Abstract
Background
Anxious youth have shown altered behavioral performance on the dot-probe task, but neural activation patterns provoked by the task remain poorly understood. In particular, neural mechanisms of threat disengagement, a clinically relevant construct, have been inadequately explored.
Method
During fMRI acquisition, 121 youth (ages 9–13; 90 with Generalized Anxiety Disorder, Separation Anxiety Disorder, and/or Social Phobia; 31 nonanxious controls) completed a dot-probe task, which required participants to identify the location of a dot replacing either a neutral or fearful face in a pair containing both faces. We assessed neural substrates of threat disengagement by comparing congruent trials (in which the dot replaces the fearful face) to incongruent trials (in which the dot replaces the neutral face).
Results
Across subjects, decreased rostrodorsal anterior cingulate cortex (rdACC) activity was observed specifically during incongruent trials. Nonanxious youth showed a convergent pattern in bilateral parahippocampal and hippocampal regions, whereas anxious youth showed an opposing pattern in these limbic areas, suggesting less integration of response across cortical and limbic areas relevant to threat appraisal. Reduced functional connectivity between rdACC and left parahippocampus/hippocampus was associated with greater anxiety.
Conclusions
In the largest dot-probe fMRI sample to date, both anxious and nonanxious youth showed a neural pattern consistent with successful disengagement of threat reactivity in the rdACC. However, anxious youth showed evidence of abnormal disengagement in bilateral parahippocampal/hippocampal clusters when attention was directed away from threat. Early interventions targeting neural mechanisms of threat disengagement may be beneficial, for example, by increasing integration across rdACC and limbic regions.
Keywords: fMRI, anxiety, pediatric, attentional bias, dot-probe task
INTRODUCTION
Selective attention toward threat has been observed in anxious youth and adults[1] and may actively contribute to the development and/or maintenance of anxiety.[2] Modification of attention bias may be a final common pathway to symptom reduction across behavioral and pharmacological treatments.[3] Consequently, recent attempts have been made to ameliorate clinical anxiety through direct modification of attentional bias, with positive preliminary findings reported in clinically anxious adults and youth.[4–6] The impact of such targeted interventions may be enhanced through a more complete understanding of neural mechanisms involved in the task, potentially allowing for further mechanistic treatment refinement (e.g., neurofeedback/neurostimulation) and synergistic treatment combinations.
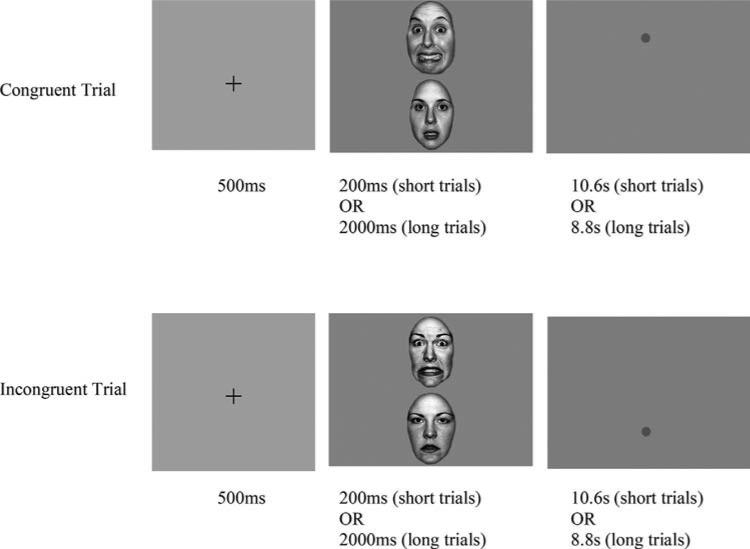
One of the most widely used measures of attentional bias is the dot-probe task[7] (Fig. 1). Early fMRI investigations of the dot-probe focused on neural responses to angry versus neutral faces, providing relevant information on brain responses to threat cues in anxious youth.[8,9] Results were convergent with a much wider literature implicating hyperreactivity in limbic/emotional processing regions (e.g., amygdala) and altered function in cognitive control regions (e.g., ventrolateral prefrontal cortex; PFC) in anxious individuals presented with threat information (e.g.,[10]).
Figure 1.
Schematic of the dot-probe task. Threat and neutral stimulus pairs are presented for a short (200 ms) or long (2,000 ms) duration, followed by a dot-probe replacing one of the two stimuli. Increased response latencies to dots in the incongruent location are frequently interpreted as an index of selective attention, or vigilance, to the threat stimulus. The terms “incongruent” and “congruent” are borrowed from the spatial attention literature. As commonly applied to the modified/emotional dot-probe task, the label “incongruent,” for trials in which the dot replaces the neutral cue, conveys the expectation that attention will tend to be more readily oriented toward emotional stimuli.
However, the behavioral index of theoretical and clinical interest in the dot-probe is calculated as a function of dot location rather than threat presence versus absence (Fig. 1). Therefore, both dot-probe performance and its correlate, real-world anxiety, may be influenced by brain responses that occur in the aftermath of threat (e.g., difficulties with threat disengagement). More recent pupil[11] and neuroimaging[12,13] data have high-lighted the importance of dot location in modulating brain responses in anxious youth, suggesting that neural indices of disengagement following dot onset may be equally relevant.
Flexible disengagement of neurocognitive resources from threat is an important feature of adaptive emotion regulation. Some argue dot-probe reaction times (RTs) primarily index difficulty disengaging from threat,[14] although RTs may also index biased initial orienting toward threat[15] and/or strategic avoidance of threat.[16] Examining the precise pattern of neural responses to incongruent versus congruent trials (e.g., using a slow event-related fMRI design) can provide complimentary and potentially disambiguating information regarding processes of neural disengagement, which might occur with or without concurrent behavioral bias in RTs (a relatively indirect index of cognitive processes). Brain deactivation, observed specifically when attention has been directed away from threat (i.e., during incongruent dots; see Fig. 1), suggests successful neural disengagement from threat following attentional manipulation.
Event-related fMRI can therefore provide new information relevant to a functional neuroanatomical model of threat disengagement that may help to better understand brain function in both healthy and clinically anxious samples. For instance, in healthy samples, rostral and dorsal regions of the anterior cingulate cortex (ACC), which are involved in dot-probe performance,[17–19] have been implicated in both regulatory control over emotional conflict[20] and, in more recent conceptualizations, threat reactivity (i.e., fear expression and appraisal).[21] These two models of ACC function lead to divergent hypotheses for the dot-probe task: if the region plays a primarily regulatory function, increased activity during incongruent trials (requiring threat disengagement) would be expected, whereas if the region reacts to threats, neural disengagement should be observed, with activation decreasing during incongruent trials. In terms of elucidating neural substrates of clinical anxiety, a recent study reported that responses to incongruent (relative to congruent) dot-probe trials in the hippocampus and parahippocampal gyrus were increased in adults at elevated risk for anxiety.[22] Examining the detailed time-course pattern of such limbic brain responses in anxious and nonanxious samples can help to clarify whether this pattern in fact represents impaired neural disengagement (i.e., attenuated decreases) during incongruent trials.
Given the purported clinical relevance of attentional bias in clinical anxiety, we examined neural substrates of dot-probe performance in the largest published dot-probe fMRI sample (in any age group) to our knowledge. We expanded on previous fMRI dot-probe findings in clinically anxious versus nonanxious youth by using a slow event-related design and focusing on neural responses to incongruent versus congruent trials, both of which include exposure to a fearful face but differ in the requirement to allocate attention toward or away from the location of threat. Because the presentation of a threat cue was held constant across all trials in the analysis, this comparison allowed for examination of neural mechanisms of flexible attentional allocation in the aftermath of a briefly presented threat, such as neural disengagement. We posited that evidence of impaired neural disengagement from threat would be observed in the brain activity of anxious patients compared to controls, for example, attenuated disengagement of regions reacting to threat. Analyses of functional connectivity and brain–behavior relationships were designed to further elaborate a neural model of attentional bias and derive potential targets for mechanistic neurocognitive intervention. Given that pediatric anxiety is associated with increased risk of emotional disorders in adulthood,[23] refinement of such targets may offer an opportunity to improve life-long trajectories of mental health.
METHODS
Dot-Probe Task
Ninety youth (ages 9–13) with DSM-IV diagnoses of Generalized Anxiety Disorder, Separation Anxiety Disorder, and/or Social Phobia and thirty-one youth with no lifetime DSM-IV disorders completed the dot-probe task (see Table 1 and Supporting Information for further details). These three prevalent diagnoses were included to allow investigation of transdiagnostic neural patterns. Informed consent and study procedures were approved by the University of Pittsburgh IRB. Participants completed the dot-probe task in the fMRI scanner using a slow event-related design, as part of a larger fMRI protocol. After an initial fixation cross presented in the middle of the screen (500 ms), a fearful and a neutral face were presented simultaneously on the top and bottom of the screen for either a short (200 ms) or long (2,000 ms) interval, followed by a probe (dot) replacing either the fearful face (“congruent” trials) or the neutral face (“incongruent” trials). Two durations of stimuli were included, as stimulus duration may moderate behavioral effects on the dot-probe.[16,24] Trials consisting of two blank ovals presented for 2,000 ms followed by a dot were included as a control condition (see Supporting Information for analysis). The dot remained on screen for the remainder of the trial (8.8–10.6 s; each trial = 11.3 s total), allowing brain responses to dissipate prior to the next trial onset. Faces were grayscale conversions of the well-validated NimStim battery,[25] half male and half female, with the same actor presented in both images in each pair. Participants completed 16 randomly interspersed trials of each type (e.g., short congruent, short incongruent, etc.) for 80 trials. Participants responded as quickly as possible to the probe, indicating its location on the screen by pressing a key for up or down. RT outliers were rescaled within and across subjects using a Winsorizing procedure (see Supporting Information).
TABLE 1.
Descriptive statistics for the anxious and nonanxious samples
| Anxious (n = 90) | Nonanxious (n = 31) | |
|---|---|---|
| Caucasian (n [%]) | 78 (86.7%) | 25 (80.6%) |
| Current diagnosisa (n [%]) | ||
| Separation anxiety disorder | 19 (21.1%) | – |
| Social phobia | 22 (24.4%) | – |
| Generalized anxiety disorder | 66 (73.3%) | – |
| Specific phobia | 13 (14.4%) | – |
| Major depressive disorder | 1 (1.1%) | – |
| Attention deficit disorder | 2 (2.2%) | – |
| Age | 10.6 (1.5) | 10.9 (1.6) |
| PARS | 20.8 (4.5) | 1.1 (2.3)* |
| Screen for child anxiety-related disorders – parent report | 35.4 (12.6) | 4.0 (3.1)* |
| Screen for child anxiety-related disorders – child report | 38.4 (12.0) | 11.6 (13.6)* |
| Mean RT, short congruent trials | 988.2 (321.6) | 1,043.0 (358.2) |
| Mean RT, short incongruent trials | 1,016.0 (332.0) | 1,023.7 (357.7) |
| Behavioral bias, short trials | 27.9 (158.9) | –19.3 (150.3) |
| Mean RT, long congruent trials | 1,050.3 (318.5) | 1,049.9 (287.8) |
| Mean RT, long incongruent trials | 1,047.7 (312.4) | 1,088.5 (358.1) |
| Behavioral bias, long trials | –2.7 (167.1) | 38.5 (177.9) |
| Overall mean behavioral bias | 12.6 (116.4) | 9.6 (112.4) |
Note: Data presented as mean (SD) unless otherwise noted. No significant differences in demographic variables according to χ2 and t tests.
Diagnostic groups are partially overlapping due to inclusion of comorbid patients. Primary/principle diagnoses were not designated, meaning that percentages for the three diagnostic inclusion groups will not sum to 100.
P < .001, t118 ≥ 9.6, Cohen's d ≥ 2.16.
fMRI Acquisition
Images were acquired using a 3 T head-only Siemens Trio scanner (Siemens Medical Systems, Ehrlangen, Germany) equipped with a fast gradient system for echoplanar imaging. A standard radiofrequency head coil was used with foam padding to restrict head motion. A 7-min 3D T1-weighted Magnetization Prepared Rapid Gradient Echo Imaging (MPRAGE) sequence was used to acquire a high-resolution anatomical scan for spatial normalization. Functional images were acquired using a posterior-to-anterior T2*-weighted echoplanar imaging sequence (TR = 1.67 s, TE = 29 ms, FOV = 205 × 205, flip = 75°, 3.2 mm isotropic voxels, 32 axial slices). Task data were collected in a single run.
fMRI Analysis
Functional volumes were corrected for slice-timing and spatially realigned to correct for motion using Analysis of Functional NeuroImaging (AFNI[26]). Participants were excluded from analysis if >30% of scans showed incremental movement >1 mm or incremental rotation >1°, or if >30% of scans showed absolute movement from baseline >5 mm or absolute rotation >5° (reported sample excludes 20 participants [14.2%]; excluded and included participants comparable on demographic and clinical variables [Ps >.2)]). Linear trends over the run were removed and outliers were Winsorized (see Supporting Information) using niscorrect from the NeuroImaging Software suite.[27] Data were temporally smoothed using a 7-point Gaussian filter (nisfilter) and converted to percentage change from the median of all task scans (custom code available upon request). Images were coregistered to the MNI Colin27 brain using a 30-parameter nonlinear automated warping algorithm[28] and spatially smoothed using a 6 mm FWHM Gaussian filter.
Data were analyzed for trials containing threat–neutral face pairs (retaining errors; see Supporting Information). Single-subject averages calculated for each condition at each scan were subjected to whole-brain model-free ANOVA tests with subject as a random factor, group (anxious/control) as a fixed factor, and congruence and scan (timepoint within trial) as fixed repeated measures. Inclusion of both groups in a single omnibus analysis reduces the potential impact of differential sample sizes on results. A moderation analysis examined further moderating effects of stimulus duration (short vs. long) on BOLD (blood oxygen–level dependent) activation in significant clusters identified in the primary whole-brain analyses (see Supporting Information for analysis details).
As the ANOVA did not account for autocorrelation across scans, significance of all identified clusters was verified in a mixed model analysis in which scan was assumed to have an autoregressive (AR1) covariance structure (nismixed). Clusters were subjected to additional robust statistical tests to protect against undue influence of individual subject- and trial-level outliers and reported only if these additional criteria were met (see Supporting Information). Type I error for voxel-wise tests was controlled using contiguity thresholds derived based on the autocorrelation of the statistical maps (AFNI's AlphaSim with smoothing estimated via 3dFWHM). Using a voxel-wise threshold of P < .001, cluster volume thresholds ranging from 38 to 44 contiguous voxels were determined necessary to hold the probability of map-wise false-positive detection at P < .01, depending on the smoothness of the data included in a particular analysis.
Brain–Behavior Regression Analysis
For each subject, behavioral bias scores were calculated as mean RT to incongruent and congruent trials and Winsorized to rescale outliers, creating a separate bias score for each of the two stimulus durations, with larger values indicating greater vigilance to threat. Whole-brain single-subject difference maps were calculated similarly by averaging signal across all scans for each condition (congruent vs. incongruent) and each stimulus duration (short vs. long) and subtracting mean signal to incongruent and congruent trials, creating two maps per subject for each of the two stimulus durations. Difference maps were Winsorized and regressed on behavioral bias scores across all subjects using AFNI's 3dRegAna command to identify clusters significantly related to behavioral bias during short and long stimulus trials. See Supporting Information for additional regressions using difference maps for fearful–neutral face pairs versus control trials.
Functional Connectivity
Time-series data were used to examine the clinical impact of individual differences in the functional integration of responses across functionally defined regions (identified in the primary ANOVA analysis). The Functional Connectivity Toolbox[29] was used to calculate, for each subject during all trials containing threat (fearful–neutral face pairs), the maximum functional canonical correlation (FCC) across pairs of regions, as described previously.[30] Fisher's r-to-z transformations were applied, and the resulting z-scores correlated with Pediatric Anxiety Rating Scale (PARS) scores.[31]
RESULTS
RTs
A 2 × 2 × 2 ANOVA with group (anxious vs. nonanxious) as a between-subjects variable, dot location (congruent vs. incongruent) and stimulus duration (short vs. long) as within-subjects variables, and Winsorized mean RTs as the dependent measure revealed a marginal Group × Congruence × Duration interaction (F1,119 = 3.36, P = .07) partially consistent with a “vigilance-avoidance” pattern – that is, anxious participants showed a more vigilant pattern than controls during short trials and a more avoidant pattern during long trials (Table 1). Behavioral bias scores were not correlated with the anxiety scales listed in Table 1, age, or gender, across all participants or in either sample alone (Ps > .1).
fMRI Effects of Congruence: All Subjects
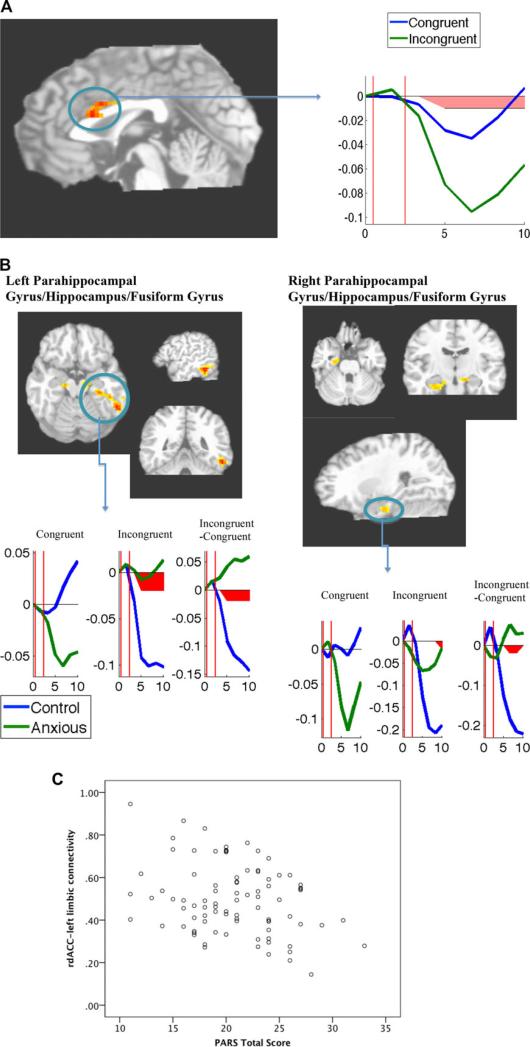
A rostrodorsal ACC (rdACC) cluster showed greater downward deflection during incongruent trials than during congruent trials (Table 2; Fig. 2A). Noidentified regions showed the opposite pattern (greater activation, or lesser deactivation, to incongruent than congruent trials).
TABLE 2.
Comparisons of BOLD signal during dot-probe trials: effects of congruence and Group × Congruence and relationship of BOLD signal to behavioral attentional bias
| Region | Location of centroidvoxel | Brodmann's areas | x | y | z | Cluster extent (mm3) | F | P | d |
|---|---|---|---|---|---|---|---|---|---|
| Congruence × Scan interactions: | |||||||||
| Incongruent > congruent (all subjects) | |||||||||
| No significant clusters | |||||||||
| Congruent > incongruent (all subjects) | |||||||||
| Rostrodorsal anterior cingulate | R anterior cingulate | 24, 32 | 1 | 23 | 21 | 11,632 | F6,714 = 3.74 | .001 | 0.35 |
| Congruence × Group × Scan interactions: | |||||||||
| Controls > anxious congruent > incongruent | |||||||||
| L parahippocampal gyrus/hippocampus/fusiform gyrus | L parahippocampal gyrus | 37, 20, 36 | –39 | –36 | –13 | 4,248 | F6,714 = 2.4 | .02 | 0.28 |
| R parahippocampal gyrus/hippocampus/fusiform gyrus | R parahippocampal gyrus | 28, 35 | 22 | –16 | –19 | 1,392 | F6,714 = 3.0 | .006 | 0.32 |
| Anxious > control congruent > incongruent | |||||||||
| No significant clusters | |||||||||
Note: Coordinates for each cluster's center-of-mass are presented in Talairach space. All findings are from unrestricted whole-brain analysis with map-wise error rate P < .01. All reported ANOVA effects interact with scan (time point) in model-free ANOVA. F, P, and Cohen's d values provided are from mixed models ANOVA performed on mean signal of all voxels in region (accounting for autocorrelation of time).
PFC, prefrontal cortex; BOLD, blood oxygen–level dependent.
Figure 2.
(A) Region more active during congruent trials than incongruent trials across all subjects. Images shown in radiological convention (left = right). Time series plot is presented for the mean of entire functional cluster. Line graphs represent mean BOLD (blood oxygen–level dependent) at each time point during congruent (blue) and incongruent (green) trials. Y-axis represents BOLD signal (in arbitrary units); X-axis represents time (in seconds). Vertical red lines indicate probe onset/face offset timepoint for short (first line) and long (second line) face durations. Post hoc decomposition of the two-way interaction performed via t tests at each time point. Time points significantly differing at P < .05 are indicated with red shading. (B) Regions showing larger deactivation to incongruent trials in control compared to anxious participants. Images shown in radiological convention (left = right). Line graphs represent mean BOLD at each time point for control (blue; n = 31) and anxious (green; n = 90) participants, with separate plots for congruent trials, incongruent trials, and the incongruent–congruent contrast. Post hoc decomposition of the three-way interactions performed via t tests at each time point. Time points significantly differing at P < .05 are indicated with red shading. (C) Scatter plot of relationship between rdACC–left parahippocampal maximum FCC values (z-transformed) and clinician-rated anxiety (PARS) in anxious sample.
fMRI Effects of Congruence Moderated by Group
Clusters in bilateral parahippocampal gyrus, extending into bilateral hippocampus, fusiform gyrus, and inferior temporal gyrus, showed Congruence × Group × Timepoint interactions in the ANOVA, characterized by larger decreases to incongruent trials in controls compared to the anxious group (Table 2; Fig. 2B).
fMRI Effects Moderated by Duration
Unlike the behavioral data, which showed a marginal moderating effect of stimulus duration, none of the fMRI clusters identified in Table 2 exhibited moderation by stimulus duration.
Individual Differences: Brain–Behavior Relationships
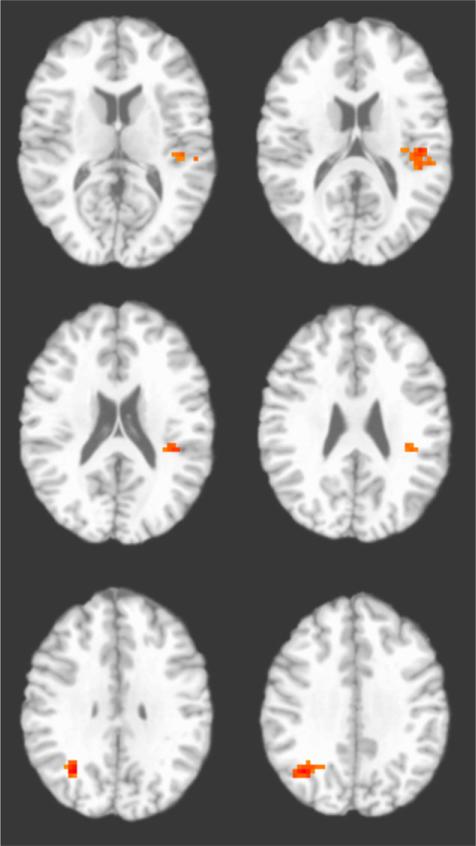
During trials with long stimulus duration, individual differences in behavioral bias were positively related to values for the incongruent > congruent contrast in the left posterior insula and right angular gyrus/precuneus (Table 3; Fig. 3). No clusters were identified showing significant brain–behavior relationships during short stimulus duration trials.
TABLE 3.
Relationship of BOLD signal during incongruent versus congruent trials to behavioral attentional bias
| Region | Location of centroid voxel | Brodmann's areas | x | y | z | Cluster extent (mm3) | b | t | P | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Trials with short (200 ms) stimulus duration: | ||||||||||
| No significant clusters | ||||||||||
| Trials with long (2 s) stimulus duration: | ||||||||||
| Positively associated with behavioral threat bias | ||||||||||
| R angular gyrus/precuneus | R angular gyrus | 39 | 35 | –59 | 33 | 1,231 | .04 | t119 = 3.85 | <.001 | .11 |
| L posterior insula | L insula | 13, 41 | –41 | –28 | 20 | 1,191 | .04 | t119 = 3.67 | <.001 | s.10 |
| Negatively associated with behavioral threat bias | ||||||||||
| No significant clusters |
Note: Coordinates for each cluster's center-of-mass are presented in Talairach space. All findings are from unrestricted whole-brain analysis with map-wise error rate P < .01.
Figure 3.
Left posterior insula and right angular gyrus regions positively associated with behavioral attention bias during long trials across all participants. Image is shown in radiological convention (left = right).
Individual Differences: Functional Connectivity Across All Face-Containing Trials
In the anxious group, greater anxiety (PARS scores) was associated with lesser connectivity (smaller maximum FCC) between rdACC and left parahippocampal/hippocampal functional clusters (r = –.29, P = .006). This relationship was nonsignificant in the control group (r = .04, P = .82) and differed significantly as a function of group (moderation effect: β = .24, P = .04). This relationship was not significant for the right parahippocampal/hippocampal FCC in either group (Ps > .82). No moderating effects of congruence were found (Supporting Information).
DISCUSSION
This study used comparisons of incongruent and congruent dot-probe trials (Fig. 1), together with a slow event-related fMRI design, to reveal mechanisms of neural disengagement following a brief threat exposure (fearful–neutral face pairs). Findings suggest that anxious youth exhibit (i) preserved ability to deactivate midline cortical (rdACC) responses and (ii) attenuated deactivation of limbic (e.g., parahippocampal/hippocampal) activity after attention is directed to a nonthreat location (i.e., during incongruent trials; Fig. 1). The divergent neural pattern in anxious youth across rdACC and limbic regions could suggest that a message received in cortical regions of the brain fails to reach subcortical emotional/stress-responsive centers. Failure to deactivate these limbic structures after threat could contribute to sustained emotional experience of the threat even after distraction. In addition to initial threat responses, emotional processing persisting in the wake of threat, even after attention has been directed elsewhere, may thus be particularly relevant to pediatric anxiety, suggesting possible targets for early intervention.
Across all subjects, incongruent trials (Fig. 1), which required orienting toward a neutral location (away from the location of the fearful face), resulted in decreased activity in the rdACC. Although early functional neuroanatomical models characterized the dorsal ACC as relevant to “cold” cognitive processes such as conflict resolution when neutral stimuli are used,[20] a more recent model suggests that, in the context of anxiety-relevant stimuli and paradigms, rostrodorsal components of the ACC are selectively involved in threat appraisal and fear expression.[21] The present pattern of findings appears to support this latter conceptualization: both anxious and nonanxious subjects showed rdACC decreases when attention was deployed away from threat, potentially consistent with successful neural disengagement and a decrease in cortical threat-related processing (e.g. appraising a context as fear-relevant).
By contrast, anxious youth showed evidence of abherrant disengagement of limbic regions during incongruent trials. Specifically, control participants deactivated portions of bilateral parahippocampal gyrus, hippocampus, and fusiform gyrus after being asked to direct attention away from threat (i.e., on incongruent trials), suggesting flexible deactivation of responses to fearful faces. Anxious youth, by contrast, showed attenuated deactivation during incongruent trials (see Fig. 2B for time-series plots and post hoc analysis). Notably, a quite similar pattern in the hippocampus and parahippocampal gyrus (i.e., greater response to incongruent dot-probe trials) was recently observed in adults carrying glucorticoid-receptor genotypes that confer risk for anxiety.[22] The precise time-course pattern revealed in the current results suggests that anxious individuals may experience difficulty disengaging limbic processing resources from fearful faces after stimuli have been removed from view and attention has been re-directed. Given that these limbic regions have been broadly implicated in contextual and memory-based processing, and specifically in emotional and facial processing and anxious responses,[19,32–34] the attenuated pattern in anxious youth may suggest residual emotional responding that does not track flexibly with task demands.
The convergent pattern of responses across rdACC and limbic regions in nonanxious youth could suggest better integration of responding across diverse brain regions involved in detection of, and response to, emotional faces. Anxious youth instead exhibited opposing responses across regions, and reduced functional connectivity between rdACC and left limbic regions (across all face-containing trials) was associated with greater anxiety in the patient sample. Recent neuroanatomical models have increasingly focused on integration of responses across networks of brain regions. Reduced cortical–limbic integration in anxious individuals, revealed through a variety of analysis methods,[35–37] is frequently interpreted as an index of impairment in the capacity for flexible, adaptive responding to a changing environment of emotional inputs. This study provides preliminary evidence for a possible breakdown in co-ordinated cortical–limbic responding in anxious youth, with midline cortical decreases failing to map onto reductions in more bottom-up stress-responsive regions, particularly in youth with the highest levels of anxiety.
Notably, brain activation in the rdACC and parahippocampal regions was unrelated to behavioral performance, and (unlike behavioral performance) activity in these regions was not moderated by stimulus duration, suggesting dot-probe RTs may not capture or reflect these specific neural effects. Thus, slow event-related fMRI may be sensitive to forms of neural disengagement difficulty not apparent at the behavioral level. In RTs, there was a marginal (P = .07) pattern of greater vigilance toward threat in anxious youth during short trials, and greater avoidance of threat during long trials, partially consistent with previous reports in adults.[24] These divergent patterns with respect to duration suggest that clinical anxiety is related to both (i) the parahippocampal deviation that is robust across stimulus durations and (ii) the behavioral pattern that is duration-dependent and that may have its own distinct neural signature. Specifically, during trials with long (but not short) stimulus duration, individual differences in behavioral bias were positively related to activity in the left posterior insula and right angular gyrus/precuneus (Table 3; Fig. 3). One possible interpretation of this brain–behavior pattern is that behavioral avoidance of relatively long (2 s) fearful faces is subserved by decreased empathic processing of fearful faces (in the right angular gyrus)[38] and/or decreased somatosensory or interoceptive processing (in the posterior insula).[39] However, as these regions have not been previously strongly linked to threat-related processing or attentional bias, their potential roles require replication and further elaboration.
Type I and II error rates for all analyses involving RTs are likely increased by considerable variability, which often characterizes dot-probe RT findings both within and across studies. RT findings in anxious youth have been mixed[1,8,9,11,12,40–42] and may be influenced by a variety of procedural variables.[43] The task design for this study was optimized for model-free assessment of the time course of neural responses, at the possible expense of RT precision. For instance, the specific stimulus presentation and long trial durations used, the relatively small total number of trials, and the unique characteristics of the scanner environment may have impacted results. Additional features including the relatively young age range of the sample,[40,42] the use of fearful rather than angry face stimuli (see limitations below), and the vertical rather than horizontal positioning of stimuli[44] may also have impacted the specific pattern observed. Given that reliability of dot-probe RTs has been repeatedly called into question in adults,[45,46] even when more optimal task designs are used, future efforts to understand attention bias as a mechanism in anxiety might benefit from inclusion of other objective indices of biased processing that could provide more reliable, proximal, sensitive, and/or fine-grained information regarding emotional information processing, such as neural responses (which appear reliable for the dot-probe task in youth[47]) or eye gaze location. In particular, although dot-probe RTs have proven useful for group-level analysis, their reliability may be inadequate for single-subject clinical applications, such as assessment and personalized treatment prescription based on attentional mechanisms. Neural measures and/or measures that closely track neural function (e.g., pupilometry) may be fruitful avenues for further research.
Previous fMRI studies of the dot-probe task in clinically anxious youth have focused exclusively on angry face processing in comparison to baseline or neutral stimulus processing. Our study extends this literature by revealing the pattern and time course of neural alterations that occur following threat (here, fearful faces), in response to attentional manipulation. Recent dot-probe studies in anxious youth using pupilometry[11] and neuroimaging[12,13] have similarly indicated altered brain responses in anxious youth that occur and/or persist after the threat stimulus (angry or fearful face) has been removed and vary as a function of dot location, although the direction of differences in brain response has not been fully consistent. Nevertheless, a consistent implication of this work is that clinical anxiety is not defined by initial neural responses to threat alone, but also relates to neural alterations persisting after attention has been experimentally manipulated. Findings could have clinical implications for understanding and improving on the beneficial effects of attention bias modification (ABM), a recently developed procedure to experimentally manipulate attention bias in anxious patients using a modified dot-probe task.[4–6] For instance, neurofeed-back, neuromodulation, or behavioral “brain-training” exercises might be usefully combined with ABM in order to pursue a neurobiological “goal state” of flexible deactivation of parahippocampal regions following attentional re-direction and/or increased consistency across ACC and limbic responses. Clinically, such a “goal state” might be characterized by an improved ability for decreased cognitive threat appraisal, neurally represented in the ACC, to efficiently translate into decreased limbic emotional/stress responses when attention is diverted from threat.
STRENGTHS AND LIMITATIONS
This fMRI study is the largest of its kind examining neural mechanisms of attention bias, a clinically relevant construct implicated in anxiety etiology and maintenance. Although the sample size reduces the risk of reporting bias and type I and II errors,[48] numerous supplementary analyses were performed in the interest of completeness, which may increase type I error risk. The use of a slow event-related design and model-free analysis allowed for unconstrained detection of activation and deactivation patterns, including patterns consistent with neural disengagement in the aftermath of threat, an important component of adaptive emotion regulation. However, the slow design included an extended dot-presentation period (up to 11 s) that reduces compatibility with the existing dot-probe literature and resulted in a relatively small number of trials per condition, potentially decreasing power. Fearful rather than angry face stimuli were used because they (i) have been specifically shown to elicit equivalent dot-probe RT and eyetracking biases as angry faces,[49] and (2) have transdiagnostic relevance to fear perception and the implication that a generic, unspecified threat is present (whereas angry faces might be construed as a specifically social form of threat). However, this design decision reduces compatibility with previous studies in youth that have primarily used angry faces, and fearful faces could have unique attentional properties (i.e., implying a threat is elsewhere rather than onscreen). Finally, this article focused on broad neural alterations relevant to attentional bias by collapsing across diagnostic groups, possibly concealing diagnosis-specific patterns (see Supporting Information for exploratory analyses). Future studies directly comparing neural function across age groups and diagnostic categories would extend these findings.
CONCLUSIONS
In summary, this study suggests an attentional manipulation away from threat (incongruent dot-location) decreases rdACC activity in both anxious and nonanxious youth, consistent with a role for this region in threat reactivity. Nonanxious youth showed a similar pattern in bilateral parahippocampal/hippocampal limbic regions implicated in emotional responses. Anxious youth, by contrast, showed attenuated deactivation of limbic regions and, in individuals with the highest levels of anxiety, decreased functional connectivity of rdACC–left parahippocampal/hippocampal time-series patterns. Thus, anxiety pathophysiology in youth may be specifically related to a failure to decrease limbic responses when attention is directed toward a neutral location, in spite of successful rdACC deactivation. More broadly, pediatric anxiety may involve a failure to successfully, and integratively, direct the brain's machinery elsewhere in the aftermath of threat-related information. Though widely used in attention bias research and, increasingly, as an experimental intervention for anxiety, mechanisms of the dot-probe task remain inadequately explored. This study provides new insight into neuroanatomical substrates that facilitate directing attention away from threat (toward neutral information), a skill that is thought to buffer against anxiety.[4–6] Mechanistic early intervention approaches (e.g., cognitive training, neurofeedback, and/or neuromodulation) could be designed to explicitly remediate these abnormalities and promote integrated deactivation of limbic regions following brief threat presentations, for example, through explicit neurofeedback. Our focus on a developmental window where anxiety confers risk for life-long patterns of chronic affective dysfunction[23] suggests such mechanistic intervention may have a particularly long-lasting and wide-reaching impact.
Acknowledgments
We acknowledge the strong contributions of the Child Anxiety Treatment Study staff in carrying out this study, and appreciate the willingness of our participants to provide data for this study.
Contract grant sponsor: National Institutes of Health; Contract grant numbers: MH080215, MH082998, MH018269, and MH100259.
Footnotes
Financial Disclosures
The authors have no conflicts of interest. Greg Siegle is an unpaid consultant for TrialIQ.
REFERENCES
- 1.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Beck AT, Emery G, Greenberg RC. Anxiety Disorders and Phobias: A Cognitive Perspective. Basic Books; New York: 1985. [Google Scholar]
- 3.Browning M, Holmes Ea, Harmer CJ. The modification of attentional bias to emotional information: a review of the techniques, mechanisms, and relevance to emotional disorders. Cogn Affect Behav Neurosci. 2010;10:8–20. doi: 10.3758/CABN.10.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. J Abnorm Psychol. 2009;118:28–33. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amir N, Beard C, Taylor CT, et al. Attention training in individuals with generalized social phobia: a randomized controlled trial. J Consult Clin Psychol. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldar S, Apter A, Lotan D, et al. Attention bias modification treatment for pediatric anxiety disorders: a randomized controlled trial. Am J Psychiatry. 2012;169:213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathews A, MacLeod C. Selective processing of threat cues in anxiety states. Behav Res Ther. 1985;23:563–569. doi: 10.1016/0005-7967(85)90104-4. [DOI] [PubMed] [Google Scholar]
- 8.Monk CS, Telzer EH, Mogg K, et al. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monk CS, Nelson EE, McClure EB, et al. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. Am J Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- 10.Bishop SJ. Neurocognitive mechanisms of anxiety: an integrative account. Trends Cogn Sci. 2007;11:307–316. doi: 10.1016/j.tics.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Price RB, Siegle G, Silk JS, et al. Sustained neural alterations in anxious youth performing an attentional bias task: a pupilometry study. Depress Anxiety. 2013;30:22–30. doi: 10.1002/da.21966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britton JC, Bar-Haim Y, Carver FW, et al. Isolating neural components of threat bias in pediatric anxiety. J Child Psychol Psychiatry. 2012;53:678–686. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telzer EH, Mogg K, Bradley BP, Ernst M, Pine DS, Monk CS. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biol Psychol. 2008;79:216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. 2004;42:1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36:809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 16.Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hypothesis. Cogn Emot. 2004;18:689–700. [Google Scholar]
- 17.Carlson JM, Beacher F, Reinke KS, et al. Nonconscious attention bias to threat is correlated with anterior cingulate cortex gray matter volume: a voxel-based morphometry result and replication. Neuroimage. 2012;59:1713–1718. doi: 10.1016/j.neuroimage.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Carlson JM, Cha J, Mujica-Parodi LR. Functional and structural amygdala – anterior cingulate connectivity correlates with attentional bias to masked fearful faces. Cortex. 2013;49:2595–2600. doi: 10.1016/j.cortex.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47:1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 20.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fani N, Gutman D, Tone EB, et al. FKBP5 and attention bias for threat: associations with hippocampal function and shape. JAMA Psychiatry. 2013;70:392–400. doi: 10.1001/2013.jamapsychiatry.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Arch Gen Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- 24.Bradley BP, Mogg K, Sara J, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: manipulation of stimulus duration. Cogn Emot. 1998;12:737–753. [Google Scholar]
- 25.Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 27.Fissell K, Tseytlin E, Cunningham D, Carter CS, Schneider W, Cohen JD. Fiswidgets: a graphical computing environment for neuroimaging analysis. Neuroinformatics. 2003;1:111–125. doi: 10.1385/ni:1:1:111. [DOI] [PubMed] [Google Scholar]
- 28.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Zhou D, Thompson WK, Siegle G. MATLAB toolbox for functional connectivity. Neuroimage. 2009;47:1590–1607. doi: 10.1016/j.neuroimage.2009.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 31.The Research Units on Pediatric Psychopharmacology Anxiety Study Group The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry. 2002;41:1061–1069. doi: 10.1097/00004583-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Lobaugh NJ, Gibson E, Taylor MJ. Children recruit distinct neural systems for implicit emotional face processing. Neuroreport. 2006;17:215–219. doi: 10.1097/01.wnr.0000198946.00445.2f. [DOI] [PubMed] [Google Scholar]
- 33.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satpute AB, Mumford JA, Naliboff BD, et al. Human anterior and posterior hippocampus respond distinctly to state and trait anxiety. Emotion. 2012;12:58–68. doi: 10.1037/a0026517. [DOI] [PubMed] [Google Scholar]
- 35.Tromp DPM, Grupe DW, Oathes DJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. 2012;69:925–934. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MJ, Whalen PJ. The structural integrity of an amygdalaprefrontal pathway predicts trait anxiety. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66:1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- 38.Saxe R, Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waters AM, Wharton TA, Zimmer-Gembeck MJ, Craske MG. Threat-based cognitive biases in anxious children: comparison with non-anxious children before and after cognitive behavioural treatment. Behav Res Ther. 2008;46:358–374. doi: 10.1016/j.brat.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Vasey MW, MacLeod C. Information-processing factors in childhood anxiety: a review and developmental perspective. In: Vasey MW, Dadds MR, editors. The Developmental Psychopathology of Anxiety. Oxford University Press; New York: 2001. pp. 27–42. [Google Scholar]
- 42.Wolters LH, de Haan E, Vervoort L, Hogendoorn SM, Boer F, Prins PJ. The time-course of threat processing in children: a temporal dissociation between selective attention and behavioral interference. Anxiety Stress Coping. 2012;25:259–273. doi: 10.1080/10615806.2011.581278. [DOI] [PubMed] [Google Scholar]
- 43.Waters AM, Lipp OV, Spence SH. Attentional bias toward fear-related stimuli: an investigation with nonselected children and adults and children with anxiety disorders. J Exp Child Psychol. 2004;89:320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Hakamata Y, Lissek S, Bar-Haim Y, et al. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmukle SC. Unreliability of the dot probe task. Eur J Personality. 2005;19:595–605. [Google Scholar]
- 46.Staugaard SR. Reliability of two versions of the dot-probe task using photographic faces. Psychol Sci Q. 2009;51:339–350. [Google Scholar]
- 47.Britton JC, Bar-Haim Y, Clementi Ma, et al. Training-associated changes and stability of attention bias in youth: implications for attention bias modification treatment for pediatric anxiety. Dev Cogn Neurosci. 2013;4:52–64. doi: 10.1016/j.dcn.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David SP, Ware JJ, Chu IM, et al. Potential reporting bias in fMRI studies of the brain. PloS One. 2013;8:e70104. doi: 10.1371/journal.pone.0070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mogg K, Garner M, Bradley BP. Anxiety and orienting of gaze to angry and fearful faces. Biol Psychol. 2007;76:163–169. doi: 10.1016/j.biopsycho.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]