Abstract
Purpose
Limited data are available on the efficacy of oral bisphosphonate therapy in breast cancer survivors. Our goal was to examine prevention of breast cancer–related bone loss in this cohort.
Patients and Methods
Eighty-seven postmenopausal women after chemotherapy for breast cancer were randomly assigned to once-weekly risedronate 35 mg or placebo for 24 months. Outcomes included bone mineral density (BMD) and turnover markers.
Results
At study initiation, 13% of patients were on an aromatase inhibitor (AI). After 24 months, there were differences of 1.6 to 2.5% (P < .05) at the spine and hip BMD between the placebo and risedronate groups. At study completion, 44% were on an AI. Adjusting for an AI, women on placebo plus AI had a decrease in BMD of (mean ± SE) 4.8% ± 0.8% at the spine and 2.8% ± 0.5% at the total hip (both P < .001). In women on risedronate + AI, the spine decreased by 2.4% ± 1.1% (P < .05) and was stable at the hip. Women in the placebo group not on an AI, maintained BMD at the spine, and had a 1.2% ± 0.5% loss at the total hip (P < .05). Women who received risedronate but no AI had the greatest improvement in BMD of 2.2% ± 0.9% (P < .05) at the total hip. Bone turnover was reduced with risedronate. There were no differences in adverse events between the groups.
Conclusion
We conclude that in postmenopausal women with breast cancer with or without AI therapy, once-weekly oral risedronate was beneficial for spine and hip BMD, reduced bone turnover, and was well tolerated.
INTRODUCTION
Although adjuvant chemotherapy has prolonged disease-free and overall survival in women with breast cancer, chemotherapy-induced early menopause is associated with bone loss and osteoporotic fractures.1–7 The Risedronate Effect on Bone Loss in Breast Cancer (REBBeCa) trial was designed to examine the efficacy of risedronate once weekly, an oral antiresorptive therapy, in the prevention of bone loss in newly postmenopausal women with breast cancer treated with chemotherapy.8 After 1 year, bone mass significantly increased at the spine and the hip with risedronate compared with placebo.8
At baseline, roughly three fourths of these patients were on tamoxifen with 13% on an aromatase inhibitor (AI). However, with the resulting new information on AIs for the prevention of breast cancer recurrence, the standard of care shifted and more women were switched from tamoxifen to an AI.1,9 The second year of the study presents the efficacy of risedronate to prevent bone loss over 24 months. However, we were also able to examine the impact of treatment with or without concomitant use of an AI.
PATIENTS AND METHODS
Newly postmenopausal women (≤ 8 years post-menopausal and verified by gonadotropin levels) with stage I–III breast cancer in the greater Pittsburgh area who were treated with chemotherapy were screened for this study as previously reported.8 Women were included with or without tamoxifen, an antiestrogen, or an AI concomitant therapy. During the 24 months of the trial, a portion of women were switched from tamoxifen to an AI or started on an AI by their private physician. Women with any illness known to affect bone mineral metabolism or on medications known to affect bone mineral metabolism were excluded. If a patient had an initial bone mineral density T-score in the osteoporotic range at the hip or spine or an adult fragility fracture they were counseled about options for therapy versus participation in the trial. Eighty-seven women were randomly assigned onto the study. The protocol was approved by the University of Pittsburgh institutional review board and all participants provided written informed consent before participation.
Study Design
The study was a double-blind, placebo-controlled, randomized clinical trial over 12 months with a 12-month extension. The prespecified sample size and analyses for year 1 have previously been reported.8 Patients were randomly assigned by computer generation to active treatment, risedronate 35 mg orally, once weekly or matching placebo. We assessed compliance by pill count.
We assessed dietary calcium intake with a validated questionnaire.10 Subjects found to have calcium intake below 1,200 mg a day received supplements containing calcium carbonate 500 mg with 200 Us of vitamin D per tablet (Oscal plus D; GlaxoSmithKline, Middlesex, United Kingdom).
Outcome Variables
The primary outcome variables for the 24-month study included change in spine and hip bone mineral density. Additional outcomes included biochemical markers of bone turnover and safety. Bone mineral density was assessed at the spine (posterior anterior and lateral), hip (femoral neck, total hip, trochanter, intertrochanter), one-third distal and total radius at baseline, 6, 12, 18, and 24 months using dual energy x-ray absorptiometry (QDR 4500A; Hologic Inc, Bedford, MA). The coefficient of bone mineral density using our densitometer is 1.3% for the PA spine and 1.4% for the total hip.11 We assessed the 25-hydroxyvitamin D by radioimmunoassay (ng/mL; Nichol Advantage; Nichols Institute Diagnostics, San Juan Capistrano, CA) and intact parathyroid hormone (pg/mL; Bayer Centaur parathyroid hormone immunoassays, Bayer, Tarrytown, NY). For bone resorption, we assessed a second morning urine for urinary N-telopeptide crosslinked collagen type 1 (NTX, nmol bone collagen equivalents/mmol creatinine; Osteomark, Ostex International, Seattle, WA). Our markers of bone formation included serum osteocalcin (ng/mL; Novacalcin; Quidel Corporation, Mountain View, CA) and serum intact N-terminal propeptide of type 1 procollagen (P1NP, ng/mL; Orion Diagnostica Inc, Espoo, Finland).
Statistical Analysis
Baseline subject characteristics were compared between the two treatment groups using independent samples t-, Wilcoxon rank sum, χ2 or Fisher’s exact tests, as appropriate, depending on the nature and distributions of variables. To compare outcomes between the two groups, we fitted mixed models using SAS MIXED (SAS Institute, Cary, NC) procedure with change from baseline in each outcome as the response variable; treatment group and time (6, 12, 18, and 24 months) as the fixed effects of interest; baseline measurement of the outcome as a covariate; and a subject random effect to account for multiple measurements from the same subjects over time.
In a secondary analysis, we examined the changes in outcomes in the two treatment groups considering the increasing use of an AI as adjuvant cancer therapy. We operationally considered a subject to have been an AI user at a given assessment if she had been on anastrozole, exemestane, or letrozole for ≥ 3 months since the previous assessment, and excluded subjects who switched back to no AI cancer therapy during the trial. Estimation-maximization algorithm12 was used to impute outcomes of those changing their cancer therapy to an AI midtrial. First, a mixed linear model was fit using only data from subjects before switching cancer therapy, with change from baseline in each outcome as the response variable; treatment group assignment, time (6, 12, 18, and 24 months), dominant cancer therapy since previous assessment (AI/no AI), and treatment group × cancer therapy interaction as fixed effects of interest; baseline measurement of the outcome as a covariate; and a subject random effect. Using this model, we estimated outcome penalties (eg, possible loss of bone mineral density) at each time point for switching cancer therapy to an AI. Second, we used these penalties to adjust outcomes after switching to an AI so that data for those subjects could be analyzed as if they had been on an AI from the start of the trial. Without such an adjustment, the possibly greater bone mineral density of those switching to an AI later in the trial would contribute to underestimating the effect of AI on bone mineral density. If there is in fact no such AI effect on bone mineral density, the estimated penalties will be very small and would not alter the results. The first and second steps mentioned were performed iteratively a large number of times, each time with an updated set of penalty estimates, until they stabilized and converged. Both raw and percent changes were used in all analyses to ensure robustness of results.
RESULTS
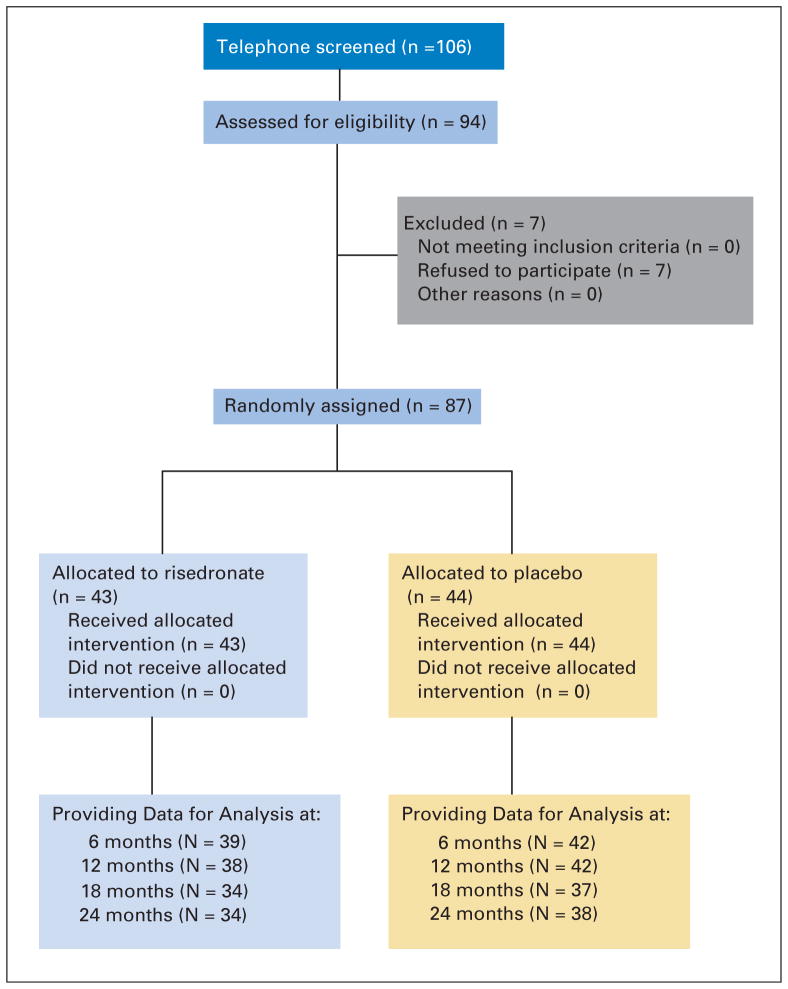
As previously described, 106 women were screened with 87 eligible for random assignment (Fig 1). At baseline, groups had similar clinical characteristics (Table 1). In the placebo group, breast cancer treatments included six women on an AI and 38 not on an AI (includes 25 on tamoxifen and one on toremifene citrate). In the risedronate group, five women were on an AI and 38 women were not on an AI (including 22 on tamoxifen, one on toremifene citrate, and one on fulvestrant). There were no differences in indices of bone mineral metabolism or density between treatment groups. Bone formation was significantly higher in the risedronate group as assessed by P1NP but not osteocalcin. The mean bone density measurements were in the normal range by WHO criteria,13 2% had osteoporosis, and the remainder was classified as having normal or low bone mass.
Fig. 1.
Flow diagram of the progress through the phases of the randomized trial.
Table 1.
Baseline Characteristics, Bone Mineral Density, and Bone Turnover
| Variable | PBO (n = 44)
|
RIS (n = 43)
|
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | 49 ± 5.9 | 50.1 ± 5.1 | .52 | ||
|
| |||||
| Years postmenopausal | 3.2 ± 2.0 | 3.4 ± 2.0 | .61 | ||
|
| |||||
| Body mass index, kg/m2 | 27.1 ± 5.2 | 28.3 ± 6.3 | .35 | ||
|
| |||||
| Dietary calcium, mg/d | 691 ± 347 | 755 ± 513 | .50 | ||
|
| |||||
| Breast cancer treatment | |||||
| Chemotherapy | 44 | 100 | 43 | 100 | NA |
| Lumpectomy | 27 | 61.4 | 28 | 65.1 | .83 |
| Mastectomy | 20 | 45.5 | 21 | 48.8 | .83 |
| Radiation therapy | 32 | 72.7 | 31 | 72.1 | 1.00 |
|
| |||||
| Tamoxifen, toremifene citrate, or fulvestrant | 30 | 68.2 | 32 | 74.4 | .64 |
| Aromatase inhibitor | 6 | 13.6 | 8 | 18.6 | .57 |
|
| |||||
| Bone metabolism | |||||
| Calcium, mg/dL | 9.1 ± 1.8 | 9.2 ± 0.4 | .57 | ||
| 25-hydroxyvitamin D, ng/mL | 29.3 ± 14.6 | 30.8 ± 18.9 | .68 | ||
| Parathyroid hormone, pg/mL | 45.3 ± 21.0 | 52.6 ± 23.9 | .14 | ||
|
| |||||
| Bone mineral density, g/cm2 | |||||
| Posteroanterior lumbar spine | 0.995 ± 0.127 | 0.985 ± 0.119 | .70 | ||
| Lateral lumbar spine | 0.737 ± 0.094 | 0.727 ± 0.098 | .64 | ||
| Total hip | 0.901 ± 0.103 | 0.910 ± 0.094 | .66 | ||
| Femoral neck | 0.768 ± 0.099 | 0.785 ± 0.105 | .43 | ||
|
| |||||
| T-score (± standard deviation) | |||||
| PA spine | −0.50 ± 1.15 | −0.61 ± 1.1 | .84 | ||
| Total hip | −0.37 ± 0.81 | −0.28 ± 0.75 | .44 | ||
| Femoral neck | −0.77 ± 0.85 | −0.59 ± 0.93 | .24 | ||
|
| |||||
| Bone turnover marker | |||||
| Urinary NTX, nmole BCE/mmol creatinine | 38.9 ± 17.6 | 46.9 ± 28.6 | .12 | ||
| Osteocalcin, ng/mL | 18.2 ± 7.3 | 20.7 ± 7.0 | .11 | ||
| P1NP, μg/mL | 49.4 ± 23.2 | 61.6 ± 23.8 | .02 | ||
Abbreviations: PBO, placebo; RIS, risedronate; PA, posterior anterior; NTX, N-telepeptide crosslinked collegen type 1; P1NP, N-terminal propeptide of type 1 procollagen.
At the end of year 1, four women in the placebo group and five women in the risedronate group declined to continue in the 12-month extension. After 24 months, 18 participants were on placebo and an AI (placebo plus AI), 20 were on risedronate and an AI (risedronate plus AI), 26 were on placebo and no AIs (placebo plus no AI), and 23 were on risedronate and no AIs (risedronate plus no AI). Three participants switched from an AI to a no AI during the trial. Compliance, as defined by taking at least 80% of the medication, was 70.5% in the placebo and 65.1% in the risedronate groups (P =.88). Thirty-four women in the risedronate and 38 in the placebo group completed the entire 24 months of the study.
Comparison of Treatment Groups
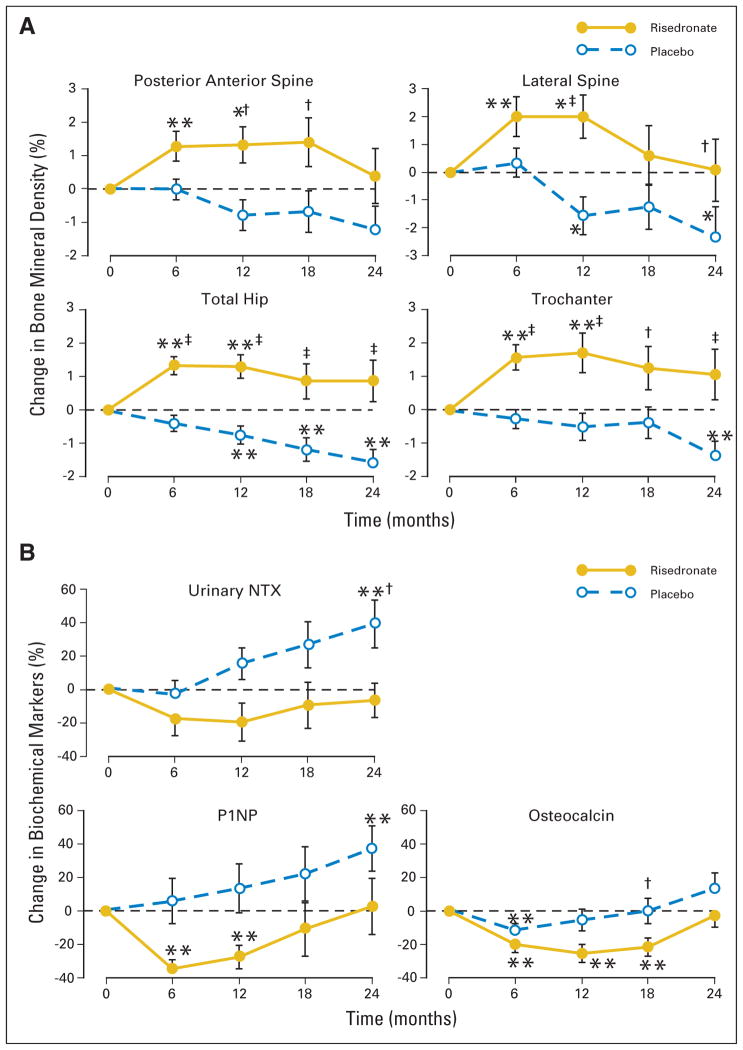
After 24 months, women in the placebo group had significant (1.4% to 2.4%) losses of bone mineral density at all sites (including lateral spine, total hip, femoral neck, trochanter, one-third distal radius, and total radius) except for the PA spine (Fig 2; Table 2). Bone mineral density remained stable in the risedronate group except for significant losses at the radial sites. Overall, bone mineral density was significantly greater in the lateral spine, total hip, trochanteric, and femoral neck sites in the women on risedronate compared with those on placebo (all P < .05; Table 2). There were no significant differences between the groups in the bone mineral density of the one-third distal radius and total radius. Urinary NTX was significantly higher in patients on placebo compared with women on risedronate (P < .05). There were no significant differences in osteocalcin or P1NP between the groups (Fig 2 and Table 2).
Fig. 2.
(A) Mean (SE) percent changes in bone mineral density from baseline to 24 months. (B) Mean (SE) percent changes in biochemical markers of bone turnover from baseline to 24 months. (*) P < .05; (**) P < .01 changes from baseline; (†) P < .05; (‡) P < .01 comparison between risedronate and placebo groups.
Table 2.
Bone Mineral Density and Bone Turnover Marker % Change at 24 Months by Treatment Groups
| Outcome | Change (% ± standard deviation)
|
P for Difference Between Groups | |||
|---|---|---|---|---|---|
| Placebo
|
Risedronate
|
||||
| Mean ± SD | P | Mean ± SD | P | ||
| Bone mineral density | |||||
| Spine | −1.2 ± 0.7 | .088 | 0.4 ± 0.8 | .63 | .082 |
| Lateral spine | −2.4 ± 1.1 | .041 | 0.1 ± 1.1 | .951 | .048 |
| Total hip | −1.6 ± 0.4 | .001 | 0.9 ± 0.6 | .176 | < .001 |
| Trochanter | −1.4 ± 0.4 | .004 | 1.1 ± 0.8 | .169 | < .001 |
| Femoral neck | −1.6 ± 0.8 | .044 | −0.0 ± 0.6 | .988 | .047 |
| 1/3 distal radius | −1.6 ± 0.5 | .004 | −1.5 ± 0.7 | .049 | .743 |
| Total radius | −2.1 ± 0.3 | < .001 | −1.7 ± 0.6 | .008 | .533 |
|
| |||||
| Bone turnover | |||||
| Urinary NTX | 39.4 ± 14.4 | .0097 | −6.5 ± 10.4 | .534 | .020 |
| P1NP | 37.2 ± 13.5 | .009 | 2.8 ± 16.7 | .870 | .195 |
| Osteocalcin | 13.7 ± 9.3 | .151 | −2.5 ± 7.4 | .736 | .1850 |
Abbreviations: SD, standard deviation; NTX, N-telepeptide crosslinked collagen type 1; P1NP, N-terminal propeptide of type 1 procollagen.
Comparisons of Treatment and Cancer Therapy Groups
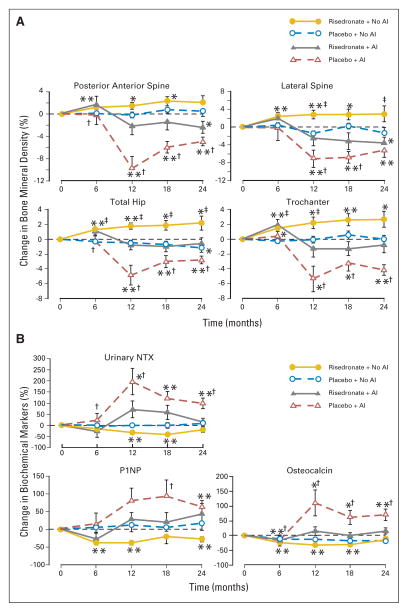
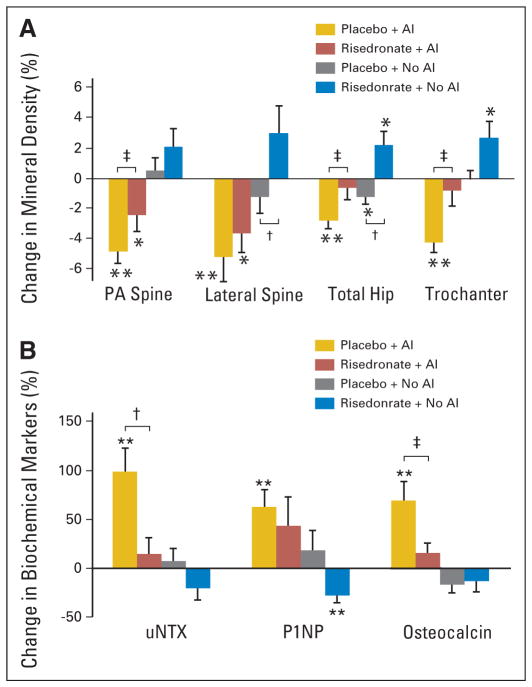
There were significant differences across these groups with respect to bone mineral density at 2 years. For example, bone density changes (mean ± SE) at the spine included a decrease of 4.8% ± 0.8% (P < .01) for placebo plus AI, a decrease of 2.4% ± 1.1% (P < .05) for risedronate plus AI, no significant change for placebo plus no AI, and an increase of 2.4% ± 0.8% (P = .011) at 18 months for risedronate plus no AI that was no longer significant at 24 months (Table 3; Figs 3 and 4). In the groups on an AI, bone mineral density loss was greater in women on placebo compared with risedronate (P < .01; Fig 3). When patients on AIs were compared, there was a difference of 2.4 ± 1.0 percentage points in spine bone density between women on placebo and risedronate (P = .025). The placebo group had greater gains in spine bone density if they were not on an AI compared with those on an AI with a difference of 5.7 ± 0.8 percentage points (P < .0001). This was also true for the risedronate group; there was a difference in spine bone mass of 5.1 ± 0.9 percentage points (P < .001) when comparing those who were on an AI compared with those who were not. Similar significant differences were noted at the lateral spine (Figs 3 and 4).
Table 3.
Bone Mineral Density and Biochemical Markers at 24 Months by Treatment and AI Use
| Parameter | Change in Outcome (%)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A
|
B
|
C
|
D
|
|||||||||
| Placebo + AI
|
Risedronate + AI
|
Placebo + No AI
|
Risedronate + No AI
|
|||||||||
| Mean | SE | P | Mean | SE | P | Mean | SE | P | Mean | SE | P | |
| Bone mineral density | ||||||||||||
| Spine | −4.8 | 0.8 | < .001 | −2.4 | 1.1 | .04 | 0.5 | 0.9 | .57 | 2.1 | 1.2 | .09 |
| Lateral spine | −5.2 | 1.6 | .004 | −3.6 | 1.3 | .01 | −1.2 | 1.1 | .29 | 3.0 | 1.8 | .12 |
| Total hip | −2.8 | 0.5 | < .001 | −0.6 | 0.8 | .44 | −1.2 | 0.5 | .04 | 2.2 | 0.9 | .04 |
| Trochanter | −4.2 | 0.7 | < .001 | −0.8 | 1.0 | .41 | −0.0 | 0.5 | .96 | 2.7 | 1.1 | .03 |
| Femoral neck | −2.4 | 1.1 | .04 | −2.5 | 0.8 | .004 | −1.4 | 1.2 | .25 | 1.9 | 0.9 | .04 |
| 1/3 radius | −2.1 | 0.6 | .003 | −2.7 | 1.1 | .03 | −1.2 | 0.9 | .19 | −0.6 | 0.8 | .45 |
| Total radius | −3.0 | 0.3 | < .001 | −3.3 | 1.0 | .003 | −1.4 | 0.5 | .02 | −0.6 | 0.6 | .32 |
|
| ||||||||||||
| Biochemical markers | ||||||||||||
| Urinary NTX | 99.4 | 24.1 | < .001 | 15.3 | 15.9 | .35 | 7.5 | 13.2 | .58 | −20.3 | 11.8 | .11 |
| P1NP | 63.5 | 17.2 | .002 | 44.0 | 29.5 | .15 | 18.3 | 20.8 | .39 | −27.4 | 7.8 | .003 |
| Osteocalcin | 69.9 | 19.5 | .002 | 15.5 | 10.4 | .15 | −16.6 | 8.3 | .06 | −13.3 | 10.5 | .23 |
Abbreviations: AI, aromatase inhibitor; NTX, N-telepeptide crosslinked collagen type 1; P1NP, N-terminal propeptide of type 1 procollagen.
Fig. 3.
(A) Mean (SE) percent changes in bone mineral density from baseline to 24 months. (B) Mean (SE) percent changes in biochemical markers of bone turnover from baseline to 24 months. (*) P < .05; (**) P < .01 changes from baseline; (†) P < .05; (‡) P < .01 comparison between risedronate plus no aromatase inhibitor (AI) and placebo plus no AI or risedronate plus AI and placebo plus AI groups.
Fig. 4.
(A) Mean (SE) percent change in bone mineral density from baseline to 24 months. (B) Mean (SE) percent change in biochemical markers of bone turnover from baseline to 24 months. (*) P < .05; (**) P < .01 changes from baseline; (†) P < .05; (‡) P < .01 comparison between placebo plus aromatase inhibitor (AI) and risedronate plus AI or placebo plus no AI and risedronate plus no AI groups.
At the total hip, placebo plus AI group had the greatest loss of bone mass of 2.8% ± 0.5% (P < .001) compared with risedronate plus AI group which maintained bone mass, or compared with the risedronate plus no AI group which gained 2.2% ± 0.9% (P < .05) at the total hip (Table 3; Figs 3 and 4). Among those on an AI, risedronate group gained 3.0 ± 0.7 percentage points more than the placebo group. Similarly, among women receiving placebo, no AI group gained 2.6 ± 0.6 percentage points (P < .001) more at the hip compared with those on an AI. In addition, those on risedronate had greater improvements in bone mass if they had not been concurrently treated with an AI compared with if they had been treated with an AI (a difference of 2.1 ± 0.6 percentage points; P < .01). Similar differences were also noted at the femoral neck and trochanteric sites (Table 3; Figs 3 and 4).
At the one-third distal radius, there were significant decreases in bone density of 2.1% ± 0.6% and 2.7% ± 1.1% (both P < .05) for women in the placebo plus AI and risedronate plus AI groups (Table 3). Bone density remained stable in women not on an AI. Among women on risedronate, those who were not on an AI, had a 2.8 ± 0.9 percentage point greater bone mineral density change at the one-third distal radius compared with those on an AI (P = .002). Similar changes were observed at the total radius.
Bone turnover was greatest in the placebo plus AI group (Table 3; Fig 3). Urinary NTX increased approximately 99% ± 24% (P < .001) in the placebo plus AI group compared with risedronate plus AI and placebo plus no AI groups, which had no significant change. Risedronate plus no AI group demonstrated a significant decrease at 12 and 18 months that was no longer significant at 24 months (Fig 3). Similar trends were noted for the markers of bone formation. Placebo plus AI group had the greatest increases in osteocalcin and P1NP over the 24 months. Risedronate plus no AI group had significant decreases in P1NP and osteocalcin observed at 6 months (Fig 3).
Risedronate was well tolerated with no differences in adverse event rates between women receiving risedronate and placebo. After 2 years, there were two fractures in the placebo and three in the risedronate group. There were no significant differences in hospitalizations, gastrointestinal symptoms, arthraligias, or recurrence of breast cancer.
DISCUSSION
Oral risedronate once weekly prevented bone loss or improved bone mass, decreased bone turnover, and was well-tolerated in postmenopausal women with chemotherapy-induced menopause with or without adjuvant hormone therapy. Several previous studies have examined the impact of intravenous or oral bisphosphonates on skeletal health. The Zometa-Femera Adjuvant Synergy Trial (Z-FAST) involved postmenopausal women with early-stage breast cancer who were started on the AI, letrozole, and randomly assigned to upfront intravenous zoledronic acid every 6 months versus delayed therapy.14 At 12 months, spine bone mineral density was 4.4% higher in the first-line group compared with the delayed group. Bone turnover was decreased in the first-line group compared with increases in the delayed group.14 Delmas et al15 randomly assigned 53 women with breast cancer–induced menopause (36 on tamoxifen) to oral risedronate versus placebo. The risedronate was given as a 30-mg daily dose for 2 weeks followed by 10 weeks off therapy. At 24 months, the difference in bone mineral density between the two groups was 2.5% at the spine and 2.6% at the femoral neck. Other studies have examined the efficacy of clondronate,16–19 which is unavailable in the United States. Our study is the first to examine the US Food and Drug Administration–approved dose of the oral bisphosphonate risedronate once weekly for this indication.
The American Society of Clinical Oncology updated guidelines for the treatment of bone health in women with a history of breast cancer in 2003.20 They expanded the recommendations from 200021 and urged oncologists to take an expanded role in the assessment of bone health.20 They suggested all women on an AI have a bone density and women with osteoporosis receive treatment. For women with low bone mass (osteopenia) or a normal bone density, they suggested lifestyle changes, calcium, vitamin D, and follow-up bone mineral density. This study provides additional data to support and further broaden these guidelines. Given the significant bone loss in the women who did not have osteoporosis at baseline, our data would support treatment in women with low bone mass in addition to those with osteoporosis. Furthermore, all women should have adequate daily intake of calcium and vitamin D.20,22,23
During the second year we noted significant differences between the two groups for osteocalcin and urinary NTX. When women were examined with and without concomitant AI therapy, risedronate decreased urinary NTX in women on an AI compared with the placebo group on an AI. A similar trend was observed for women not on an AI. Other investigators have examined biochemical markers in women with breast cancer on aromatase inhibitors versus tamoxifen. Banerjee et al24 examined women treated with anastrozole, tamoxifen alone, or the combination and reported increases in bone resorption in women on anastrozole compared with no change in women on tamoxifen or the combination of both. Similar results were reported in the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial.25 They found increases in bone turnover markers in the women on the AI compared with decreases in women on tamoxifen. In a recent surveillance study, women with breast cancer were observed after 1 year of treatment with an AI.26 In women treated with risedronate, there were decreases in bone resorption, compared with increases in bone resorption in the women who were on AI but not on risedronate. Our study extends these findings in a double-blind, randomized trial. We found the greatest increases in bone resorption in women who were on an AI in the placebo group with lower levels if they were on risedronate. There was relative maintenance of bone turnover in women who were on placebo and not an AI and a further decrease in the women who were on risedronate and no AI. These findings suggest that risedronate is able to reduce bone turnover with or without concomitant treatment of an AI.
This study had several limitations. We were not powered to examine fracture efficacy. Because on average women had a bone density classification in the normal range, it was unlikely that we would see a fracture in this study. During the course of the study, due to a shift in the standard of care, women were switched from tamoxifen to AIs or started on an AI by their physicians. Although this made the analysis more challenging, we were able to examine the impact of an AI on bone and the response to risedronate. This makes the results more generalizable. Because studies are ongoing regarding the use of AIs or estrogen agonist/antagonists in this population, future recommendations may be modified.
This study has several advantages. Our study was performed at a single center and bone density assessments were performed by the same technologist on the same machine. The dose and schedule of risedronate is the US Food and Drug Administration–approved dose for the prevention and treatment of postmenopausal osteoporosis and has been shown to reduce hip, vertebral, and nonvertebral fractures.27,28 Moreover, although a meta-analysis of drug therapy for osteoporosis reported that up to one half of patients do not take medications as directed,29 we observed a compliance rate of 65% to 70% at 2 years. There were no significant differences in adverse outcomes associated with oral bisphosphonates. Furthermore, because it was not an intravenous bisphosphonate, it was less likely to have concern for osteonecrosis of the jaw or atrial fibrillation which has recently been reported with intravenous bisphosphonates.30 Finally, the changes in biochemical markers in bone turnover reflect a mirror image of the change noted in bone mass. Previous studies suggest that markers are an independent risk factor for fractures.31 The reductions in markers of bone turnover with risedronate would suggest an additional advantage for fracture prevention in these women.
In summary, we found that once-weekly oral risedronate was successful at maintaining or improving bone mass in postmenopausal women with cancer-related bone loss. This medication was well-tolerated and proved to be effective with or without the use of an AI. Further studies are needed to determine whether these improvements in bone mass and decreases in bone turnover translate to fracture reduction for these patients.
Acknowledgments
Supported by the National Institutes of Health (NIH)/National Institute of Diabetes and Digestive and Kidney Diseases (Grant No. K24 DK062895-03), a Procter and Gamble and Sanofi-aventis noncompany-sponsored trial grant, and the University of Pittsburgh Montefiore University Hospital Clinical Translational Research Center (NIH/National Center for Research Resources [NCRR]/Clinical Translational Science Institute Grant No. UL1 RR024153 and NIH/NCRR/GCRC Grant No. M01RR000056).
We thank the University of Pittsburgh Clinical Translational Research Center staff and the data safety monitoring board members.
Footnotes
This study appears in the ClinicalTrials.gov registry with the identifier NCT00118508.
Clinical Trials repository link available on JCO.org.
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO’s conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Susan L. Greenspan, Procter and Gamble (C); Victor G. Vogel, Procter and Gamble (C) Stock Ownership: None Honoraria: Susan L. Greenspan, Procter and Gamble; Rajib Bhattacharya, Procter and Gamble Research Funding: Susan L. Greenspan, Procter and Gamble; Rajib Bhattacharya, Procter and Gamble; Victor G. Vogel, Procter and Gamble Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Susan L. Greenspan, Rajib Bhattacharya, Karen T. Vujevich, Susan M. Sereika, Victor G. Vogel
Financial support: Susan L. Greenspan Administrative support: Susan L. Greenspan
Provision of study materials or patients: Susan L. Greenspan, Adam Brufsky, Barry C. Lembersky, Victor G. Vogel
Collection and assembly of data: Susan L. Greenspan, Adam Brufsky, Rajib Bhattacharya, Karen T. Vujevich, Susan M. Sereika
Data analysis and interpretation: Susan L. Greenspan, Subashan Perera, Victor G. Vogel
Manuscript writing: Susan L. Greenspan, Adam Brufsky, Subashan Perera
Final approval of manuscript: Susan L. Greenspan, Adam Brufsky, Barry C. Lembersky, Rajib Bhattacharya, Karen T. Vujevich, Subashan Perera, Susan M. Sereika, Victor G. Vogel
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Pfeilschifter J, Diel IJ. Osteoporosis due to cancer treatment: Pathogenesis and management. J Clin Oncol. 2000;18:1570–1593. doi: 10.1200/JCO.2000.18.7.1570. [DOI] [PubMed] [Google Scholar]
- 3.del Mastro L, Venturini M, Sertoli MR, et al. Amenorrhea induced by adjuvant chemotherapy in early breast cancer patients: Prognostic role and clinical implications. Br Cancer Res Treat. 1997;43:183–190. doi: 10.1023/a:1005792830054. [DOI] [PubMed] [Google Scholar]
- 4.Schilsky RL, Lewis BJ, Sherins RJ, et al. Gonadal dysfunction in patients receiving chemotherapy for cancer. Ann Intern Med. 1980;93:109–116. doi: 10.7326/0003-4819-93-1-109. [DOI] [PubMed] [Google Scholar]
- 5.Shapiro CL, Manola J, LeBoff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19:3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Maricic M, Bassford TL, et al. Fracture risk among breast cancer survivors: Results from the Women’s Health Initiative Observational study. Arch Intern Med. 2005;165:552–558. doi: 10.1001/archinte.165.5.552. [DOI] [PubMed] [Google Scholar]
- 7.Chien AJ, Goss PE. Aromatase inhibitors and bone health in women with breast cancer. J Clin Oncol. 2006;24:5305–5312. doi: 10.1200/JCO.2006.07.5382. [DOI] [PubMed] [Google Scholar]
- 8.Greenspan SL, Bhattacharya RK, Sereika SM, et al. Prevention of bone loss in survivors of breast cancer: A randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2007;92:131–136. doi: 10.1210/jc.2006-1272. [DOI] [PubMed] [Google Scholar]
- 9.Althuis MD, Dozier JM, Anderson WF, et al. Global trends in breast cancer incidence and mortality 1973–1997. Int J Epidemiol. 2005;34:405–412. doi: 10.1093/ije/dyh414. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Jacques P, Shipp C. Dietary calcium intake and bone loss from the spine in healthy postmenopausal women. Am J Clin Nutr. 1987;46:685–687. doi: 10.1093/ajcn/46.4.685. [DOI] [PubMed] [Google Scholar]
- 11.Towers AL, Clay CA, Sereika SM, et al. Skeletal integrity in patients with nail patella syndrome. J Clin Endocrinol. 2005;90:1961–1965. doi: 10.1210/jc.2004-0997. [DOI] [PubMed] [Google Scholar]
- 12.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via EM algorithm. J Royal Stat Soc Series B: Stat Methodology. 1977;39:1–38. [Google Scholar]
- 13.Kanis JA for the WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 14.Brufsky A, Harker WG, Beck JT, et al. Zoledronic acid inhibits adjuvant letrozole-induced bone loss in postmenopausal women with early breast cancer. J Clin Oncol. 2007;25:829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 15.Delmas PD, Balena R, Confravreux E, et al. Bisphosphonate risedronate prevents bone loss in women with artificial menopause due to chemotherapy of breast cancer: A double blind, placebo controlled study. J Clin Oncol. 1997;15:955–962. doi: 10.1200/JCO.1997.15.3.955. [DOI] [PubMed] [Google Scholar]
- 16.Saarto T, Elomas I. Chemical castration induced by adjuvant cyclophosphamide, methotrexate, and fluorouracil chemotherapy causes rapid bone loss that is reduced by clodronate: A randomized study in premenopausal breast cancer patients. J Clin Oncol. 1997;15:1341–1347. doi: 10.1200/JCO.1997.15.4.1341. [DOI] [PubMed] [Google Scholar]
- 17.Vehmanen L, Saarto T, Risteli J, et al. Short-term intermittent intravenous clodronate in the prevention of bone loss related to chemotherapy-induced ovarian failure. Breast Cancer Res Treat. 2004;87:181–188. doi: 10.1023/B:BREA.0000041624.00665.4e. [DOI] [PubMed] [Google Scholar]
- 18.Vehmanen L, Saarto T, Elomaa I, et al. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients: The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37:2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 19.Powles TJ, McCloskey E, Patterson AHG, et al. Oral clodronate and reduction in loss of bone mineral density in women with operable primary breast cancer. J Natl Can Inst. 1998;90:704–708. doi: 10.1093/jnci/90.9.704. [DOI] [PubMed] [Google Scholar]
- 20.Hillner BE, Ingle JN, Chlebowki RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21:4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Hillner BE, Ingle JN, Berenson JR, et al. American Society of Clinical Oncology guideline on the role of bisphosphonates in breast cancer. J Clin Oncol. 2000;18:1378–1391. doi: 10.1200/JCO.2000.18.6.1378. [DOI] [PubMed] [Google Scholar]
- 22.Gralow JR. Bone density in breast cancer: When to intervene? J Clin Oncol. 2007;25:3194–3197. doi: 10.1200/JCO.2007.12.3430. [DOI] [PubMed] [Google Scholar]
- 23.Perez EA, Weilbaecher K. Aromatase inhibitors and bone loss. Oncology. 2006;20:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee S, Smith IE, Folkerd L, et al. Comparative effects of anastrozole, tamoxifen alone and in combination on plasma lipids and bone-derived resorption during neoadjuvant therapy in the IMPACT trial. Ann Oncol. 2005;16:1632–1638. doi: 10.1093/annonc/mdi322. [DOI] [PubMed] [Google Scholar]
- 25.Eastell R, Hannon RA, Cuzick J, et al. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial. J Bone Miner Res. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 26.Confavreux CB, Fontana A, Guastalla JP, et al. Estrogen-dependent increase in bone turnover and bone loss in postmenopausal women with breast cancer treated with anastrozole. prevention with bisphophonates. Bone. 2007;41:346–352. doi: 10.1016/j.bone.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 27.McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–340. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 28.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: A randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 29.Kothawala P, Badamgarav E, Ryu S, Miller RM, et al. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–1501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 30.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 31.Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: The EPIDOS prospective study. J Bone Miner Res. 1996;11:1531–1538. doi: 10.1002/jbmr.5650111021. [DOI] [PubMed] [Google Scholar]