Abstract
Significant advances have been made in the identification of key molecular pathways that play pivotal roles in the initiation and progression of pancreatic ductal adenocarcinoma (PDAC). Among the common genetic and epigenetic changes, oncogenic mutations in Kras and upregulation of the c-Myc oncogene are frequent events in PDAC. Using genetically defined in vivo models, several studies have recently demonstrated that expression of mutant Kras and c-Myc are equally important for the initiation and maintenance of pancreatic cancer. The targeted downregulation of a single oncogene resulted in cancer cell death at primary and metastatic sites. These findings are very encouraging and provide a strong rationale for the development of targeted therapies against these oncogenic drivers. Despite what appeared to be a complete response to the ablation of the oncogene, a few dormant cancer cells remained present, and it was demonstrated that they are a cellular reservoir for a swift relapse of pancreatic cancer following oncogene reactivation. This review summarizes the basic principles of cancer dormancy and the applicability of the novel genetic models for reversible metastatic PDAC to elucidate the role of cancer stem cells as well as biological and molecular mechanisms that mediate the survival of dormant tumor cells.
Keywords: Pancreatic Cancer, Pancreatic Ductal Adenocarcinoma, Oncogenes, c-Myc, Disease Progression, Metastasis, Cancer Cell Dormancy, Cancer-initiating Cells, Genetically-engineered Mice, Cre recombinase, Tetracycline-controlled transactivation
Introduction
Three of the main goals of cancer therapy as defined by the National Cancer Institute are the complete eradication of known tumors, preventing cancer recurrence, and blocking a dissemination of malignant cells to distant sites (http://training.seer.cancer.gov/treatment/). The success of radiation, targeted or cytotoxic therapy and other types of cancer treatment are typically measured by the complete or partial remission of the malignant cells within primary tumors and their metastatic descendants. Even without obvious signs of cancer in patients and what is believed to be a complete response to therapy, minimal residual disease may still be present depending on the sensitivity of the assay that is being employed to detect remaining cancer cells. The residual malignant cells can either remain proliferative or enter a quiescent state where they stop dividing. In the first scenario, which is defined as tumor mass dormancy (1), the remaining small tumor reservoir is a result of equilibrium between cell proliferation and cell death that restricts the physical expansion of the neoplasm. This might be a result of an immune response, lack of blood and nutrient supply or cell intrinsic mechanisms that promote apoptosis during or following cell division. In contrast to this particular type of tumor growth arrest, cancer dormancy is more frequently referred to the condition where residual cancer cells stop dividing and enter a quiescent state. It should be noted that cancer cell dormancy may not only be a manifestation of minimal residual disease following therapy. It may also be a consequence of an earlier dissemination of cancer cells prior to therapy that remained quiescent at distant sites because they have not yet fully adapted to the new microenvironment (2, 3). In both cases, the residual cancer cells can persist in a dormant stage, and cancer stays asymptomatic for a prolonged period, sometimes for decades. Upon receiving favorable cell intrinsic or extrinsic cues, residual malignant cells can switch to a fast-dividing mode and grow back a clinically overt disease (1). Given the significant implication of dormant cancer cells that serve as a major cellular basis for cancer relapse, understanding their unique biology and elucidating the underlying mechanisms for the maintenance of quiescence will be critical for developing rational therapies against these cells and to reduce the risk of cancer recurrence.
Modeling cancer dormancy
The rate of relapse and formation of metastases following chemotherapy is the most obvious indication that a significant subset of patients still has minimal residual disease. Pusztai et al. (4) for instance examined the time and rate of breast cancer recurrence before and after complete clinical response to anthracycline-based combination chemotherapy, and they reported that more than 85% of patients with metastatic cancer had relapsed within 13 years. Detection and characterization of residual tumor cells, which may be in the order of 1 cancer cell among a million normal cells in the bone marrow (5), is very challenging in a clinical setting, and it is therefore essential to utilize model systems to study the biology of cancer dormancy.
Cell cycle arrest and expression of ABC transporters in a subset of neoplastic cells are two widely accepted mechanisms that promote a selection of drug-resistant and dormant cancer cells during chemotherapy (1, 6, 7). For example, Naumov and coworkers (8) established a metastatic breast cancer model in mice using the transplantation of GFP-labeled mammary carcinoma cell lines, and they clearly demonstrated that treatment with doxorubicin is ineffective in targeting non-dividing cancer cells. In addition to xenograft models, recent efforts have been made to model cancer dormancy in vitro based on 3-D co-cultures of breast cancer cells with cell types predominant in bone marrow (9). Besides elucidating cancer cell intrinsic factors, these novel organotypic model systems have been applied to define the role of the microvasculature as well as the fibrous stroma in tumor cell dormancy and the reawakening of cancer cells from a quiescent state in response to changes in the growth factor milieu (10, 11). Recent advances in modeling multistage carcinogenesis have also verified the importance of adaptive immunity for tumor cell growth arrest, which contributes to cancer dormancy (12).
Novel therapies directed against cancer-specific, molecular targets (i.e., targeted cancer therapies) hold the promise of being more selective for cancer cells, and unlike cytotoxic agents, they should also eradicate quiescent cells. However, studies in patients with chronic myeloid leukemia (CML) have shown that quiescent leukemia-initiating cells survive even after years of treatment with imatinib, and these cells are responsible for disease relapse upon therapy discontinuation (13). In line with this notion, Hamilton et al. (14) have recently demonstrated using mouse models that CML stem cells do not require Bcr/Abl expression for their survival. These observations clearly suggest that cancer cell dormancy is not a phenomenon specific for cytotoxic interventions and will remain a challenging problem following the advent of targeted therapies. Another important implication of these findings is that biologically relevant functions of oncogenes and putative therapeutic targets are restricted to particular cancer cell subtypes. Experimental evidence for this notion was provided in 1996 by Ewald et al. (15) using the first doxycycline-inducible model for reversible tumorigenesis. In this model, expression of the cancer-initiating oncogene (i.e., SV40 large T) was only required for certain stages of tumorigenesis. Although subsequent studies using a similar experimental approach have demonstrated that primary and even metastatic cancer cells can remain “addicted” to the expression of genes like c-Myc, mutant Kras, and ErbB2 (16, 17, 18, 19, 20, 21), some types of cancers quickly reemerge following reactivation of the oncogene after, what appeared to be, a complete remission upon the initial ablation of the oncogenic driver [for a more comprehensive reviews on this subject see (22, 23)]. Collectively, these studies in ligand-regulated tumor models may have provided experimental proof that a few cancer cells can remain dormant following the targeted inhibition of a single oncogene. Not all of these studies, however, clearly discriminate cancer cell dormancy from de novo transformation events that both can result in cancer recurrence.
Evidence for the presence of pancreatic cancer stem cells that can cause cancer dormancy in genetic models of targeted therapy
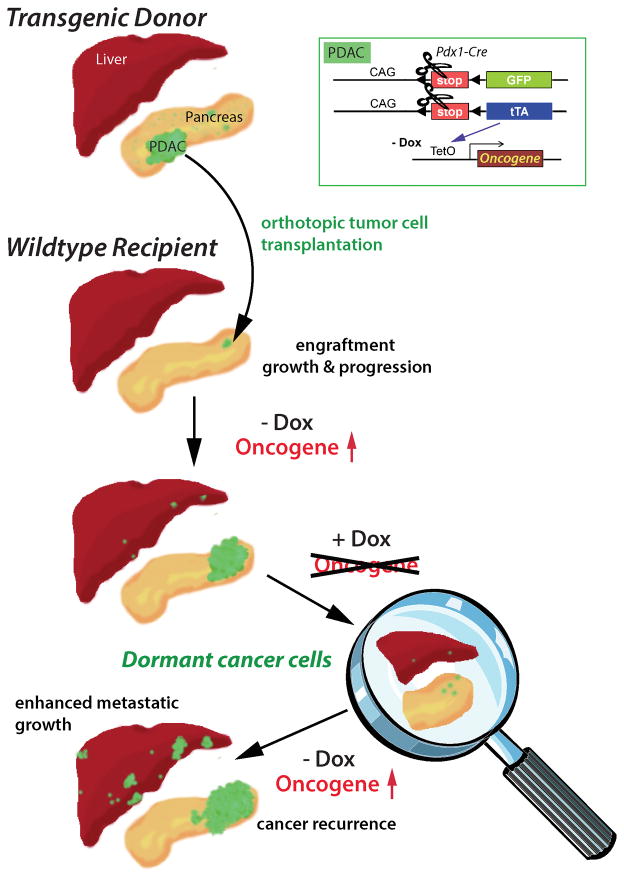
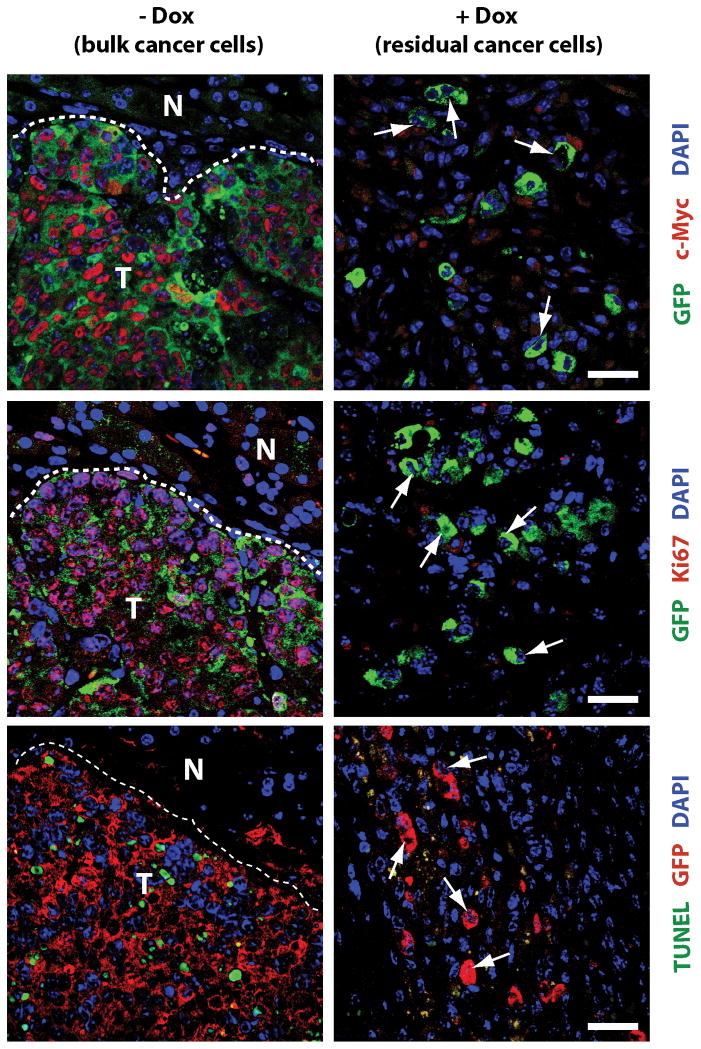
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human malignancies, and approximately 80% of the patients have metastatic disease at the time of diagnosis. There is currently no effective therapy to treat PDAC, and chemo- and radiotherapies are merely a part of palliative care (24). As the majority of pancreatic cancer cells carry activating mutations in the Kras gene (25), its encoded GTPase is an attractive protein for targeted therapy. The importance of this protein as a therapeutic target is emphasized by the recent launch of the ‘RAS initiative’ of the National Cancer Institute in the summer of 2013. In line with this notion, two independent research groups have recently shown that expression of mutant Kras is equally required for the initiation as well as maintenance of primary and metastatic PDAC (26, 27, 28). Although these studies may herald a very effective strategy to treat pancreatic cancer through inhibition of Kras, the rapid reemergence of primary and metastatic disease following reactivation of this oncogenic driver might be indicative for the existence of dormant cancer cells in this animal model (28). Our team has shown recently that many human PDAC cases as well as Kras-induced pancreatic tumors in mice overexpress c-Myc (29). In contrast to other PDAC models, upregulation of c-Myc in pancreatic progenitors is entirely sufficient to induce metastatic pancreatic cancer after a very short latency. A persistent expression of c-Myc is required for cancer cell survival at primary and metastatic sites regardless of whether neoplastic cells express or lose expression of wildtype p53 in response to Cdkn2a deficiency. Although we observed a macroscopically complete remission of all cancers in response to the downregulation of c-Myc, it was evident that residual cancer cells were still present at primary and metastatic sites. In subsequent transplantation experiments, we confirmed that cancer recurrence following re-expression of c-Myc was caused by residual neoplastic cells and not a de novo transformation of normal cells in the pancreas. In order to gain better insight into minimal residual disease in this genetic model, we used a Cre/lox-based, cell-fate mapping technique to visualize and isolate GFP-expressing cancer cells from primary and metastatic sites that survived the ablation of the transforming oncogene. The general principle of this labeling method and its application in models for reversible pancreatic cancer to trace dormant neoplastic cells is shown in Fig. 1. Using immunofluorescence staining on histological sections, we validated that residual tumor cells did not express transgenic or upregulate endogenous c-Myc. In addition, they were neither proliferating nor undergoing cell death, suggesting that these cells were quiescent, which is a main characteristic for cancer cell dormancy (Fig. 2). The flow cytometric analysis of GFP-labeled, residual c-Myc-negative tumor cells in comparison to bulk tumor cells expressing exogenous c-Myc revealed that the dormant population was enriched for cells that express cancer stem cell markers (CD24, CD44, CD133, and Sca-1) (29). Following orthotopic re-transplantation, we observed a better engraftment and a higher rate of tumor initiation in recipient mice that had received the residual cancer cell population as compared to an equal number of bulk tumor cells. Hence, dormant cancer cells in this model might be enriched for cancer stem cells based on the higher rate of engraftment as well as expression of specific cell surface markers.
Fig. 1. Genetic labeling of cancer cells with ligand-controlled oncogene expression and its application in transplant models for reversible pancreatic cancer to trace dormant neoplastic cells in primary and metastatic sites.
Expression of Cre recombinase under the regulation of the Pdx1 promoter (Pdx1-Cre) induces a constitutive expression of the tetracycline-controlled transactivator (tTA) and GFP in pancreatic progenitors and their normal and neoplastic descendants. The pancreas-specific expression of the tTA subsequently induces the activation of a TetO-driven responder transgene encoding an oncogene of choice, which leads to PDAC. An orthotopic transplantation of tumor cells into wildtype recipients is performed to discriminate true cancer dormancy in response to oncogene ablation from de novo transformation of normal cells following oncogene re-activation and cancer recurrence. The expression of the oncogene can be tightly controlled in a temporal manner though administration of doxycycline (Dox); Tet-OFF system. In contrast, the constitutive expression of GFP allows a cell lineage tracing throughout all stages of tumorigenesis, including metastatic dissemination in viable and in fixed tissues.
Fig. 2. Dormant cancer cells do not express c-Myc, lack nuclear staining of Ki67, and are TUNEL negative.
Upper panels: Immunofluorescence (IF) staining of c-Myc (red, nuclear) and GFP (green, cytoplasmic) in pancreatic cancers (−Dox, left) and residual cancer cells following tumor regression in response to downregulation of c-Myc (+Dox, right); arrows in the right panel indicate c-Myc-negative nuclei within residual cancer cells; T, tumor; N, adjacent normal tissue (left panels). Middle panels: IF staining of Ki67 (red, nuclear) and GFP (green, cytoplasmic), arrows indicate Ki67-negative nuclei within residual cancer cells. Lower panels: TUNEL labeling (green) of nuclei of apoptotic cells and IF staining against GFP (red, cytoplasmic), arrows indicate TUNEL-negative nuclei within residual cancer cells; bars in all panels represents 25 μm.
As there are currently no cytotoxic, systemic, or targeted therapies to effectively treat metastatic pancreatic cancer, it is evident that treating this disease does not instigate a biological response or progression that parallels many other cancer types such as that of the breast, prostate, or hematopoietic system. Cancer dormancy therefore does not (yet) appear to be a clinically relevant phenomenon for the majority of pancreatic cancers, at least not for unresectable cases. The dismal prognosis combined with a high frequency of oncogenic Kras mutations are compelling reasons why this disease moves to center stage in our persistent efforts aimed at developing targeted therapies against Ras proteins and their downstream effectors, including c-Myc. The biological response to the targeted ablation of Kras and c-Myc in established pancreatic tumors of genetically engineered mouse models clearly highlight the importance of this signal transducer and transcription factor for the maintenance of metastatic disease. However, these novel genetic models also herald a manifestation of residual disease despite complete oncogene inhibition. This may be a lingering challenge in the development of targeted therapies for PDAC. To accomplish two of the main goals of cancer therapy stated earlier, i.e., the complete eradication of tumors and preventing cancer recurrence, it is therefore essential to elucidate intrinsic and extrinsic factors that can contribute to residual disease in pancreatic cancer. The future will tell whether this assumption is premature, but from our collective knowledge about the biology and natural history of other types of cancer such as CML, melanoma, and breast cancer, cancer cell dormancy and recurrence in response to targeted therapy is very likely scenario for PDAC.
Unanswered questions and future directions
The initial molecular analysis of dormant pancreatic cancer cells in the c-Myc-induced tumor model revealed that they do not carry mutations in Kras (29). Therefore, oncogenic Kras does not seem to mediate the survival of cancer cells in the absence of c-Myc expression as previously reported in a mammary tumor model (20). This notion is clearly supported by the fact that cancer cells also remained dormant following the ligand-mediated downregulation of mutant Kras in PDAC (26, 28). Future studies might elucidate whether there are common underlying molecular mechanisms that mediate the survival of dormant tumor cells in the absence of c-Myc or mutant Kras as oncogenic drivers. Although it is likely that cell intrinsic mechanisms play a significant role, it is equally important to experimentally address whether the stroma provides a suitable microenvironment that facilitates cancer cell dormancy and disease recurrence. It was surprising to note that the majority of fibrous stromal cells survived the selective attrition of carcinoma cells in the c-Myc-induced tumor model (29). The dormant tumor cells, on the other hand, were always in close proximity or entirely embedded in the fibrous stroma in both the primary site and metastatic lesions. It is therefore reasonable to propose that, similar to CML-initiating cells (30), signaling from the microenvironment is an essential component of cancer dormancy. Among various cell types within the stroma, cancer-associated myofibroblasts (CAFs) and stellate cells are known to facilitate the stemness of cancer cells in GI tumors and PDAC (31, 32). These cells produce various growth factors such as Wnt ligands and HGF that play a crucial role in the maintenance of normal stem cells and cancer-initiating cells in these tissues (33, 34). Therefore, targeting these signaling pathways in the tumor-associated stroma might be an adjuvant strategy to eradicate dormant, cancer-initiating cancer cells that are refractory to cancer cell-specific therapy.
An important step towards determining common mechanisms in dormant pancreatic cancer cells will be the isolation of these residual cells from other cancer models with an inducible expression of mutant Kras using either Cre/lox-mediated cell lineage tracing (29) or the H2B-GFP-based label retention method to mark long-term quiescent cells (35). Using these experimental approaches to viably isolate dormant cells, it might be possible in the future to study in more detail the degree of heterogeneity in this residual cancer cell population using conventional flow cytometry with stem cell markers or high-throughput single-cell genomics (36, 37). The fact that not all residual tumor cells seem to express all individual cell surface markers (i.e., CD44 and CD133 in addition to CD24 or Sca1) is first evidence that the dormant cancer cells might consist of, to some degree, a heterogeneous cell population. Another important question is whether the cancer stem cells that are already present in the bulk of the tumor are the specific cell population that survives a targeted therapy and contributes to cancer dormancy. As an alternative mechanism to a simple enrichment of cancer stem cells during cancer remission, it might also be possible that actively dividing cells enter a quiescent state following oncogene ablation and adapt characteristics of stem cells. It has been proposed that the microenvironment plays a crucial role in cancer stem cell reprogramming, epithelial-mesenchymal transition (EMT) as well as tumor invasion and metastasis (31). Tumor stem cell enrichment and cancer cell reprograming might actually co-exist during cancer regression and contribute to a certain degree to the heterogeneity within the residual cancer cell population.
Acknowledgments
Financial support: PHS grant R21 CA155175 (K.-U.W) from the National Cancer Institute; and the Nebraska Cancer and Smoking Disease Research Program NE DHHS LB506 2011-36 (K.-U.W.).
This work was supported, in part, by the Public Health Service grant CA155175 (K.-U.W.) from the National Cancer Institute. Additional financial support was provided to K.-U.W. by the Nebraska Cancer and Smoking Disease Research Program (NE DHHS LB506 2011-36). W.-C.L. and N.R. were supported through a research assistantship from the UNMC Graduate Studies Office. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclosure statement: The authors have nothing to disclose.
References
- 1.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5:1799–807. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paez D, Labonte MJ, Bohanes P, Zhang W, Benhanim L, Ning Y, et al. Cancer dormancy: a model of early dissemination and late cancer recurrence. Clin Cancer Res. 2012;18:645–53. doi: 10.1158/1078-0432.CCR-11-2186. [DOI] [PubMed] [Google Scholar]
- 4.Pusztai L, Asmar L, Smith TL, Hortobagyi GN. Relapse after complete response to anthracycline-based combination chemotherapy in metastatic breast cancer. Breast Cancer Res Treat. 1999;55:1–8. doi: 10.1023/a:1006161906667. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix M. Significance, detection and markers of disseminated breast cancer cells. Endocr Relat Cancer. 2006;13:1033–67. doi: 10.1677/ERC-06-0001. [DOI] [PubMed] [Google Scholar]
- 6.Demicheli R. Tumour dormancy: findings and hypotheses from clinical research on breast cancer. Semin Cancer Biol. 2001;11:297–306. doi: 10.1006/scbi.2001.0385. [DOI] [PubMed] [Google Scholar]
- 7.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 8.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. 2003;82:199–206. doi: 10.1023/B:BREA.0000004377.12288.3c. [DOI] [PubMed] [Google Scholar]
- 9.Marlow R, Honeth G, Lombardi S, Cariati M, Hessey SM, Pipili A, et al. A novel model of dormancy for bone metastatic breast cancer cells. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-13-0991. [DOI] [PubMed] [Google Scholar]
- 10.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13:507–18. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Cortes J, O’Brien S, Kantarjian H. Discontinuation of imatinib therapy after achieving a molecular response. Blood. 2004;104:2204–5. doi: 10.1182/blood-2004-04-1335. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton A, Helgason GV, Schemionek M, Zhang B, Myssina S, Allan EK, et al. Chronic myeloid leukemia stem cells are not dependent on Bcr-Abl kinase activity for their survival. Blood. 2012;119:1501–10. doi: 10.1182/blood-2010-12-326843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ewald D, Li M, Efrat S, Auer G, Wall RJ, Furth PA, et al. Time-sensitive reversal of hyperplasia in transgenic mice expressing SV40 T antigen. Science. 1996;273:1384–6. doi: 10.1126/science.273.5280.1384. [DOI] [PubMed] [Google Scholar]
- 16.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 17.Fisher GH, Wellen SL, Klimstra D, Lenczowski JM, Tichelaar JW, Lizak MJ, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–62. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 19.Shachaf CM, Kopelman AM, Arvanitis C, Karlsson A, Beer S, Mandl S, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431:1112–7. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 20.Boxer RB, Jang JW, Sintasath L, Chodosh LA. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell. 2004;6:577–86. doi: 10.1016/j.ccr.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 21.Podsypanina K, Politi K, Beverly LJ, Varmus HE. Oncogene cooperation in tumor maintenance and tumor recurrence in mouse mammary tumors induced by Myc and mutant Kras. Proc Natl Acad Sci U S A. 2008;105:5242–7. doi: 10.1073/pnas.0801197105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonkers J, Berns A. Oncogene addiction: sometimes a temporary slavery. Cancer Cell. 2004;6:535–8. doi: 10.1016/j.ccr.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Shachaf CM, Felsher DW. Tumor dormancy and MYC inactivation: pushing cancer to the brink of normalcy. Cancer Res. 2005;65:4471–4. doi: 10.1158/0008-5472.CAN-05-1172. [DOI] [PubMed] [Google Scholar]
- 24.Sultana A, Tudur SC, Cunningham D, Starling N, Neoptolemos JP, Ghaneh P. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer: results of secondary end points analyses. Br J Cancer. 2008;99:6–13. doi: 10.1038/sj.bjc.6604436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardeesy N, DePinho RA. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 26.Collins MA, Bednar F, Zhang Y, Brisset JC, Galban S, Galban CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012 doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying H, Kimmelman AC, Lyssiotis CA, Hua S, Chu GC, Fletcher-Sananikone E, et al. Oncogenic Kras Maintains Pancreatic Tumors through Regulation of Anabolic Glucose Metabolism. Cell. 2012;149:656–70. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collins MA, Brisset JC, Zhang Y, Bednar F, Pierre J, Heist KA, et al. Metastatic pancreatic cancer is dependent on oncogenic Kras in mice. PLoS One. 2012;7:e49707. doi: 10.1371/journal.pone.0049707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin WC, Rajbhandari N, Liu C, Sakamoto K, Zhang Q, Triplett AA, et al. Dormant cancer cells contribute to residual disease in a model of reversible pancreatic cancer. Cancer Res. 2013;73:1821–30. doi: 10.1158/0008-5472.CAN-12-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traer E, MacKenzie R, Snead J, Agarwal A, Eiring AM, O’Hare T, et al. Blockade of JAK2-mediated extrinsic survival signals restores sensitivity of CML cells to ABL inhibitors. Leukemia. 2012;26:1140–3. doi: 10.1038/leu.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borovski T, De Sousa E Melo, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–9. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 32.Lonardo E, Frias-Aldeguer J, Hermann PC, Heeschen C. Pancreatic stellate cells form a niche for cancer stem cells and promote their self-renewal and invasiveness. Cell Cycle. 2012;11:1282–90. doi: 10.4161/cc.19679. [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–80. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen L, De Sousa E Melo, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12:468–76. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 35.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalisky T, Quake SR. Single-cell genomics. Nat Methods. 2011;8:311–4. doi: 10.1038/nmeth0411-311. [DOI] [PubMed] [Google Scholar]
- 37.Dalerba P, Kalisky T, Sahoo D, Rajendran PS, Rothenberg ME, Leyrat AA, et al. Single-cell dissection of transcriptional heterogeneity in human colon tumors. Nat Biotechnol. 2011;29:1120–7. doi: 10.1038/nbt.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]