Abstract
Prior studies have shown that performance on standardized measures of memory in children with autism spectrum disorder (ASD) is substantially reduced in comparison to matched typically developing controls (TDC). Given reported deficits in face processing in autism, the current study compared performance on an immediate and delayed facial memory task for individuals with ASD and TDC. In addition, we examined volumetric differences in classic facial memory regions of interest (ROI) between the two groups, including the fusiform, amygdala, and hippocampus. We then explored the relationship between ROI volume and facial memory performance. We found larger volumes in the autism group in the left amygdala and left hippocampus compared to TDC. In contrast, TDC had larger left fusiform gyrus volumes when compared with ASD. Interestingly, we also found significant negative correlations between delayed facial memory performance and volume of the left and right fusiform and the left hippocampus for the ASD group but not for TDC. The possibility of larger fusiform volume as a marker of abnormal connectivity and decreased facial memory is discussed.
Keywords: autism, facial memory, fusiform gyrus, amygdala, hippocampus
1. Introduction
Prior studies have shown that performance on standardized measures of memory in children with autism spectrum disorders (ASD) is substantially reduced in comparison to matched typically developing controls (TDC) [1,2,3,4,5,6]. This is not surprising given speculation of various white matter temporal lobe abnormalities in ASD [7] and the role that temporal structures play in memory and learning [8]. One measure that has demonstrated multiple differences in memory functioning in those with ASD compared to TDC [5,9] is the Test of Memory and Learning (TOMAL) [10]. The TOMAL is comprised of various verbal and non-verbal subtests, including the Facial Memory subtest. Performance on the TOMAL Facial Memory subtests may be of particular interest in studying memory impairments in ASD because of associated deficits in face processing [11] and atypicalities in the fusiform gyrus (For simplicity, throughout the paper we will often just refer to the fusiform and not fusiform gyrus, gyral, or volume.) of the temporal lobe [12]. Despite the large amount of research pertaining to facial processing in ASD, the literature examining facial memory is more limited, and a comprehensive understanding of facial memory functioning in this population is lacking [13,14,15].
1.1. Facial Processing and Memory as a Deficit in Autism
Facial processing has been recognized as a specific deficit in autism [16,17,18]. Individuals with ASD exhibit impairments in perception of facial affect [19,20,21], direction of eye gaze [22], eye contact [23,24], and attention to eyes [25,26]. Specific behavioral impairments in ASD help provide clues to the mechanisms of abnormal facial perception. For example, individuals with ASD tend to fixate longer on objects than faces (similar to typically developing individuals), but are less likely to scan regions of the face outside the primary facial features (i.e., eyes, nose, mouth) [27,28,29,30]. Research also suggests that individuals with ASD may rely on individual features during facial processing rather than taking a holistic approach [31] and also may tend not to benefit from face orientation during facial recognition tasks [20,32]. These results provide evidence for atypicalities in how individuals with ASD attend to and process facial stimuli.
Individuals with ASD have also been found to perform more poorly on facial recognition tasks relative to object recognition [13,14,33,34,35]. This has been suggested to be due to abnormal scanning of facial regions during encoding, which certainly could be the basis for impaired facial memory [29]. In a review of studies of facial perception in ASD, Weigelt et al. [36] reported that quantitative deficits in facial perception on behavioral tasks are much more impaired in tasks with memory demands, although other hypotheses have been proposed as well. For example, impaired facial perception may also be due to greater visuospatial effort required for facial processing [37], the inherent social content of face stimuli [38], or impaired gaze fixation [25,39]. Indeed, research on facial processing in ASD has revealed that individuals with ASD have impaired prototype formation of faces [11], which may help explain why once faces are attended to, they may be categorized and consolidated in memory very distinctly in ASD.
Specific to facial memory, children with ASD are particularly impaired in their memory for faces [15], which may be less apparent in adolescence [14] and least apparent in adulthood [14,15]. Also, Kuusikko-Gauffin and colleagues [35] found that facial memory improved with age, with significant differences in facial memory between children, but not adolescents or adults. In addition, parents of ASD individuals had poorer facial memory performance than control parents.
1.2. Neuroanatomical Correlates of Facial Processing and Memory
Functional neuroimaging studies have identified a face-specific region in the fusiform gyrus of the temporal lobe termed the fusiform face area (FFA) [40]. The FFA is responsible for processing both facial features (e.g., nose, mouth, eyes), as well as the spatial relation among face parts [41,42,43,44,45,46,47]. Disruption of the fusiform face area in the fusiform gyrus may help explain why individuals with ASD have deficits in facial processing and facial memory.
Kleinhans et al. [48], Anderson et al. [49], and Khan et al. [50] have shown reduced functional connectivity in ASD not only between the fusiform and other cortical areas but also between left and right fusiform gyri, and within the fusiform gyrus itself during face processing. Studies that have examined individuals with identifiable lesions to the fusiform have also demonstrated similar facial processing impairments [51,52,53]. Generally, the literature supports abnormalities in face-processing networks involving the fusiform, including reduced long-range and local functional connectivity (i.e., within the fusiform face area) [50], rather than only a region specific abnormality [39,54,55].
The fusiform is also functionally related to both the amygdala and the hippocampus, two structures critical for memory and emotional processing. Studies looking at face processing, rather than memory, have found abnormal pathway microstructure [56] and connectively [50] between the hippocampus/amygdala and fusiform. All three of these regions show reduced activation during task-based fMRI studies of facial processing in autism [25,57,58,59]. Hypoactivation of the amygdala and fusiform is often observed in ASD individuals relative to TDC [55]. It is also important to consider that the lack of activation in these brain regions in individuals with ASD compared to TDC may also relate to differences in processing emotional intensity [60] and dynamic versus static facial stimuli [54,61]. Both facets of facial processing (i.e., emotional intensity and dynamic expressions) have more ecological validity pertaining to social interaction deficits than the simple viewing of pictures of facial expressions (i.e., static stimuli). Thus, a multitude of factors may influence face processing and facial memory.
Several studies have examined fusiform gyral volume comparing controls to those with ASD. Volume in TDC individuals is considered a marker of structural integrity, albeit a coarse indicator of brain development [62]. ASD studies that have examined fusiform gyral volume have reported differences in size [63,64,65,66,67,68,69,70]. However, the direction of the differences, including hemispheric effects, varies. In a meta-analysis, Cauda et al. [71] reported a larger fusiform associated with autism, but underscored the variability of reported differences across studies. Inconsistencies in the volumetric literature in autism also exist for other temporal lobe structures such as the amygdala and hippocampus. In a sample of individuals with Asperger syndrome, Murphy et al. [72] found larger amygdala but not hippocampal volume. In contrast, Hasan, Walimuni and Frye [73] reported larger hippocampal volume in autism. The lack of a consistent direction to volume differences in the fusiform may be a reflection of the heterogeneity of ASD and associated morphological differences that may also result in varied cognitive impairments.
Additionally, variability in volumetric findings of temporal lobe structures in ASD likely has to do with age, developmental, and maturation effects. For example, some volumetric studies have implicated early overgrowth followed by normalization of amygdala volumes in middle childhood with either normalization or persistence of hippocampal enlargements [56]. Some studies only examined adults, like Dziobek, Bahnemann, Convit, and Heekeren [68], who found that the relationship between amygdala volume and fusiform thickness was actually smaller in autism compared with TDC, providing further evidence for disrupted neural networks. Still others have found reduced volume of the hippocampal–amygdala complex in autism in adolescents and adults [63,74]. As such, variability in reported volumetric findings of temporal lobe structures in ASD likely reflects differences in age and heterogeneity of the disorder. Pelphrey, Shultz, Hudac, and Vander Wyk [55] propose that “ASD begins with a failure in the emergence of the specialized functions of one or more of the set of neuroanatomical structures involved in social information processing. This failure happens early in ontogeny, within the first nine months to one year of life, if not earlier. In turn, because the affected regions do not generate the normal stream of both intrinsic and stimulus driven signals, the normal developmental pattern of connections among these brain regions is greatly altered” (p. 4). Thus, examining volumetrics, although not a direct measure of neural connectivity, represents a logical place to begin in understanding how it might influence abnormal functioning of specialized neuroanatomical structures (and subsequently connectivity and functionality).
How fusiform morphology may contribute to impairments in facial memory is not yet known. However, it would seem to be a logical structure for examination, given the role the fusiform plays in face processing. Likewise, because of the important role that the medial temporal lobe plays in memory—particularly the hippocampus and, to a certain extent, the amygdala—it would be important for any facial memory study to volumetrically assess these regions, as well. Accordingly, the current study investigated whether hippocampal, amygdala, or fusiform gyral volume related to performance on the TOMAL Facial Memory task, both immediate and 30-minute delayed recall, in children 5 to 19 years of age with ASD compared to TDC age-matched individuals. The format for assessing TOMAL Facial Memory includes an immediate recognition recall trial where previously observed faces have to be identified amidst foils not seen. With each trial, the number of target faces and foils increases. Because performance on this initial trial requires face processing, individuals with ASD would be expected to perform more poorly, possibly just because of the challenges specific to processing facial information.
The delayed recognition trial occurs 30 min after the immediate recall trial and is composed of faces that have been previously viewed along with foils that have not. The child has no opportunity for rehearsal during the 30-min interval. Since the previously seen face has already been initially processed, this delayed aspect of the TOMAL Facial Memory Task taps consolidation. As a contrast to facial memory, the TOMAL also utilizes visual memory tasks such as Visual Selective Reminding that has no aspect of face processing but rather visual spatial retention, using both immediate and delayed recall. Also, the Object Recall task assesses immediate retention of visually presented line drawings of common objects including a single drawing of a generic face as one of 24 stimuli. Object recall does not have a delayed retention measure. By comparing ASD and TDC participants on the TOMAL Facial Memory subtest with visually processed memory tasks like the Visual Selective Reminding and Object Recall provides the comparison of how specific an impairment in facial memory may be or whether more general non-verbal, visual memory impairments may be associated with ASD. Also, of importance is whether these TOMAL memory measures relate to fusiform, hippocampal and amygdala volume.
We examined several hypotheses about the role of fusiform gyral, hippocampal, and amygdala volume in TOMAL Facial Memory performance. First, it was hypothesized that facial memory performance would be significantly lower for individuals with autism than for TDC participants. Second, given the literature supporting volume differences in these temporal lobe structures in ASD, it was hypothesized that the fusiform gyral, amygdala, and hippocampal volumes would be larger for the ASD group when compared with TDC participants and, furthermore, that these structures would be negatively correlated with TOMAL Facial Memory performance, both immediate and with a 30-min delay; however, that fusiform gyral volume would not correlate with TOMAL Performance on the Visual Selective Reminding and Object Recognition.
2. Method
2.1. Ascertainment
Autism and TDC participants were recruited predominantly from community sources, including parent support groups, youth groups, and schools, and from clinic social skills groups, as described by Bigler et al. [75] and Alexander et al. [76]. The subjects in this study are a subset of participants in a longitudinal investigation of late brain development from three years of age through early adulthood. The subset for this investigation was selected from the larger sample based on age within the reference norms of the TOMAL, having complete TOMAL data from the time of initial assessment, and closeness of group matching on age, PIQ, handedness, and head circumference. All facets of this investigation were undertaken with the understanding and written consent of each subject or legal guardian, with the approval of the University of Utah and Brigham Young University Institutional Review Boards, where testing was performed, and in compliance with national legislation and the Code of Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association.
2.2. Subject Groups
All subjects were males, 5–19 years of age. The ASD group had a total of 56 participants and the TDC group a total of 31 participants with complete neuropsychological and neuroimaging datasets. Potential sex differences in memory were not examined.
2.3. Idiopathic Autism Sample
Autism was diagnosed rigorously. The subject’s parent was interviewed using the Autism Diagnostic Interview–Revised (ADI-R) [77], a semi-structured, investigator-based interview with good reliability and validity. All subjects with autism were also directly assessed using the Autism Diagnostic Observation Schedule–Generic (ADOS-G) [78], a semi-structured play and interview session designed to elicit social, communication, and stereotyped repetitive behaviors characteristic of autism. All autistic subjects met ADI–R, ADOS–G, and the Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM–IV) criteria for autistic disorder [79]. History, physical exam, fragile X gene testing, and karyotype, performed on all subjects, excluded medical causes of autism. In regards to medications, fifteen individuals with ASD were on psychotropic medications, including five participants on stimulant medications. None of these individuals represent outliers in the data.
2.4. Control Sample
Typically developing control subjects had no developmental, neurological, or clinical history of major psychiatric disorders. Control subjects likewise completed an assessment with the ADOS-G and were assessed rigorously for autism spectrum disorders to ensure none met any criterion. Two participants in the control group were on allergy medications.
2.5. IQ
In the current study, IQ was used as a selection and descriptive variable, to ensure that both controls and ASD participants met the criterion. Because of age differences at the time of recruitment, different versions of intellectual tests were used over the 10 years of subject accrual to the parent project. Summary IQ findings were based on one of the following: Wechsler Intelligence Scale for Children–Third Edition (WISC–III), Wechsler Adult Intelligence Scale–Third Edition (WAIS-III), Wechsler Abbreviated Scale of Intelligence (WASI; VIQ and PIQ indexes) [80,81,82], or Differential Ability Scales (DAS) [83]. IQ was not used as a covariate because IQ and memory performance are highly interrelated making it an inappropriate covariate in neurodevelopmental study such as this because it would over-control the dependent memory variable [84].
2.6. Head Circumference and Handedness
No unusual developmental anomaly was found in the sample that may relate to cognitive outcome [85]. Macrocephaly occurs with a greater frequency in autism for ~20% of children [86]; the control sample was group-matched for head circumference. Handedness was measured using the Edinburgh Handedness Inventory [87]. A score of +100 signifies complete right-handedness and –100 indicates complete left-handedness.
2.7. Neuroimaging
Volumetric studies were based on magnetic resonance images acquired on a Siemens Trio 3.0 Tesla scanner at the University of Utah. A 12-channel RF head coil was used to obtain 3D T1-weighted image volumes with 1 mm isotropic resolution using an MP-RAGE sequence (TI = 900 msec, TR = 2300 msec, TE = 2.91 msec, flip angle = 9 degrees, sagittal, field of view = 25.6 cm, matrix = 256 × 256 × 160).
2.8. Volumetric Image Analysis
All analyses were performed with FreeSurfer, version 5.1 (http://surfer.nmr.mgh.harvard.edu/), following the methods detailed by Bigler et al. [88], and included automated volume calculations of the following temporal lobe regions of interest (ROI): fusiform gyrus, amygdala and hippocampus along with total intracranial volume (TICV). It should be noted that hippocampus segmentations are more reliable than amygdala segmentations [89]. TICV was used as a matching variable and covariate.
2.8.1. Memory
Although the entire TOMAL was administered, and generally samples various domains of memory in children and adolescents, ages 5 years 0 months through 20 years 0 months, this study focused specifically on the Facial Memory subtest [10]. Details of overall TOMAL performance in autism have been reported by Southwick et al. [5]; however, that study did not explore any brain correlates. The Facial Memory subtest was administered according to standard methods with delayed retention assessed at 30 min after the immediate recognition trials. The Facial Memory subtest is a nonverbal subtest requiring recognition and identification of previously viewed faces from a set of distracters: black-and-white photos of various ages, males, and females, and various ethnic backgrounds. There is an immediate recognition score, as well as a 30-min delayed score. Visual Selective Reminding is a nonverbal free-recall task in which the examinees point to specified dots on a card, after a demonstration of the examiner, and are reminded only of items recalled incorrectly. Trials are continued until mastery is achieved or through eight trails. Visual Selective Reminding also has a delayed recall task after 30 min. During Object Recall, the examinees are presented with a series of named pictures and have to recall them across four trials. There is no delayed portion of the Object Recall subtest.
2.8.2. Statistical Analysis
Multivariate analysis of covariance (MANCOVA) was employed to describe group means for autism and control subjects on the TOMAL, with TICV as a covariate for volumetric analyses. Additionally, MANCOVA was employed to describe group means for autism and control subjects on volumetric analyses, with TICV and age as covariates. Lastly, partial correlations were run to examine the relationship between TOMAL performance and ROI volume, controlling for age and TICV.
3. Results
3.1. Sample Characteristics
As shown in Table 1, no significant differences were found between groups for group-matching variables (age, head circumference, handedness index) except for IQ.
Table 1.
Demographic Information.
| ASD (n = 56) | Typically-developing (n = 31) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | p | |
| Age in years | 12.00 | 4.37 | 5.00–19.75 | 11.98 | 4.01 | 5.25–19.33 | 0.02 | 0.98 |
| Head Circumference (cm) | 54.71 | 5.81 | 50.70–60.50 | 55.34 | 2.13 | 51.80–60.50 | –0.00 | 0.99 |
| Total Intracranial Volume (TICV,cm3) | 1672.32 | 157.84 | 1268.66–2063.84 | 1675.94 | 176.45 | 1399.97–2171.21 | 0.10 | 0.92 |
| Handedness Inventory | 61.45 | 54.78 | –100–100 | 65.28 | 44.97 | –80–100 | 0.01 | 0.99 |
| Wechsler FIQ | 98.26 | 16.63 | 61–137 | 115.24 | 15.57 | 87–152 | 4.53 ** | < 0.001 |
| Wechsler PIQ | 102.41 | 16.00 | 66–138 | 116.13 | 15.46 | 90–155 | 3.88 ** | < 0.001 |
| Wechsler VIQ | 95.57 | 20.92 | 55–145 | 110.94 | 16.14 | 74–140 | 3.54 ** | < 0.001 |
* = p < 0.05; ** = p < 0.01.Edinburgh Handedness Inventory on a scale from –100 (left-handed) to 100 (right-handed).
3.2. Facial Memory Performance in Autism and Controls
As shown in Table 2, a MANCOVA revealed that individuals in the autism group performed significantly worse on the Facial Memory subtest F(1, 82) = 32.76, p < 0.01 and the Facial Memory Delayed subtest F(1, 82) = 14.32, p < 0.01. ASD participants also performed significantly more poorly on the Visual Selective Reminding immediate and delayed recall, a task with only abstract visual stimuli, as well as with the Object Recall, which had only a simple line drawing of a single face amongst numerous other common and familiar objects.
Table 2.
Mean Scaled Score TOMAL Performance by Group (MANOVA).
| Measure | Mean (SD) ASD n = 56 | Mean (SD) TDC n = 31 | F | p | ρη2 |
|---|---|---|---|---|---|
| Facial Memory | 7.29 (2.36) | 10.52 (2.53) | 32.76 | < 0.001 | 0.28 |
| Facial Memory Delayed | 7.64 (2.73) | 9.83 (2.17) | 14.32 | < 0.001 | 0.15 |
| Object Recall | 5.98 (3.45) | 9.37 (2.70) | 21.57 | < 0.001 | 0.21 |
| Visual Selective Reminding (Immediate) | 7.48 (3.30) | 9.87 (2.56) | 11.76 | < 0.001 | 0.12 |
| Visual Selective Reminding (Delayed) | 8.75 (2.03) | 10.10 (1.52) | 10.06 | < 0.001 | 0.11 |
Note: TOMAL subtest scores are age-corrected scaled scores. ρη2 = partial eta squared. Partial eta squared is an effect size measure that shows the variance explained by the predictor (TOMAL subtest) after excluding variance explained by other predictors (TICV).
To insure that memory performance differences were related to autism and not simply to generalized lower cognitive functioning, we individually matched (±7 points) participants with autism to typical developing controls on IQ. All TOMAL subtests remained significantly different between the groups even after IQ matching.
3.3. ROI Volumes in Autism and Controls
MANCOVA was used to compare facial memory ROI volumes (Table 3), including the left and right fusiform gyrus, amygdala, and hippocampus. Upon controlling for TICV, the left amygdala and the left hippocampus were significantly larger in ASD than in controls, while the left fusiform gyrus was significantly larger in controls. Effect sizes were minimal and no other volume differences were found between the groups.
Table 3.
ROI volume multivariate analysis controlling for TICV and Age.
| Structure | Mean (SD) ASD n = 56 | Mean (SD) TDC n = 31 | F | P | ρη2 |
|---|---|---|---|---|---|
| center Fusiform | 12.12 (1.89) | 12.95 (2.03) | 4.91 * | 0.03 | 0.06 |
| Right Fusiform | 11.81 (1.79) | 11.64 (1.67) | 0.25 | 0.62 | 0.00 |
| center Amygdala | 1.78 (0.30) | 1.67 (0.24) | 4.07 * | 0.049 | 0.05 |
| Right Amygdala | 1.78 (0.28) | 1.72 (0.23) | 1.21 | 0.27 | 0.01 |
| center Hippocampus | 4.62 (0.61) | 4.39 (0.66) | 4.15 * | 0.048 | 0.05 |
| Right Hippocampus | 4.61 (0.65) | 4.56 (0.46) | 0.40 | 0.53 | 0.01 |
Note: ROI volumes are measured in centimeters cubed. TDC = typically-developing controls; ASD = Autism Spectrum Disorder. * = p <0.05. ρη2 = partial eta squared. Partial eta squared is an effect size measure that shows the variance explained by the predictor (TOMAL subtest) after excluding variance explained by other predictors (TICV, age).
3.4. Relationship between ROI Volume and Facial Memory Performance
Using partial correlations controlling for TICV (see Table 4), the Facial Memory subtest was not significantly correlated with any ROI volume. However, Facial Memory Delayed was significantly negatively correlated with left fusiform gyrus and right hippocampal volume (r = –0.29, p = 0.042 and r = –0.28, p = 0.046, respectively) in the autism group, indicating that as left fusiform gyrus and right hippocampal volumes increased, performance on the delayed facial memory decreased. TOMAL Facial Memory performance (immediate and delayed) was not significantly correlated with any ROI volume in controls.
Table 4.
Partial Correlations between facial memory ROI volumes and TOMAL performance–controlling for TICV and age.
| Structure | Facial Memory | Facial Memory Delayed | ||
|---|---|---|---|---|
| ASD | TDC | ASD | TDC | |
| center Fusiform | 0.10 | 0.05 | –0.28 * | –0.21 |
| Right Fusiform | 0.13 | 0.12 | –0.15 | –0.09 |
| center Amygdala | 0.04 | –0.01 | –0.02 | 0.09 |
| Right Amygdala | 0.07 | –0.04 | –0.11 | –0.07 |
| center Hippocampus | –0.05 | 0.06 | –0.11 | 0.08 |
| Right Hippocampus | –0.17 | 0.17 | –0.28 * | 0.14 |
Note: The correlations presented are Pearson’s r scores. ASD = Autism Spectrum Disorder; TDC = typically-developing controls; * = p < 0.05.
To test the specificity of the observed significant relationship between Delayed Facial Memory and fusiform gyrus volume, ROI volume comparisons were subsequently performed for Object Recall and Visual Selective Reminding, immediate and delayed, as shown in Table 5. No significant correlations were observed (p > 0.05).
Table 5.
Partial correlations between facial memory ROI volumes and TOMAL performance—controlling for TICV and age.
| Structure | Object Recall | Visual Selective Reminding (Immediate) | Visual Selective Reminding (Delayed) | |||
|---|---|---|---|---|---|---|
| ASD | TDC | ASD | TDC | ASD | TDC | |
| Left Fusiform | 0.08 | –0.08 | 0.13 | –0.24 | 0.11 | 0.13 |
| Right Fusiform | –0.01 | 0.01 | –0.14 | –0.07 | 0.10 | 0.26 |
| Left Amygdala | –0.14 | 0.01 | –0.13 | –0.11 | 0.06 | 0.10 |
| Right Amygdala | 0.03 | –0.04 | –0.14 | –0.14 | 0.14 | 0.12 |
| Left Hippocampus | –0.20 | 0.19 | –0.02 | –0.18 | 0.07 | –0.16 |
| Right Hippocampus | –0.16 | 0.17 | –0.24 | –0.18 | 0.08 | 0.26 |
Note: The correlations presented are Pearson’s r scores. ASD = Autism Spectrum Disorder; TDC = typically-developing controls.
4. Discussion
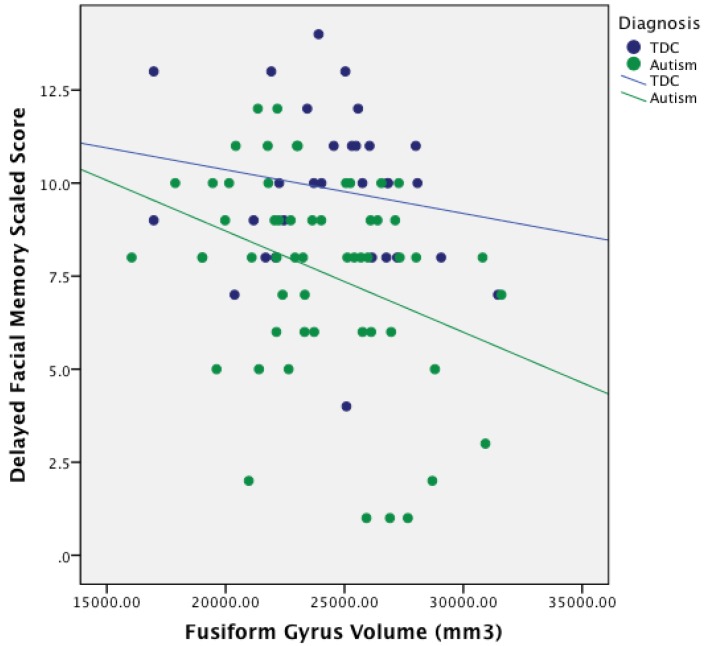
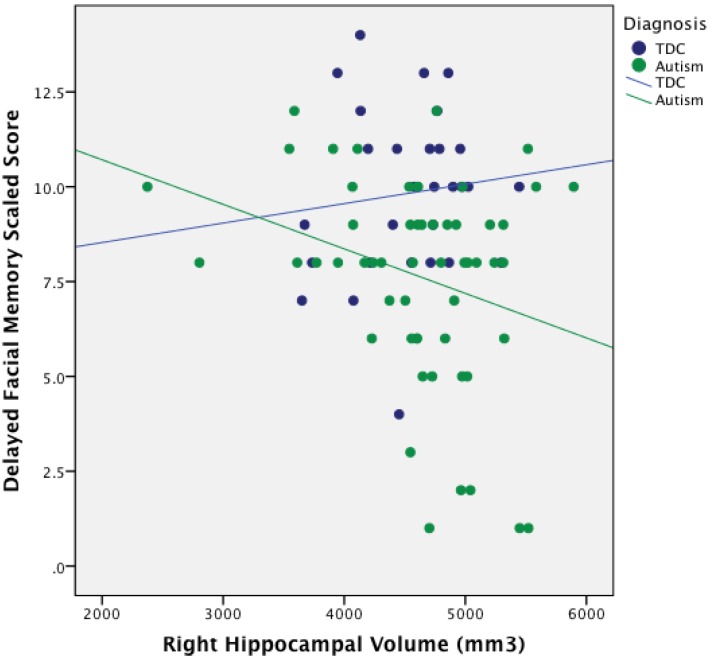
Anatomically, ASD participants had significantly smaller left fusiform but larger left amygdala and hippocampal volumes compared to TDC participants. With regards to memory performance, ASD participants performed significantly lower than TDCs on the TOMAL Facial Memory, Object Recall, and Visual Selective Reminding Test, consistent with other studies that have reported similar memory deficits in autism [1,2,5,6]. However, only the delayed component of the TOMAL Facial Memory test related to fusiform and hippocampal volume (see Figure 1). Interestingly, the fusiform correlation with Facial Memory was negative and significant only on the left and only for the delayed component. The significant hippocampal volume relation with delayed Facial Memory was also negative, but only on the right (see Figure 2). As can be seen in Figure 1, all but one of the participants with the largest fusiform volumes and poorest Delayed Facial Memory scores were in the ASD group. Likewise, those ASD participants with the lowest performance on the delayed Facial Memory task had the largest right hippocampal volumes. This association of larger size of the fusiform and hippocampus with poorer consolidation of facial memory implicates aberrant development of these structures in ASD, but only within a subset of individuals with ASD.
Figure 1.
Relationship between Fusiform Gyrus Volume and Delayed Facial Memory Performance in ASD.
Note: TDC = Typically Developing Controls. mm3 = millimeters cubed.
Figure 2.
Relationship between Right Hippocampal Volume and Delayed Facial Memory Performance in ASD
Note: TDC = Typical Developing Controls. mm3 = millimeters cubed.
A number of functional neuroimaging studies have shown that the working memory aspect of face processing and immediate retention involves bilateral fusiform and hippocampal areas [90,91]. However, given the generally non-verbal nature of facial memory, more right ventral and medial temporal lobe participation may be involved [92,93,94,95]. Given the potential rightward bias for facial memory, the larger right-sided hippocampus findings in ASD being associated with poorer delayed TOMAL Facial Memory performance appears somewhat straightforward. Larger size may reflect neural irregularities within the hippocampus that are associated with impaired face memory. Explanation for why larger size of only the left fusiform was associated with poorer delayed Facial Memory is perplexing and requires more inference. The TOMAL Facial Memory task is considered part of the non-verbal core of memory tasks but there are no subject restrictions that would preclude the participant from using verbal cues (i.e., that face is round with a small mouth) in performing the task, which may be a factor in the relation between left fusiform volume and facial memory performance. In fact, Grossman, Klin, Carter, and Volkmar [130] found that when ASD children are presented with facial emotional processing tasks, they perform better with words for emotions rather than faces. Also, Brown and Lloyd-Jones [96] have shown that subjects who verbally describe a face before performing the non-verbal face recognition task performed better. As a group, the ASD subjects had smaller overall fusiform volume, but it was its larger size that related to poorer consolidation of face memory.
4.1. Fusiform Gyrus Volumes in Autism
Abnormal size of the fusiform gyrus in autism has been previously reported as reviewed in the introduction [63,64,65,66,67,68,69,70]. In the current study, only the left fusiform was significantly smaller. Using a voxel-based morphometry technique, Toal et al. [67] observed that individuals with autism had decreased volumes in medial temporal and fusiform gyrus regions relative to controls. Reduced fusiform gyral volumes may be related to age as evidenced by Wallace et al. [69] who found thinner fusiform gyrus cortex in autism that appeared to increase with age. Raznahan et al. [70] also found a relationship between size and age, revealing smaller cortical volumes and thickness in fusiform and middle temporal gyri in children with ASD. Similarly, van Kooten et al. [97], in a post-mortem histological study involving 7 individuals with ASD, found fewer and smaller neurons in the fusiform gyrus in autism. How neuronal count actually relates to neuroimaging measured volume is not known at this time. However, this may be a key area for future study. In contrast, using voxel-based morphometry, Waiter et al. [64] found right-side fusiform gyral volume to be significantly larger. Likewise, in adults, larger fusiform gyral volumes were found in ASD [70], and still other studies have found no anatomical differences in fusiform gyrus volumes associated with autism [63]. Regardless of these differences and inconsistencies related to fusiform volume and ASD, the TDC and ASD participants in this sample did not differ in age yet, as shown in Figure 1, larger fusiform on the left was associated with poorer consolidation of facial memory.
While not understood at this time, one reason for neuroanatomical variability in autism may largely be due to the clinical and genetic heterogeneity of ASD and cross-sectional age-related developmental differences in neuroanatomical size between childhood and adulthood. Variability is also expected because of different methods used in volume quantification. Possibly one key to understanding this rather confusing picture about fusiform volume, development, and ASD may be that volumetric differences, regardless of direction, exist within these medial and ventral temporal lobe structures in ASD that reflects heterogeneity of fusiform development. Clearly, these regions participate in memory, social cognition, and face processing [64] but also dynamically change with maturation [70]. Thus, the answer for what fusiform volume and laterality may mean in facial memory likely will require prospective, longitudinal investigations.
4.2. Atypical Structure–Function Relationship of Fusiform Gyrus Morphometry in Autism
When attempting to interpret structure- and size-function relations, there has been much discussion across all of biology as to a possible “Goldilocks” effect—just the right size or amount for maximal function [98,99,100]. As such, optimal size–function relations are often reflected in positive associations [101]. In partial support for the “bigger is better” hypothesis, Gautam et al. [102] examined structure–function relations that differ by age. In aging, larger cortical volumes often equate to larger remaining portions of functional neural substrates (i.e., brain reserve) and hence lead to better functionality and resistance to the effects of age-related degeneration and onset of neurodegenerative disorders. However, in autism, larger development may signal early overgrowth—an indication of aberrant connectivity. Overgrowth provides no advantage as has been shown in aging and neurodegenerative studies of non-ASD individuals [103,104,105]. Thus positive correlations between volume loss and neuropathology, such as in Alzheimer’s and mild cognitive impairment (MCI) [102,106,107], have implications about cognitive functioning that are vastly different than the relationship between size and function in typical child development and ASD [108]. As can be seen in both Figure 1, Figure 2 the negative correlations were driven by a subset of ASD subjects with the largest volumes yet poorest Facial Memory scores. A number of ASD participants had Facial Memory scores and fusiform and hippocampal volumes that completely overlapped with TDC participants, underscoring that this larger size relationship with poorer memory represents yet another heterogeneous finding in ASD.
Interestingly, although facial memory was not specifically examined, Dziobek, Bahnemann, Convit, and Heekeren [68] observed that a region of increased cortical thickness within the fusiform gyrus was associated with impairments in face processing in autism. There is some speculation that these aberrant neural growth patterns in autism may reflect some failure in cellular pruning, which may be regionally manifested [109].
In our sample of ASD participants, it is possible that the larger size of the fusiform in a subset reflects regional abnormalities in pruning with associated errors in connectivity and this is why some ASD subjects (as shown in Figure 1) who had the largest fusiform volume also had the poorest consolidation of memory for faces. Overall, a failure in growth regulation (whether it be undershooting or overshooting), is a perplexing factor associated with autism and may be why greater size is related to poorer function in this sample of individuals with ASD. Brain growth rate abnormalities have been inferred in ASD [110,111], and would fit with the negative correlation between size and performance in ASD. These abnormalities likely reflect disrupted developmental neurobiology in ASD, and bolsters the notion that size-function relationships in ASD are an important piece to understanding the disorder. Indeed, dysfunction in one brain region likely affects development and functioning of related brain regions, leaving complex and individualized neurodevelopmental patterns among ASD individuals.
With regards to memory in general, the fusiform has been implicated as an important region in visual memory consolidation processes [112,113]. Specific to face-location and face-name associations, functional neuroimaging studies have demonstrated that the region of the fusiform gyrus plays a role in memory consolidation [91,114]. Consistent with its putative role in consolidation, the current findings suggest that larger fusiform volume uniquely affects consolidation of face memory in ASD.
4.3. Relationship between Structure Size and Connectivity
How can evidence of the pathology of increased size be reconciled with evidence of pathological functional under-connectivity both within (short-range) and between (long-range) brain structures in autism [50]? Courchesne and Pierce [115] have outlined some of the morphological differences seen in the developing brain in autism, where altered developmental trajectories may result in different volumes depending on age; differences in volume likely reflect different organization within a structure as well as its connectivity with other structures [116]. Likewise, any disruption of early brain development, even if a given structure eventually normalizes in volume with age, has the potential to significantly influence circuitry and resultant function [117]. The relation between abnormal developmental brain growth trajectories and abnormal connectivity in autism has been implicated in a number of studies [118,119], with disrupted connectivity representing major theories of autism [115,117,120,121,122,123,124]. Hazlett et al. [119] observed larger temporal lobe volume in young ASD subjects out to age 6. Although they did not specifically assess the fusiform gyrus, lobular volume is typically highly positively related to individual gyral volumes [88]. Gyral volumes outside of some optimal size may be an indicator of abnormal connectivity, helping to explain the negative correlation between fusiform volume and delayed facial memory performance in the current study. Additionally, Casanova et al. [125,126,127] have shown that increases in cortical gray matter may relate to aberrant increases in white matter via increased white matter projections necessary to maintain the connectivity of the increased number of cortical cells in the autistic brain. However, increased number of white matter connections in autism may not result in greater and more efficient functional connectivity.
Given the research suggesting that facial processing may change with age in typical individuals [128], and cross-sectional age-related research suggesting atypical trajectories of fusiform volume and cortical thickness in autism [70,71], examination of facial memory from a longitudinal approach will hopefully answer some of these questions. Additionally, given the potential importance of the rapid subcortical face detection and attention system [18], as well as the cortical face processing system involving the fusiform gyrus in autism, coordinated longitudinal multimodal examination of both systems may help discern causal pathways in brain development leading to impaired face processing. Given the current findings, it may be especially important to examine consolidation of face memory in relation to face processing and brain development. One can only imagine the extraordinarily negative impact impaired facial memory has on understanding and navigating the social world across the lifespan. As such, unraveling the neuroanatomical basis to face processing and facial memory impairments in autism represents an important topic of investigation.
As discussed in the introduction, the fusiform, hippocampus and amygdala are all connected both intra- and inter-hemispherically. A major limitation of the current investigation is that only volumes of these structures were examined and not their functional connectivity with regards to facial memory. As Frith [130] has pointed out, only limited inferences can be made when only one dimension—like volume—of a neural system is examined in disorders such as autism. Accordingly, a next step in this line of research will need to not only explore volumes of these regions but additional indicators of their morphology and functional connectivity in retention of facial memory.
5. Conclusions
The objective of the current study was to examine facial memory in autism and to explore the relation of facial memory performance with anatomical ROI volumes known to be involved in face processing. Larger volumes in the autism group in the left amygdala and left hippocampus compared to TDC were found in this sample. In contrast, TDC had larger left fusiform gyral volumes when compared with ASD. These differences did not relate to TOMAL Facial Memory for the immediate trials but negative correlations between delayed Facial Memory performance and the left fusiform and right hippocampus for the autism group but not for TDC were found. It is possible that larger fusiform gyrus and hippocampal volumes may be a marker of abnormal connectivity and functionality in facial memory.
Acknowledgments
The project described was supported by Grant Numbers RO1 MH080826 (JEL, EDB, ALA, NL), RO1 MH084795 (JEL, PTF, NL), and KO8 MH092697 (JSA) from the National Institute Of Mental Health; Grant Numbers T32 HD07489 and the Hartwell Foundation (BGT), P30 HD003352-45 (Waisman Center Core Grant), and CHRCDA K12 HD001410 (BAZ) from the Eunice Kennedy Shriver NICHD, The Hartwell Foundation (BGT), and the Primary Children’s Medical Center Foundation (BAZ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institute of Child Health & Development, or the National Institutes of Health. We thank former members of the Utah Autism CPEA for their assistance during the early stages of this project. We sincerely thank the children, adolescents, and adults with autism and the individuals with typical development, who participated in this study, and their families. The brain Imaging and Behavior Laboratory at BYU is partially supported by a donation from the Poelman Foundation. Although Dr. Bigler is the co-author of the TOMAL, he receives no royalties and reports no conflict of interest. The assistance of Tracy J. Abildskov and Jo Ann Petrie, is gratefully acknowledged.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bowler D.M., Gardiner J.M., Grice S.J. Episodic memory and remembering in adults with Asperger syndrome. J. Autism Dev. Disord. 2000;30:295–304. doi: 10.1023/A:1005575216176. [DOI] [PubMed] [Google Scholar]
- 2.Gardiner J.M., Bowler D.M., Grice S.J. Further evidence of preserved priming and impaired recall in adults with Asperger’s syndrome. J. Autism Dev. Disord. 2003;33:259–269. doi: 10.1023/A:1024450416355. [DOI] [PubMed] [Google Scholar]
- 3.Millward C., Powell S., Messer D., Jordan R. Recall for self and other in autism: Children’s memory for events experienced by themselves and their peers. J. Autism Dev. Disord. 2000;30:15–28. doi: 10.1023/A:1005455926727. [DOI] [PubMed] [Google Scholar]
- 4.Russell J., Jarrold C., Henry L. Working memory in children with autism and with moderate learning difficulties. J. Child. Psychol. Psyc. 1996;37:673–686. doi: 10.1111/j.1469-7610.1996.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 5.Southwick J.S., Bigler E.D., Froehlich A., Dubray M.B., Alexander A.L., Lange N., Lainhart J.E. Memory functioning in children and adolescents with autism. Neuropsychology. 2011;25:702–710. doi: 10.1037/a0024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams D.L., Goldstein G., Minshew N.J. The profile of memory function in children with autism. Neuropsychology. 2006;20:21–29. doi: 10.1037/0894-4105.20.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange N., DuBray M.B., Lee J.E., Froimowitz M.P., Froehlich A., Adluru N., Wright B., Ravichandran C., Fletcher P.T., Bigler E.D., et al. Atypical diffusion tensor hemispheric asymmetry in autism. Autism Res. 2010;3:350–358. doi: 10.1002/aur.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeneson A., Squire L.R. Working memory, long-term memory, and medial temporal lobe function. Learn. Mem. 2011;19:15–25. doi: 10.1101/lm.024018.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lajiness-O’Neill R.R., Beaulieu I., Titus J.B., Asamoah A., Bigler E.D., Bawle E.V., Pollack R. Memory and learning in children with 22q11.2 deletion syndrome: Evidence for ventral and dorsal stream disruption? Child. Neuropsychol. 2005;11:55–71. doi: 10.1080/09297040590911202. [DOI] [PubMed] [Google Scholar]
- 10.Reynolds C.R., Bigler E.D. Test of Memory and Learning. Pro-ed; Austin, TX, USA: 1994. [Google Scholar]
- 11.Gastgeb H.Z., Wilkinson D.A., Minshew N.J., Strauss M.S. Can individuals with autism abstract prototypes of natural faces? J. Autism Dev. Disord. 2011;41:1609–1618. doi: 10.1007/s10803-011-1190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiner K.S., Grill-Spector K. The improbable simplicity of the fusiform face area. Trends Cog. Sci. 2012;16:251–254. doi: 10.1016/j.tics.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Hauck M., Fein D., Maltby N., Waterhouse L., Feinstein C. Memory for faces in children with autism. Child. Neuropsychol. 1998;4:187–198. doi: 10.1076/chin.4.3.187.3174. [DOI] [Google Scholar]
- 14.Williams D.L., Goldstein G., Minshew N.J. Impaired memory for faces and social scenes in autism: Clinical implications of memory dysfunction. Arch. Clin. Neuropsychol. 2005;20:1–15. doi: 10.1016/j.acn.2002.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Wilkinson D.A., Best C.A., Minshew N.J., Strauss M.S. Memory awareness for faces in individuals with autism. J. Autism Dev. Disord. 2010;40:1371–1377. doi: 10.1007/s10803-010-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adolphs R., Spezio M.L., Parlier M., Piven J. Distinct face-processing strategies in parents of autistic children. Curr. Biol. 2008;18:1090–1093. doi: 10.1016/j.cub.2008.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dawson G., Webb S.J., McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Dev. Neuropsychol. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- 18.Kleinhans N.M., Richards T., Johnson L.C., Weaver K.E., Greenson J., Dawson G., Aylward E. fMRI evidence of neural abnormalities in the subcortical face processing system in ASD. Neuroimage. 2011;54:697–704. doi: 10.1016/j.neuroimage.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hobson R.P. The autistic child’s appraisal of expressions of emotion: A further study. J. Child. Psychol. Psychiatry. 1986;27:671–680. doi: 10.1111/j.1469-7610.1986.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 20.Hobson R.P., Ouston J., Lee A. What’s in a face? The case of autism. Br. J. Psychol. 1988;79:441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- 21.Bormann-Kischkel C., Vilsmeier M., Baude B. The development of emotional concepts in autism. J. Child. Psychol. Psychiatry. 1995;36:1243–1259. doi: 10.1111/j.1469-7610.1995.tb01368.x. [DOI] [PubMed] [Google Scholar]
- 22.Baron-Cohen S., Baldwin D.A., Crowson M. Do children with autism use the speaker’s direction of gaze strategy to crack the code of language? Child. Dev. 1997;68:48–57. doi: 10.2307/1131924. [DOI] [PubMed] [Google Scholar]
- 23.Phillips W., Baron-Cohen S., Rutter M. The role of eye contact in goal detection: Evidence from normal infants and children with autism or mental handicap. Dev. Psychopathol. 1992;4:8. [Google Scholar]
- 24.Hobson R.P., Lee A. Hello and goodbye: A study of social engagement in autism. J. Autism Dev. Disord. 1998;28:117–127. doi: 10.1023/A:1026088531558. [DOI] [PubMed] [Google Scholar]
- 25.Dalton K.M., Nacewicz B.M., Johnstone T., Schaefer H.S., Gernsbacher M.A., Goldsmith H.H., Alexander A.L., Davidson R.J. Gaze fixation and the neural circuitry of face processing in autism. Nat. Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klin A., Jones W., Schultz R., Volkmar F., Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch. Gen. Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- 27.Moore D.J., Heavey L., Reidy J. Attentional processing of faces in ASD: A dot-probe study. J. Autism Dev. Disord. 2012;42:2038–2045. doi: 10.1007/s10803-012-1449-4. [DOI] [PubMed] [Google Scholar]
- 28.Pelphrey K.A., Sasson N.J., Reznick J.S., Paul G., Goldman B.D., Piven J. Visual scanning of faces in autism. J. Autism Dev. Disord. 2002;32:249–261. doi: 10.1023/A:1016374617369. [DOI] [PubMed] [Google Scholar]
- 29.Snow J., Ingeholm J.E., Levy I.F., Caravella R.A., Case L.K., Wallace G.L., Martin A. Impaired visual scanning and memory for faces in high-functioning autism spectrum disorders: It’s not just the eyes. J. Int. Neuropsychol. Soc. 2011;17:1021–1029. doi: 10.1017/S1355617711000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright B., Alderson-Day B., Prendergast G., Bennett S., Jordan J., Whitton C., Gouws A., Jones N., Attur R., Tomlinson H., et al. Gamma activation in young people with autism spectrum disorders and typically-developing controls when viewing emotions on faces. PLoS One. 2012;7:e41326. doi: 10.1371/journal.pone.0041326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weeks S.J., Hobson R. The salience of facial expression for autistic children. J. Child. Psychol. Psychiatry. 1987;28:137–151. doi: 10.1111/j.1469-7610.1987.tb00658.x. [DOI] [PubMed] [Google Scholar]
- 32.Tantam D., Monaghan L., Nicholson H., Stirling J. Autistic children’s ability to interpret faces: A research note. J. Child. Psychol. Psychiatry. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 33.Blair R.J., Frith U., Smith N., Abell F., Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40:108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- 34.Boucher J., Lewis V., Collis G. Familiar face and voice matching and recognition in children with autism. J. Child. Psychol. Psychiatry. 1998;39:171–181. doi: 10.1111/1469-7610.00311. [DOI] [PubMed] [Google Scholar]
- 35.Kuusikko-Gauffin S., Jansson-Verkasalo E., Carter A., Pollock-Wurman R., Jussila K., Mattila M., Rahko J., Ebeling H., Pauls D., Moilanen I. Face memory and object recognition in children with high-functioning autism or asperger syndrome and in their parents. Res. Autism Spectr. Disord. 2011;5:622–628. doi: 10.1016/j.rasd.2010.07.007. [DOI] [Google Scholar]
- 36.Weigelt S., Koldewyn K., Kanwisher N. Face identity recognition in autism spectrum disorders: A review of behavioral studies. Neurosci. Biobehav. Rev. 2012;36:1060–1084. doi: 10.1016/j.neubiorev.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Hubl D., Bolte S., Feineis-Matthews S., Lanfermann H., Federspiel A., Strik W., Poustka M.D., Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.WNL.0000091862.22033.1A. [DOI] [PubMed] [Google Scholar]
- 38.Bookheimer S.Y., Wang A.T., Scott A., Sigman M., Dapretto M. Frontal contributions to face processing differences in autism: Evidence from fMRI of inverted face processing. J. Int. Neuropsychol. Soc. 2008;14:922–932. doi: 10.1017/S135561770808140X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hadjikhani N., Joseph R.M., Snyder J., Chabris C.F., Clark J., Steele S., McGrath L., Vangel M., Aharon I., Feczko E., et al. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. NeuroImage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Kanwisher N., McDermott J., Chun M.M. The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barton J.J., Press D.Z., Keenan J.P., O’Connor M. Lesions of the fusiform face area impair perception of facial configuration in prosopagnosia. Neurology. 2002;58:71–78. doi: 10.1212/WNL.58.1.71. [DOI] [PubMed] [Google Scholar]
- 42.Harris A., Aguirre G.K. Neural tuning for face wholes and parts in human fusiform gyrus revealed by FMRI adaptation. J. Neurophysiol. 2010;104:336–345. doi: 10.1152/jn.00626.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris A., Aguirre G.K. The representation of parts and wholes in face-selective cortex. J. Cogn. Neurosci. 2008;20:863–878. doi: 10.1162/jocn.2008.20509. [DOI] [PubMed] [Google Scholar]
- 44.Maurer D., O’Craven K.M., Le Grand R., Mondloch C.J., Springer M.V., Lewis T.L., Grady C.L. Neural correlates of processing facial identity based on features versus their spacing. Neuropsychologia. 2007;45:1438–1451. doi: 10.1016/j.neuropsychologia.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Rhodes G., Michie P.T., Hughes M.E., Byatt G. The fusiform face area and occipital face area show sensitivity to spatial relations in faces. Eur. J. Neurosci. 2009;30:721–733. doi: 10.1111/j.1460-9568.2009.06861.x. [DOI] [PubMed] [Google Scholar]
- 46.Rotshtein P., Geng J.J., Driver J., Dolan R.J. Role of features and second-order spatial relations in face discrimination, Face recognition, And individual face skills: Behavioral and functional magnetic resonance imaging data. J. Cogn. Neurosci. 2007;19:1435–1452. doi: 10.1162/jocn.2007.19.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yovel G., Kanwisher N. Face perception: Domain specific, not process specific. Neuron. 2004;44:889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 48.Kleinhans N.M., Richards T., Sterling L., Stegbauer K.C., Mahurin R., Johnson L.C., Greenson J., Dawson G., Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- 49.Anderson J.S., Druzgal T.J., Froehlich A., DuBray M.B., Lange N., Alexander A.L., Abildskov T., Nielsen J.A., Cariello A.N., Cooperrider J.R., et al. Decreased interhemispheric functional connectivity in autism. Cereb. Cortex. 2011;21:1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khan S., Gramfort A., Shetty N.R., Kitzbichler M.G., Ganesan S., Moran J.M., Lee S.M., Gabrieli J.D., Tager-Flusberg H.B., Joseph R.M., et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc. Natl. Acad. Sci. USA. 2013;110:3107–3112. doi: 10.1073/pnas.1214533110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damasio A.R., Damasio H., Van Hoesen G.W. Prosopagnosia: Anatomic basis and behavioral mechanisms. Neurology. 1982;32:331–341. doi: 10.1212/WNL.32.4.331. [DOI] [PubMed] [Google Scholar]
- 52.Sergent J., Signoret J.L. Varieties of functional deficits in prosopagnosia. Cereb. Cortex. 1992;2:375–388. doi: 10.1093/cercor/2.5.375. [DOI] [PubMed] [Google Scholar]
- 53.Uttner I., Bliem H., Danek A. Prosopagnosia after unilateral right cerebral infarction. J. Neurol. 2002;249:933–935. doi: 10.1007/s00415-002-0710-8. [DOI] [PubMed] [Google Scholar]
- 54.Sato W., Toichi M., Uono S., Kochiyama T. Impaired social brain network for processing dynamic facial expressions in autism spectrum disorders. Neuroscience. 2012;13:99. doi: 10.1186/1471-2202-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pelphrey K.A., Shultz S., Hudac C.M., Vander Wyk B.C. Research review: Constraining heterogeneity: The social brain and its development in autism spectrum disorder. J. Child. Psychol. Psychiatry. 2011;52:631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conturo T.E., Williams D.L., Smith C.D., Gultepe E., Akbudak E., Minshew N.J. Neuronal fiber pathway abnormalities in autism: An initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J. Int. Neuropsychol. Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Critchley H., Daly E., Phillips M., Brammer M., Bullmore E., Williams S., Van Amelsvoort T., Robertson D., David A., Murphy D. Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Hum. Brain Mapp. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deeley Q., Daly E.M., Surguladze S., Page L., Toal F., Robertson D., Curran S., Giampietro V., Seal M., Brammer M.J., et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biol. Psychiatry. 2007;62:207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 59.Ishitobi M., Kosaka H., Omori M., Matsumura Y., Munesue T., Mizukami K., Shimoyama T., Murata T., Sadato N., Okazawa H., et al. Differential amygdala response to lower face in patients with autistic spectrum disorders: An fMRI study. Res. Autism Spectr. Disord. 2011;5:910–919. doi: 10.1016/j.rasd.2010.10.005. [DOI] [Google Scholar]
- 60.Ashwin C., Baron-Cohen S., Wheelwright S., O’Riordan M., Bullmore E.T. Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2006;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Pelphrey K.A., Morris J.P., McCarthy G., LaBar K.S. Perception of dynamic changes in facial affect and identity in autism. Soc. Cogn. Affect. Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stiles J., Jernigan T.L. The basics of brain development. Neuropsychol. Rev. 2010;20:327–348. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pierce K., Muller R.A., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform “face area” in autism: Evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- 64.Waiter G.D., Williams J.H., Murray A.D., Gilchrist A., Perrett D.I., Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Rojas D.C., Peterson E., Winterrowd E., Reite M.L., Rogers S.J., Tregellas J.R. Regional gray matter volumetric changes in autism associated with social and repetitive behavior symptoms. BMC Psychiatry. 2006;6:56. doi: 10.1186/1471-244X-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neeley E.S., Bigler E.D., Krasny L., Ozonoff S., McMahon W., Lainhart J.E. Quantitative temporal lobe differences: Autism distinguished from controls using classification and regression tree analysis. Brain Dev. 2007;29:389–399. doi: 10.1016/j.braindev.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Toal F., Daly E.M., Page L., Deeley Q., Hallahan B., Bloemen O., Cutter J., Brammer M.J., Curran S., Robertson D., et al. Clinical and anatomical heterogeneity in autistic spectrum disorder: A structural MRI study. Psychol. Med. 2010;40:1171–1181. doi: 10.1017/S0033291709991541. [DOI] [PubMed] [Google Scholar]
- 68.Dziobek I., Bahnemann M., Convit A., Heekeren H.R. The role of the fusiform-amygdala system in the pathophysiology of autism. Arch. Gen. Psychiatry. 2010;67:397–405. doi: 10.1001/archgenpsychiatry.2010.31. [DOI] [PubMed] [Google Scholar]
- 69.Wallace G.L., Dankner N., Kenworthy L., Giedd J.N., Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133:3745–3754. doi: 10.1093/brain/awq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raznahan A., Toro R., Daly E., Robertson D., Murphy C., Deeley Q., Bolton P.F., Paus T., Murphy D.G. Cortical anatomy in autism spectrum disorder: An in vivo MRI study on the effect of age. Cereb. Cortex. 2010;20:1332–1340. doi: 10.1093/cercor/bhp198. [DOI] [PubMed] [Google Scholar]
- 71.Cauda F., Geda E., Sacco K., D’Agata F., Duca S., Geminiani G., Keller R. Grey matter abnormality in autism spectrum disorder: An activation likelihood estimation meta-analysis study. J. Neurol. Neurosurg. Psychiatry. 2011;82:1304–1313. doi: 10.1136/jnnp.2010.239111. [DOI] [PubMed] [Google Scholar]
- 72.Murphy C.M., Deeley Q., Daly E.M., Ecker C., O’Brien F.M., Hallahan B., Toal F., Reed S., Hales S., Robertson D.M., et al. Anatomy and aging of the amygdala and hippocampus in autism spectrum disorder: An in vivo magnetic resonance imaging study of Asperger syndrome. Autism Res. 2012;5:3–12. doi: 10.1002/aur.227. [DOI] [PubMed] [Google Scholar]
- 73.Hasan K.M., Walimuni I.S., Frye R.E. Global cerebral and regional multimodal neuroimaging markers of the neurobiology of autism: Development and cognition. J. Child. Neurol. 2013;28:874–885. doi: 10.1177/0883073812452917. [DOI] [PubMed] [Google Scholar]
- 74.Aylward E.H., Minshew N.J., Goldstein G., Honeycutt N.A., Augustine A.M., Yates K.O., Barta P.E., Pearlson G.D. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/WNL.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 75.Bigler E.D. Neurobiology and neuropathology underlie the neuropsychological deficits associated with traumatic brain injury. Arch. Clin. Neuropsychol. 2003;18:595–621; discussion 623–627. [PubMed] [Google Scholar]
- 76.Alexander A.L., Lee J.E., Lazar M., Boudos R., DuBray M.B., Oakes T.R., Miller J.N., Lu J., Jeong E., McMahon W.M., et al. Diffusion tensor imaging of the corpus callosum in autism. NeuroImage. 2007;34:61–73. doi: 10.1016/j.neuroimage.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 77.Lord C., Rutter M., Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 78.Lord C., Risi S., Lambrecht L., Cook E.H., Jr., Leventhal B.L., DiLavore P.C., Pickles A., Rutter M. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 2000;30:205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- 79.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. APA; Washington, DC, USA: 1994. [Google Scholar]
- 80.Wechsler D. Wechsler Intelligence Scale for Children-III (WISC-III) The Psychological Corporation; San Antonio, TX, USA: 1991. [Google Scholar]
- 81.Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) The Psychological Corporation; San Antonio, TX, USA: 1997. [Google Scholar]
- 82.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX, USA: 1999. [Google Scholar]
- 83.Elliot C.D. Differential Ability Scales. 2nd ed. Harcourt Assessment; San Antonio, TX, USA: 2007. [Google Scholar]
- 84.Dennis M., Francis D.J., Cirino P.T., Schachar R., Barnes M.A., Fletcher J.M. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J. Int. Neuropsychol. Soc. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.White S., O’Reilly H., Frith U. Big heads, Small details and autism. Neuropsychologia. 2009;47:1274–1281. doi: 10.1016/j.neuropsychologia.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 86.Lainhart J.E., Bigler E.D., Bocian M., Coon H., Dinh E., Dawson G., Deutsch C.K., Dunn M., Estes A., Tager-Flusberg H., et al. Head circumference and height in autism: A study by the collaborative program of excellence in autism. Am. J. Med. Genet. A. 2006;140:2257–2274. doi: 10.1002/ajmg.a.31465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oldfield R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 88.Bigler E.D., Abildskov T.J., Wilde E.A., McCauley S.R., Li X., Merkley T.L., Fearing M.A., Newsome M.R., Scheibel R.S., Hunter J.V., et al. Diffuse damage in pediatric traumatic brain injury: A comparison of automated versus operator-controlled quantification methods. Neuroimage. 2010;50:1017–1026. doi: 10.1016/j.neuroimage.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Hanson J.L., Suh J.W., Nacewicz B.M., Sutterer M.J., Cayo A.A., Stodola D.E., Burghy C.A., Wang H., Avants B.B., Yushkevich P.A., et al. Robust automated amygdala segmentation via multi-atlas diffeomorphic registration. Front. Neurosci. 2012;6:166. doi: 10.3389/fnins.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hau X., Berg A.C., Oh H., Samaras D., Leung H.C. Multi-voxel pattern analysis of selective representation of visual working memory in ventral temporal and occipital regions. NeuroImage. 2013;73:8–15. doi: 10.1016/j.neuroimage.2013.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Robinson-Long M., Eslinger P.J., Wang J., Meadowcroft M., Yang Q.X. Functional MRI evidence for distinctive binding and consolidation pathways for face-name associations: Analysis of activation maps and BOLD response amplitudes. Top. Magn. Reson. Imag. 2009;20:271–278. doi: 10.1097/RMR.0b013e3181e8f1f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor M.J., Mills T., Pang E.W. The development of face recognition; hippocampal and frontal lobe contributions with MEG. Brain Topogr. 2011;24:261–270. doi: 10.1007/s10548-011-0192-z. [DOI] [PubMed] [Google Scholar]
- 93.Atri A., O’Brien J.L., Sreenivasan A., Rastegar S., Salisbury S., DeLuca A.N., O’Keefe K.M., LaViolette P.S., Rentz D.M., Locascio J.J., et al. Test-retest reliability of memory task functional magnetic resonance imaging in Alzheimer disease clinical trials. Arch. Neurol. 2011;68:599–606. doi: 10.1001/archneurol.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Majerus S., D’Argembeau A., Martinez Perez T., Belayachi S., Van der Linden M., Collette F., Salmon E., Seurinck R., Fias W., Maquet P. The commonality of neural networks for verbal and visual short-term memory. J. Cogn. Neurosci. 2010;22:2570–2593. doi: 10.1162/jocn.2009.21378. [DOI] [PubMed] [Google Scholar]
- 95.Koshino H., Kana R.K., Keller T.A., Cherkassky V.L., Minshew N.J., Just M.A. fMRI investigation of working memory for faces in autism: Visual coding and underconnectivity with frontal areas. Cereb. Cortex. 2008;18:289–300. doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown C., Lloyd-Jones T.J. Verbal facilitation of face recognition. Mem. Cognit. 2005;33:1442–1456. doi: 10.3758/BF03193377. [DOI] [PubMed] [Google Scholar]
- 97.Van Kooten I.A., Palmen S.J., von Cappeln P., Steinbusch H.W., Korr H., Heinsen H., Hof P.R., van Engeland H., Schmitz C. Neurons in the fusiform gyrus are fewer and smaller in autism. Brain. 2008;131:987–999. doi: 10.1093/brain/awn033. [DOI] [PubMed] [Google Scholar]
- 98.Yamasaki L. Balancing proliferation and apoptosis in vivo: The Goldilocks theory of E2F/DP action. Biochim. Biophys. Acta. 1999;1423:M9–M15. doi: 10.1016/s0304-419x(99)00003-7. [DOI] [PubMed] [Google Scholar]
- 99.Little A.G. The “Goldilocks” principle. Chest. 2005;128:13–14. doi: 10.1378/chest.128.1.13. [DOI] [PubMed] [Google Scholar]
- 100.Yun T.J., Bevan M.J. The Goldilocks conditions applied to T cell development. Nat. Immunol. 2001;2:13–14. doi: 10.1038/83118. [DOI] [PubMed] [Google Scholar]
- 101.Koscik T.R., Tranel D. Brain evolution and human neuropsychology: The inferential brain hypothesis. J. Int. Neuropsychol. Soc. 2012;18:394–401. doi: 10.1017/S1355617712000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Guatam P., Cherbuin N., Sachdev P.S., Wen W., Anstey K.J. Relationships between cognitive function and frontal grey matter volumes and thickness in middle aged and early old-aged adults: The PATH through life study. NeuroImage. 2011;55:845–855. doi: 10.1016/j.neuroimage.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 103.Bartrés-Faz D., Solé-Padullés C., Junque C., Rami L., Bosch B., Bargallo N., Falcon C., Sanchez-Valle R., Molinuevo J.L. Interactions of cognitive reserve with regional brain anatomy and brain function during a working memory task in healthy elders. Biol. Psychol. 2009;80:256–259. doi: 10.1016/j.biopsycho.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Solé-Padullés C., Bartrés-Faz D., Junque C., Vendrell P., Rami L., Clemente I.C., Bosch B., Villar A., Bargallo N., Jurado M.A., et al. Brain structure and function related to cognitive reserve variables in normal aging, Mild cognitive impairment and Alzheimer’s disease. Neurobiol. Ag. 2009;30:1114–1124. doi: 10.1016/j.neurobiolaging.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 105.Stern Y., Habeck C., Moeller J., Scarmeas N., Anderson K.E., Hilton H.J., Flynn J., Sackeim H., van Heertum R. Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex. 2005;15:394–402. doi: 10.1093/cercor/bhh142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Baxter L.C., Sparks D.L., Johnson S.C., Lenoski B., Lopez J.E., Connor D.J., Sabbagh M.N. Relationship of cognitive measures and gray and white matter in Alzheimer’s disease. J. Alzheimers. Dis. 2006;9:253–260. doi: 10.3233/jad-2006-9304. [DOI] [PubMed] [Google Scholar]
- 107.Braak E., Braak H. Alzheimer’s disease: Transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol. 1997;93:323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- 108.Giedd J.N., Rapoport J.L. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thomas M.S., Knowland V.C., Karmiloff-Smith A. Mechanisms of developmental regression in autism and the broader phenotype: A neural network modeling approach. Psychol. Rev. 2011;118:637054. doi: 10.1037/a0025234. [DOI] [PubMed] [Google Scholar]
- 110.Hua X., Thompson P.M., Leow A.D., Madsen S.K., Caplan R., Alger J.R., O’Neill J., Joshi K., Smalley S.L., Toga A.W., et al. Brain growth rate abnormalities visualized in adolscents with autism. Brain Mapp. 2013;34:425–436. doi: 10.1002/hbm.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nordahl C.W., Lange N., Li D.D., Barnett L.A., Lee A., Buonocore M.H., Simon T.J., Rogers S., Ozonoff S., Amaral D.G. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc. Natl. Acad. Sci. USA. 2011;108:20195–20200. doi: 10.1073/pnas.1107560108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mainy N., Kahane P., Minotti L., Hoffmann D., Bertrand O., Lachaux J.P. Neural correlates of consolidation in working memory. Hum. Brain Mapp. 2007;28:183–193. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang K., Jiang T., Yu C., Tian L., Li J., Liu Y., Zhou Y., Xu L., Song M., Li K. Spontaneous activity associated with primary visual cortex: A resting-state fMRI study. Cereb. Cortex. 2008;18:697–704. doi: 10.1093/cercor/bhm105. [DOI] [PubMed] [Google Scholar]
- 114.Van Dongen E.V., Takashima A., Barth M., Fernandez G. Functional connectivity during light sleep is correlated with memory performance for face-location associations. Neuroimage. 2011;57:8. doi: 10.1016/j.neuroimage.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 115.Courchesne E., Pierce K. Brain overgrowth in autism during a critical time in development: Implications for frontal pyramidal neuron and interneuron development and connectivity. Int. J. Dev. Neurosci. 2005;23:153–170. doi: 10.1016/j.ijdevneu.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 116.Courchesne E., Carper R., Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290:337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- 117.Polsek D., Jagatic T., Cepanec M., Hof P.R., Simic G. Recent Developments in neuropathology of autism spectrum disorders. Transl. Neurosci. 2011;2:256–264. doi: 10.2478/s13380-011-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis J.D., Theilmann R.J., Fonov V., Bellec P., Lincoln A., Evans A.C., Townsend J. Callosal fiber length and interhemispheric connectivity in adults with autism: Brain overgrowth and underconnectivity. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hazlett H.C., Poe M.D., Gerig G., Styner M., Chappell C., Smith R.G., Vachet C., Piven J. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch. Gen. Psychiatry. 2011;68:467–476. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boersma M., Kemner C., de Reus M.A., Collin G., Snijders T.M., Hofman D., Buitelaar J.K., Stam C.J., van den Heuvel M.P. Disrupted functional brain networks in autistic toddlers. Brain Connect. 2013;3:41–49. doi: 10.1089/brain.2012.0127. [DOI] [PubMed] [Google Scholar]
- 121.Belmonte M.K., Allen G., Beckel-Mitchener A., Boulanger L.M., Carper R.A., Webb S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004;24:9228–9231. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Just M.A., Cherkassky V.L., Keller T.A., Minshew N.J. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 123.Minshew N.J. Brief report: Brain mechanisms in autism: Functional and structural abnormalities. J. Autism Dev. Disord. 1996;26:205–209. doi: 10.1007/BF02172013. [DOI] [PubMed] [Google Scholar]
- 124.Minshew N.J., Williams D.L. The new neurobiology of autism: Cortex, Connectivity, And neuronal organization. Arch. Neurol. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Casanova M.F., Buxhoeveden D., Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: Implications for autism. Neuroscientist. 2003;9:496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- 126.Casanova M.F., Buxhoeveden D.P., Brown C. Clinical and macroscopic correlates of minicolumnar pathology in autism. J. Child. Neurol. 2002;17:692–695. doi: 10.1177/088307380201700908. [DOI] [PubMed] [Google Scholar]
- 127.Casanova M.F., van Kooten I.A., Switala A.E., van Engeland H., Heinsen H., Steinbusch H.W., Hof P.R., Trippe J., Stone J., Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol. 2006;112:287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 128.Tehrani-Doost M., Salmanian M., Ghanbari-Motlagh M., Shahrivar Z. Delayed face recognition in children and adolescents with autism spectrum disorders. Iran. J. Psychiatry. 2012;7:52–56. [PMC free article] [PubMed] [Google Scholar]
- 129.Frith C. What do imaging studies tell us about the neural basis of autism? Novartis Found. Symp. 2003;251:149–197. doi: 10.1002/0470869380.ch10. [DOI] [PubMed] [Google Scholar]
- 130.Grossman J.B., Klin A., Carter A.S., Volkmar F.R. Verbal bias in recognition of facial emotions in children with Asperger syndrome. J. Child. Psychol. Psyc. 2003;41:369–379. [PubMed] [Google Scholar]