ELISA are increasingly used now-a-days because these tests can detect recent infection.[12,13,14] However, limitations of these tests are their low sensitivity.
Molecular methods like PCR are now widely being employed in the diagnosis of many infectious diseases including the parasitic such as amoebiasis, malaria, filariasis, etc.[5] PCR has been employed for diagnosis of CE using hydatid cyst fluid samples.[15] The present study attempts to find the role of PCR on serum and urine in the diagnosis of CE. Only 5 out of 10 (50%) surgically confirmed cases were found to be having E. granulosus specific DNA sequences in the serum. All the five cases presented with ruptured hydatid cyst, which might be the most probable reason for the presence of E. granulosus specific DNA in their serum. As most of the CE patients harbor an intact cyst, serum might not be a suitable sample for the molecular diagnosis of CE.
It has been observed from earlier studies that the hydatid antigens are excreted in the urine of patients with CE. However, the use of urine as a sample for antigen detection may not help in the diagnosis of CE due to the low sensitivity and specificity.[6,16] As the excretion of antigen in urine has been well-documented in CE, there was a suspicion that the parasitic DNA might also be excreted. However, in the present study it was observed that DNA of E. granulosus was not excreted in urine as none of the urine samples were positive by PCR. The most likely explanation for the low sensitivity of PCR in urine could be the infrequency of E. granulosus DNA in urine. A similar finding has been reported when a urine-based PCR assay was used for the diagnosis of histoplasmosis.[17]
CONCLUSIONS
The present study is the first study of the kind where in PCR was employed to detect E. granulosus specific nucleic acid in serum and urine. The results of the present study show that PCR is highly sensitive for detection of E. granulosus specific DNA in hydatid cyst fluid and not so when tested with serum or urine. An interesting observation made was the presence of E. granulosus specific DNA in patients with ruptured hydatid cyst, indicating that E. granulosus specific DNA does not enter the circulation unless it is ruptured. As in most patients the cyst remains intact, the study also reveals that serum and urine are not useful specimens to detect E. granulosus. In the present study, EITB to detect anti-echinococcal antibodies in serum performed better when compared to antibody detection ELISA or DNA detection in serum. The PCR performed equally to antigen detection ELISA and both had a high degree of sensitivity and specificity while testing hydatid cyst fluid samples.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Williams JF, López Adaros H, Trejos A. Current prevalence and distribution of hydatidosis with special reference to the Americas. Am J Trop Med Hyg. 1971;20:224–36. doi: 10.4269/ajtmh.1971.20.224. [DOI] [PubMed] [Google Scholar]
- 2.Matossian RM. The immunological diagnosis of human hydatid disease. Trans R Soc Trop Med Hyg. 1977;71:101–4. doi: 10.1016/0035-9203(77)90070-0. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia BB, Pathak KM. Echinococcosis. In: Parija SC, Pathak KM, editors. Review of Parasitic Zoonoses. Delhi: AITBS Publ; 1990. pp. 268–80. [Google Scholar]
- 4.Parija SC. Text Book of Medical Parasitology. 2nd ed. Chennai: All India Publishers and Distributors; 2004. pp. 268–80. [Google Scholar]
- 5.Parija SC, Devi S. Current concepts in the diagnosis of cystic echinococcosis in humans and livestock and intestinal echinococcosis in Canine hosts. J Vet Parasitol. 1999;13:93–102. [Google Scholar]
- 6.Chaya D, Parija SC. Evaluation of a newly designed sandwich enzyme linked immunosorbent assay for the detection of hydatid antigen in serum, urine and cyst fluid for diagnosis of cystic echinococcosis. Trop Parasitol. 2013;3:125–31. doi: 10.4103/2229-5070.122131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning; A Laboratory Manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 8.Chowdhury N. Helminths of domesticated animals in Indian subcontinent. In: Chowdhury N, Tada I, editors. Helminthology. New Delhi: Narosa Publishing House; 1994. pp. 73–144. [Google Scholar]
- 9.Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev. 2003;16:18–36. doi: 10.1128/CMR.16.1.18-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravinder PT, Parija SC. Countercurrent immuno-electrophoresis test for detection of hydatid antigen in the fluid from hydatid cysts: A preliminary report. Acta Trop. 1997;66:169–73. doi: 10.1016/s0001-706x(97)00036-3. [DOI] [PubMed] [Google Scholar]
- 11.Gottstein B. An immunoassay for the detection of circulating antigens in human echinococcosis. Am J Trop Med Hyg. 1984;33:1185–91. doi: 10.4269/ajtmh.1984.33.1185. [DOI] [PubMed] [Google Scholar]
- 12.Shariff M, Parija SC. Counter-current immuno-electrophoresis test for serodiagnosis of hydatid disease by detection of circulating hydatid antigen. J Microbiol Methods. 1991;14:71–6. [Google Scholar]
- 13.Shariff M, Parija SC. Co-agglutination (Co-A) test for circulating antigen in hydatid disease. J Med Microbiol. 1993;38:391–4. doi: 10.1099/00222615-38-6-391. [DOI] [PubMed] [Google Scholar]
- 14.Kanwar JR, Kanwar RK, Grewal AS, Vinayak VK. Significance of detection of immune-complexed 8 kDa hydatid-specific antigen for immunodiagnosis of hydatidosis. FEMS Immunol Med Microbiol. 1994;9:231–6. doi: 10.1111/j.1574-695X.1994.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 15.Gottstein B. Molecular and immunological diagnosis of echinococcosis. Clin Microbiol Rev. 1992;5:248–61. doi: 10.1128/cmr.5.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parija SC, Ravinder PT, Rao KS. Detection of hydatid antigen in urine by countercurrent immunoelectrophoresis. J Clin Microbiol. 1997;35:1571–4. doi: 10.1128/jcm.35.6.1571-1574.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang YW, Li H, Durkin MM, Sefers SE, Meng S, Connolly PA, et al. Urine polymerase chain reaction is not as sensitive as urine antigen for the diagnosis of disseminated histoplasmosis. Diagn Microbiol Infect Dis. 2006;54:283–7. doi: 10.1016/j.diagmicrobio.2005.10.008. [DOI] [PubMed] [Google Scholar]


