Abstract
Background
HIV-infected prisoners have a high prevalence of alcohol use disorders and commonly relapse to alcohol soon after release to the community which is linked to high morbidity, poor antiretroviral therapy (ART) adherence and increased sexual risk-taking behaviors. Extended-release naltrexone (XR-NTX) effectively reduces relapse to alcohol in alcohol dependent persons, yet it remains unexamined among criminal justice system (CJS) populations transitioning to the community.
Methods
A randomized double-blind, placebo-controlled trial of XR-NTX to improve HIV treatment outcomes via reducing relapse to alcohol use after prison release for HIV-infected hazardous drinking and alcohol dependent prisoners is discussed.
Results
Acceptability of study participation is high with 86% of those referred who met eligibility criteria and 85% of those who were able to receive injections prior to release accepted injections, yet important implementation issues are identified and addressed during the study and are discussed in this paper.
Conclusion
Medication-assisted therapies for prevention of relapse to alcohol use for CJS populations transitioning to the community, especially for HIV-infected patients, are urgently needed in order to reduce alcohol relapse after release and improve HIV treatment outcomes and contribute to improved individual and public health.
Keywords: Alcohol Use Disorder, Hazardous Drinking, HIV, Extended-Release Naltrexone, prisoners, randomized controlled trial
Introduction1
Incarceration in the United States has become epidemic with 1 in every 100 Americans currently behind bars [1]. Compared to the general population, the U.S. criminal justice system (CJS) disproportionately houses individuals with significant medical and substance use disorders (SUDs); specifically, the prevalence for HIV/AIDS is 3–4-fold [2] and Hepatitis C (HCV) is 13-fold higher that surrounding communities [1]. Similarly, it is estimated that the prevalence of alcohol dependence and problematic drinking is 40–60% among prisoners [3]. Alcohol use negatively impacts the health outcomes for individuals infected with HIV, HCV or both [4, 5].
Similar to the case for infectious diseases, prisoners transitioning to the community are at high risk for negative consequences from SUDs, including overdose, death, relapse to alcohol and drug use, and discontinuity from chronic care – in particular HIV care [5–10]. Naltrexone (NTX), an FDA-approved and evidence-based pharmacotherapy used to treat alcohol dependence, is available in both oral and the injectable extended-release-formulation (XR-NTX). In the most comprehensive, prospective, randomized controlled trial (RCT) of alcohol treatment pharmacotherapies, the COMBINE trial affirmed oral NTX as superior to acamprosate, including with or without adjunctive cognitive behavioral counseling [11, 12]. XR-NTX also effectively prevents relapse and decreases heavy drinking in alcohol-dependent people without HIV [12–14]. Despite no head-to-head comparisons, monthly XR-NTX is perceived to have an adherence advantage over oral naltrexone [15]. Despite people living with HIV/AIDS (PLWHA) having a high prevalence of alcohol use disorders and that alcohol negatively impacts HIV treatment outcomes, no RCTs of available pharmacotherapies have focused on HIV-infected patients. Moreover, no trials directly examine the impact of alcohol treatment on HIV rather than on alcohol treatment outcomes with the hypothesis that reductions in alcohol use would improve HIV treatment outcomes – specifically retention in care, antiretroviral therapy (ART) adherence and HIV risk behaviors. Moreover, where HIV and alcohol are concentrated within the CJS, NTX in either formulation has not been empirically tested to assess its impact on HIV treatment and criminal justice outcomes. The current study specifically uses a placebo-controlled design to examine if XR-NTX administered before release reduces alcohol consumption and thereby improves ART adherence, viral suppression and reduction in HIV risk behaviors in HIV-infected patients – the only patients that can transmit HIV.
Methods
Study design
Project INSPIRE is a prospective, double-blind randomized, placebo-controlled trial of XR-NTX among HIV-infected prisoners with alcohol use disorders (alcohol dependence or hazardous drinking) who are transitioning to the community. The study design is depicted in Figure 1.
Figure 1.
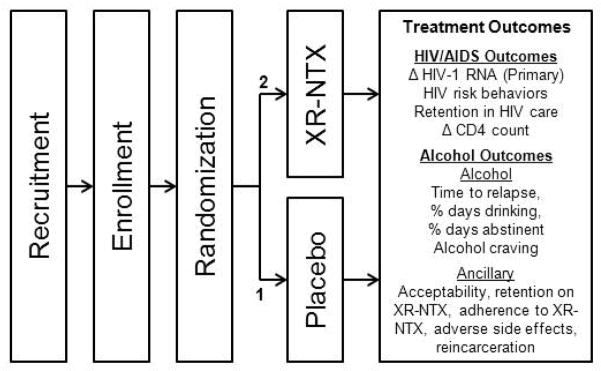
Study Design
Ethical Oversight
All procedures were reviewed and approved by Institutional Review Boards (IRB) at Yale University and Connecticut Department of Correction (CTDOC) Research Advisory Committee and it was registered at www.clinicaltrials.gov (NCT10177310). Because the trial involved prisoners with substance use disorders, additional protections were afforded by the Office of Human Research Protections (OHRP) at the Department of Health and Human Services and a Certificate of Confidentiality was obtained from the National Institutes of Health (NIH).
Research Goals
Because alcohol consumption, especially heavy drinking, negatively impacts numerous HIV treatment outcomes, including poor linkage and retention in care, ART adherence and viral suppression [16], the primary aim of this study is to examine if an evidence-based alcohol treatment pharmacotherapy (XR-NTX used here due to its adherence advantage and avoidance of pill burden) effectively improves HIV treatment outcomes. Thus for HIV-infected persons, we focused primarily on the most distal HIV treatment outcome, viral suppression (HIV-1 RNA<400 copies/mL) 6-months post-release, since it is a surrogate for both retention in care and optimal ART adherence. Viral suppression, even in the absence of suboptimal condom use, also markedly reduces HIV transmission among heterosexual adults [17]. The secondary HIV treatment outcomes include maximal viral suppression (HIV-1 RNA<50 copies/mL) and XR-NTX’s effect on HIV risk behaviors. Other secondary outcomes include those related to alcohol relapse (time to alcohol relapse, percent days drinking or heavy drinking and alcohol craving), and XR-NTX toxicity when combined with ART, especially hepatotoxicity and injection site reactions.
Sample Size and Power Calculations
We calculated the sample size needed to detect the difference in primary outcome with at least 80% power and a two-sided significance level of p<0.05. We assumed alpha=0.05, beta=0.20, and a compound symmetry true correlation structure of 0.5 (the most conservative, based on our results from earlier studies where our prison-release data suggested that 59% of HIV+ inmates leave prison with viral suppression, defined as HIV-1 RNA < 400 copies/mL [18], and where the proportion of HIV-infected inmates with alcohol use disorders leave prison with a non-detectable HIV-1 RNA is 28% [19]). Given the safety concerns about combining XR-NTX with ART, we proposed to oversample those receiving XR-NTX and therefore randomized 2:1 to XR-NTX and placebo. We also assumed retention in the study to be 80% over the first six months, thereby requiring a total sample of 125 (XR-NTX=83 and placebo=42).
Study Procedures
Recruitment and Screening
Subjects were recruited directly within the CTDOC or from a community-based organization that initiates transitional case management 90 days pre-release. The Infectious Disease Control Nurse (IDCN) coordinates all HIV-related care, including discharge planning, and referrals to post-release services and research. All 90-day pre-release HIV-infected inmates who either had documented alcohol problems or responded affirmatively to “did you ever have ≥4 drinks (women) or ≥5 drinks (men) on any day in the month prior to incarceration?” were asked to sign a release of information (ROI) to learn more about a research study that could help them transition home.
Eligibility Process
After completing the ROI, the IDCN referred patients to our Research Assistant (RA) who would then schedule a meeting with the patient in a confidential prison setting and assess for eligibility and if eligible, describe the details of the study. The inclusion criteria included: 1) documented HIV-infection; 2) transitioning to New Haven or Hartford areas; 3) met criteria for alcohol dependence (Mini International Neuropsychiatric Interview (MINI)) [20–22] or hazardous drinking (Alcohol Use Disorders Identification Test (AUDIT)) [23, 24]; 4) able to provide informed consent; 5) could speak English or Spanish; and 6) 18 years or older. Ineligibility included: 1) prescription of opioid pain medications or expressing a need for them; 2) grade 3 aspartate aminotransferase (AST) or alanine aminotransferase (ALT) elevations (>5x upper limit of normal); 3) evidence of Child’s Pugh Class C Cirrhosis; 4) breastfeeding, pregnant or unwilling to use contraception (women); or 5) enrolled in another pharmacological or adherence research study.
Subjects who were considered eligible for XR-NTX therapy for alcohol dependence or problem drinking met the criteria for alcohol dependence by the MINI [20–22] or hazardous drinking according to the AUDIT, a screening tool developed by the World Health Organization [23, 24], (score ≥4 for women and ≥8 for men) [25].
Informed Consent and Enrollment
After establishing eligibility, the RA completed the informed consent procedures and assessed the participant’s interest in participation, including willingness to receive their first injection within prison one week before release. On the day of release, and ideally after the initial injection if the participant was not released first, the RA would meet the participant in New Haven or Hartford to reaffirm the informed consent to assure that there was no real or perceived coercion within the prison, and inquire about any side effects from the initial injection. During the enrollment process, participants are also asked to complete additional ROIs, allowing study staff to contact the CTDOC, their community primary care providers, substance abuse treatment programs, area hospitals and the Medicaid office for information regarding medical conditions, prescription refill information and laboratory results.
Covariates and Outcomes Measures
Screening and Intervention Measures
All enrolled participants underwent baseline assessments, follow-up interviews and laboratory assessments monthly for 12 months. Please refer to Table 1 for the measures, main outcomes assessed, and the study timeline.
Table 1.
Study measures and time of assessment.
| Study Measure | Interview Time | |||
|---|---|---|---|---|
| Baseline | Day of Release | Quarterly (12, 24, 36, 48) | Weeks (4, 8, 16, 20, 28, 32, 40, 44) | |
|
| ||||
| Demographic Information | ||||
| Demographic Questions | X | |||
| Housing Questions | X | X | X | X |
|
| ||||
| Health Care Status | ||||
| HIV quality of life (SF-36) [47] | X | X | ||
| Current Medications | X | X | X | X |
| Prescription Refill | X | X | X | X |
| Prison Medical Record (Medication, ART regimen, HCV antibody, mediation allergies) | X | |||
| Visual Analog Scale [48] | X | X | X | X |
| Previous Experience with Alcohol and Drug Treatment | X | |||
|
| ||||
| Mental Health | ||||
| Mini International Neuropsychiatric Interview (MINI) [20–22] | X | |||
| Correctional Medical Record Diagnoses | X | |||
| Brief Symptom Inventory (BSI) [49] | X | X | ||
|
| ||||
| Drug and Alcohol Use | ||||
| Addiction Severity Index [50, 51] | X | X | ||
| Drug Urine Toxicology Screening (opioids, methadone, cocaine, marijuana, benzodiazepines, oxycodone, buprenorphine, and amphetamines) | X | X | X | X |
|
| ||||
| Alcohol Use | ||||
| Alcohol Use Disorder Identification Test [23–25] | X | |||
| Timeline Follow-back [52, 53] | X | X | X | X |
| Alcohol Craving | X | X | X | X |
| DSM-IV Criteria using MINI [21] | ||||
| Alcohol Short Index of Problems (SIP-A) [54] | X | X | ||
| Blood Alcohol Content via Breathalyzer | X | X | X | |
| Phosphatidyl Ethanol Testing (PEth) [55–59] | ||||
|
| ||||
| Criminal Justice Status (CJS) | ||||
| Current CJS Involvement | X | X | X | X |
| Entry and Release dates from CJS | X | X | X | X |
| Time to Reincarceration | X | X | ||
| Days of Reincarceration | X | X | ||
|
| ||||
| HIV Risk Behaviors | ||||
| Sexual Risk Behaviors [60] | X | X | X | X |
|
| ||||
| HIV Biological Outcome Measures | ||||
| HIV RNA level | X | X | ||
| CD4 Count | X | X | ||
| HIV genotype | X | Week 48 | ||
|
| ||||
| Other Laboratory Tests | ||||
| Liver Function Tests (AST, ALT, GGT) | X | X | X | X |
| Renal Function Tests (BUN, Creatinine) | X | X | X | X |
|
| ||||
| Side Effects | ||||
| Systemic Assessment for Treatment Emergent | X | Weeks 12, 24 | Weeks 4, 8, 16, 20 | |
| Effects Intervention (SAFTEE) [26, 61] | ||||
|
| ||||
| Payments | ||||
| Research Interviews | $20 upon release | $20 | $40 Weeks 12, 24; $60 Weeks 36, 48 | $20 Weeks 4, 8, 16, 20; $30 Months 28, 32, 40, 44 |
| Clinical Interviews | $10 | $10 Week 12 | $10 Weeks 4, 8, 16, 20 | |
Legend: ART=antiretroviral therapy; CJS=criminal justice system; ALT=alanine aminotransferase; AST=aspartate aminotransferase; GGT=gamma-glutamyl transpeptidase; BUN=blood urine nitrogen
Process Measures
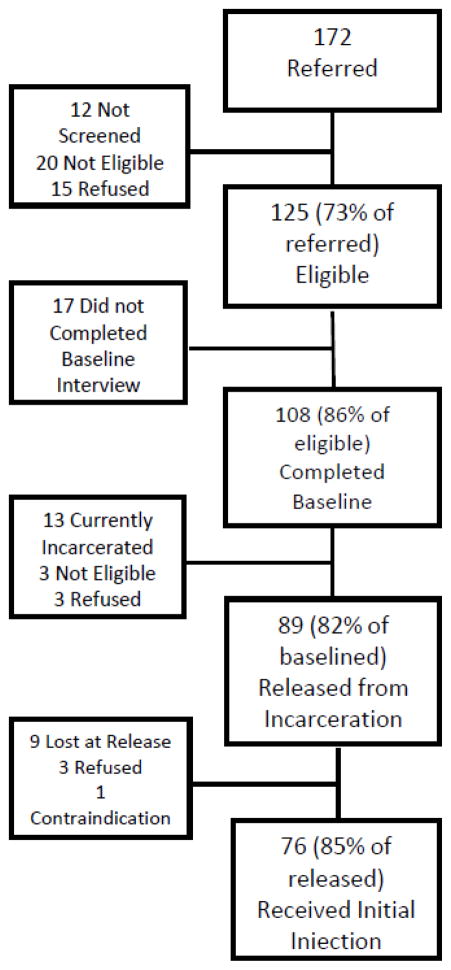
In addition to standardized individual-level outcomes as noted in Table 1, a number of intangible qualitative components of the intervention were also obtained once appropriate ROIs were completed. These included (1) communication with staff, participants and CTDOC personnel, clinicians from research meeting notes; (2) tolerability and adverse event monitoring assessed monthly during the 6 month intervention utilizing the Systemic Assessment For Treatment Emergent Effects Intervention (SAFTEE) [26]; 3) acceptance of study participation, specifically focusing on importance of treating alcohol problems with an injectable medication and recognition that alcohol contributes to poor outcomes was assessed through evaluating those who met eligibility criteria for participation and who signed consent and completed the baseline interview (N=108/125, 86%) and by the number who completed baseline who were to be released to the community and accepted the initial injection prior to release (N=76/89, 85%) as depicted in the Flow Chart in Figure 2; (4) ancillary encounters with the alcohol treatment clinicians and counseling staff and case management services (e.g. assistance with basic needs such as food, shelter or safety, disability insurance, medical appointments, etc); (5) attrition from intervention over time, irrespective of their ability to remain in the assigned study arm will be recorded, to learn about their drug use and adherence behavior; and (6) acceptability of the intervention, with a focus on acceptance of a monthly injection.
Figure 2.
Study Flow of Acceptability
Randomization
Randomization 2:1 to XR-NTX: placebo was used to sufficiently address tolerability issues of receiving XR-NTX rather than placebo. Covariate adaptive randomization [27] assured that covariates potentially associated with outcomes were controlled for in the randomization [28, 29]. This randomization approach produces less imbalance than other conventional randomization methods (e.g., stratified, block, simple) [28]. Covariates included in the randomization included: 1) presence or absence of opioid dependence; and 2) being prescribed or not prescribed ART.
Intervention
Study Procedures
Due to the complexity of working with prison inmates before release and following them into the community, the study intervention procedures are detailed below and specific implementation issues regarding these procedures needed to be overcome prior to beginning the actual study and are discussed later and in Table 2.
Table 2.
Study Implementation Issues
| Permissions and Access in Facilities | ||
|---|---|---|
| Obstacle | Action Taken | Knowledge Gained |
| Unrestricted personnel access to the facilities | Staff training by DOC as professional visitors, annual training renewals, background checks, and study approval by Research Advisory Committee. | Collaboration with the DOC is key to the studies success; all staff will be trained upon hire. |
| Permission to enter the facilities with computers, medications, and needles | A letter was obtained from the DOC central office allowing us to bring study computer and medication packages into each facility. Each warden was provided a copy of this letter. Default settings for the computers must be set to DOC standards and medications were to be sealed from the manufacture. | To ensure DOC policy compliance study computer specifics were sent to DOC before ordering. A meeting with samples of packaged medication, along with any applicable letters from the manufacture for suggested syringes used with medication. |
| Confidential space for interviews and injections in the facilities | When scheduling interviews space was requested in the professional visitors area or in the medical unit allowing staff members the confidentiality needed to conduct interviews and injections with the safety of with correctional officers nearby. | Requests for professional and medical visits were faxed to the appropriate offices approximately 7 days prior to the requested date to ensure a confidential room is reserved. Medical visits were for hours that were after peak working to accommodate the limited space |
| Recruitment and Facility Staff Trainings | ||
| Obstacle | Action Taken | Knowledge Gained |
| Obtaining signed Releases of Information | At the start and periodically throughout the study, information sessions were held for medical staff with new information about addiction medicine including the use of XR-NTX and information about the studies including contact information and how to refer inmates. | Educate and meet with medical staff from all the facilities to inform them of XR-NTX and the study. Releases of Information were needed to refer inmates to the study and to allow study staff to obtain needed medical information. |
| Training all DOC nurses and CRs on proper use and safety concerns of XR-NTX | To ensure the safe delivery of the injections all CRs were trained by an Alkermes Inc. representative. To ensure all DOC and CMHC employees were aware of the study, medication, and possible side effects, special education sessions were performed by the study PIs. We obtained approval from the DOC to place notes in the DOC chart to acknowledge the participants who were had received an injection and the number to call for any side effects concerns. | All injections are performed by CRs who are CTDOC credentialed nurses who have obtained training on the appropriate manner on how to administer the study drug. Additionally all medical staff were educated on the side effects and use of XR-NTX in the community in the form of lectures and handouts. |
| Informed Consent Process | Due to the elevated concerns of coercion of inmates in the criminal justice setting, all study staff are trained with the increased awareness of this special populations needs. Additionally, upon release participants undergo the informed consent process to safeguard against coercion during their incarceration. | In addition to standard human subjects training, additional training for special populations increased the awareness of coercion. To reduce the risk of coercion participants underwent the informed consent process for a second time upon release. |
| Post Release Concerns and Contact | ||
| Obstacle | Action Taken | Knowledge Gained |
| Having an on-call system for dealing with adverse consequences | To ensure all medical side effect or questions from a participant or their provider were addressed 24 hours a day, a toll free number was provided. | We obtained a toll free number that was always staffed in case of questions or concerns about the injections that DOC nurses or medical clinicians could call as well as the clients themselves could utilize when they were released. |
| Continuous contact between study staff and participants | Cellular phones were given to all participants at time of release from prison and jail as of September, 2012 and after approval by the NIAAA, Yale IRB, in order to improve retention in care after release and allow participants to easily contact the RA, study clinician or a counselor if they had any questions or concerns about the study or study medication | The increase in communication and availability increased the study retention. |
| Reincarceration after enrollment | As a way to capture real life situations and data, contact with the participant throughout the year of participation a request was made to the CTDOC to continue with study interviews should someone become reincarcerated. | To continue data collection if a participant was reincarcerated we obtained approval by the CTDOC to reenter the prison and jail facilities if the participants were reincarcerated to obtain study interviews. |
| Recruitment | ||
| Obstacle | Action Taken | Knowledge Gained |
| Potential willingness to accept treatment (e.g., medication or an injection) for an alcohol use disorder | Prison staff was not involved in the recruitment process aside from identifying “heavy drinking” in the 30 days before incarceration. When the RA met with potentially eligible clients, considerable time discussing AUDs and their potential impact on health, HIV treatment outcomes and on public health were discussed as part of the initial visit. | Prison staff only identified individuals with severe alcohol dependence so it was important not only to educate them about the various levels of AUDs, but to leave this discussion to potential participants so that they could ask more detailed questions by a trained research staff member. |
| Increase enrollment and obtain other criminal justice settings | There was a decrease in the number of HIV+ persons in the correctional system, thus to increase enrollment and involve other criminal justice setting the eligibility criteria were expanded to include those on parole and probation, but within 30 days of release, in March 2013. | Expanding the criteria of participants who were under community supervision by the criminal justice system allowed the opportunity to gain knowledge of the behaviors of those under probation and parole, and expand the generalizability of the studies findings. |
| Changes in early release policies within the CJS | The CTDOC Referrals Liaison and facility ID nurses played a critical role in monitoring possible release dates, transitional housing status and policy changes. Additional communication was required to monitor these changes. | Policies are continuously changing with the needs of each state. A good relationship is needed between the research staff and correctional staff to monitor policy changes and the implications it will have on the inmates and study. |
Legend: CTDOC=Connecticut Department of Correction; CR=Clinical Researcher; CMHC=Correctional Managed Health Care; XR-NTX=Extended-release naltrexone; NIAAA=National Institute on Alcohol Abuse and Alcoholism; IRB=Internal Review Board; RA=Research Assistant; AUD=alcohol use disorder.
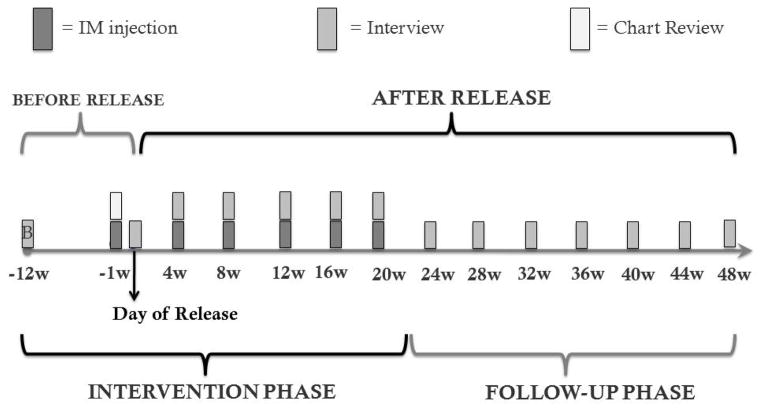
(1) Pre-Release
The baseline interview was conducted using a laptop computer with a computer-assisted survey (CASI) [30, 31] immediately after initial consent procedures. CASI was selected to allow inmates to answer questions confidentiality and avoid prison officers from overhearing responses to private issues like HIV risk behaviors and alcohol and drug consumption as previously described [32]. Approximately 1–2 weeks pre-release, patients were randomized using baseline interview data and received their first study medication injection. Please refer to Figure 3 for Injection and Study Procedure Timeline. Study medication allocation was sent to the Yale Investigational Drug Service (YIDS) where study medication and placebo are stored and distributed and labeled by the pharmacist using the participant’s study number. A specialized “order” was placed in the medical record indicating the patient had received a study medication injection and if the patient complained of any adverse side effects, the study clinician would be called at a toll-free number. In unusual cases where a participant was released without an injection, it was administered in the community if within 30 days post-release.
Figure 3.
Study Procedure Timeline
(2) Day of Release
To facilitate retention, RAs met and transported participants to a site of their preference (home, shelter, short-term housing, etc.) and inquired about any other local spots where they are likely to spend time during the course of the study. All participants, as noted previously, underwent consent again after release to ensure that no coercion took place within prison, along with a brief survey to assess the within-prison injection experience within the prison, and undergo phlebotomy, breathalyzer, urine toxicology, update contact information, and their health and behaviors within the prison. Participants were paid an “enhanced” incentive for showing up immediately after release to improve retention. As of September 2012, study participants were provided with an inexpensive mobile phone to maintain a direct line of communication with the staff and medical providers. Participants that were active in study or enrolled on or after September 2012 were provided with study cell phones during their participation. If the participant already had a phone and preferred to use their own cell phone then we reimbursed them for their cell phone usage, provided they responded to our phone calls and/or text messages.
(3) Monthly Research Visits
All monthly CASI interviews were conducted in private rooms along with phlebotomy, urine toxicology tests, urine pregnancy tests for female participants, alcohol breathalyzer, ART adherence assessments in the form of the Visual Analog Scale [33], Phosphatidylethanol (PEth) tests (quarterly), and periodic prescription refill data collection through the CT Medicaid office (see Table 1). During the first six months when injections were administered, adverse side effects were assessed and all participants received a brief medical management counseling intervention [34].
(4) Injection Procedures
Injections occurred monthly for five months post-release for a total of 6 injections, followed by 7 additional follow-up months without injections. The Clinician Researcher (CR) assessed the prior month’s adverse side effects, confirmed and documented the injection of the study medication allocation and recorded it with the participant’s study number. This was done immediately after the monthly interview by the RA.
(5) Counseling visits
All subjects, irrespective of randomization, received standardized monthly 15–20 minute Medical Management (MM) counseling intervention for alcohol dependence [25, 34] concurrent with the 6 monthly injections. The brief MM counseling intervention has been documented to be equivalent to lengthier cognitive behavior counseling intervention used in the COMBINE trial [35]. MM briefly reviews medication information including alcohol pharmacotherapy, laboratory results, drinking status and prior counseling in addition to briefly counseling patients about the hazards of drinking. For all participants, irrespective of randomization, they were referred to more intensive community-based counseling if perceived be failing the existing treatment program.
Payments
Please refer to Table 1 for subject compensation and time points.
Specific Safety Protocols
All medications were prepared and packaged by Alkermes, Inc. and dispensed to YIDS where a licensed pharmacist dispensed medications, blinded to randomization, to the CRs who performed the injections after subject randomization. No experimental medications were used. Subjects were monitored closely at monthly visits for side effects, liver and renal toxicities as well as for relapse to alcohol or other drug use. Prior to administering the monthly injection, current medications and medical diagnoses were reviewed from information gleaned from the correctional medical record. Also, urine toxicology, alcohol breathalyzer, review of liver and renal function, and an assessment for active alcohol and other drug use was completed; a urine pregnancy screen was obtained for all women. Grade 4 hepatotoxicity (defined as liver function tests >10 x upper limit of normal with clinical symptoms or signs of hepatotoxicity), development of Childs-Pugh Class C cirrhosis, and pregnancy were immediate reasons to stop the study and unblind the participant.
Analytic Plan
HIV Treatment Outcomes
The primary outcome for this study was a comparison of the proportion achieving a non-detectable (<400 copies/mL and <50 copies/mL) HIV-1 RNA level at the end of the intervention (6 months post-release) for both treatment groups. Missing values were imputed as failure and assessed via logistic regression.
Other secondary HIV treatment measures included mean change in HIV-1 RNA levels over 6 (intervention effect) and 12 months (post-intervention effect) after release, including values at 3 and 9 months. Changes in log10 HIV-1 RNA level from baseline will be fitted to a linear regression with interval censoring using the SAS procedure LIFEREG with the dist=normal option. This robustly accounts for the large number of censored values owing to viral loads at the lower limits of detection at baseline and at follow-up. Missing values are imputed as zero change from baseline. Normal probability plots confirmed that data fit the parametric assumptions of the regression. A general linear model including baseline CD4 lymphocyte count (CD4) and HIV-1 RNA level as covariates will assess mean change in the log10 HIV RNA level from baseline to the 6-month and 12-month follow-up. In order to further inform our virologic outcome approach, we will also conduct a repeated measure outcome of viral load that incorporates mean change from baseline throughout the 12 months of the study. This allows a smaller sample size to reflect a difference and accounts for the changing nature of subjects’ lives, for example housing [36] and concomitant drug use [37] during the follow-up period. For instance, a single time point may not reflect how one did in the past or will do in the future since alcohol use is a chronic relapsing condition. Univariate chi-square tests and odds ratios with 95% confidence intervals will be used to assess differences in the proportion of participants in each group who meet each endpoint. Kaplan-Meier and log-rank tests will be used to assess differences in the time to endpoints in each group.
Similarly, CD4 counts have been strongly associated with survival and risk for development of opportunistic infections. It will not, however, be a primary endpoint as CD4 benefits may persist after loss of adherence. Using the same arguments made for HIV-1 RNA in this patient population, we will use multiple measures. Kruskal-Wallis testing and a Dunn multiple comparison test will be performed to determine any statistically significant changes observed in CD4 count over the course of the study between groups. Analysis of change in mean/median CD4 count from baseline to weeks 24 and 48 will use the Wilcoxon rank test, stratified by variables such as ART experience. Spearman’s rank correlation will test for associations between a wide range of variables with a binomial distribution. Outcomes of interest include: 1) Averaged change in CD4 count from baseline over time (repeated measures); and 2) Proportion who increase their CD4 count by >50 (and >100).
Alcohol Use Outcomes
Alcohol use outcomes will also be a secondary outcome since the purpose of this study is to examine how alcohol consumption impacts ART adherence and ultimately viral suppression. The TimeLine Follow-Back (TLFB) uses calendar-based methods and collects specific daily amounts of alcohol consumption using anchor dates to improve accuracy. A 10-point Likert scale assesses alcohol craving and the ASI assesses “severity” of alcohol-related problems. These standardized alcohol outcome measures are previously described [38] and widely used in previous alcohol studies, including both Project MATCH [39] and COMBINE studies [35]. TLFB assesses alcohol relapse [35, 38, 39] and: 1) time to relapse to heavy drinking (men: ≥5 drinks/drinking day; women: ≥4 drinks/drinking day) will be assessed using Kaplan Meier survival analysis, using the log rank test and Wilcoxin statistics and stratifying by XR-NTX and placebo; 2) both percent of days drinking and percent of days abstinent over the six-month intervention period comparing XR-NTX and placebo after transforming the outcome to means and compared statistically using Mantel-Haenszel Chi Square; and 3) alcohol craving where a mean change in total score will be compared as a repeated measure between treatment groups and a comparison of means will be analyzed using the Mantel-Haenszel Chi Square [40].
Implementation Issues
Working with criminal justice populations requires considerable experience, logistical problem-solving and ethical oversight. Some important barriers to conducting this study, all of which were successfully overcome, merit inclusion to help guide other researchers working with criminal justice populations and we have included these below and in Table 2.
All staff had to be trained and approved to enter and leave facilities that required CTDOC clearance after obtaining study approval from the Research Advisory Committee; then a letter had to be obtained from the Director of the Research Unit, shown by the RA at each visit that they were allowed to enter the facility.
Bringing a needle into the facility: All clinical staff had to be authorized by the CTDOC to bring in the pre-packaged XR-NTX and placebo injections that included the manufacturer’s provided and recommended needles to draw up the medication and another to administer the medication to the participants. This required a separate evaluation process.
Confidential space to interview but allowing prison officers access for safety concerns: We had to obtain access to a guaranteed space to perform the eligibility screen, informed consent process, and baseline interviews for the RAs as well as a separate clinical private space for the nurses to provide the injections.
Computer entry: We had to obtain separate approval to bring laptop computers into the prisons and jails in order to record the responses to the participants’ interviews. This included ensuring that all computers had the Internet and cameras disabled. The rationale for using computers and the CASI method was to avoid prison staff from hearing responses to survey questions and thus maintaining confidentiality.
Necessity of obtaining signed ROIs: This was not only to allow IDCNs to refer patients to the study, but also for study staff to review their medical records as part of the screening process and for obtaining information regarding HIV treatment, HCV status and baseline HIV VL, CD4, reasons for incarceration, and baseline liver and renal function.
Informed Consent Process: Due to the work with prisoners, informed consent was obtained at time of baseline assessment and on day of release when prisoners would be re-consented in order to avoid coercion.
The process of giving a medication (XR-NTX or Placebo) WHILE participants were still incarcerated: This required authorization by CTDOC for us to place a note in the patients’ medical DOC record identifying that the patients were enrolled in the study and had received and injection that might be XR-NTX and a number to call if the participant should have side effects as delineated in educational formats as described above.
Training all DOC nurses and clinicians on proper use and safety concerns of XR-NTX: All injections are performed by study clinicians who are CTDOC credentialed nurses in the 20 state prisons and jails within CT and who have obtained training on the appropriate manner on how to administer the study drug via the Alkermes, Inc. representatives. Additionally in order for all medical staff to understand the side effects and use of XR-NTX in the community, Drs. Springer and Altice (Principal Investigators) and other research staff provided education to CTDOC and Correctional Managed Health Care (CMHC) employees in the form of lectures and handouts.
Having an on-call system for dealing with adverse consequences: we obtained a toll free number that was always staffed in case of questions or concerns about the injections that DOC nurses or medical clinicians could call as well as the clients themselves could utilize when they were released.
Cellular phones were given to all participants at time of release from prison and jail as of September, 2012 and after approval by the NIAAA, Yale IRB, in order to improve retention in care after release and allow participants to easily contact the RA, study clinician or a counselor if they had any questions or concerns about the study or study medication.
Reduction in HIV populations in the CJS: there has been a decrease in the number of HIV+ inmates in the correctional system. Thus, to increase enrollment and capture other aspects of the CJS, enrollment was expanded to include those leaving prison or jail and under community supervision through the parole or probation offices.
Reincarceration interviews: We obtained approval by the CTDOC to reenter the prison and jail facilities if the participants were reincarcerated to obtain study interviews.
Summary
This study is the first double-blind, placebo-controlled randomized trial designed to explore a novel prison-release alcohol treatment intervention for HIV-infected prisoners meeting DSM-IV criteria for alcohol dependence or hazardous drinking who are transitioning to the community. The novelty of this study is the use of XR-NTX, one of the most effective evidence-based treatments of alcohol dependence that is not hindered by adherence problems, among a group profoundly impacted by alcohol use: released HIV-infected prisoners. Importantly among this population, the majority of study participants are prescribed ART and if found to be effective, will contribute greatly to HIV treatment as prevention [41]. Little attention has addressed or provided empirical data for treatment as prevention among criminal justice-involved persons [42]. The additional use of a placebo-controlled strategy further strengthens any findings that should be demonstrated in the study. The use of XR-NTX has not been evaluated in HIV-infected patients who have pre-incarceration indicators of serious alcohol problems. Importantly, all participants were abstinent from alcohol for many months before initiating treatment and addresses one of the comparative effectiveness concerns raised by the PREDICT trial in Germany that sought to deploy the COMBINE trial methodology, but did not require pre-treatment abstinence [43]. This trial also proactively addresses alcohol problems as a chronic relapsing condition and prophylactically treats it before a patient has spiraled into more severe alcohol problems. Novel in this trial is the strategy of using a placebo-controlled design to determine the extent to which treating alcohol use disorders improves HIV treatment outcomes. This is crucial since patients who are able to be adherent to HIV medications and maintain viral suppression are unlikely to transmit HIV to others, even in the absence of using condoms [44]. Without the use of the placebo-control design, it would be impossible to determine if the pharmacotherapy had any effect. This clinical trial will provide a road map to refine further the use of XR-NTX, if proven efficacious, and provide empirical support for implementation science strategies to expand treatment more broadly to CJ-involved HIV-infected patients. This will not only be crucial for HIV prevention strategies in the U.S., but likely to have greater implications for areas like Eastern Europe, Central Asia and South America where the interface between alcohol use disorders and HIV is even greater [32, 42, 45, 46].
Acknowledgments
The funding for this manuscript is provided by NIAAA R01AA018944 (SAS and FLA) for research and supported by NIDA for career development awards (K02 DA032322 for SAS and K24 DA032322 for FLA). Guidance in study design and assistance with the project was provided by Kendall Bryant, Ph.D., and Deidre Roach, Ph.D. Both study medication and placebo were provided free from an investigator-initiated grant from Alkermes, Inc.
Footnotes
Abbreviations: Criminal justice system (CJS); substance use disorders (SUDs); Naltrexone (NTX); Extended-release naltrexone (XR-NTX); randomized controlled trial (RCT); People living with HIV/AIDS (PLWHA); Connecticut Department of Correction (CTDOC); Internal Review Board (IRB); Infectious Disease Control Nurse (IDCN); release of information (ROI); Research Assistant (RA); Mini International Neuropsychiatric Interview (MINI); Alcohol Use Disorders Identification Test (AUDIT); Systemic Assessment For Treatment Emergent Effects Intervention (SAFTEE); computer-assisted survey (CASI); Yale Investigational Drug Service (YIDS); Phosphatidylethanol (PEth); Clinician Researcher (CR); Medical Management (MM); TimeLine Follow-Back (TLFB); Correctional Managed Health Care (CMHC).
Clinical Trial number :NCT01077310
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hammett TM, Harmon MP, Rhodes W. The burden of infectious disease among inmates of and releasees from US correctional facilities, 1997. American journal of public health. 2002;92:1789–94. doi: 10.2105/ajph.92.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM. HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PloS one. 2009;4:e7558. doi: 10.1371/journal.pone.0007558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mumola C. Justice UDo, editor. Substance Abuse and Treatment, State and Federal Prisoners, 1997. 1999. pp. 1–16. [Google Scholar]
- 4.Serfaty L, Poujol-Robert A, Carbonell N, Chazouilleres O, Poupon RE, Poupon R. Effect of the interaction between steatosis and alcohol intake on liver fibrosis progression in chronic hepatitis C. Am J Gastroenterol. 2002;97:1807–12. doi: 10.1111/j.1572-0241.2002.05793.x. [DOI] [PubMed] [Google Scholar]
- 5.Springer SA, Azar MM, Altice FL. HIV, alcohol dependence, and the criminal justice system: a review and call for evidence-based treatment for released prisoners. The American journal of drug and alcohol abuse. 2011;37:12–21. doi: 10.3109/00952990.2010.540280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, et al. Release from prison--a high risk of death for former inmates. The New England journal of medicine. 2007;356:157–65. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99:361–8. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- 8.Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N, et al. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug and alcohol dependence. 2006;84:188–94. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Palepu A, Tyndall MW, Li K, Yip B, O’Shaughnessy MV, Schechter MT, et al. Alcohol use and incarceration adversely affect HIV-1 RNA suppression among injection drug users starting antiretroviral therapy. Journal of urban health: bulletin of the New York Academy of Medicine. 2003;80:667–75. doi: 10.1093/jurban/jtg073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer SA, Spaulding AC, Meyer JP, Altice FL. Public Health Implications for Adequate Transitional Care for HIV-Infected Prisoners: Five Essential Components. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53:469–79. doi: 10.1093/cid/cir446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial.[see comment] JAMA: the journal of the American Medical Association. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 12.Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, et al. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2005;293:1617–25. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 13.O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol-dependent patients who are abstinent before treatment. Journal of clinical psychopharmacology. 2007;27:507–12. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- 14.Pettinati HM, Gastfriend DR, Dong Q, Kranzler HR, O’Malley SS. Effect of extended-release naltrexone (XR-NTX) on quality of life in alcohol-dependent patients. Alcoholism, clinical and experimental research. 2009;33:350–6. doi: 10.1111/j.1530-0277.2008.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR. Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization. Addiction. 2008;103:1801–8. doi: 10.1111/j.1360-0443.2008.02345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and alcohol dependence. 2010;112:178–93. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Springer SA, Pesanti E, Hodges J, Macura T, Doros G, Altice FL. Effectiveness of antiretroviral therapy among HIV-infected prisoners: reincarceration and the lack of sustained benefit after release to the community. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2004;38:1754–60. doi: 10.1086/421392. [DOI] [PubMed] [Google Scholar]
- 19.Saber-Tehrani AS, Springer SA, Qiu J, Herme M, Wickersham J, Altice FL. Rationale, study design and sample characteristics of a randomized controlled trial of directly administered antiretroviral therapy for HIV-infected prisoners transitioning to the community - a potential conduit to improved HIV treatment outcomes. Contemp Clin Trials. 2012;33:436–44. doi: 10.1016/j.cct.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amorim P, Lecrubier Y, Weiller E, Hergueta T, Sheehan D. DSM-IH-R Psychotic Disorders: procedural validity of the Mini International Neuropsychiatric Interview (MINI). Concordance and causes for discordance with the CIDI. European psychiatry: the journal of the Association of European Psychiatrists. 1998;13:26–34. doi: 10.1016/S0924-9338(97)86748-X. [DOI] [PubMed] [Google Scholar]
- 21.Sheehan D, Lecrubier Y, Harnett-Sheehan K, Janavs J, Weiller E, Bonors L, et al. Reliability and Validity of the MINI International Neuropsychiatric Interview (M.I.N.I.): According to the SCID-P. Eur Psychiat. 1997;12:232–41. [Google Scholar]
- 22.Lecrubier Y, Sheehan DV, Weiller E, Amorim P, Bonora I, Sheehan KH, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: Reliability and validity according to the CIDI. Eur Psychiat. 1997;12:224–31. [Google Scholar]
- 23.Barbor ea. The AUDIT, Guidelines for use in primary care. World health organization; 2001. [Google Scholar]
- 24.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 25.National Institute on Alcohol Abuse and Alcoholism; NIAAA, editor. Helping Patients Who Drink Too Much: A Clinician’s Guide. 2005. NIAAA; 2005. [Google Scholar]
- 26.Levine J, Schooler NR. SAFTEE: a technique for the systematic assessment of side effects in clinical trials. Psychopharmacology bulletin. 1986;22:343–81. [PubMed] [Google Scholar]
- 27.International Harm Reduction Development Program (IHRD) of the Open Society Institute (OSI); Open Society Institute (OSI), editor Barriers to Access: Medication-Assisted Treatment and Injection-Driven HIV Epidemics. New York, NY: Open Society Institute; 2008. pp. 1–5. http://wwwsorosorg/initiatives/health/focus/ihrd/articles_publications/publications/barriers_20080215/barriersfootnotes040808pdf. [Google Scholar]
- 28.Scott NW, McPherson GC, Ramsay CR, Campbell MK. The method of minimization for allocation to clinical trials. a review. Control Clin Trials. 2002;23:662–74. doi: 10.1016/s0197-2456(02)00242-8. [DOI] [PubMed] [Google Scholar]
- 29.Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43:215–21. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tideman RL, Chen MY, Pitts MK, Ginige S, Slaney M, Fairley CK. A randomised controlled trial comparing computer-assisted with face-to-face sexual history taking in a clinical setting. Sexually transmitted infections. 2007;83:52–6. doi: 10.1136/sti.2006.020776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caldwell DH, Jan G. Computerized assessment facilitates disclosure of sensitive HIV risk behaviors among African Americans entering substance abuse treatment. The American journal of drug and alcohol abuse. 2012;38:365–9. doi: 10.3109/00952990.2012.673663. [DOI] [PubMed] [Google Scholar]
- 32.Azbel L, Wickersham JA, Grishaev Y, Dvoryak S, Altice FL. Burden of infectious diseases, substance use disorders, and mental illness among Ukrainian prisoners transitioning to the community. PloS one. 2013;8:e59643. doi: 10.1371/journal.pone.0059643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amico KR, Fisher W, Cornman D, Shuper P, Redding C, Konkle-Parker D, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr Hum Retrovirol. 2006;42:455–9. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 34.Pettinati H, Weiss R, Miller WR, Donovan D, Ernst D, BJR NIAAA, editor. Medical Management Treatment Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence. Bethesda, MD: DHHS; 2004. [Google Scholar]
- 35.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2006;295:2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 36.Zelenev A, Marcus R, Kopelev A, Cruzado-Quinones J, Spaulding A, Desabrais M, et al. Patterns of Homelessness and Implications for HIV Health After Release from Jail. AIDS and behavior. 2013 doi: 10.1007/s10461-013-0472-6. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krishnan A, Wickersham JA, Chitsaz E, Springer SA, Jordan AO, Zaller N, et al. Post-release substance abuse outcomes among HIV-infected jail detainees: results from a multisite study. AIDS and behavior. 2013;17 (Suppl 2):S171–80. doi: 10.1007/s10461-012-0362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anton RE, Randall CL. Measurement and choice of drinking outcome variables in the COMBINE Study. J Stud Alcohol Suppl. 2005:104–9. doi: 10.15288/jsas.2005.s15.104. discussion 92–3. [DOI] [PubMed] [Google Scholar]
- 39.Project MATCH Research Group. Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. Journal of studies on alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- 40.Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Archives of general psychiatry. 1996;53:225–31. doi: 10.1001/archpsyc.1996.01830030047008. [DOI] [PubMed] [Google Scholar]
- 41.Mayer K, Gazzard B, Zuniga JM, Amico KR, Anderson J, Azad Y, et al. Controlling the HIV epidemic with antiretrovirals: IAPAC consensus statement on treatment as prevention and preexposure prophylaxis. Journal of the International Association of Providers of AIDS Care. 2013;12:208–16. doi: 10.1177/2325957413475839. [DOI] [PubMed] [Google Scholar]
- 42.Vagenas P, Azbel L, Polonsky M, Kerimi N, Mamyrov M, Dvoryak S, et al. A review of medical and substance use co-morbidities in Central Asian prisons: Implications for HIV prevention and treatment. Drug and alcohol dependence. 2013;132 (Suppl 1):S25–31. doi: 10.1016/j.drugalcdep.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann K, Lemenager T, Hoffmann S, Reinhard I, Hermann D, Batra A, et al. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addiction biology. 2013;18:937–46. doi: 10.1111/adb.12012. [DOI] [PubMed] [Google Scholar]
- 44.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ludford KT, Vagenas P, Lama JR, Peinado J, Gonzales P, Leiva R, et al. Screening for Drug and Alcohol Use Disorders and Their Association with HIV-Related Sexual Risk Behaviors among Men Who Have Sex with Men in Peru. PloS one. 2013;8:e69966. doi: 10.1371/journal.pone.0069966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vagenas P, Ludford KT, Gonzales P, Peinado J, Cabezas C, Gonzales F, et al. Being Unaware of Being HIV-Infected is Associated with Alcohol Use Disorders and High-Risk Sexual Behaviors Among Men Who have Sex with Men in Peru. AIDS and behavior. 2013 doi: 10.1007/s10461-013-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brazier J, Jones N, Kind P. Testing the validity of the Euroqol and comparing it with the SF-36 health survey questionnaire. Qual Life Res. 1993;2(3):169–180. doi: 10.1007/BF00435221. [DOI] [PubMed] [Google Scholar]
- 48.Amico KR, Fisher W, Cornman D, Shuper P, Redding C, Konkle-Parker D, Barta W, Fisher J. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr Hum Retrovirol. 2006;42(4):455–459. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 49.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med. 1983;13:596–605. [PubMed] [Google Scholar]
- 50.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 51.Rosen C, Henson B, Finney J, Moos R. Consistency of self-administered and interview-based Addiction Severity Index composite scores. Addiction. 2000;95(3):419–425. doi: 10.1046/j.1360-0443.2000.95341912.x. [DOI] [PubMed] [Google Scholar]
- 52.Sobell LC, Toneatto T, Sobell MB, Leo GI, Johnson L. Alcohol abusers’ perceptions of the accuracy of their self-reports of drinking: implications for treatment. Addict Behav. 1992;17(5):507–511. doi: 10.1016/0306-4603(92)90011-j. [DOI] [PubMed] [Google Scholar]
- 53.Sobell LC, Sobell MB. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association; 2000. Alcohol Timeline Followback (TLFB) pp. 477–479. [Google Scholar]
- 54.Alterman AI, Cacciola JS, Ivey MA, Habing B, Lynch KG. Reliability and validity of the alcohol short index of problems and a newly constructed drug short index of problems. J Stud Alcohol Drugs. 2009;70(2):304–307. doi: 10.15288/jsad.2009.70.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hahn JA, Dobkin LM, Mayanja B, Emenyonu NI, Kigozi IM, Shiboski S, Bangsberg DR, Gnann H, Weinmann W, Wurst FM. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcohol Clin Exp Res. 2012;36(5):854–862. doi: 10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aradottir S, Asanovska G, Gjerss S, Hansson P, Alling C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol Alcohol. 2006;41(4):431–437. doi: 10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- 57.Stewart SH, Law TL, Randall PK, Newman R. Phosphatidylethanol and alcohol consumption in reproductive age women. Alcohol Clin Exp Res. 2010;34(3):488–492. doi: 10.1111/j.1530-0277.2009.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartmann S, Aradottir S, Graf M, Wiesbeck G, Lesch O, Ramskogler K, Wolfersdorf M, Alling C, Wurst FM. Phosphatidylethanol as a sensitive and specific biomarker: comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addict Biol. 2007;12(1):81–84. doi: 10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- 59.Wurst FM, Thon N, Aradottir S, Hartmann S, Wiesbeck GA, Lesch O, Skala K, Wolfersdorf M, Weinmann W, Alling C. Phosphatidylethanol: normalization during detoxification, gender aspects and correlation with other biomarkers and self-reports. Addict Biol. 2010;15(1):88–95. doi: 10.1111/j.1369-1600.2009.00185.x. [DOI] [PubMed] [Google Scholar]
- 60.Fisher JD, Fisher WA, Cornman DH, Amico RK, Bryan A, Friedland GH. Clinician-delivered intervention during routine clinical care reduces unprotected sexual behavior among HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):44–52. doi: 10.1097/01.qai.0000192000.15777.5c. [DOI] [PubMed] [Google Scholar]
- 61.Johnson BA, Ait-Daoud N, Roache JD. The COMBINE SAFTEE: a structured instrument for collecting adverse events adapted for clinical studies in the alcoholism field. J Stud Alcohol Suppl. 2005;(15):157–167. doi: 10.15288/jsas.2005.s15.157. discussion 140. [DOI] [PubMed] [Google Scholar]