Abstract
Object
While many centers place bilateral DBS systems simultaneously, unilateral STN DBS followed by a staged contralateral procedure has emerged as a treatment option for many patients. However little is known about whether the preoperative phenotype predicts when staged placement of a DBS electrode in the opposite subthalamic nucleus will be required. We aimed to determine whether preoperative clinical phenotype predicts early staged placement of a second subthalamic deep brain stimulation (DBS) electrode in patients who undergo unilateral subthalamic DBS for Parkinson's disease (PD).
Methods
Eighty-two consecutive patients with advanced PD underwent unilateral subthalamic DBS contralateral to the most affected hemibody and had at least 2 years of follow-up. Multivariate logistic regression determined preoperative characteristics that predicted staged placement of a second electrode in the opposite subthalamic nucleus. Preoperative measurements included aspects of the Unified Parkinson Disease Rating Scale (UPDRS), motor asymmetry index, and body weight.
Results
At 2 years follow-up, 28 of the 82 patients (34%) had undergone staged placement of a contralateral electrode while the remainder chose to continue with unilateral stimulation. Statistically significant improvements in UPDRS total and part 3 scores were retained at the end of the 2 year follow-up period in both subsets of patients. Multivariate logistic regression showed that the most important predictors for early staged placement of a second subthalamic stimulator were low asymmetry index (odds ratio 13.4; 95% confidence interval 2.8, 64.9), high tremor subscore (OR 7.2; CI 1.5, 35.0), and low body weight (OR 5.5; CI 1.4, 22.3).
Conclusions
This single center study provides evidence that elements of the preoperative PD phenotype predict whether patients will require early staged bilateral subthalamic DBS. These data may aid in the management of patients with advanced PD who undergo subthalamic DBS.
Keywords: deep brain stimulation, predictors, Parkinson’s disease
Object
Although bilateral deep brain stimulation (DBS) of the subthalamic nucleus (STN) is superior to best medical therapy for the management of the motor symptoms of advanced Parkinson’s disease (PD), it is associated with a higher incidence of serious adverse events 7,37. While bilateral surgery likely provides greater motor benefit than unilateral surgery, the relative merits of these two surgical approaches have been a matter of debate 1,28. The motor symptoms of PD are asymmetric, and a prior study suggests that the electrode contralateral to the more severely affected hemibody is responsible for most of the motor benefit following staged bilateral subthalamic DBS 28. Additionally, a number of studies have shown significant motor and quality of life benefits following unilateral subthalamic DBS for advanced PD at up to 1 year post-operatively 1,3,6,11,31,36, leading authors to conclude that unilateral DBS followed by a staged bilateral procedure (when needed) is a viable option for many patients 1,26.
Advanced PD patients show heterogeneous phenotypes, including relative degrees of motor asymmetry, tremor, gait dysfunction, dyskinesias, and other symptoms 14–16,18,20,22,23,25,32–35. Despite this, little is known about whether specific phenotypes predict the need for staged placement of a contralateral subthalamic electrode 32. Such knowledge could be valuable to patients and clinicians who seek to maximize motor benefit and minimize potential risks associated with the intervention. Additionally, centers implanting either bilateral or unilateral stimulators might alter their surgical plan in selected patients, taking into account specific motor phenotypes in the context of age, cognitive function, speech and swallowing dysfunction, and other comorbidities. We hypothesized that elements of the preoperative phenotype would predict whether staged bilateral subthalamic DBS occurred within 2 years postoperatively in patients with advanced PD.
Methods
Participants
We evaluated 82 consecutive patients with advanced PD who underwent unilateral subthalamic DBS between March 2004 and December 2008 and were followed for at least 2 years. All patients received unilateral subthalamic DBS contralateral to the most severely affected hemibody and had the option to electively undergo contralateral surgery later if and when clinically warranted. No patients during that time period underwent initial bilateral subthalamic DBS or pallidal DBS.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board (IRB) of the University of Alabama at Birmingham. The IRB approved a waiver of consent for collection of these data as part of routine clinical care and quality control.
Surgical Procedure
Electrodes (Model 3387; Medtronic, Minneapolis, MN) were implanted under local anesthesia, guided by magnetic resonance imaging (MRI) frame-based stereotaxy and microelectrode recordings, and all surgical procedures were performed by the same neurosurgeon (BLG). In all cases, post-operative MRI was performed within 24 hours of surgery to verify lead placement and assess for potential surgical complications. Further details of the surgical procedure have been described elsewhere 4. No formal criteria were established a priori for when or whether patients underwent staged placement of the contralateral subthalamic DBS. Rather, the decision to proceed with the second surgery was determined by a real-world clinical risk/benefit assessment based upon patient preference in combination with advice of the treating clinicians. Considerations for the decision included overall quality of life, residual or progressive disability due to motor symptoms, and non-motor symptoms. At the end of the 2 year observation period, we recorded whether or not subjects had undergone staged bilateral subthalamic DBS.
Clinical Outcome Assessment
The Unified Parkinson’s Disease Rating Scale (UPDRS) is the standard clinical metric for pre- and post-operative assessment of PD patients 9. We prospectively measured and recorded preoperative motor function and other baseline characteristics in consecutive patients in a database (Table 1). Tremor subscore was the sum of ‘on’ medication and ‘practically defined off’ medication tremor items on UPDRS Parts 2 and 3 (questions 16, 20, and 21). Dyskinesia subscore was the sum of the three dyskinesia items in UPDRS Part 4 (questions 32–34). Asymmetry index was calculated as the absolute difference between the total of the items corresponding to each side of the body divided by the sum of the items for both sides ([L−R]/[L+R]) 22. Patients in this clinical series had reassessment of total UPDRS scores postoperatively at 3, 6, and 12 months and every 6 months thereafter, as described in our prior report of clinical outcomes of unilateral subthalamic DBS at 12 months follow-up 36.
Table 1.
Non-parametric analysis comparing baseline variables in patients receiving unilateral or staged bilateral STN DBS
| Variable | All Subjects at Baseline |
Unilateral STN DBS | Staged Bilateral STN DBS | p-value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | N | Mean ± SD | N | Mean ± SD | ||
| Age at First Surgery | 59.9 ± 9.8 | 51 | 60.2 ± 9.5 | 28 | 59.3 ± 10.4 | 0.8421 |
| Weight (kg) | 81.5 ± 19.9 | 48 | 86.0 ± 18.6 | 23 | 72.2 ± 18.8 | 0.0076* |
| Total UPDRS | 71.3 ± 15.5 | 53 | 69.0 ± 14.4 | 27 | 75.9 ± 16.7 | 0.0883 |
| UPDRS Part 1 | 2.6 ± 1.8 | 54 | 2.6 ± 1.6 | 27 | 2.7 ± 2.2 | 0.9273 |
| UPDRS Part 2 ‘off’ | 24.3 ± 6.1 | 54 | 23.7 ± 5.8 | 27 | 25.6 ± 6.6 | 0.1940 |
| UPDRS Part 2 ‘on’ | 8.8 ± 5.1 | 53 | 8.6 ± 4.9 | 27 | 9.3 ± 5.5 | 0.5833 |
| UPDRS Part 3 ‘off’ | 36.6 ± 10.8 | 54 | 34.8 ± 9.8 | 28 | 40.0 ± 11.8 | 0.0645 |
| UPDRS Part 3 ‘on’ | 12.7 ± 7.5 | 53 | 12.4 ± 6.9 | 28 | 13.3 ± 8.5 | 0.8312 |
| UPDRS Part 4 | 8.2 ± 2.5 | 53 | 8.2 ± 2.5 | 27 | 8.2 ± 2.6 | 0.9632 |
| Tremor Subscore | 6.8 ± 6.2 | 53 | 5.9 ± 4.9 | 27 | 7.4 ± 5.1 | 0.1770 |
| Dyskinesia Subscore | 2.4 ± 2.0 | 53 | 2.6 ± 2.1 | 27 | 2.0 ± 1.7 | 0.3420 |
| Asymmetry index | 0.4 ± 0.2 | 53 | 0.5 ± 0.2 | 28 | 0.3 ± 0.2 | 0.0047* |
Statistical Analysis
Multivariate logistic regression determined the relationship between the following preoperative predictor variables: UPDRS total score, UPDRS Part 3 “off” total, UPDRS asymmetry index, UPDRS tremor subscore, UPDRS dyskinesia subscore, weight, gender, initial surgery side, and the dichotomous outcome variable of whether staged bilateral subthalamic DBS occurred within 24 months of the initial electrode placement. Cutoff points for the multivariate logistic regression on predictor variables were determined by the sample mean when normally distributed (UPDRS total, UPDRS part 3 ‘off,’ UPDRS asymmetry index, and body weight) and by empiric clinical criteria (i.e., presence or absence of the trait) when a normal distribution was not present (for the tremor and dyskinesia subscores). Additionally, non-parametric analyses were performed to compare the preoperative UPDRS total score, UPDRS parts 1, 2, 3, and 4, UPDRS tremor subscore, UPDRS dyskinesia subscore, UPDRS asymmetry index, weight, age, and gender between the patients who remained with unilateral stimulation versus those who underwent staged bilateral surgery within two years of the initial procedure.
Results
At 2 years postoperatively, 34% (N = 28) of the patients had undergone staged bilateral subthalamic DBS. Table 1 shows the demographic data for the total sample of patients as well as the non-parametric analyses comparing baseline variables in subjects receiving unilateral or staged bilateral subthalamic DBS within 2 years of follow-up. Adverse events for the total sample of patients is shown in Table 2. Combining the 28 patients who had staged bilateral surgery and the 54 who had unilateral surgery yields a total of 110 subthalamic DBS placements in 82 patients. There were 13 total surgical complications for a rate of 11.8% for the whole study sample. Of the 13 complications, 10 occurred after the first side was placed (10/82 = 12.2%), and 3 occurred after the second side was placed (3/28 = 10.7%). The preoperative phenotypes that were most statistically different between the groups were the asymmetry index (0.5 and 0.3, respectively, p = 0.0047) and body weight (86.0 and 72.2 kg, respectively, p = 0.0076).
Table 2.
Surgical Adverse Events for First STN DBS Surgery (All Subjects) and Second STN DBS Surgery (Staged Bilateral Subjects Only)
| Type of Adverse Event | After first side STN DBS (N=110) |
After second side STN DBS (N=28) |
Total |
|---|---|---|---|
| Implanted Pulse Generator (IPG) infection | 1 | 1 | 2 |
| Lead adjustment for scalp erosion | 3 | 0 | 3 |
| Lead infection | 2 | 1 | 3 |
| Lead reposition | 2 | 0 | 2 |
| Lead malfunction | 1 | 1 | 2 |
| Subdural hematoma | 1 | 0 | 1 |
| Total | 10 | 3 | 13 |
Multivariate logistic regression yielded odds ratios (OR) with 95% confidence intervals for predicting whether staged bilateral subthalamic DBS occurred within two years. The preoperative characteristics most predictive of staged bilateral subthalamic DBS within 2 years of the first surgery were asymmetry index ≤ 0.4 (OR 13.4; CI 2.8, 64.9), tremor subscore ≥ 8 (OR 7.2; CI 1.5, 35.0), and body weight ≤ 80 kilograms (OR 5.5; CI 1.4, 22.3, see Figure 1A). UPDRS total score, UPDRS Part 3 “off,” initial surgery side, gender, and dyskinesia subscore were not significantly predictive of undergoing the staged surgery within 2 years of the initial unilateral procedure. Histograms for each of the potential predictive factors are provided in Figure 1B. Both the unilateral and staged bilateral subthalamic DBS patients showed statistically significant improvements in UPDRS scores at 2 years follow-up (Table 3).
Figure 1.
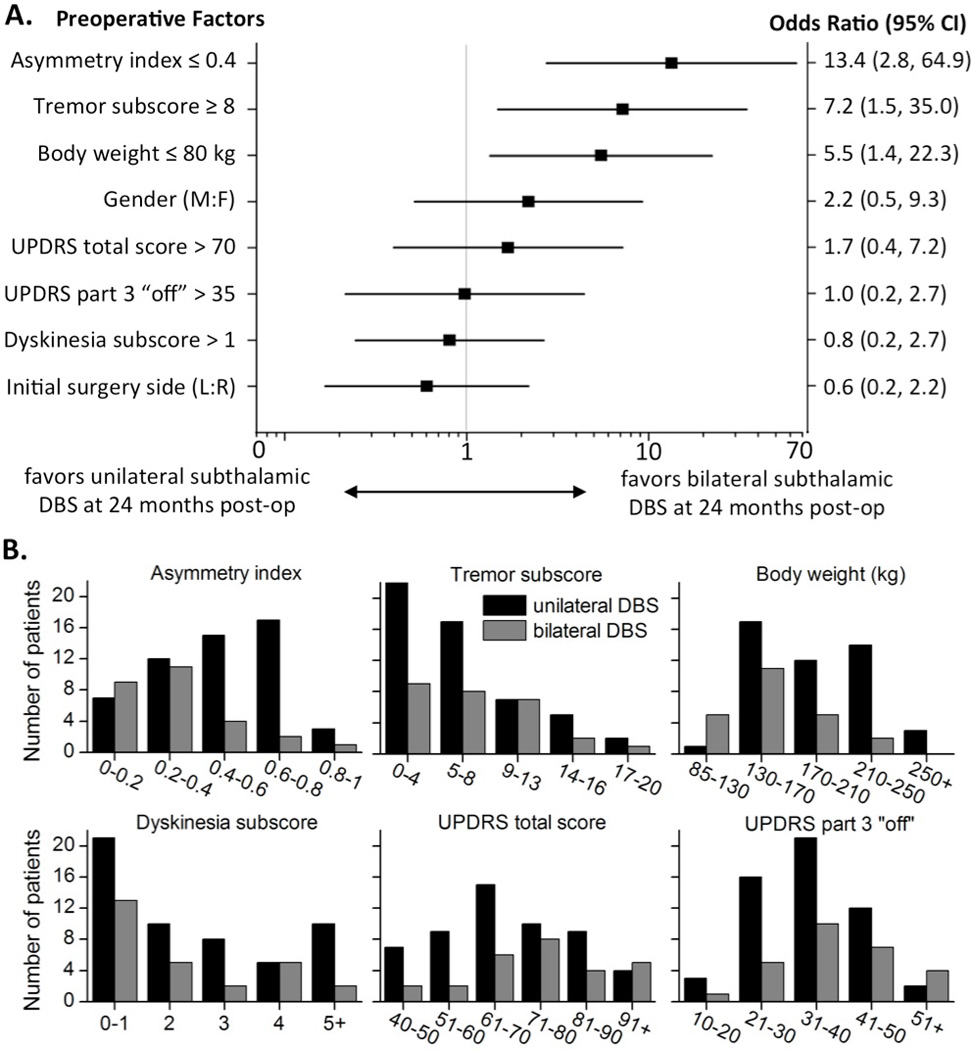
Odds Ratios Predicting Likelihood of Having Staged Bilateral DBS Within 24 Months
(A) Logistic regression analyses of preoperative predictors for staged bilateral subthalamic DBS within 2 years of the initial unilateral procedure. (B) Histograms of the preoperative phenotypes, organized by unilateral or bilateral subthalamic DBS at 2 years follow-up.
Table 3.
Motor Outcomes for Unilateral and Bilateral STN DBS Patients
| Baseline (X±SD) |
24 Month (X±SD) |
p-value | ||
|---|---|---|---|---|
| Bilateral (N =28 ) | ||||
| Total UPDRS | 75.9 ± 16.7 | 61.5 ± 12.9 | 0.0007* | |
| Part 1 | 2.7 ± 2.2 | 2.9 ± 2.3 | 0.9098 | |
| Part 2 OFF | 25.6 ± 6.6 | 17.2 ± 3.8 | 0.0076* | |
| Part 2 ON | 9.3 ± 5.5 | 9.2 ± 3.0 | 0.9645 | |
| Part 3 OFF | 40.0 ± 11.8 | 24.2 ± 9.8 | 0.0086* | |
| Part 3 ON | 13.3 ± 8.5 | 13.7 ± 8.3 | 0.5196 | |
| Part 4 | 8.2 ± 2.6 | 3.5 ± 1.7 | <0.0001* | |
| Unilateral (N =54 ) | ||||
| Total UPDRS | 69.0 ± 14.4 | 56.8 ± 15.5 | 0.0004* | |
| Part 1 | 2.6 ± 1.6 | 3.3 ± 2.3 | 0.4489 | |
| Part 2 OFF | 23.7 ± 5.8 | 20.0 ± 6.7 | 0.0325* | |
| Part 2 ON | 8.6 ± 4.9 | 9.4 ± 6.4 | 0.1787 | |
| Part 3 OFF | 34.8 ± 9.8 | 24.4 ± 8.9 | 0.0104* | |
| Part 3 ON | 12.4 ± 6.9 | 12.4 ± 6.9 | 0.1250 | |
| Part 4 | 8.2 ± 2.5 | 3.8 ± 1.4 | <0.0001* | |
Discussion
Heterogenous phenotypes drive patients with advanced PD toward invasive therapies such as DBS. This single center observational study of 82 consecutive patients who underwent unilateral subthalamic DBS suggests that motor symmetry, high tremor subscore, and low body weight were most predictive of early staged placement of a contralateral subthalamic electrode. Symmetric bilateral motor symptoms were the strongest single predictor for early staged contralateral surgery, indicating conversely that highly asymmetric patients were more likely to remain with unilateral stimulation at 2 years follow-up. This is the most straightforward of our findings as it stands to reason that symmetric symptoms would be more likely to require bilateral surgery. This finding also agrees with a prior study that pooled results in PD patients who were randomized to either unilateral globus pallidus interna or subthalamic DBS 32. Additionally, we show that patients with a high degree of tremor underwent the staged contralateral surgery sooner. This likely reflects the highly visible nature of tremor in some patients and its tendency to be refractory to dopaminergic medications 8,21. Though we did not use the independent scale developed by Schiess et al 29 to classify patients as being of the tremor-predominant subtype, this scale was derived from UPDRS part 3 much like our method. The clinical relevance of tremor as a predictor is highlighted by the recent research demonstrating that patients with tremor predominant PD have a distinct subtype of PD from those with predominantly postural instability and gait disorder (PIGD) and less tremor 12,27,30. Finally, low body weight at the time of the initial surgery was also one of the significant predictors for early staged bilateral subthalamic DBS. This is perhaps the least intuitive of the three predictors, though perhaps there is a correlation between symmetric PD symptoms, and/or high degree of tremor, and weight loss. Regardless of its underlying cause, the clinical importance of low weight as a predictor is supported by the finding that unintended weight loss is a common manifestation of many neurodegenerative diseases 5. Additionally, we and others have described progressive weight loss associated with PD and weight gain after subthalalmic DBS 14,15,18,20,25,33–35.
Factors less predictive of early staged bilateral subthalamic DBS warrant further discussion as well. Interestingly, UPDRS total score and UPDRS part 3 “off” scores were not significant predictors for the early staged surgery, arguing that asymmetry and tremor predominance are specific aspects of the motor phenotype that are most strongly associated with early bilateral surgery. Furthermore, severe dyskinesias associated with levodopa frequently necessitate DBS therapy, and in contrast to our findings for tremor, markedly dyskinetic patients did not seek early bilateral surgery and in fact trended towards remaining with unilateral stimulation over the study period. We were somewhat surprised by this finding, particularly considering that expert opinion in the field has generally held that highly dyskinetic patients will require bilateral subthalamic DBS immediately 16,23. Importantly, the observed improvement in motor fluctuations and dyskinesias at 2 years follow-up measured by the UPDRS Part 4 argues that patients remained with unilateral stimulation because their dyskinesias were improved by unilateral stimulation. Consistent with this interpretation, prior studies have shown significant improvement in motor fluctuations and activities of daily living following unilateral subthalamic DBS at up to 1 year postoperatively 6,11,19,24,36. In another study, subjects reported by questionnaire that they had elected to remain with unilateral stimulation because of symptomatic improvement 32. Other aspects of the preoperative phenotype, such as gender and initial side of surgery, were not predictive of early staged surgery, further suggesting that the factors that were significant predictors were not observed by chance alone.
Interestingly, only 38% of these patients elected to have the staged bilateral procedure over the 2 years following their initial surgery. Our finding of significant motor improvement in patients who underwent both unilateral and bilateral subthalamic DBS for PD suggests that many patients with advanced PD retain significant motor improvement following unilateral STN DBS at up to 2 years postoperatively. Considering that motor symptoms of PD are typically asymmetric, responsive to dopaminergic medications, and mildly improved in the arm ipsilateral to the subthalamic DBS electrode 11,31, our current findings suggest that a subset of PD patients who undergo unilateral subthalamic DBS will have sustained improvement in symptoms for years following unilateral surgery. Although prospective, randomized studies comparing unilateral and bilateral stimulation evaluating motor outcomes and potential adverse events are needed, there are several studies suggesting that bilateral subthalamic DBS is associated with decreased verbal fluency and speech dysfunction 2,10,13. Additionally, although it has not been studied, it is reasonable to expect shorter surgery times, lower cost, and fewer perioperative complications with placement of one rather than two DBS electrodes, at least with respect to the initial lead placement 17.
This observational study has several strengths and some potential limitations. Selection bias is minimized because this was a consecutive series of patients who underwent unilateral DBS at a single stereotactic target contralateral to the most severely affected hemibody. No patients were offered bilateral DBS or unilateral DBS at another target during the observational period. Additionally, careful characterization allowed detailed analyses of the motor phenotype and assessment of outcomes at 2 years follow-up. Although there were no rigid criteria for when to pursue the second surgery, this is a potential strength of the study in that the decision reflected a real-world clinical decision made by individual patients with the advice of caregivers, based on the multi-faceted aspects of their disease. Though we anticipate that many or most of these patients will eventually require the staged surgery because of the progressive nature of the disease, our data still provide valuable information about the two year postoperative interval. Though our data do not address whether staged surgery will be needed over longer time intervals, they do suggest that many patients derive significant clinical benefit for years after the initial intervention, as manifested by the significant improvements in the global UPDRS, but specifically in the UPDRS part 4 (motor complications). These data in particular argue that significant benefit in dyskinesias and fluctuations is achieved with unilateral stimulation in many patients. These are among the most common clinical symptoms that drive patients to this more invasive therapy. However, since we did not perform a questionnaire asking patients why they did or did not pursue the staged surgery, it does leave open the possibility that some patients chose not to proceed with the staged surgery because of their knowledge and experience with the first surgery. Though the sustained clinical benefit at 2 years in the patients who remained with unilateral DBS argues somewhat against this, follow-up studies would be strengthened by the presence of a questionnaire asking the reasons for pursuing or not pursuing a staged surgery.
Despite the relatively large sample of patients, we cannot exclude the possibility that some of the potential predictors (i.e., UPDRS total and UPDRS part 3 “off”) might reach statistical significance with a larger sample of patients. Indeed, the most extreme total UPDRS scores reflect a trend towards bilateral surgery (Figure 1B). Although it is possible that non-motor features including cognitive decline associated with advanced PD or psychiatric symptoms might also influence whether some patients elected to have the second surgery, the significant predictive factors were still observed despite these potential confounds. The extent to which these and other non-motor aspects of the disease alter clinical decision making in the perioperative period should be addressed more specifically in future studies. Although it is reasonable to suspect fewer perioperative complications from unilateral versus bilateral surgery, our data do not address this issue directly. There are likely distinct adverse event profiles associated with unilateral electrode placement followed by staged surgery when needed; unilateral followed immediately by a staged procedure; and bilateral simultaneous lead placement in a single initial procedure. The adverse event rates between the first and second surgeries were similar, but the total number of adverse events in our sample is too small to make an adequate comparison between the unilateral and staged bilateral groups. Finally, patient evaluations were not blinded, which might lead to placebo effects or to sources of bias from the rater or the patient when outcome is assessed. Regardless, the observed predictors for early staged subthalamic DBS reflect an important clinical outcome derived from real world clinical decision-making in patients with advanced PD.
In summary, our findings suggest that PD patients with certain preoperative clinical features, including highly symmetric motor symptoms, tremor predominance, and/or low body weight are more likely to require staged contralateral subthalamic DBS surgery sooner than patients without those clinical features. The motor symptoms of PD are typically asymmetric and levodopa-responsive, and we demonstrate that many patients derive significant and enduring improvement in motor function from unilateral subthalalmic DBS. Unilateral subthalamic DBS followed by staged placement of a contralateral stimulator (when needed) is a reasonable treatment plan for many patients with PD, particularly those with prominent asymmetry or comorbidities that might increase the risk associated with bilateral surgery. These results may provide useful information for patients and caregivers who are planning to undergo this intervention that can dramatically alter quality of life.
Acknowledgments
Study funding: Supported by NIH (K23 NS067053-01, H.W.)
Footnotes
Financial disclosure/conflict of interest: The authors have no disclosures or conflicts of interest to report regarding the research covered in this manuscript.
Portions of this work were presented in poster form at the 14th International Movement Disorder Congress, Buenos Aires, Argentina, June 16, 2010.
References
- 1.Alberts JL, Hass CJ, Vitek JL, Okun MS. Are two leads always better than one: An emerging case for unilateral subthalamic deep brain stimulation in Parkinson's disease. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberts JL, Voelcker-Rehage C, Hallahan K, Vitek M, Bamzai R, Vitek JL. Bilateral subthalamic stimulation impairs cognitive-motor performance in Parkinson's disease patients. Brain. 2008;131:3348–3360. doi: 10.1093/brain/awn238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker HC. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in Parkinson's disease. Parkinsonism Relat Disord. 18:63–68. doi: 10.1016/j.parkreldis.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amirnovin R, Williams ZM, Cosgrove GR, Eskandar EN. Experience with microelectrode guided subthalamic nucleus deep brain stimulation. Neurosurgery. 2006;58:ONS96–ONS102. doi: 10.1227/01.NEU.0000192690.45680.C2. discussion ONS196-102. [DOI] [PubMed] [Google Scholar]
- 5.Aziz NA, van der Marck MA, Pijl H, Olde Rikkert MG, Bloem BR, Roos RA. Weight loss in neurodegenerative disorders. J Neurol. 2008;255:1872–1880. doi: 10.1007/s00415-009-0062-8. [DOI] [PubMed] [Google Scholar]
- 6.Chung SJ, Jeon SR, Kim SR, Sung YH, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson's disease. Eur Neurol. 2006;56:127–132. doi: 10.1159/000095704. [DOI] [PubMed] [Google Scholar]
- 7.Deuschl G, Schade-Brittinger C, Krack P, Volkmann J, Schafer H, Botzel K, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 8.Elble RJ. Tremor and dopamine agonists. Neurology. 2002;58:S57–S62. doi: 10.1212/wnl.58.suppl_1.s57. [DOI] [PubMed] [Google Scholar]
- 9.Fahn S, Elton RL. Members of the UPDRS Development Committee: The Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Caine DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 10.Funkiewiez A, Ardouin C, Caputo E, Krack P, Fraix V, Klinger H, et al. Long term effects of bilateral subthalamic nucleus stimulation on cognitive function, mood, and behaviour in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2004;75:834–839. doi: 10.1136/jnnp.2002.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 12.Herman T, Rosenberg-Katz K, Jacob Y, Auriel E, Gurevich T, Giladi N, et al. White matter hyperintensities in Parkinson's disease: do they explain the disparity between the postural instability gait difficulty and tremor dominant subtypes? PLoS One. 2013;8:e55193. doi: 10.1371/journal.pone.0055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hershey T, Wu J, Weaver PM, Perantie DC, Karimi M, Tabbal SD, et al. Unilateral vs. bilateral STN DBS effects on working memory and motor function in Parkinson disease. Exp Neurol. 2008;210:402–408. doi: 10.1016/j.expneurol.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacques C, Davies R, Friedman JH. Weight loss is often a problem in late PD. J Neurosci Nurs. 1998;30:342. [PubMed] [Google Scholar]
- 15.Kashihara K. Weight loss in Parkinson's disease. J Neurol. 2006;253(Suppl 7):VII38–VII41. doi: 10.1007/s00415-006-7009-0. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner-Fisman G, Fisman DN, Sime E, Saint-Cyr JA, Lozano AM, Lang AE. Long-term follow up of bilateral deep brain stimulation of the subthalamic nucleus in patients with advanced Parkinson disease. J Neurosurg. 2003;99:489–495. doi: 10.3171/jns.2003.99.3.0489. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner-Fisman G, Herzog J, Fisman DN, Tamma F, Lyons KE, Pahwa R, et al. Subthalamic nucleus deep brain stimulation: summary and meta-analysis of outcomes. Mov Disord. 2006;21(Suppl 14):S290–S304. doi: 10.1002/mds.20962. [DOI] [PubMed] [Google Scholar]
- 18.Korczyn AD, Gurevich T. Parkinson's disease: before the motor symptoms and beyond. J Neurol Sci. 289:2–6. doi: 10.1016/j.jns.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Linazasoro G, Van Blercom N, Lasa A. Unilateral subthalamic deep brain stimulation in advanced Parkinson's disease. Mov Disord. 2003;18:713–716. doi: 10.1002/mds.10407. [DOI] [PubMed] [Google Scholar]
- 20.Lorefalt B, Ganowiak W, Palhagen S, Toss G, Unosson M, Granerus AK. Factors of importance for weight loss in elderly patients with Parkinson's disease. Acta Neurol Scand. 2004;110:180–187. doi: 10.1111/j.1600-0404.2004.00307.x. [DOI] [PubMed] [Google Scholar]
- 21.Marjama-Lyons J, Koller W. Tremor-predominant Parkinson's disease. Approaches to treatment. Drugs Aging. 2000;16:273–278. doi: 10.2165/00002512-200016040-00003. [DOI] [PubMed] [Google Scholar]
- 22.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Rudolph A, et al. Survival in Parkinson disease: thirteen-year follow-up of the DATATOP cohort. Neurology. 2005;64:87–93. doi: 10.1212/01.WNL.0000148603.44618.19. [DOI] [PubMed] [Google Scholar]
- 23.Moro E, Allert N, Eleopra R, Houeto JL, Phan TM, Stoevelaar H. A decision tool to support appropriate referral for deep brain stimulation in Parkinson's disease. J Neurol. 2009;256:83–88. doi: 10.1007/s00415-009-0069-1. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura K, Christine CW, Starr PA, Marks WJ., Jr Effects of unilateral subthalamic and pallidal deep brain stimulation on fine motor functions in Parkinson's disease. Mov Disord. 2007;22:619–626. doi: 10.1002/mds.21300. [DOI] [PubMed] [Google Scholar]
- 25.Novakova L, Ruzicka E, Jech R, Serranova T, Dusek P, Urgosik D. Increase in body weight is a non-motor side effect of deep brain stimulation of the subthalamic nucleus in Parkinson's disease. Neuro Endocrinol Lett. 2007;28:21–25. [PubMed] [Google Scholar]
- 26.Papapetropoulos S, Salcedo AG, Singer C, Gallo BV, Jagid JR. Staged unilateral or bilateral STN-DBS? Mov Disord. 2008;23:775. doi: 10.1002/mds.21916. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg-Katz K, Herman T, Jacob Y, Giladi N, Hendler T, Hausdorff JM. Gray matter atrophy distinguishes between Parkinson disease motor subtypes. Neurology. 2013 doi: 10.1212/WNL.0b013e31828cfaa4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samii A, Kelly VE, Slimp JC, Shumway-Cook A, Goodkin R. Staged unilateral versus bilateral subthalamic nucleus stimulator implantation in Parkinson disease. Mov Disord. 2007;22:1476–1481. doi: 10.1002/mds.21554. [DOI] [PubMed] [Google Scholar]
- 29.Schiess MC, Zheng H, Soukup VM, Bonnen JG, Nauta HJ. Parkinson's disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis. Parkinsonism Relat Disord. 2000;6:69–76. doi: 10.1016/s1353-8020(99)00051-6. [DOI] [PubMed] [Google Scholar]
- 30.Skeie GO, Muller B, Haugarvoll K, Larsen JP, Tysnes OB. Differential effect of environmental risk factors on postural instability gait difficulties and tremor dominant Parkinson's disease. Mov Disord. 2010;25:1847–1852. doi: 10.1002/mds.23178. [DOI] [PubMed] [Google Scholar]
- 31.Slowinski JL, Putzke JD, Uitti RJ, Lucas JA, Turk MF, Kall BA, et al. Unilateral deep brain stimulation of the subthalamic nucleus for Parkinson disease. J Neurosurg. 2007;106:626–632. doi: 10.3171/jns.2007.106.4.626. [DOI] [PubMed] [Google Scholar]
- 32.Taba HA, Wu SS, Foote KD, Hass CJ, Fernandez HH, Malaty IA, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 113:1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 33.Teasdale N, Hue O, Simoneau M, Tremblay A, Marceau P, Marceau S. Predictors of weight loss in Parkinson's disease: is weight loss the chicken or the egg? Mov Disord. 2007;22:436–437. doi: 10.1002/mds.21203. [DOI] [PubMed] [Google Scholar]
- 34.Uc EY, Struck LK, Rodnitzky RL, Zimmerman B, Dobson J, Evans WJ. Predictors of weight loss in Parkinson's disease. Mov Disord. 2006;21:930–936. doi: 10.1002/mds.20837. [DOI] [PubMed] [Google Scholar]
- 35.Walker HC, Lyerly M, Cutter G, Hagood J, Stover NP, Guthrie SL, et al. Weight changes associated with unilateral STN DBS and advanced PD. Parkinsonism Relat Disord. 2009;15:709–711. doi: 10.1016/j.parkreldis.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson's disease at 1 year. Neurosurgery. 2009;65:302–309. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 309–310. [DOI] [PubMed] [Google Scholar]
- 37.Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, Jr, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. Jama. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]


