Abstract
Public health initiatives focused on obesity prevention and lifestyle intervention programmes for patients with obesity have struggled to contain the obesity epidemic to date. In recent years, antiobesity drug therapies have had a limited role in clinical treatment algorithms for patients with obesity. Indeed, a number of high-profile antiobesity drug suspensions have markedly impacted upon the landscape of obesity pharmacotherapy. In this review, we discuss the advent of an increasing array of pharmacotherapeutic agents, which are effective both in inducing weight loss and in maintaining weight loss achieved by lifestyle measures. The development of these drugs as antiobesity agents has followed varying paths, ranging from lorcaserin, a selective serotonin agent, exploiting the beneficial central actions of fenfluramine but without the associated systemic side effects, to liraglutide, a gut hormone already used as a glucose-lowering drug but with appetite-suppressant properties, or the novel drug combination of phentermine/topiramate, two ‘old’ drugs used in lower doses than with previous therapeutic uses, resulting in an additive effect on weight loss and fewer side effects. We summarize the key findings from recent randomized controlled trials of these three drugs. Although these agents lead to clinically important weight loss when used as monotherapy, the use of antiobesity drugs as adjunctive therapy post intensive lifestyle intervention could prove to be the most successful strategy. Moreover, a progressive approach to obesity pharmacotherapy perhaps offers the best opportunity to finally address the obesity crisis on a mass scale.
Keywords: appetite suppressant, liraglutide, lorcaserin, obesity, pharmacotherapy, phentermine, topiramate, weight loss
Introduction
Obesity is a serious and increasing threat to the health of populations globally. The high burden of comorbidities, such as type 2 diabetes (T2D), cardiovascular disease and certain cancers, associated with obesity heighten the severity of this obesity crisis [Whitlock et al. 2009]. Globally, over 200 million men and almost 300 million women were obese, defined by body mass index (BMI ≥ 30 kg/m2) in 2008, which represents an approximate doubling of the prevalence of obesity since 1980 [Finucane et al. 2011]. The prevalence of obesity is already considered to have reached epidemic proportions in western societies in particular, where in some countries, despite a recent plateau a prevalence of greater than 30% has been documented [Flegal et al. 2012]. Severe obesity, in particular, imposes disproportionately high healthcare and economic burdens at individual and societal levels [Grieve et al. 2013]. Public health initiatives to date have struggled to contain increasing obesity incidence, and are hampered by failures in measuring effects of preventative strategies, as revealed in a recent US Institute of Medicine report (http://iom.edu/Reports/2013/Evaluating-Obesity-Prevention-Efforts-A-Plan-for-Measuring-Progress.aspx accessed 21 August 2013). While prevention of obesity is the strategic imperative, treatment of patients with obesity is also an immediate priority. In this review, we discuss the advent of an increasing array of pharmacotherapeutic agents, which are effective both in inducing weight loss and in maintaining weight loss achieved by lifestyle measures. Unlike bariatric surgery, pharmacotherapy offers an opportunity for improving treatment outcomes to the many millions of patients who can benefit from weight loss.
Position of pharmacotherapy in clinical obesity management
Lifestyle modification remains the cornerstone of weight management. Lifestyle intervention programmes which may include dietetic, exercise or psychological aspects, are effective in reducing weight in the short to medium term [Finer, 2001; Loveman et al. 2011], as are more intensive meal replacements or very low energy diets for patients with severe obesity (BMI ≥ 35 kg/m2) [Saris, 2001; Lean et al. 2013a]. However in the long term, most will regain much of their lost weight [Anderson et al. 2001]. Currently, bariatric surgery is reserved for patients with severe or complex obesity (BMI ≥ 40 kg/m2 or ≥ 35 kg/m2 in the presence of at least one obesity-related comorbidity), and is the most effective treatment in this population, both in terms of amount of weight loss achieved and durability of weight loss or ‘weight maintenance’, as well as amelioration of obesity-related comorbidities [Sjostrom et al. 2007, 2012; Kashyap et al. 2013]. The Swedish Obese Subjects study has shown that, compared with conservative management, bariatric surgery is also associated with a long-term reduction in all-cause mortality [Sjostrom et al. 2007]. However, surgery will never be able to be performed in anything more than a miniscule proportion of those with obesity, and carries risks, although low, of surgical complications [Hutter et al. 2011] and weight regain [Karmali et al. 2013]. Therefore, an effective alternative therapeutic approach is urgently required. The standard approach has been to consider pharmacological treatment for obesity only after dietary, exercise or behavioural interventions have been initiated and their effects assessed (http://publications.nice.org.uk/obesity-cg43 accessed 21 August 2013). However, a dual-pronged approach, using pharmacological agents as adjuvant therapy after lifestyle intervention to maintain the weight loss achieved, may ultimately prove to be the most useful paradigm [Finer et al. 1992; Richelsen et al. 2007; Bray, 2013; Wadden et al. 2013].
Challenges in obesity pharmacotherapy
Expectations and goals
Patient and clinician expectations from antiobesity drugs may differ and often are hard to fulfil. The clinician would consider that an ideal antiobesity drug would selectively reduce body fat stores, preferably visceral, by normalizing the regulatory or metabolic disturbances involved in the pathogenesis of obesity, to the extent that obesity-related comorbidities, medical and psychological, were ameliorated or ‘cured’, with subsequent reductions in mortality and improvement in quality of life. Furthermore, the ideal antiobesity agent should exhibit only minor, if any, side effects, be preferentially administered orally for long-term use, and be widely accessible at an affordable price. However, an incomplete understanding of the aetiopathogenesis of obesity has hitherto precluded the development of specific targeted therapies for obesity [O’Rahilly and Farooqi, 2008]. The success of leptin replacement therapy for the exceedingly rare congenital leptin deficiency, which results in extreme early onset obesity, is the only example of such a targeted approach [Paz-Filho et al. 2011]. A chronic imbalance between energy intake and expenditure undoubtedly underpins the accumulation of excess body fat [Speakman et al. 2011]. Indeed, the increasing ease of food accessibility over the last 30 years is widely acknowledged to be a major driver for the obesity epidemic [Swinburn et al. 2011]. The high heritability of obesity suggests a strong genetic component to the pathophysiology [O’Rahilly and Farooqi, 2008]. While substantial advances have been made in the study of genetic factors underlying obesity susceptibility [Berndt et al. 2013; Magi et al. 2013], including the identification of increasing numbers of genetic variants associated with the risk of obesity [Wheeler et al. 2013], translation of these discoveries into preventive and therapeutic measures of direct clinical benefit has proven to be extremely challenging [McCarthy, 2010], and ‘personalized’ obesity therapy currently remains beyond tangible reach [El-Sayed Moustafa and Froguel, 2013].
From a patient perspective, weight loss per se may be viewed as an important outcome and often expectations therein may be unrealistic [Fabricatore et al. 2007; Wee et al. 2013]. While sustained moderate weight loss of 5–10% would result in health gain [Caterson et al. 2012; Unick et al. 2013], few patients regard this as a successful weight loss outcome [Foster et al. 1997]. Furthermore, results of the LookAHEAD study in obese patients with T2D suggest that 8% weight reduction achieved through an intensive lifestyle intervention is insufficient to reduce cardiovascular disease [Wing et al. 2013]. Overall, there is a lack of data on whether benefits of lifestyle and pharmacological interventions are sustained and translate into longer term prevention of obesity-related comorbidities [Dunkley et al. 2012].
Regulatory approval
The US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) differ in their stances on efficacy of antiobesity drugs. The FDA 2007 Draft Obesity Drug Guidance requires achievement of a statistically significant difference of at least 5% in mean weight loss between drug candidate and placebo at 1 year and that the proportion of subjects who lose at least 5% from baseline of body weight in the drug-candidate group should be 35% or more, and be approximately twice the proportion in the placebo-treated group. In contrast, the EMA regards weight reduction from baseline as being more clinically relevant than placebo-subtracted weight loss and requires evidence for weight loss of at least 10% of baseline body weight at 1 year, which must also be at least 5% greater than that achieved on placebo. The EMA guideline also states that when the clinical response is at least 10% weight loss at the end of 1 year, the proportions of responders in various treatment arms could be considered as an alternative primary efficacy criterion. Although FDA and EMA cite weight reduction as their primary efficacy criterion, weight reduction needs to be accompanied by commensurate improvements in cardiovascular risk factors. One critical point for both agencies is that the above criteria are guidelines, not definitive arbitrators of success or failure, and both FDA and EMA retain the absolute right to reach their final decisions about the efficacy and safety of each new drug candidate on a case-by-case basis. Furthermore, there is currently no regulatory pathway to license a drug for ‘weight loss maintenance’ or ‘prevention of weight regain’.
Two recent events have substantially changed the regulatory and marketing landscape for the development, registration and commercialization of novel drugs for the treatment of obesity. The first was the suspension of the marketing authorization for rimonabant from the European market in October 2008 due to an increasing number of reports of psychiatric adverse events and suicidality (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500014774.pdf accessed 21 August 2013).
The second was the withdrawal of sibutramine in Europe (http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2010/01/WC500069995.pdf accessed 21 August 2013) and the USA as a fallout from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial [James et al. 2010] that showed a higher frequency of nonfatal myocardial infarction and stroke in subjects on sibutramine treatment compared with placebo. Rimonabant and sibutramine were the latest in a long line of centrally acting antiobesity drugs (Table 1) that have been linked with major safety concerns [Rodgers et al. 2012]. With this background, and the fact that obesity pharmacotherapy is likely to be used widely and long term, there is an appropriate demand from regulatory authorities for evidence of a very favourable benefit–risk for any new drug. The FDA (formally) and the EMA (in practice) now demand postmarketing studies, including long-term cardiovascular outcome trials to assess the effect of a drug on the risk of major adverse cardiac events. As for any drug, an understanding of both unwanted on-target effects of antiobesity agents (e.g. sympathomimetic for sibutramine and mood depression for rimonabant) and characterization of off-target effects is necessary to ensure safety.
Table 1.
Suspension of licensing for antiobesity drugs.
| Drug | Year | Reason for suspension |
|---|---|---|
| Dinitrophenol | 1938 | Dermatitis, neuropathy, agranulocytosis, visual impairment, death |
| Aminorex | 1968 | Chronic pulmonary hypertension |
| Amphetamines (schedule II) | 1971 | Addiction, hypertension, myocardial toxicity |
| Fenfluramine/dexfenfluramine alone or in combination with phentermine | 1997 | Valvular heart disease |
| Phenylpropanolamine | 2000 | Hemorrhagic stroke |
| Rimonabant | 2009 | Psychiatric disorders, depression, suicidal ideation |
| Sibutramine | 2010 | Risk of major cardiovascular events |
Drug development
Aside from the obstacles in getting approval from regulatory agencies, the obesity pharmacotherapy market has not proved to be as financially rewarding as expected. Sales of drugs have failed to meet expectations or projections, with low uptake and short duration of use by patients [Hemo et al. 2011]. In the USA, the reluctance of medical insurers to provide reimbursement for antiobesity drugs is often cited as the main reason why sales of new antiobesity drugs are disappointing. Among patients with severe obesity in the USA, interest in obesity treatments is high but coverage and receipt of treatment is low [Arterburn et al. 2013]. However, in the UK, market penetration is also low even when patients are routinely reimbursed for antiobesity drug costs. High expectations of prescribers and patients, and a lack of specialist or primary care physician expertise in clinical obesity treatment are all too common scenarios [Wass and Finer, 2013]. These unmet needs and disconnects will have to be addressed before any new drug entering the antiobesity market may stand a reasonable chance of being a commercial success.
Novel obesity pharmacotherapies
Antiobesity drugs can be considered under two broad categories: central-acting appetite suppressants or satiety enhancers which may also have peripheral actions; and peripherally acting agents, for example orlistat, which is an inhibitor of gastric and pancreatic lipases that blocks fat absorption from the gut. Given the role of the brain in controlling appetite and evidence of high expression levels of ‘obesity susceptibility genes’ in brain [Willer et al. 2009], centrally acting agents appear to hold the most promise. Currently sympathomimetic drugs such as phentermine and diethylpropion are approved only in the USA for short-term (less than 3 months) treatment and thus do not fit into a rational paradigm for treatment of a chronic disorder. However, the FDA has recently approved the serotonin agonist lorcaserin (Belviq; Arena/Eisai, Tokyo, Japan) and a combination of low-dose phentermine/topiramate (Qsymia; Vivus Inc., Mountain View, CA, USA), raising the possibility of improving current paradigms for treatment of obesity. In Europe, both drugs were rejected primarily over concerns that safety had not been demonstrated, a stance that has been criticized [Astrup et al. 2013]. The development of the glucagon-like peptide 1 (GLP-1) analogue, liraglutide, at doses higher than used for managing hyperglycaemia, as an antiobesity agent is also promising. In this review, we summarize important findings from recent randomized controlled trials (Table 2) that investigated the weight loss associated with these three agents, including discussion of their side effects.
Table 2.
Summary of randomized placebo-controlled antiobesity drug trials.
| Drug | Reference | N (T2D) | BMI (mean) | Age (mean) | 1-year Δ%WL (mean) | % with >5% WL (versus placebo) | Frequent side effects | Uncommon side effects |
|---|---|---|---|---|---|---|---|---|
| Lorcaserin | [Smith et al. 2010] (BLOOM) | 3182 (0) | 36.2 | 44.1 | 3.7% | 45% versus 20% | Dry mouth | Nausea |
| Fatigue | Urinary tract infection | |||||||
| Lorcaserin | [Fidler et al. 2011] (BLOSSOM) | 4008 (0) | 35.9 | 43.8 | 3.0% | 47% versus 25% | Dizziness | Constipation/diarrhoea |
| Headache | Hypoglycaemia (in patients with T2D) | |||||||
| Lorcaserin | [O’Neil et al. 2012] (BLOOM-DM) | 604 (604) | 36.0 | 52.4 | 3.5% | 45% versus 16% | ||
| Phentermine/ topiramate | [Allison et al. 2012] (EQUIP) | 1267 (0) | 42.2 | 42.6 | 9.4% | 67% versus 17% | Paraesthesia | Palpitations |
| Dry mouth | Disturbances in attention | |||||||
| Phentermine/ topiramate | [Gadde et al. 2011] (CONQUER) | 2487 (393) | 36.6 | 51.1 | 8.6% | 70% versus 21% | Constipation | Alopecia |
| Headache | Diarrhoea | |||||||
| Dysgeusia | Anxiety and irritability | |||||||
| Insomnia | Depression/fatigue | |||||||
| Dizziness | Blurred vision | |||||||
| Glaucoma | ||||||||
| Liraglutide | [Astrup et al. 2012] | 398 (21) | 34.8 | 45.9 | 4.9% | 73% v 28% | Nausea | Pancreatitis |
| Vomiting | ||||||||
| Liraglutide* | [Wadden et al. 2013] | 422 (0) | 35.6 | 46.2 | 6.1% | 51% versus 21% | Constipation | |
| Diarrhoea | ||||||||
| Headache |
Patients in this study were randomized after a run-in on a low calorie diet during which mean weight loss was 6%.
BMI, body mass index; T2D, type 2 diabetes mellitus; WL, weight loss.
Lorcaserin
Lorcaserin is a serotonin type 2C receptor (5HT2CR) agonist, developed to exploit the 5HT2CR-specific beneficial effects of fenfluramine while removing the unwanted effects of 5-HT2A and 5-HT2B receptor agonism [Fiorella et al. 1995]. Lorcaserin was officially launched in the USA in June 2013. The efficacy and safety of lorcaserin were evaluated in three separate randomized, double-blind, placebo-controlled phase III trials [Smith et al. 2010; Fidler et al. 2011; O’Neil et al. 2012]. In the 2-year Behavioural modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial [Smith et al. 2010], 3182 subjects with a BMI of 30–45 kg/m2 or 27–45 kg/m2 and at least one weight-related comorbidity were randomized to receive placebo twice daily or lorcaserin 10 mg twice daily, with completers being randomized 1:1 to remain on lorcaserin treatment or switched to placebo. The primary efficacy endpoints evaluated percentage weight loss at 1 year and weight maintenance through 2 years in subjects achieving at least 5% weight loss at 1 year. The 1-year Behavioural modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial [Fidler et al. 2011] randomized 4008 subjects with a BMI of 30–45 kg/m2 or 27–29.9 kg/m2 and at least one weight-related comorbidity to placebo, lorcaserin 10 mg every day or lorcaserin 10 mg twice a day. The placebo-subtracted efficacy of lorcaserin at 1 year was 3.6 kg. On its own, lorcaserin produces only modest weight loss, as summarized in a recent meta-analysis taking into account five randomized controlled studies, with a mean weight loss of 3.2 kg at 1 year and BMI reduction of 1.2 kg/m2 compared with placebo [Chan et al. 2013].
Of note, patients in the BLOOM trial experienced weight regain during the second year of active treatment (Figure 1), suggesting that tolerance to lorcaserin may have developed. The primary year 2 endpoint was to determine, in patients who had lost at least 5% of baseline body weight at 52 weeks, the proportion who maintained this degree of weight loss at the end of the second year of treatment. Although the rate of weight regain in subjects continuing lorcaserin therapy was slower than observed in subjects who had been switched to placebo, the slope of the upward trajectory was much steeper than that observed in the placebo/placebo group of subjects. Consistent with the emergence of pharmacological tolerance, the difference between the percentage of patients achieving more than a 5% weight loss on this drug versus placebo decreased from 27.2% at week 52 to 17.6% at week 104. On the basis of the trial data, the product licence requires that if after 12 weeks of treatment with lorcaserin, a patient has not lost at least 5% of the baseline body weight, use of the drug should be discontinued since it is unlikely that the patient will achieve meaningful weight loss with continued treatment.
Figure 1.
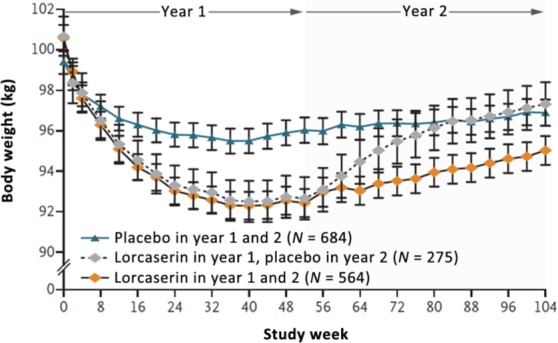
Effects of lorcaserin on body weight during years 1 and 2 among only those patients who continued the study past year 1. (Reprinted with permission from Smith et al. [2010]. Copyright © 2010 Massachusetts Medical Society.)
A trial of lorcaserin in patients who were overweight or obese and had T2D treated with sulphonylurea or metformin (BLOOM-DM) [O’Neil et al. 2012] found a mean weight loss of 5.0 ± 0.35% on lorcaserin 10 mg daily, 4.5 ± 0.35% on lorcaserin 10 mg twice daily versus –1.5 ± 0.36% on placebo. Haemoglobin A1c improved by approximately 1% on lorcaserin. Of patients on lorcaserin 10 mg twice and once daily, symptomatic hypoglycaemia was reported in 7.4% and 10.5% of patients compared with 6.3% in those on placebo and was more common in those on sulphonylurea. No patient in any treatment group reported severe hypoglycaemia, an episode that resulted in confusion, loss of consciousness or treatment with parenteral agents, and none withdrew from the study because of hypoglycaemia.
The European Committee on Human Medicinal Products (CHMP) had concerns about the potential risk of tumours, particularly with long-term use, and the potential risk of psychiatric disorders and valvulopathy (http://www.ema.europa.eu/docs/en_GB/document_library/Medicine_QA/2013/05/WC500143811.pdf accessed 21 August 2013). As a result of this unfavourable report, Arena Pharmaceuticals withdrew its application for European Union approval for the product. The cancer risk in animal studies had been a concern of the FDA when it rejected lorcaserin the first time around but they ultimately concluded that, in females rats, the incidence of mammary adenocarcinoma increased at plasma exposures 87 times the daily human clinical dose, the incidence of mammary fibroadenoma was increased in female rats at all doses with no safety margin to the clinical dose, but that the relevance to humans of this increased incidence of mammary adenocarcinomas and fibroadenomas in rats is unknown (http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022529lbl.pdf accessed 21 August 2013).
As regards valvulopathy, an analysis of three trials [Weissman et al. 2013] found that the 1-year rate of echocardiographic FDA-defined valvulopathy was 2.04% in the placebo group and 2.37% in the lorcaserin group when missing values were imputed, or 2.45% and 2.57% for 1-year completers. The differences between the placebo and lorcaserin groups were small and statistically not significant, with point estimates of risk 1.16 [95% confidence interval (CI) 0.81–1.67] in the randomized-echo population and 1.03 (95% CI 0.68–1.57) in the completer population.
Other concerns are the potential for interaction with other selective serotonin reuptake inhibitors (e.g. many antidepressants) or with monoamine oxidase inhibitors to cause serotonin syndrome and neuroleptic malignant syndrome. Commonly occurring adverse events associated with lorcaserin were consistent with its serotonergic agonist mechanism of action, that is, increased incidences of blurred vision, dizziness, somnolence, headache, gastrointestinal disturbance and nausea. Lorcaserin is contraindicated in pregnancy due to a risk of teratogenicity. The future development strategy for lorcaserin in Europe remains ‘under evaluation’.
Phentermine/topiramate
A second agent recently approved by the FDA exploits the principle of combining two drugs with synergistic effects allowing a dose reduction of each drug and thus less toxicity without a loss in efficacy. Phentermine and extended-release topiramate (Qnexa/Qsiva/Qsymia) was launched in September 2012. Phentermine, a nonselective stimulator of synaptic noradrenaline, dopamine and serotonin release, has been widely used (mainly outside of Europe) as a short-term appetite suppressant since the 1960s [Ryan and Bray, 2013]. Topiramate is an anticonvulsant drug and following anecdotal reports of weight loss occurring in patients with epilepsy, it was evaluated as a potential antiobesity drug in clinical trials [Astrup et al. 2004a]. Topiramate produced a very substantial weight reduction in subjects with obesity, particularly when administered after initial weight loss induced with a very low energy diet [Astrup et al. 2004b], but an indication as a monotherapy for obesity was abandoned due to dose-dependent neuropsychiatric and cognitive adverse events, such as memory and concentration impairment, language difficulties and mood changes [Nathan et al. 2011; Sommer et al. 2013]. Although the exact mechanism of action for weight loss with topiramate is not known, animal experiments suggest that topiramate-induced weight loss results from increased energy expenditure, decreased energetic efficiency and decreased caloric intake as an appetite suppressant [Richard et al. 2000].
The phentermine/topiramate combination is a once-daily formulation designed to provide an immediate release of phentermine and a delayed release of topiramate that would not be achieved by simply combining the two drugs already marketed (i.e. the drug combination produces peak exposure to phentermine in the morning and a peak concentration of topiramate in the evening). Phentermine/topiramate has been evaluated in clinical trials at three different dosages, that is, phentermine/topiramate 3.75/23 mg (low dose), 7.5/46 mg (intermediate dose) and 15/92 mg (full dose), doses which are substantially lower than when either is used as a monotherapy [Gadde et al. 2011; Allison et al. 2012; Garvey et al. 2012]. Two clinical studies provided efficacy and safety data that formed the basis for approval of the medication. EQUIP [Allison et al. 2012] enrolled 1267 subjects up to 70 years of age with BMI of at least 35 kg/m2. Most subjects in this study were women (82.9%) and white (80%), and the mean age of subjects was 42.6 years with a mean weight of 116.1 kg and a mean BMI of 42.1 kg/m2. EQUIP required blood pressure to be controlled (≤140/90 mmHg using up to two antihypertensive medications), fasting blood glucose up to 110 mg/dl and triglycerides up to 200 mg/dl using none or one lipid-lowering medication. This study compared placebo with the low-dose and the full-dose combination. Patients in the placebo, low- and high-dose groups lost 1.6%, 5.1% and 10.9% of baseline body weight respectively at 56 weeks. In categorical analysis, 17.3% of patients on placebo, 44.9% of those in the low-dose group and 66.7% of those in the full-dose group lost at least 5% of baseline body weight at 56 weeks.
CONQUER [Gadde et al. 2011] enrolled 2487 adults up to 70 years of age with BMI of 27–45 kg/m2 (no lower BMI limit in the case of T2D) and also required patients with two comorbidities (hypertension, hypertriglyceridaemia, impaired fasting glucose, impaired glucose tolerance, T2D or visceral adiposity). Thus, the patient population represents those with higher risk profiles from the consequences of excess weight. Again, most subjects were women (69.7%) and white (86%), with a baseline mean weight of 103.1 kg and a mean BMI of 36.6 kg/m2. In this population, the mid and high doses of phentermine/topiramate were evaluated for efficacy and safety (Figure 2). A titration period is required for phentermine/topiramate, starting at a dosage of 3.75/23 mg. In these studies, the titration period was 4 weeks, while the recommendation for use is at least 2 weeks. All subjects in these studies received a lifestyle modification programme based on the LEARN Program for Weight Management [Brownell, 2000]. Primary outcome was percentage weight loss. Seventy percent of patients on high-dose phentermine/topiramate lost at least 5% of their body weight compared with 62% on mid-dose phentermine/topiramate and 21% on placebo. The average percentage weight lost was 9.8% in the high-dose group, 7.8% in the mid-dose group and 1.2% in the placebo group. Interestingly, the corresponding proportions of patients achieving at least a 10% weight loss were 48%, 37% and 7% respectively. This combination medication has produced much higher weight loss than previously seen in clinical trials of obesity medications, such as the fenfluramines, sibutramine, orlistat or rimonabant. The CONQUER study was extended for a second year of observation, with patients keeping their treatment assignment. This has been published as the SEQUEL study (676 subjects) [Garvey et al. 2012]. At the end of the second year of treatment, patients completing the trial taking the mid-dose combination maintained a weight loss of 9.3% below baseline, and those on the high dose maintained a 10.7% weight loss from baseline.
Figure 2.
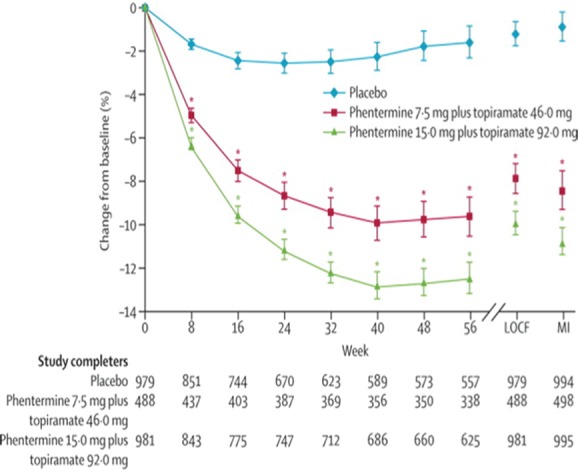
Effects of phentermine plus topiramate on bodyweight. LOCF, last observation carried forward; MI, myocardial; infarction. (Reprinted with permission from Gadde et al. [2011]. Copyright © 2011 Elsevier Science Ltd.)
Adverse events associated with phentermine/topiramate treatment were generally consistent with those reported for phentermine (i.e. dry mouth, constipation, insomnia and palpitations) and for topiramate (i.e. dizziness, paraesthesia, disturbances in attention, metabolic acidosis and renal calculi), together with headache, dysgeusia (distortion of sense of taste), alopecia and hypokalaemia. Other potentially serious safety concerns regarding phentermine/topiramate include teratogenicity, elevations in resting heart rate and anxiety/depression (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292315.pdf accessed 21 August 2013). The drug can increase the risk of acute myopia and secondary angle closure glaucoma. The FDA report highlights previous findings that women who received topiramate during pregnancy (for its existing epilepsy indication) were more likely to have infants born with an orofacial cleft. In the phentermine/topiramate obesity trials, 34 pregnancies were reported and the drug was discontinued soon after pregnancy became known; 19 pregnancies were carried to term, 15 births had exposure to topiramate and there were no fetal adverse outcomes. To reduce the risk of teratogenicity, women of childbearing potential should have a negative pregnancy test prior to starting phentermine/topiramate, and at monthly intervals thereafter. An additional concern is that topiramate may interfere with the pharmacokinetics of oral contraceptives, leading to failure of contraceptive protection, although such risk is unlikely at doses greater than 200 mg/day. Accordingly, the approval of phentermine/topiramate required a risk evaluation and mitigation strategy, so that patients and prescribers may be best informed of the teratogenic risk. If a patient becomes pregnant while taking phentermine/topiramate, treatment should be immediately discontinued.
In the CONQUER study, treatment with phentermine/topiramate at the mid and high doses was associated with mean increases in heart rate of 0.6 and 1.6 beats per minute (bpm) respectively compared with placebo [Gadde et al. 2011]. However, study participants treated with these doses had greater mean reductions in blood pressure than participants given placebo. A higher proportion of phentermine/topiramate-treated patients also experienced a categorical increase in heart rate compared with placebo-treated patients (>20 bpm: 13.5% mid dose, 19.6% full dose versus 11.9% placebo). At 2 years, a small heart rate increase persisted with phentermine/topiramate treatment, although the difference versus placebo was not statistically significant [Garvey et al. 2012]. Whereas phentermine/topiramate treatment led to a small increase in heart rate, systolic and diastolic blood pressure decreased and there have been no increases in the total number of major adverse cardiac events with phentermine/topiramate compared with placebo. Cognitive dysfunction, with difficulty with language, memory, confusion or word-finding abilities is also a concern [Shin and Gadde, 2013]. If this develops, patients should be advised not to drive and that the medication should be stopped. Phentermine/topiramate must not be used in patients being treated with a monoamine oxidase inhibitor. Taking into account the magnitude of weight loss and the favourable changes in blood pressure, the FDA concluded that the benefit–risk balance was positive and supported the approval of phentermine/topiramate (http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM292315.pdf accessed 21 August 2013). The drug’s labelling recommends regular heart rate monitoring and recommends against use in patients with recent or unstable cardiac or cerebrovascular disease since its use in these patients has not been studied. Similarly to lorcaserin, the FDA insists that if after 12 weeks of treatment with phentermine/topiramate at the mid dose, a patient has not lost at least 3% of the baseline weight, either the drug should be discontinued or the dose increased. If the latter option is chosen and the patient does not lose at least 5% of the baseline weight during an additional 12 weeks of treatment, the drug should be discontinued, because the patient is unlikely to achieve meaningful weight loss with continued treatment.
The European CHMP again took a contrary view and recommended against accepting the marketing authorization application of phentermine/topiramate in the European Union in October 2012 due to concerns over potential long-term cardiovascular and central nervous system effects, teratogenic potential, and the possibility of use by patients for whom this combination therapy is not indicated (http://www.ema.europa.eu/docs/en_GB/document_library/Summary_of_opinion_-_Initial_authorisation/human/002350/WC500134085.pdf accessed 21 August 2013). The EMA confirmed this decision in February 2013 and it seems that the results of a planned cardiovascular outcome trial will be needed as a preapproval condition.
GLP-1 receptor agonists
Given the relative dearth of effective antiobesity agents and lack of prospects for new drug development, obesity researchers and clinicians are increasingly turning to a drug repositioning strategy in order to expand therapeutic options. The advent of GLP-1 receptor (GLP-1R) agonists and related incretin therapies has already substantially enhanced the therapeutic armamentarium for T2D [Lovshin and Drucker, 2009]. Now, the appetite-suppressant properties of such agents are being exploited in an approach to managing obesity even in patients without diabetes [Holst, 2013]. A recent meta-analysis of the effects of GLP-1R agonists on weight in clinical trials of patients who are overweight or obese with or without T2D showed a greater weight loss was achieved in patients treated with GLP-1R compared with control groups (weighted mean difference −2.9 kg, 95% CI −3.6 to −2.2; 21 trials, 6411 participants) [Vilsboll et al. 2012]. However, weight was a secondary endpoint in most of the trials analyzed in this study.
Liraglutide (Victoza, Novo Nordisk, Bagsvaerd, Denmark) is a long-acting GLP-1R agonist that has around 97% homology to native GLP-1. Liraglutide is also metabolized by dipeptidyl peptidase IV, but at a much slower rate [Malm-Erjefalt et al. 2010], thus resulting in a long duration of action. Licensed for treatment of T2D, it has no current indication as a weight loss agent. However, accumulating evidence from trials at existing ‘diabetes’ doses and higher doses of up to 3.0 mg suggest that that liraglutide could assume a primary role in clinical management of obesity in the near future. Several studies of liraglutide in patients with obesity, outside of glucose-lowering clinical trials, have been now been reported.
In a randomized phase II dose-ranging trial, the effects of liraglutide in adults with obesity (n = 564, BMI 30–40 kg/m2) were investigated [Astrup et al. 2009]. Participants were advised to follow a diet calculated to provide a 500 kcal daily energy deficit and to increase physical activity. Mean weight loss with liraglutide at doses 1.2, 1.8, 2.4 and 3.0 mg were 4.8 kg, 5.5 kg, 6.3 kg and 7.2 kg respectively compared with 2.8 kg with placebo and 4.1 kg with orlistat given as an active comparator, and was 2.1–4.4 kg greater than that with placebo. The percentage of individuals who lost more than 5% weight with liraglutide 3.0 mg was 76% (n = 70) compared with placebo (30%, n = 29) or orlistat (44%, n = 42). In a partially open-label extension to the trial, participants on liraglutide 2.4/3.0 mg for 2 years (n = 184) maintained a 2-year weight loss of 7.8 kg from the time of study run in and lost 3.0 kg (1.3–4.7) more weight than those on orlistat (n = 95) [Astrup et al. 2012]. Preliminary results from the SCALE trial investigating the potential of liraglutide to induce and maintain weight loss in people without diabetes who are obese or overweight with comorbidities such as prediabetes, hypertension and dyslipidaemia have reported that the average weight loss for people treated with liraglutide 3 mg at 56 weeks was 8.0% compared with 2.6% for people treated with placebo. The proportion of people achieving a weight loss of at least 5% was 64% for liraglutide 3 mg and 27% for placebo. The proportion of people achieving a weight loss of at least 10% was 33% for liraglutide 3 mg and 10% for placebo treatment (http://www.novonordisk.com/include/asp/exe_news_attachment.asp?sAttachmentGUID=3f254ec7-1b91-4334-bf0d-572d4e6a4c3e accessed 15 December 2013).
Wadden and colleagues provided further insights into the therapeutic potential of liraglutide in obesity management [Wadden et al. 2013]. In a randomized phase III trial, which included an initial diet and exercise run-in period, treatment of participants who achieved at least 5% weight loss during the run in (n = 551, BMI ≥ 30 kg/m2 or ≥27 kg/m2 with comorbidities) with either liraglutide or placebo showed that liraglutide treatment not only maintained the weight loss achieved with the lifestyle intervention, but also resulted in an additional 6% weight loss over 56 weeks (n = 159 in the liraglutide-treated group) compared with 0.2% in the control group (n = 146) (Figure 3). Thus, liraglutide treatment subsequent to a diet and exercise intervention could represent a successful therapeutic strategy for ‘successful weight losers’. Another recent trial in patients who were overweight or obese with prediabetes [Kim et al. 2013] found that subjects who continued to use liraglutide 1.8 mg daily (n = 24) lost twice as much weight as those using placebo (n = 27; 6.8 versus 3.3 kg; p < 0.001). Liraglutide-treated subjects also had a significant improvement in steady state plasma glucose concentration during an insulin suppression test (–3.2 versus 0.2 mmol/liter; p 0.001) and significantly (p ≤ 0.04) greater lowering of systolic blood pressure (–8.1 versus –2.6 mmHg), fasting glucose (–0.5 versus 0 mmol/liter) and triglyceride (–0.4 versus –0.1 mmol/liter) concentration.
Figure 3.
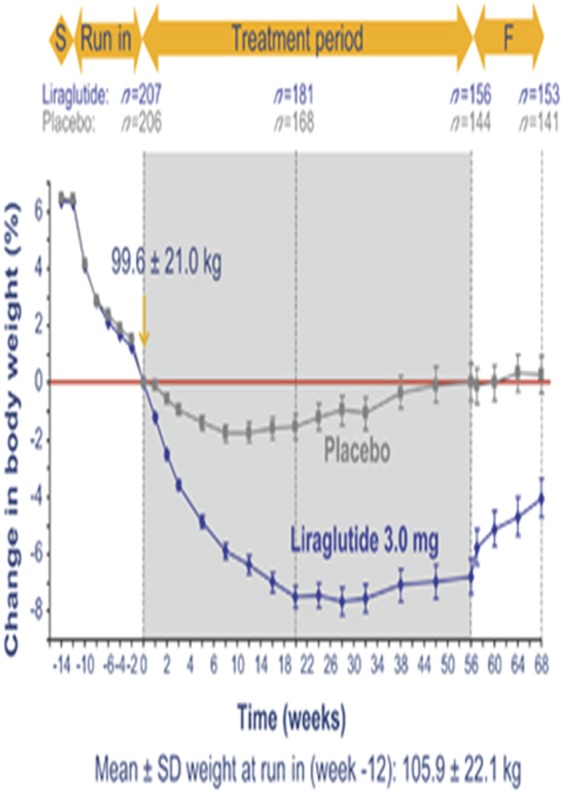
Effects of liraglutide on body weight. (Reprinted with permission Wadden et al. [2013]. Copyright © 2013 Macmillan Publishers Ltd.)
Liraglutide is generally well tolerated. Mild to moderate nausea and vomiting are the main side effects and are often transient [Astrup et al. 2012], but may contribute to weight loss as shown in an analysis of the Astrup randomized trial; those who experienced transient nausea or vomiting lost approximately 3 kg more weight than those who did not [Lean et al. 2013b]. Furthermore, mechanistic studies in patients with T2D suggest that the weight loss may involve combined effects on energy intake and energy expenditure [Horowitz et al. 2012]. As with all GLP-1R agonists, long-term safety data are not yet established, however in a burgeoning field of research, cardioprotective properties of incretin therapies have been demonstrated in animal models and in humans [Ussher and Drucker 2012]. Concerns on an association between GLP-1 agonists and pancreatitis and pancreatic cancer have been raised [Butler et al. 2013; Cohen, 2013]. The EMA concluded earlier in 2013 that ‘while there are still some uncertainties with respect to long term pancreatic safety’, ‘no new data has emerged that implies that this risk is higher compared to what has previously been concluded’ (http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/08/WC500147026.pdf accessed 15 December 2013), a view with which the FDA concurred (http://www.publichealthwatchdog.com/fda-agrees-with-ema-on-incretin-mimetic-diabetes-drugs/ accessed 15 December 13).
Other drugs in earlier development stages
There are many drugs in development, including agents targeting central hypothalamic pathways (e.g. RM-493, a melanocortin type 4 receptor agonist) or peripheral metabolism (e.g. beloranib, increases fat oxidation). Other drug combinations are also in clinical trials: the combination of bupropion and naltrexone (Contrave, Orexigen Therapeutics Inc. La Jolla, CA, USA) has recently been resubmitted to the FDA as a new drug application.
Conclusion
The recent advent of novel therapies and approaches in the pharmacotherapeutics of obesity provides an opportunity to meet the demand of the current obesity epidemic. Centrally acting agents may play an important role in treating patients with obesity; however, there are substantial barriers to successfully integrating these agents into weight management practice, particularly in Europe, and the future position of antiobesity pharmacological agents in obesity management remains uncertain. The use of current antiobesity drugs in isolation is unlikely to be successful and alternative therapeutic paradigms should be developed. In particular, adjunctive pharmacological therapy post intensive lifestyle intervention is a strategy, which in some patients can achieve outcomes that rival bariatric surgery. Despite the many deficiencies, a progressive approach to obesity pharmacotherapy perhaps offers the best opportunity to finally address the obesity crisis on a mass scale.
Footnotes
Funding: SM is funded by the Rosetrees Trust and supported by the National Institute of Health Research University College London Hospitals Biomedical Research Centre. AP is supported by the University of Pisa, Italy.
Conflict of interest statement: N Finer has provided paid consultancy to NovoNordisk, Arena and Vivus Inc.
Contributor Information
Sean Manning, UCLH Centre for Weight Loss, Metabolic and Endocrine Surgery, University College London Hospitals, London, UK.
Andrea Pucci, UCLH Centre for Weight Loss, Metabolic and Endocrine Surgery, University College London Hospitals, London, UK.
Nicholas Finer, UCLH Centre for Weight Loss, Metabolic and Endocrine Surgery, University College London Hospitals, Ground Floor West Wing, 250 Euston Road, London NW1 2PG, UK.
References
- Allison D., Gadde K., Garvey W., Peterson C., Schwiers M., Najarian T., et al. (2012) Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring) 20: 330–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J., Konz E., Frederich R., Wood C. (2001) Long-term weight-loss maintenance: a meta-analysis of US studies. Am J Clin Nutr 74: 579–584 [DOI] [PubMed] [Google Scholar]
- Arterburn D., Westbrook E., Terrell A. (2013) Weight control practices of severely obese patients who are not seeking bariatric surgery. Obesity (Silver Spring) 21: 1509-1513 [DOI] [PubMed] [Google Scholar]
- Astrup A., Carraro R., Finer N., Harper A., Kunesova M., Lean M., et al. (2012) Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes (Lond) 36: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrup A., Caterson I., Zelissen P., Guy-Grand B., Carruba M., Levy B., et al. (2004b) Topiramate: long-term maintenance of weight loss induced by a low-calorie diet in obese subjects. Obes Res 12: 1658–1669 [DOI] [PubMed] [Google Scholar]
- Astrup A., Rossner S., Finer N., Van Gaal L. (2013) Obesity in Europe – does anybody care? Expert Opin Pharmacother 14: 971–973 [DOI] [PubMed] [Google Scholar]
- Astrup A., Rossner S., Van Gaal L., Rissanen A., Niskanen L., Al Hakim M., et al. (2009) Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet 374: 1606–1616 [DOI] [PubMed] [Google Scholar]
- Astrup A., Toubro S. (2004a) Topiramate: a new potential pharmacological treatment for obesity. Obes Res 12(Suppl.): 167S-173S [DOI] [PubMed] [Google Scholar]
- Berndt S., Gustafsson S., Magi R., Ganna A., Wheeler E., Feitosa M., et al. (2013) Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 45: 501-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray G. (2013) Why do we need drugs to treat the patient with obesity? Obesity (Silver Spring) 21: 893–899 [DOI] [PubMed] [Google Scholar]
- Brownell K. (2000) The Learn Program for Weight Management. Euless, TX: Amer Health Pub Co [Google Scholar]
- Butler P., Elashoff M., Elashoff R., Gale E. (2013) A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care 36: 2118–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson I., Finer N., Coutinho W., Van Gaal L., Maggioni A., Torp-Pedersen C., et al. (2012) Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular Outcomes (SCOUT) Trial. Diabetes Obes Metab 14: 523–530 [DOI] [PubMed] [Google Scholar]
- Chan E., He Y., Chui C., Wong A., Lau W., Wong I. (2013) Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev 14: 383–392 [DOI] [PubMed] [Google Scholar]
- Cohen D. (2013) Has pancreatic damage from glucagon suppressing diabetes drugs been underplayed? BMJ 346: f3680. [DOI] [PubMed] [Google Scholar]
- Dunkley A., Charles K., Gray L., Camosso-Stefinovic J., Davies M., Khunti K. (2012) Effectiveness of interventions for reducing diabetes and cardiovascular disease risk in people with metabolic syndrome: systematic review and mixed treatment comparison meta-analysis. Diabetes Obes Metab 14: 616–625 [DOI] [PubMed] [Google Scholar]
- El-Sayed Moustafa J., Froguel P. (2013) From obesity genetics to the future of personalized obesity therapy. Nat Rev Endocrinol 9: 402–413 [DOI] [PubMed] [Google Scholar]
- Fabricatore A., Wadden T., Womble L., Sarwer D., Berkowitz R., Foster G., et al. (2007) The role of patients’ expectations and goals in the behavioral and pharmacological treatment of obesity. Int J Obes (Lond) 31: 1739–1745 [DOI] [PubMed] [Google Scholar]
- Fidler M., Sanchez M., Raether B., Weissman N., Smith S., Shanahan W., et al. (2011) A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab 96: 3067–3077 [DOI] [PubMed] [Google Scholar]
- Finer N. (2001) Low-calorie diets and sustained weight loss. Obes Res 9(Suppl. 4): 290S-294S [DOI] [PubMed] [Google Scholar]
- Finer N., Finer S., Naoumova R. (1992) Drug therapy after very-low-calorie diets. Am J Clin Nutr 56: 195S-198S [DOI] [PubMed] [Google Scholar]
- Finucane M., Stevens G., Cowan M., Danaei G., Lin J., Paciorek C., et al. (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377: 557–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D., Helsley S., Lorrain D., Rabin R., Winter J. (1995) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. III: The mechanistic basis for supersensitivity to the LSD Stimulus following serotonin depletion. Psychopharmacology (Berl) 121: 364–372 [DOI] [PubMed] [Google Scholar]
- Flegal K., Carroll M., Kit B., Ogden C. (2012) Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497 [DOI] [PubMed] [Google Scholar]
- Foster G., Wadden T., Vogt R., Brewer G. (1997) What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol 65: 79–85 [DOI] [PubMed] [Google Scholar]
- Gadde K., Allison D., Ryan D., Peterson C., Troupin B., Schwiers M., et al. (2011) Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (conquer): a randomised, placebo-controlled, phase 3 trial. Lancet 377: 1341–1352 [DOI] [PubMed] [Google Scholar]
- Garvey W., Ryan D., Look M., Gadde K., Allison D., Peterson C., et al. (2012) Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr 95: 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve E., Fenwick E., Yang H., Lean M. (2013) The disproportionate economic burden associated with severe and complicated obesity: a systematic review. Obes Rev 14: 883-894 [DOI] [PubMed] [Google Scholar]
- Hemo B., Endevelt R., Porath A., Stampfer M., Shai I. (2011) Adherence to weight loss medications; post-marketing study from HMO pharmacy data of one million individuals. Diabetes Res Clin Pract 94: 269–275 [DOI] [PubMed] [Google Scholar]
- Holst J. (2013) Incretin hormones and the satiation signal. Int J Obes (Lond) 37: 1161-1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M., Flint A., Jones K., Hindsberger C., Rasmussen M., Kapitza C., et al. (2012) Effect of the once-daily human GLP-1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res Clin Pract 97: 258–266 [DOI] [PubMed] [Google Scholar]
- Hutter M., Schirmer B., Jones D., Ko C., Cohen M., Merkow R., et al. (2011) First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 254: 410–420; discussion 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W., Caterson I., Coutinho W., Finer N., Van Gaal L., Maggioni A., et al. (2010) Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 363: 905–917 [DOI] [PubMed] [Google Scholar]
- Karmali S., Brar B., Shi X., Sharma A., De Gara C., Birch D. (2013) Weight recidivism post-bariatric surgery: a systematic review. Obes Surg 23: 1922-1933 [DOI] [PubMed] [Google Scholar]
- Kashyap S., Bhatt D., Wolski K., Watanabe R., Abdul-Ghani M., Abood B., et al. (2013) Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 36: 2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Abbasi F., Lamendola C., Liu A., Ariel D., Schaaf P., et al. (2013) Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care 36: 3276-3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean M., Brosnahan N., McLoone P., McCombie L., Higgs A., Ross H., et al. (2013a) Feasibility and indicative results from a 12-month low-energy liquid diet treatment and maintenance programme for severe obesity. Br J Gen Pract 63: e115–e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean M., Carraro R., Finer N., Hartvig H., Lindegaard M., Rossner S., et al. (2013b) Tolerability of nausea and vomiting, and associations with weight loss, in a randomized trial of liraglutide in obese, non-diabetic adults. Int J Obes (Lond) 14 August (epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveman E., Frampton G., Shepherd J., Picot J., Cooper K., Bryant J., et al. (2011) The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess 15: 1–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovshin J., Drucker D. (2009) Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 5: 262–269 [DOI] [PubMed] [Google Scholar]
- Magi R., Manning S., Yousseif A., Pucci A., Santini F., Karra E., et al. (2013) Contribution of 32 GWAS-identified common variants to severe obesity in European adults referred for bariatric surgery. PLoS One 8: e70735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malm-Erjefalt M., Bjornsdottir I., Vanggaard J., Helleberg H., Larsen U., Oosterhuis B., et al. (2010) Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Dispos 38: 1944–1953 [DOI] [PubMed] [Google Scholar]
- McCarthy M. (2010) Genomics, type 2 diabetes, and obesity. N Engl J Med 363: 2339–2350 [DOI] [PubMed] [Google Scholar]
- Nathan P., O’Neill B., Napolitano A., Bullmore E. (2011) Neuropsychiatric adverse effects of centrally acting antiobesity drugs. CNS Neurosci Ther 17: 490–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil P., Smith S., Weissman N., Fidler M., Sanchez M., Zhang J., et al. (2012) Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 20: 1426–1436 [DOI] [PubMed] [Google Scholar]
- O’Rahilly S., Farooqi I. (2008) Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes 57: 2905–2910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Filho G., Wong M., Licinio J. (2011) Ten years of leptin replacement therapy. Obes Rev 12: e315–e323 [DOI] [PubMed] [Google Scholar]
- Richard D., Ferland J., Lalonde J., Samson P., Deshaies Y. (2000) Influence of topiramate in the regulation of energy balance. Nutrition 16: 961–966 [DOI] [PubMed] [Google Scholar]
- Richelsen B., Tonstad S., Rossner S., Toubro S., Niskanen L., Madsbad S., et al. (2007) Effect of orlistat on weight regain and cardiovascular risk factors following a very-low-energy diet in abdominally obese patients: a 3-year randomized, placebo-controlled study. Diabetes Care 30: 27–32 [DOI] [PubMed] [Google Scholar]
- Rodgers R., Tschop M., Wilding J. (2012) Anti-obesity drugs: past, present and future. Dis Model Mech 5: 621–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan D., Bray G. (2013) Pharmacologic treatment options for obesity: what is old is new again. Curr Hypertens Rep 15: 182–189 [DOI] [PubMed] [Google Scholar]
- Saris W. (2001) Very-low-calorie diets and sustained weight loss. Obes Res 9(Suppl. 4): 295S-301S [DOI] [PubMed] [Google Scholar]
- Shin J., Gadde K. (2013) Clinical utility of phentermine/topiramate (Qsymia) combination for the treatment of obesity. Diabetes Metab Syndr Obes 6: 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom L., Narbro K., Sjostrom C., Karason K., Larsson B., Wedel H., et al. (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357: 741–752 [DOI] [PubMed] [Google Scholar]
- Sjostrom L., Peltonen M., Jacobson P., Sjostrom C., Karason K., Wedel H., et al. (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307: 56–65 [DOI] [PubMed] [Google Scholar]
- Smith S., Weissman N., Anderson C., Sanchez M., Chuang E., Stubbe S., et al. (2010) Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 363: 245–256 [DOI] [PubMed] [Google Scholar]
- Sommer B., Mitchell E., Wroolie T. (2013) Topiramate: effects on cognition in patients with epilepsy, migraine headache and obesity. Ther Adv Neurol Disord 6: 211–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman J., Levitsky D., Allison D., Bray M., De Castro J., Clegg D., et al. (2011) Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech 4: 733–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn B., Sacks G., Hall K., McPherson K., Finegood D., Moodie M., et al. (2011) The global obesity pandemic: shaped by global drivers and local environments. Lancet 378: 804–814 [DOI] [PubMed] [Google Scholar]
- Unick J., Beavers D., Bond D., Clark J., Jakicic J., Kitabchi A., et al. (2013) The long-term effectiveness of a lifestyle intervention in severely obese individuals. Am J Med 126: 236–242, 242.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ussher J., Drucker D. (2012) Cardiovascular biology of the incretin system. Endocr Rev 33: 187–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsboll T., Christensen M., Junker A., Knop F., Gluud L. (2012) Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ 344: d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden T., Hollander P., Klein S., Niswender K., Woo V., Hale P., et al. (2013) Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the scale maintenance randomized study. Int J Obes (Lond) 37: 1443-1451 [DOI] [PubMed] [Google Scholar]
- Wass J., Finer N. (2013) Action on obesity: comprehensive care for all. Clin Med 13: 4–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee C., Hamel M., Apovian C., Blackburn G., Bolcic-Jankovic D., Colten M., et al. (2013) Expectations for weight loss and willingness to accept risk among patients seeking weight loss surgery. JAMA Surg 148: 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman N., Sanchez M., Koch G., Smith S., Shanahan W., Anderson C. (2013) Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ Cardiovasc Imaging 6: 560–567 [DOI] [PubMed] [Google Scholar]
- Wheeler E., Huang N., Bochukova E., Keogh J., Lindsay S., Garg S., et al. (2013) Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nat Genet 45: 513-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock G., Lewington S., Sherliker P., Clarke R., Emberson J., Halsey J., et al. (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373: 1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer C., Speliotes E., Loos R., Li S., Lindgren C., Heid I., et al. (2009) Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing R., Bolin P., Brancati F., Bray G., Clark J., Coday M., et al. (2013) Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369: 145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]


