Abstract
The post-thaw recovery of mouse embryonic stem cells (mESCs) is often assumed to be adequate with current methods. However as this publication will show, this recovery of viable cells actually varies significantly by genetic background. Therefore there is a need to improve the efficiency and reduce the variability of current mESC cryopreservation methods. To address this need, we employed the principles of fundamental cryobiology to improve the cryopreservation protocol of four mESC lines from different genetic backgrounds (BALB/c, CBA, FVB, and 129R1 mESCs) through a comparative study characterizing the membrane permeability characteristics and membrane integrity osmotic tolerance limits of each cell line. In the companion paper, these values were used to predict optimal cryoprotectants, cooling rates, warming rates, and plunge temperatures, and then these predicted optimal protocols were validated against standard freezing protocols.
Keywords: ES cells, embryonic stem cells, embryonic stem, cryopreservation, fundamental cryobiology, membrane permeability, mouse, osmotic tolerance limits, osmotically inactive cell volume
Introduction
Coordinated projects, such as the Knockout Mouse Project (KOMP) [5], Canada’s North American Conditional Mouse Mutagenesis Program (NorCOMM, http://norcomm.org), and the European Conditional Mouse Mutagenesis Program (EUCOMM, http://www.eucomm.org) [6], will create thousands of mutant mouse embryonic stem cell (mESC) lines as a step towards producing thousands of genetically modified mice for biomedical research. They will also create the logistical problem of animal storage and maintenance. Storage and maintenance of live animal lines when not actively under research are highly impractical [18], but cryo-banking of mouse lines as embryonic stem cells (ESC) is cost-effective, and the restoration of mESCs into live, reproductively viable mice is routine [49,53]. The efficiency of this process is greatly enabled when cryopreservation and thawing methods produce healthy, rapidly dividing, germ-line competent cells.
Percent post-thaw recovery (PTR) following cell cryopreservation has been demonstrated to vary widely across and within species, and ESCs are no exception. Human and non-human primate ESCs are notoriously difficult to cryopreserve and there have been numerous studies designed to improve PTR using variations of non-equilibrium and equilibrium-cooling methods [8,29,31,32,34,39,40,50,51,56,61]. Mouse ESC cryopreservation, on the other hand, has generally been regarded as successful [30] and relatively few reports have been published that center around post-thaw recovery following cryopreservation [16,45,54,55]. However, it is difficult to compare estimates of mESC recovery between reports such as those of Ure et al. [55] and Udy et al. [54], which count recovery of colonies as opposed to individual cells, and reports such as that by Miszta-Lane et al. [45] and Kashuba Benson et al. [35], where the percent of single cells are reported. Additionally, the report by Miszta-Lane et al. [45] centers on the 129R1 mESC line, which is one cell line that cryopreserves satisfactorily. Anecdotal reports (personal communication, D. Nielsen, Stem Cell Technologies technical support, 2004; personal communication, Xin Yu, University of California-Davis, 2004) and a recent publication from Kashuba Benson et al. [35] concerning the C57BL/6 cell line show PTR of mESCs to be highly variable across cell lines.
A fundamental approach to improving cryopreservation methods is based on Mazur’s Two Factor Hypothesis [42], in which the ideal freezing protocol is one that optimally balances two key damaging forces of intracellular ice formation and solute effects. Systematic analysis of key cryobiological parameters of a cell enables the description of the total cell response to water and solute (in particular, cryoprotective agents (CPAs)) movement across the cell membrane and the temperature dependence of this process. These parameters include osmotic tolerance limits (OTL), osmotically inactive cell volume (Vb), hydraulic conductivity (Lp), the CPA permeability of the cell membrane (Ps), and the activation energy (Ea) for Lp and Ps. Quantification of these parameters enables the computer modeling and subsequent testing in our companion manuscript where we estimate optimal cooling and warming rates as well as optimal plunge temperatures with the goal of maintaining intracellular supercooling below two Kelvin to minimize potential damaging intracellular ice formation [42], while cooling and warming quickly to minimize damaging solute effects.
We previously designed a method to improve post-thaw recovery for a C57BL/6 mESC line by which ESC lines could be systematically analyzed in order to derive optimal cooling and warming rates as well as plunge temperatures [35]. In the present study, we analyzed and compared four mESC lines of different genetic backgrounds (BALB/c, CBA, FVB, and 129R1) in order to determine fundamental cryobiological factors that are responsible for the observed wide variation in PTR. With these data, we can determine protocols individualized to each cell line that optimize PTR or gain clues as to whether a “one-size fits all” protocol could be designed to optimize PTR in all mESC lines or in groups of mESC lines with common characteristics. Therefore the purpose of the present study is two-fold: to standardize post-thaw recovery data across multiple cell lines, and to investigate the hypothesis that the variation of cryopreservation success among mESC lines can be explained in part by biophysical variation.
Materials & Methods
Embryonic stem cells
The following mESC lines were acquired at early passage: BALB/c (Thromb-X Group, Chemicon International, Temecula, CA, now part of Millipore, Billerica, MA), CBA (Thromb-X Group), FVB/N (Thromb-X Group), and 129R1 (A. Nagy, Mount Sinai Hospital, Toronto, Canada). Mouse ESC cultures were negative for all pathogens (IMPACT I test, Research Animal Diagnostic Laboratory, Columbia, Missouri; www.radil.missouri.edu/info/index.asp).
Cell culture
Embryonic stem cells were cultured on primary mouse embryonic fibroblast cells (PMEF) (Millipore, Billerica, MA) at 37 °C and 5% CO2. Culture media for the 129R1 mESC line and BALB/c mESC line (R1/C culture medium) contained 15% Defined FBS (Hyclone, Logan, UT), 0.1mM non-essential amino acids (GIBCO/ Invitrogen, Carlsbad, CA), 1.0 mM sodium pyruvate (GIBCO), 100 μM beta-mercaptoethanol (Sigma Aldrich, St. Louis, MO), 50 IU/mL penicillin (GIBCO), 50 μg/mL streptomycin (GIBCO), and 1000 U ESGRO/mL (Millipore) in high glucose DMEM (Millipore). CBA and FVB mESCs were cultured in RESGRO culture medium (Millipore). Embryonic stem cells were passaged and/or collected every 2 days or at approximately 80% confluence.
Embryonic stem cells were used within 10 passages from the original, and were of normal karyotype at highest passage. Cell counts were performed using a hemacytometer and Trypan blue stain (Sigma Aldrich) for membrane integrity for all standard cultures.
Separation of mESCs from feeders by differential sedimentation
For all experiments, mESCs were separated from the feeder cells using a differential sedimentation technique previously described by Doetschman [19]. The separation of PMEF from mESCs is routine, and there are many variations of the basic method exploiting the difference in the rate at which fibroblast feeder cells and mESCs settle and adhere to culture dishes [28,46,52]. Briefly, trypsinized ESC cultures containing PMEF were centrifuged, resuspended in 10 mL of culture medium, and plated on the original 100 mm cell culture dish for 30 min at 37 °C. Following incubation, the cell suspension was transferred to a second culture dish for one-hour incubation at 37 °C in order to remove remaining fibroblast feeders. Following the second incubation, the cell suspension was removed, and these collected ESCs were counted, centrifuged, and resuspended in either DPBS or culture medium for experimentation. Our application of the Doetschman sedimentation method resulted in greater than 99% removal of contaminating feeder cells from the ES cell suspension (data not shown).
Prior to cryopreservation, post-thaw analysis, or any experiments requiring single cell suspension, visual confirmation of single cell suspensions was performed using a compound microscope.
Standard cryopreservation method
For the standard equilibrium cooling method, cells were resuspended in freezing medium (1.3 M (10%) Me2SO (Sigma Aldrich), 50% defined fetal bovine serum (Hyclone), and 40% culture medium) in 1mL aliquots in cryovials (Nalgene Nunc International, Rochester, NY). Cryovials were transferred to a commercially available freezing kit (Nalgene), refrigerated at -80 °C overnight (a process which cools at a rate of approximately 1 °C/minute), and subsequently transferred to liquid nitrogen (LN2).
Percent post-thaw recovery by cell line
For all cell lines, mESCs in culture were trypsinized into single cell suspensions, feeder cells were removed, and mESCs were resuspended at 1 × 106 cells/mL in standard freezing medium containing prepared R1/C culture medium. Membrane integrity prior to cryopreservation was assessed by flow cytometry analysis (FACScan, BD Biosciences, San Jose, CA) of propidium iodide staining of suspended cells in 1X PBS. Immediately post-thaw, ESCs were diluted drop-wise over a one minute time period by 5 volumes of culture media, centrifuged, resuspended in 1X PBS, stained with propidium iodide, and analyzed by flow cytometry for membrane integrity. Percent post-thaw recovery was expressed with consideration for both total cell count and percent membrane intact cells before and after cryopreservation:
Electronic particle counter
Using the method previously described by Kashuba Benson et al. [35], a modified [14] electronic particle counter (EPC) (Coulter Counter model ZM, Beckman Coulter, Inc., Fullerton, CA) was used for all cellular volumetric measurements with volume calibration using standard nominal 10 μm polystyrene latex particles (Beckman Coulter, Inc., Fullerton, CA) at 0, 6, 12, and 22 °C. Raw volumetric data were exported into Mathematica (Wolfram Research Inc., Champaign, Illinois) computing package for processing and analysis.
Determination of osmotic tolerance limits
Osmotic tolerance limits were defined by the maintenance of plasma membrane integrity, as indicated by propidium iodide exclusion in 80% of the ESC population following exposure to anisosmotic conditions using sodium chloride as the impermeable solute, in the manner previously described by Kashuba Benson et al. [35]. Briefly, solutions of varying osmolality were prepared using Dulbecco’s Phosphate Buffered Saline (DPBS) that was adjusted to the appropriate osmolality by the addition of either double distilled water or sodium chloride (Sigma Aldrich). The solutions were adjusted to pH 7.1 using sodium hydroxide or hydrochloric acid as necessary. The osmolality of each solution was verified using a vapor pressure osmometer (Wescor, Logan, UT). On three separate days for each ESC line, ESCs were trypsinized and separated from fibroblast feeders. Equal numbers of cells were then exposed to solutions of 37, 75, 150, 600, 1200, 2400 and 4800 mOsm (n=3 for each solution) for 10 minutes at room temperature. Cells were abruptly returned to isosmotic by the abrupt addition of appropriate volumes of hyperosmotic solution in the case of hypoosmotic conditions, and hypoosmotic solution in the case of hyperosmotic conditions. Cells were then centrifuged for five minutes at 200g and resuspended in isosmotic solution. Cells exposed to anisosmotic conditions were compared to controls in which the same quantities of cells were exposed to isosmotic conditions (285 mOsm) following the same protocol. Plasma membrane integrity was assessed by flow cytometry analysis (FACScan, Becton Dickinson, San Jose, CA) of propidium iodide exclusion.
Measurement of cell osmotic response
Embryonic stem cell volumetric response to variable osmolality was measured at 22 °C using an EPC, as previously described [11,20,21,35,44,48]. Mean cell volume response was measured in real time following abrupt exposure to 206, 285, 600, 900, 1350, and 2880 mOsm solutions prepared from 10X PBS (Sigma) and Milli-Q water and adjusted to pH 7.1 with hydrochloric acid. The osmolality of the solutions was verified using a vapor pressure osmometer (Wescor). Data were averaged over 100 ms intervals prior to analysis. Three replicates were performed for each experimental condition. For representative plot of the output, please refer to Figure 1 in this manuscript, and Figure 2.1A from [35]. Electronic particle counter data can be subject to noise due to cellular debris and ambient electrostatic variation. Custom software was utilized that used an approach similar to that of Armitage and Juss [3] but with maximum likelihood estimates of locations of population means [13] to avoid influence of non-uniformly distributed noise (see Figure 1 for characteristic processing output).
Figure 1.
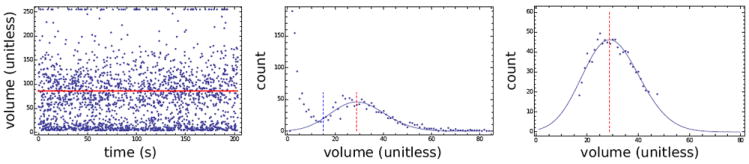
Output of data reduction algorithm for Boyle Van’t Hoff volumetric data. The left panel shows raw uncalibrated data from the Coulter counter. The middle and right panels show histograms for the data from the left panel. The algorithm first determines the approximate minimum that occurs between the cell volume signals and the low volume debris and noise, (left (blue) dashed line, center panel), removes the data corresponding to volumes less than this line, and removes large volumes above the same “count” to reduce influence from doublet cells and large debris (see Armitage and Juss [3] for a similar approach). The algorithm then uses a maximum likelihood estimate based on the normal distribution on the remaining data (shown in the right panel) to determine sample mean (red line, all panels). The solid lines are associated plots of the resulting Gaussian normal probability distribution function. Note that due to processing, the uncalibrated volumes from the left panel one do not align with those of the other panels.
Figure 2.
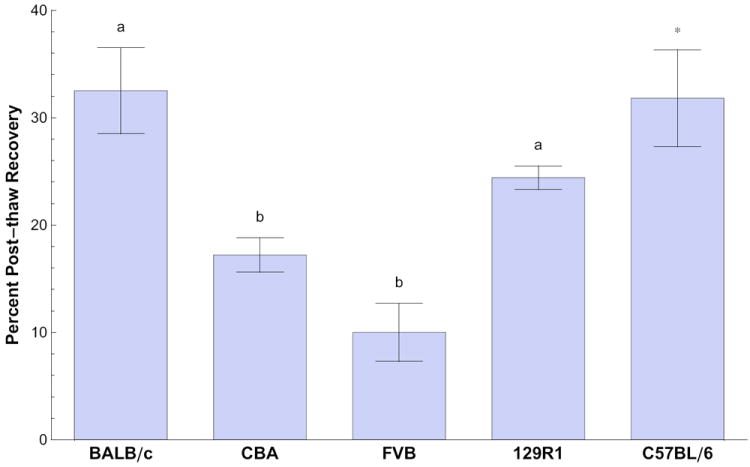
Percent post-thaw recoveries of membrane-intact BALB/c, CBA, FVB, and 129R1 (R1) mouse embryonic stem cells frozen in cryovials in standard freezing conditions (106 cells/mL, 1 °C/minute cooling rate, 1.0 M Me2SO, -80 °C plunge temperature). Percent post-thaw recovery is expressed as mean ± SEM. Different superscripts indicate significantly different means (p<0.05). Previously published results for C57BL/6 mouse embryonic stem cells cryopreserved under the same conditions (indicated with the asterisk) are included for reference [35], but are not included in the statistical analysis.
Equilibrated cell volumes were normalized to their respective isotonic values, and plotted against the reciprocal of normalized osmolality in accordance with the Boyle Van’t Hoff relationship [36,47]. Linear regression was performed using Mathematica to fit the Boyle Van’t Hoff equation to the data. This equation is defined by
where V is total cell volume at osmolality π, Wiso is isotonic cell water volume, πiso is isotonic osmolality, and Vb is the osmotically inactive cell volume. Vb was determined by performing a linear regression of volume as a function of the reciprocal of osmolality and extrapolating to infinite osmolality (i.e. 1/π=0)—note the regression line was not forced through isosmotic volume [12].
Determination of permeability parameters
As previously described by Kashuba Benson et al. [35], cell volume changes over time were measured by an EPC following abrupt addition of cells to 1.0 M CPA in 1X PBS. Volumetric changes were measured in the presence of 1.0 M Me2SO, 1.0 M EG, and 1.0 M PG at 0, 6, 12, 22, and 34 °C. For a representative plot of the experimental output, please refer to Figure 2.1B [35]. Measurements of cells in the presence of 1.0 M glycerol were determined at 22 °C. Three replicates were performed for each treatment on 3 different days.
Data were fit to the following two-parameter mass transport model [36] to determine membrane permeability coefficients for cryoprotective agents (Ps) and hydraulic conductivity in the presence of cryoprotectants (Lp) at all temperatures:
Here, subscripts s and n indicate permeating and non-permeating quantities, respectively, A is the volume independent spherical surface area at Viso, ρ ≈1 kg/L is the density of water to within 1% error. We assume the relationship N = Wiso πiso. The Arrhenius relationship (see [43])
where P0 is the value at some absolute reference temperature T0, R is the gas constant and Ea is the activation energy for the process, expressed in kcal/mol, was used to determine the temperature dependence for the parameters P(T)=Lp and P(T)=Ps. The parameter Ea was determined as the slope of the linear regression of R ln(P(T)) versus 1/T.
Statistical analysis
For all comparisons, standard two-way analysis of variance (ANOVA) was performed with the SAS General Linear Models program (SAS Institute, Inc., Cary, NC) using a value of p<0.05 for significance. In situations where a plot of the residuals of a data set was not normally distributed, data were normalized using either natural logarithm or square root transformation. Based on the results of the ANOVA for Vb and lower OTL values, Fisher’s least significant difference tests were conducted to evaluate significant differences between mESC lines (p<0.05). All values are presented as mean ± SEM, unless stated otherwise. It is important to note that cell line and genetic background are completely confounded variables; when reporting statistical significance in the Results section, we used the term, “genetic background”.
Results
Percent post-thaw recovery varied by cell line under standard conditions
Percent post-thaw recovery of mESC lines varied significantly across genetic background (p<0.05) under standard freezing conditions. Percent post-thaw recovery was 32.5 ± 4.0% (average ± SEM) for the BALB/c line, 17.2 ± 1.6% for the CBA line, 10.0 ± 2.7% for the FVB line, and 24.4 ± 1.1% for the 129R1 line (Figure 2). Values were significantly different between the BALB/c, CBA, and FVB lines and between the FVB and 129R1 mESC lines (p<0.05).
Osmotically inactive and isosmotic cell volume
Isosmotic volumes were 744 ± 5 μm3, 960 ± 20 μm3, 690 ± 5 μm3, and 719 ± 6 μm3 (mean ± SE), for the BALB/c, CBA, FVB, and 129R1 cell lines, respectively. There was a significant effect of cell line and day (p<0.05) on isosmotic volume. Mouse ESCs from all lines behaved as ideal osmometers (W π = constant) over a range of 200 mOsm to 2800 mOsm. Extrapolation of the regression line to infinite osmolality yielded osmotically inactive fractions (Vb/Viso) of 0.432 ± 0.024 for the BALB/c line, 0.391 ± 0.033 for the CBA line, 0.526 ± 0.024 for the FVB line, and 0.520 ± 0.045 for the 129R1 line. There was a significant main effect of genetic background on Vb (p < 0.05), with Vb of the CBA mESC line significantly lower than that of the FVB or 129R1 mESC line (p < 0.05). The previously published Vb value for the C57BL/6 mESC line [35] is listed for comparative purposes with values for the cell lines in this study in Table 2, and linear regressions are shown in Figure 3.
Table 2.
Osmotically inactive cell volume fraction (Vb/Viso) and isosmotic volume of five mouse embryonic stem cell lines.
| Cell line | Vb/Viso | Viso (um3) |
|---|---|---|
| C57BL/6* | 0.497 ± 0.013 | 695±4 |
| BALB/c | 0.432 ± 0.024a,b | 744±5a |
| CBA | 0.391 ± 0.033a | 960±20b |
| FVB | 0.526 ± 0.024b | 690±5c |
| 129R1 | 0.520 ± 0.045b | 719±6a |
Vb is expressed as a volume fraction of isosmotic cell volume ± SEM. Different superscripts indicate statistically significant differences within columns (p<0.05), i.e. similar superscripts indicate statistically indistinguishable groups within columns (p<0.05).
The Vb/Viso value for the C57BL/6 mESC line is previously published [35] and listed for ease of comparison; Viso for the C57BL/6 mESC line was not previously published. Neither value was included in statistical analyses.
Figure 3.
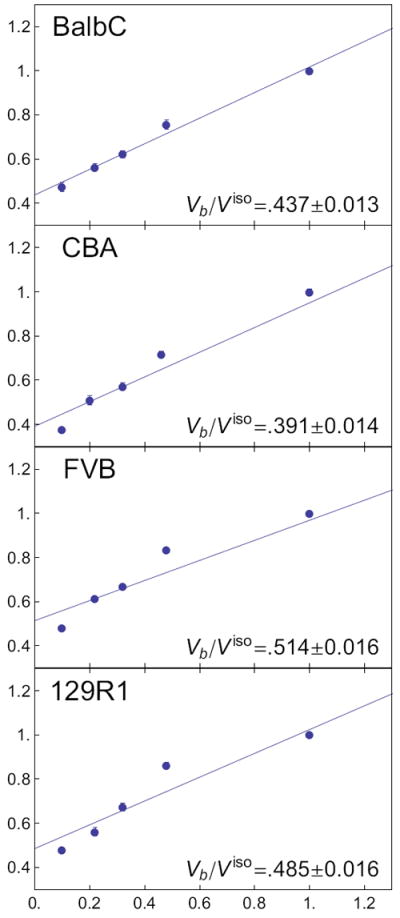
Boyle van’t Hoff plots for all cell lines with corresponding linear regressions and infinite-osmolality intercept Vb/Viso ± standard error. Error bars indicate standard error of the mean at each osmolality.
Osmotic tolerance
The effects of anisosmotic conditions on mESC membrane integrity, as determined by propidium iodide (PI) exclusion, are shown along with previously published 80% osmotic tolerance limits for the C57BL/6 mESC line [35] for illustrative purposes, in Figure 4. Interpolation between data points was used to determine upper and lower osmolalities where 80% of the mESC population retained membrane integrity for each cell line and each day. This resulted in estimates of between 142 ± 5 mOsm (mean over experimental days ± SEM) and 816 ± 40 mOsm (1.6 and 0.6 × Viso, BALB/c), 153 ± 5 mOsm and 784 ± 10 mOsm (1.6 and 0.6 × Viso, CBA), 163 ± 5 mOsm and 671 ± 80 mOsm (1.4 and 0.7 × Viso, FVB), and 143 ± 5 mOsm and 1003 ± 141 mOsm (1.5 and 0.7 × Viso, 129R1). There was a significant main effect of genetic background on the lower OTL (p<0.05), in which the lower OTL of the FVB cell line was significantly higher (p<0.05) than that of the 129R1 or BALB/c mESC line. Genetic background was not a significant main effect for upper OTL. Genetic background was not a main effect for the range of OTL.
Figure 4.
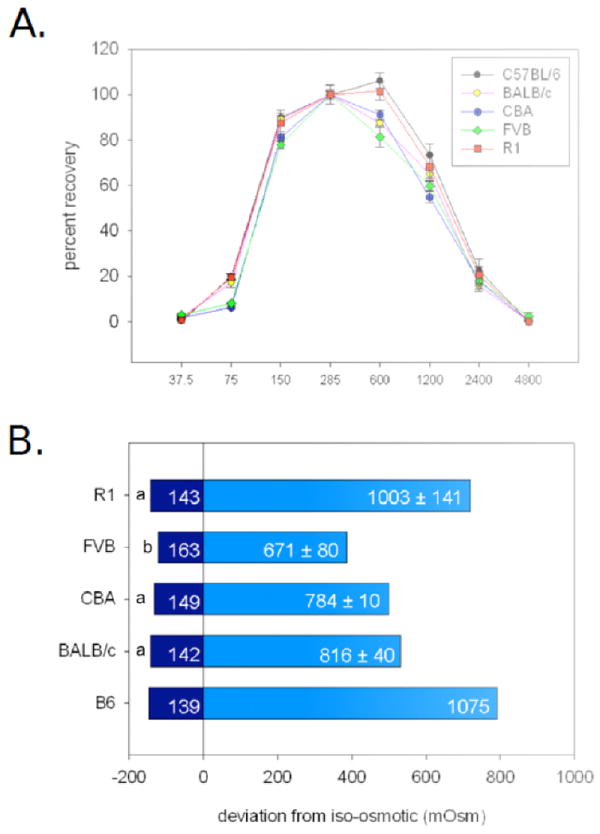
Osmotic tolerance limits (OTL) of five mouse embryonic stem cell (mESC) lines as determined by plasma membrane integrity. Previously published data for the C57BL/6 mESC line [35] are included for comparative purposes but were not included in statistical analyses. Equal quantities of mESCs were exposed to solutions of 38, 75, 150, 600, 1200, 2400, and 4800 mOsm for 10 minutes at room temperature, and compared with controls exposed to isosmotic solution (285 mOsm) in the same manner. Plasma membrane integrity was assessed by flow cytometry analysis of propidium iodide exclusion. A. Inverted U-shaped osmotic tolerance curves of BALB/c, C57BL/6 (B6), CBA, FVB, and 129R1 (R1) mESC lines. B. OTL, defined as the range of osmolalities in which 80% of cells maintained plasma membrane integrity, for each of 5 different mESC lines (n=3). “0” represents isosmotic (285 mOsm). Distance from isosmotic is in terms of mOsm. Inset numbers indicate the extrapolated mean osmolalities (through linear regression) ± SEM at which 80% of mESCs retained membrane integrity. SEM for all lower OTL was ± 5 mOsm. Different lower case superscripts indicate statistically significant differences in lower OTL (p<0.05).
Permeability parameters
Changes in mESC volume in the presence of 1.0 M CPA, measured over time by electronic particle counter (EPC) at 0, 6, 12, 22, and 34 °C, were fitted to compute Lp and Ps. Room temperature (RT) values of Lp, Ps, and their associated activation energies ( and ) are shown in Tables 3,4, and 5. For all cell lines, Lp and Ps values in the presence of glycerol were significantly lower (p<0.05) than either Lp or Ps values in the presence of Me2SO, PG, or EG. Based upon this finding, glycerol was deemed inappropriate for equilibrium freezing of these mESC lines and Ea values were not assessed.
Table 3.
Room temperature hydraulic conductivity (Lp) for five mouse embryonic stem cell (mESC) lines in the presence of 1.0 M cryoprotective agent (CPA).
| Genetic background | Dimethyl sulfoxide | Ethylene glycol | 1,2-propanediol | Glycerol |
|---|---|---|---|---|
| C57BL/6* | 0.41 ± 0.03 | 0.48 ± 0.06 | 0.42 ± 0.03 | 0.14 ± 0.01 |
| BALB/c | 0.15 ± 0.01a,1 | 0.15 ± 0.01a,1 | 0.18 ± 0.01a,1 | 0.05 ± 0.01 b,1 |
| CBA | 0.21 ± 0.01a,1 | 0.15 ± 0.01a,b,1 | 0.19 ± 0.01a,1 | 0.09 ± 0.01b,2 |
| FVB | 0.39 ± 0.04a,2 | 0.24 ± 0.03b,1,2 | 0.22 ± 0.04b,1,2 | 0.10 ± 0.01c,2 |
| 129R1 | 0.53 ± 0.12a,2 | 0.35 ± 0.02a,2 | 0.33 ± 0.04a,2 | 0.10 ± 0.01b,2 |
Hydraulic conductivity values are expressed as mean ± SEM μm·min-1·atm-1. Different superscripts indicate statistically significant effects (p<0.05): of CPA within each genetic background on Lp values (letters, rows), or of genetic background within each CPA group on Lp values (numbers, columns).
Values for the C57BL/6 mESC line are previously published [35] and listed for ease of comparison, but were not included in statistical analyses.
Table 4.
Room temperature cryoprotectant permeability (Ps) for five mouse embryonic stem cell (mESC) lines in the presence of 1.0 M cryoprotective agent (CPA).
| Genetic background | Dimethyl sulfoxide | Ethylene glycol | 1,2-propanediol | Glycerol |
|---|---|---|---|---|
| C57BL/6* | 4.59 ± 0.41 | 4.17 ± 0.24 | 6.58 ± 0.38 | 1.05 ± 0.07 |
| BALB/c | 8.62 ± 0.53a,1 | 11.74 ± 0.84b,1 | 10.51 ± 0.67a,b,1 | 1.99 ± 0.251,c |
| CBA | 9.10 ± 0.60a,1 | 8.41 ± 0.28a,1 | 9.40 ± 0.61a,2 | 2.32 ± 0.281,b |
| FVB | 4.53 ± 0.37a,2 | 5.32 ± 0.50a,2 | 6.92 ± 0.64a,3 | 1.33 ± 0.371,b |
| 129R1 | 4.00 ± 0.48a,2 | 3.92 ± 0.23a,2 | 5.24 ± 0.27a,4 | 0.99 ± 0.071,b |
Cryoprotectant permeability values are expressed as mean ± SEM μm·min-1. Different superscripts indicate statistically significant effects (p<0.05): of CPA within each genetic background on Ps values (letters, rows), or of genetic background within each CPA group on Ps values (numbers, columns).
Values for the C57BL/6 mESC line are previously published [35] listed for ease of comparison but were not included in statistical analyses.
Table 5.
Activation energies of hydraulic conductivity ( ) and of cryoprotectant permeability ( ) for five mouse embryonic stem cell (mESC) lines in the presence of 1.0 M cryoprotective agent (CPA).
| Genetic background |
|
|
||||||
|---|---|---|---|---|---|---|---|---|
| Me2SO | EG | PG | Me2SO | EG | PG | |||
|
|
|
|
||||||
| C57BL/6* | 14.29 | 15.35 | 14.12 | 15.47 | 13.19 | 14.08 | ||
| BALB/c | 12.33 ± 0.60a,1,2 | 12.94 ± 0.49a,1 | 13.80 ± 0.52a,1 | 13.44 ± 0.81a,1 | 13.04 ± 0.21a,1 | 15.15 ± 1.11a,1 | ||
| CBA | 10.82 ± 1.61a,1 | 13.01 ± 1.09a,1 | 11.51 ± 0.45a,1 | 17.35 ± 2.37a,1 | 16.75 ± 1.16a,1 | 19.79 ± 0.46a,1 | ||
| FVB | 12.29 ± 1.42a,1,2 | 13.01 ± 1.09a,1 | 10.09 ± 1.34a,1 | 16.94 ± 1.47a,1 | 14.95 ± 0.66a,1 | 19.00 ± 1.99a,1 | ||
| 129R1 | 16.82 ± 1.98a,2 | 14.52 ± 0.50a,1 | 12.94 ± 1.03a,1 | 13.80 ± 1.78a,1 | 15.85 ± 0.63a,1 | 18.17 ± 0.70a,1 | ||
Activation energy is expressed as mean±SEM kcal·mol-1. Different superscripts indicate statistically significant effects (p<0.05) (i.e., similar superscripts indicate statistically indistinguishable groups, p<0.05): of CPA within each genetic background on or values (letters, rows), or of genetic background within each CPA group on or values (numbers, columns).
Values for the C57BL/6 mESCline are previously published values [35] listed for ease of comparison but were not included in statistical analyses. Me2SO, dimethyl sulfoxide; EG, ethylene glycol; PG, 1,2-propanediol.
a. Hydraulic conductivity at room temperature
Two-way ANOVA revealed that genetic background and CPA type, as well as the interaction between these factors all had a significant effect on the room temperature (22 °C) water permeability parameter Lp (p<0.05). Highest to lowest Lp values were: 129R1, FVB, CBA, and BALB/c. To examine differences between genetic backgrounds, we first made comparisons of data that had been grouped across CPA types, in particular Lp in the presence of glycerol was significantly lower than Lp in the presence of Me2SO, PG or EG (p<0.05). Overall, Lp in the presence of Me2SO was significantly higher than values in the presence of EG and PG at room temperature (p<0.05). Please refer to Table 3 for a list of Lp values.
To obtain more detailed comparisons between genetic backgrounds, we compared the water permeability parameters for each cell line within a given CPA type at 22 °C. Values for Lp in the presence of Me2SO were significantly lower (p<0.05) for the CBA and BALB/c cell lines relative to the FVB or 129R1 mESC lines. In EG, room temperature Lp values of the 129R1 mESC line were significantly higher (p<0.05) than those of the BALB/c and CBA mESC lines. In PG, values of Lp were significantly higher (p<0.05) for the 129R1 mESC line relative to the BALB/c and CBA mESC lines. In glycerol, among all Lp values, those for the BALB/c line were significantly lower (p<0.05) than those of the CBA, FVB, and 129R1 mESC lines; however there was no significant difference between CBA, FVB, and 129R1 mESC lines.
Finally, the effect of CPA on Lp values was analyzed for each genetic background. In keeping with the main effects described above, within the BALB/c, FVB, and 129R1 lines, Lp in glycerol was significantly lower than Lp for all other CPAs (p<0.05), and within the CBA line, Lp in glycerol was significantly lower than Lp in Me2SO and PG (p<0.05). There was no significant difference between Lp in Me2SO, PG, and EG within the BALB/c, CBA, or 129R1 mESC lines. However, for the FVB line, Lp in Me2SO was significantly higher than Lp in the presence of EG, PG, or glycerol.
b. Temperature dependence of hydraulic conductivity
Two-way ANOVA revealed a main effect of genetic background on (p<0.05), but no main effect of CPA. Fisher LSD comparisons of genetic background indicated that values for the 129R1 line were significantly higher than those of the CBA and FVB lines (p<0.05). There was no significant main effect of the interaction of genetic background and CPA on . However, for comparisons of values for specific CPA among strains, post-tests showed that in the presence of Me2SO, was significantly higher for the 129R1 line than for that of the CBA line (p<0.05). Please refer to Table 5 for mean values and their standard errors, and Figure 5 for their corresponding Arrhenius plots.
Figure 5.
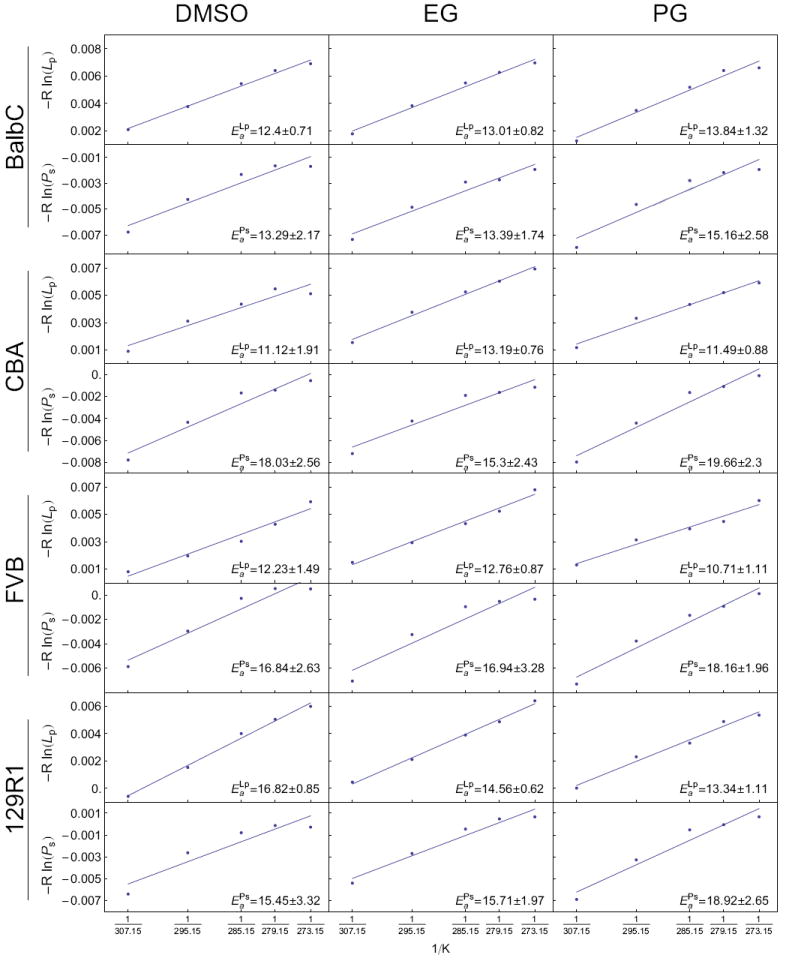
Arrhenius plots of water and solute permeability as a function of temperature for all cell lines and CPAs with resulting best-fit parameters from a linear regression represented as best fit of the regression slope ± the predicted standard error of the slope. Variables and their units are given in Table 1.
c. Solute permeability at room temperature
Two-way ANOVA revealed that genetic background and CPA type, as well as the interaction between these factors all had a significant effect on the room temperature (22 °C) solute permeability parameter Ps (p<0.05). Using Fisher LSD post-test multiple comparisons among strains, we found significant differences (p<0.05) in overall Ps values between cell lines of all genetic backgrounds with the exception of between the CBA and BALB/c mESC lines. In this case the order from lowest to highest Ps values was: 129R1, FVB, (CBA or BALB/c). As stated previously, Ps for glycerol was significantly lower than it was for Me2SO, EG, and PG. Overall, the PG Ps value was significantly higher that for Me2SO (p<0.05); however there was no significant difference between Ps in EG and in either Me2SO or PG.
Looking at the effects within each CPA group, we found differences among genetic backgrounds for Ps values. In particular, room temperature Ps values for Me2SO and PG did not significantly differ between the FVB and 129R1 cell lines or between the CBA and BALB/c cell lines; however Me2SO Ps values for the FVB and 129R1 mESC lines were significantly lower (p<0.05) than those of the BALB/c and CBA lines. Room temperature EG Ps values did not differ significantly between the FVB and 129R1 cell lines. However, there were significant differences with other cell lines comparisons. Highest to lowest EG Ps by cell line was: BALB/c, CBA, (FVB or 129R1) (p<0.05). Finally, there were no significant differences in glycerol Ps values between genetic backgrounds.
To examine the interaction of CPA and strain, the effect of CPA on Ps values was analyzed within each genetic background. For all genetic backgrounds, glycerol Ps was significantly lower (p<0.05) than Ps for the other CPAs. Within the CBA, 129R1, and FVB cell lines, there was no significant difference between Ps for Me2SO, EG, and PG. Within the BALB/c cell line, Ps for Me2SO was significantly lower than that for EG (p<0.05). Please refer to Table 4 for a list of Ps values.
d. Temperature dependence of solute permeability
Two-way ANOVA indicated that both genetic background and CPA significantly affected . Fisher post-test analysis of strain effects revealed that BALB/c line values were significantly lower (p<0.05) than those of the FVB and CBA lines. Moreover, Fisher post-test analysis of CPA effects indicated that values were significantly higher (p<0.05) in the presence of PG than in the presence of Me2SO or EG. For interactions, we found that the BALB/c in the presence of EG was significantly lower (p<0.05) than that of the CBA line in the presence of PG. Please refer to Table 5 for a list of values and Figure 5 for their corresponding Arrhenius plots.
Discussion
Post-thaw recovery of cells following cryopreservation has been demonstrated to vary widely across cell types as well as among and within species. These differences can be attributed to wide-ranging differences in fundamental cryobiological parameters specific to individual cell types and species [25,58]. This variability even extends to individuals; a good example being bull spermatozoa, where not only is there variability from individual to individual, but also from sample to sample from the same individual [17]. Embryonic stem cells have demonstrated tremendous variability in post-thaw recovery from species to species. Embryonic stem cell post-thaw recovery using 1 M Me2SO, a cooling rate of 1 °C/minute, and plunge temperature of -80°C can range from 0.1 to 1% in human ESCs [22,33], from 0.4 to 5% in non-human primate ESCs [22] (personal communication, Shoukhrat Mitalipov, Oregon National Primate Research Center, 2004), and anywhere from 10% to 90 percent with mESCs [35,45] (personal communication, Deanna Nielsen, Stem Cell Technologies technical support, 2004; personal communication, Xin Yu, University of California-Davis, 2004). With the exception of the 2007 report of an 88% post-thaw recovery rate in the 129R1 mESC line by Miszta-Lane et al. [45], and a 31.9% post-thaw recovery rate in a C57BL/6 mESC line by Kashuba Benson et al. [35], reports of variability in post-thaw recovery of mESCs have been largely anecdotal or confined to laboratory observation. This study examined the post-thaw recoveries of four mESC lines from differing genetic backgrounds and describes the fundamental cryobiological cell parameters that would contribute to such differences. Defining such parameters allows us to predict cell volume excursions during the addition and removal of CPAs and estimate the degree of dehydration cells undergo during cooling [1], and accordingly develop cryopreservation protocols that maximize post-thaw recovery by minimizing damaging intracellular ice formation and solute effects.
As expected, with the standard cryopreservation protocol (1 M Me2SO, 1 °C/minute cooling rate to -80 °C, plunge into liquid nitrogen, then warming in a 37 °C water bath), the percent post-thaw recoveries of membrane-intact cells of BALB/c, CBA, FVB, and 129R1 mESC lines were shown to vary significantly by genetic background, with a range of 10.0 to 32.5% recovery (Figure 2). Interestingly, the post-thaw recovery of 129R1 mESCs of 24.4 ± 1.2% was dramatically different from the 88% recovery rate reported by Miszta-Lane et al. [45] under similar conditions. Our definition of post-thaw recovery may have been stricter, resulting in lower percentages, as we considered only the single-cell population during FACScan analysis and excluded cell clusters. Importantly, our method accounted for cell lysis during cryopreservation and warming by considering the total number of cells, membrane-intact and not, both prior to and following cryopreservation. Additionally, while cell counts pre-and post-centrifugation were similar (data not shown), it is possible that the centrifugation step prior to measurement of membrane integrity also resulted in reduced cell recovery and lower post-thaw numbers. However, this centrifugation step is part of standard culture methodology [46] as a post-thaw means to remove remaining CPA and as such, mimics the practical laboratory situation. Cells with damaged membranes are potentially lost during such a process; however the same cells, if not lost, would also be excluded from viable cell counts using the propidium iodide-exclusion membrane integrity assay. The variable of cell loss during centrifugation was also controlled by the application of this step to all treatment groups in our experiments, therefore this centrifugation step was not considered to have a large effect on post-thaw cell recovery.
For this study we chose to use a membrane integrity test instead of long-term end point assays such as a measure of apoptosis or colony formation. While the latter assays are potentially more appropriate indicators of long-term post-thaw survival, determining appropriate apoptotic assays for post-thaw mouse embryonic stem cells is beyond the scope of this primarily biophysical study, and these extended assays would be extremely impractical for our large comparative study. The classical biophysical approach we used considers primarily mechanical damage phenomena (e.g. death due to intracellular ice formation, exceeding osmotic tolerance limits) and therefore a propidium iodide-exclusion membrane-integrity assay is an appropriate test of these phenomena and a baseline indicator of cell survival.
Osmotic tolerance ranges differed significantly at the lower limits, and there were some differences at upper osmotic tolerance range in the absence of an overall effect of genetic background. However, with the broad range of osmotic tolerance limits displayed by all cell lines in this study (Figure 4), these differences were not great enough to warrant changing the standard freezing media CPA concentration of 10%, or roughly 1 M, for any individual line as the addition of 1 M CPA, especially drop-wise [27], should not have a damaging osmotic effect. In fact, osmotic tolerance limits such as these would possibly enable even higher concentrations of CPAs to be used in vitrification protocols if necessary, as long as the CPAs were added in stepwise fashion. In general, all cell lines displayed a relatively wide range of osmotic tolerance, expressed as factors of Viso, ranging from lower volume limits of 0.6-0.7 × Viso to upper limits of 1.4 - 1.6 × Viso. These ranges are consistent with previous findings with the C57BL/6 mESC line (0.6-1.5 × Viso)[35] and are also comparable to limits of other cell types such as canine pancreatic islets (0.6-1.52 × Viso)[62], human spermatozoa (0.75-1.02 × Viso)[24], human umbilical cord blood CD34+ cells (0.6-1.52 × Viso)[59], and human granulocytes (0.7-1.68 × Viso)[4]. For consistency with our previous study [35], we used NaCl as the major osmolyte to create hyperosmotic solutions. However, there have been reports of differential damage upon exposure to ionic or nonionic solutes used to induce anisosmolality [23], possibly due to increased ion permeability [16,41,57]. While this effect is not universal [60], it is possible that the use of NaCl had a confounding effect on our osmotic tolerance experiments.
The Boyle van’t Hoff regressions in Figure 3 show consistently high residual at the 600 mOsm level, echoing the results from our previous work with the C57BL/6 mESC line [35]. In fact, discarding either the isosmotic volume point or the 600 mOsm volume point, one would arrive at considerably lower osmotically inactive volumes (data not shown). The consistency of this phenomenon across cell lines suggests that there may be active cell volume regulation in mESC and further investigation into this phenomenon is warranted.
Recently, Katkov [37] published an analysis of parameter estimation in the cryobiological literature, stating that it may be incorrect to assume that the temperature dependence of the solute permeability parameter Ps follows the Arrhenius relationship (with activation energy ). Katkov’s argument is that Ps is actually a lumped parameter ωRT, where ω is the “solute mobility” term which should have an Arrhenius-like dependence on temperature (with activation energy ). Our study was instigated before this argument was made and therefore optimal freezing protocols were produced with predictions of subzero Ps made with . Because the solute permeability data were fit (with a high correlation coefficient) with the assumption that Ps follows the Arrhenius model of temperature dependence, the error induced by using this model is minimized at temperatures near the range in which measurements were made. Much below these temperatures, say less than -10 °C, Ps is small enough so that the effects of error in parameter values are greatly reduced, and the effects on optimal cooling rates are effectively zero.
There has been some recent discussion on the appropriateness of using suprazero biophysical parameters to predict subzero behavior (see e.g. [2,7]). Unfortunately, there are considerable challenges in measuring subzero biophysical parameters putting this beyond the scope of this study. This potential source of error has the potential for influencing both optimal cooling rate and optimal plunge temperature. However, we note that this extrapolation technique has been recently very successfully applied in mESCs [35].
The combination of the significant effects of genetic background on Vb, permeability parameters, and Ea strongly suggests that optimal cooling rates will vary with genetic background, and that a “one-size fits all” protocol may not suffice. Additionally, significant overall effects of CPA on permeability parameters and suggest that optimal cooling rates will also vary by CPA. As was previously determined with the C57BL/6 mESC line [35], glycerol was determined to be unsuitable for equilibrium cooling for all mESC lines studied due to its markedly lower room temperature values of Lp and Ps. Future studies will include theoretical simulations to predict optimal cooling rates for cryopreservation methods involving Me2SO, EG, and PG. Experimental validation of the predicted optimal cooling rates as well as predicted optimal plunge temperatures will be performed with Me2SO and PG. 1,2-propanediol will be preferentially studied due to its more stable glass forming properties relative to EG and Me2SO [9,10] and due to overall propylene glycol Ps being significantly higher than that for Me2SO Ps at room temperature across the four mESC lines studied [35], which would enable the most rapid addition and removal of CPA at room temperature with minimal damage to the cell membrane [35,26]. Predicted optimal cooling rates of Me2SO will be explored for comparative purposes, as it is the CPA used in standard cryopreservation protocols.
Table 1.
Definition of major symbols and terms
| Symbol or abbreviation | Description | Units | Value |
|---|---|---|---|
| s,n,w | Subscripts: s, solute; n, non-permeating; w, water | None | None |
| Lp | Hydraulic conductivity in the presence of cryoprotectant | μm min-1 atm-1 | Parameter |
| Ps | Solute permeability | μm min-1 | Parameter |
| Ea | Activation energy | kcal mol-1 | Parameter |
| T | Temperature | K | 295 |
| A | Cell surface area | μm2 | Parameter |
| V | Total cell volume | μm3 | Variable |
| Vb | Osmotically inactive cell volume | μm3 | Parameter |
| W | Intracellular water volume | μm3 | Variable |
| S | Moles of internal permeating solute at time t | Moles | Variable |
| t | Time | Seconds | Variable |
| πiso | Osmolality of initial (isotonic) intracellular nonpermeating solutes | Osm kg-1 H2O | 0.290 |
| πn | Osmolality of extracellular nonpermeating solutes | Osm(kg H2O)-1 | Variable |
| Ms | Molality of extracellular permeating solutes | Mol (kg H2O)-1 | Variable |
| N | Osmoles of intracellular salts | Osm | Variable |
| V̄ | Partial molal volume of solute | L mole-1 | Variable |
| R | The universal gas constant | kcal mole-1 K-1 | 1.987 × 10-3 |
Acknowledgments
The authors (CMK and JDB) wish to state that John Critser played a critical role in the design and analysis of the present study, and made significant contributions to the initial drafts. This work was initially undertaken while we were both students in his lab, and his insight and enthusiasm for meaningful cryobiological discovery through basic science still shines in these manuscripts.
Funding: This work was supported, in part, by grants from the National Institutes of Health, National Center for Research Resources (U42 RR1482, T32-RR07004, R41RR025927) and the Cryobiology Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agca Y, Liu J, McGrath JJ, Peter AT, Critser ES, Critser JK. Membrane permeability characteristics of metaphase II mouse oocytes at various temperatures in the presence of Me2SO. Cryobiology. 1998;36:287–300. doi: 10.1006/cryo.1998.2088. [DOI] [PubMed] [Google Scholar]
- 2.Akhoondi M, Oldenhof H, Wolkers WF. Water transport processes during cooling of mammalian cells. Cryobiology. 2011;63:311. [Google Scholar]
- 3.Armitage WJ, Juss BK. Osmotic response of mammalian cells: effects of permeating cryoprotectants on nonsolvent volume. J Cell Physiol. 1996;168:532–8. doi: 10.1002/(SICI)1097-4652(199609)168:3<532::AID-JCP5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 4.Armitage WJ, Mazur P. Osmotic tolerance of human granulocytes. Am J Physiol. 1984;247:C373–C381. doi: 10.1152/ajpcell.1984.247.5.C373. [DOI] [PubMed] [Google Scholar]
- 5.Austin CP, Battey JF, Bradley A, Bucan M, Capecchi M, Collins FS, Dove WF, Duyk G, Dymecki S, Eppig JT, Grieder FB, Heintz N, Hicks G, Insel TR, Joyner A, Koller BH, Lloyd KCK, Magnuson T, Moore MW, Nagy A, Pollock JD, Roses AD, Sands AT, Seed B, Skarnes WC, Snoddy J, Soriano P, Stewart DJ, Stewart F, Stillman B, Varmus H, Varticovski L, Verma IM, Vogt TF, von Melchner H, Witkowski J, Woychik RP, Wurst W, Yancopoulos GD, Young SG, Zambrowicz B. The knockout mouse project. Nat Genet. 2004;36(9):921–4. doi: 10.1038/ng0904-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auwerx J, et al. The European dimension for the mouse genome mutagenesis program. Nat Genet. 2004;36:925–927. doi: 10.1038/ng0904-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balasubramanian SA, Wolkers WF, Bischof JC. Membrane hydration correlates to cellular biophysics during freezing in mammalian cells, 2009. Biochim Biophys Acta-Biomembr. 2009;1788:945–953. doi: 10.1016/j.bbamem.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Baran SW, Ware CB. Cryopreservation of rhesus macaque embryonic stem cells. Stem Cells Dev. 2007;16:339–344. doi: 10.1089/scd.2007.900-de. [DOI] [PubMed] [Google Scholar]
- 9.Baudot A, Alger L, Boutron P. Glass-forming tendency in the system water-dimethyl sulfoxide. Cryobiology. 2000;40:151–158. doi: 10.1006/cryo.2000.2234. [DOI] [PubMed] [Google Scholar]
- 10.Baudot A, Odagescu V. Thermal properties of ethylene glycol aqueous solutions. Cryobiology. 2004;48:283–294. doi: 10.1016/j.cryobiol.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Benson CT, Liu CT, Gao DY, Critser ES, Critser JK. Determination of the osmotic characteristics of hamster pancreatic islets and isolated pancreatic islet cells. Cell Transplant. 1993;2:461–465. doi: 10.1177/096368979300200604. [DOI] [PubMed] [Google Scholar]
- 12.Benson JD. Some comments on recent discussion of the Boyle van’t Hoff relationship. Cryobiology. 2012;64:118–120. doi: 10.1016/j.cryobiol.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Benson JD, Gallia JM, Chicone CC, Critser JK. Coulter counter data reduction and parameter estimation. Cryobiology. 2006;53:430–431. [Google Scholar]
- 14.Benson JD, Haidekker MA, Kashuba Benson CM, Critser JK. Mercury free operation of the Coulter counter MultiSizer II sampling stand. Cryobiology. 2005;51:344–347. doi: 10.1016/j.cryobiol.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Chan SY, Evans MJ. In situ freezing of embryonic stem cells inmultiwell plates. Trends Genet. 1991;7:76. doi: 10.1016/0168-9525(91)90274-T. [DOI] [PubMed] [Google Scholar]
- 16.Chan HC, Nelson DJ. Chloride-dependent cation conductance activated during cellular shrinkage. Science. 1992;257:669–71. doi: 10.1126/science.1379742. [DOI] [PubMed] [Google Scholar]
- 17.Chaveiro A, Liu J, Engel B, Critser JK, Woelders H. Significant variability among bulls in the sperm membrane permeability for water and glycerol: possible implications for semen freezing protocols for individual males. Cryobiology. 2006;53:349–59. doi: 10.1016/j.cryobiol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Critser JK, Mobraaten LE. Cryopreservation of murine spermatozoa. ILAR J. 2000;41:197–206. doi: 10.1093/ilar.41.4.197. [DOI] [PubMed] [Google Scholar]
- 19.Doetschman T. Gene Targeting in Embryonic Stem Cells: I. History and Methodology. In: Pinkert CA, editor. Transgenic Animal Technology: A Laboratory Handbook. Second Edition. Academic Press; San Diego: 2002. [Google Scholar]
- 20.Ebertz SL, McGann LE. Cryoprotectant permeability parameters for cells used in a bioengineered human corneal equivalent and applications for cryopreservation. Cryobiology. 2004;49:169–180. doi: 10.1016/j.cryobiol.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Fedorow C, McGann LE, Korbutt GS, Rayat GR, Rajotte RV, Lakey JR. Osmotic and cryoprotectant permeation characteristics of islet cells isolated from the newborn pig pancreas. Cell Transplant. 2001;10:651–659. [PubMed] [Google Scholar]
- 22.Fujioka T, Yasuchika K, Nakamura Y, Nakatsuji N, Suemori H. A simple and efficient cryopreservation method for primate embryonic stem cells. Int J Dev Biol. 2004;48:1149–1154. doi: 10.1387/ijdb.041852tf. [DOI] [PubMed] [Google Scholar]
- 23.Gao DY, Ashworth E, Watson PF, Kleinhans FW, Mazur P, Critser JK. Hyperosmotic tolerance of human spermatozoa: separate effects of glycerol, sodium chloride, and sucrose on spermolysis. Biol Reprod. 1993;49:112–23. doi: 10.1095/biolreprod49.1.112. [DOI] [PubMed] [Google Scholar]
- 24.Gao DY, Liu J, Liu C, McGann LE, Watson PF, Kleinhans FS, Mazur P, Critser ES, Critser JK. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum Reprod. 1995;10:1109–1122. doi: 10.1093/oxfordjournals.humrep.a136103. [DOI] [PubMed] [Google Scholar]
- 25.Gao DY, Mazur P, Critser JK. Fundamental Cryobiology of Mammalian Sperm. In: Karow AM, Critser JK, editors. Reproductive Tissue Banking: Scientific Principles. Academic Press; San Diego: 1997. pp. 263–328. [Google Scholar]
- 26.Gilmore JA, Du J, Tao J, Peter AT, Critser JK. Osmotic properties of boar spermatozoa and their relevance to cryopreservation. J Reprod Fertil. 1996;107:87–95. doi: 10.1530/jrf.0.1070087. [DOI] [PubMed] [Google Scholar]
- 27.Gilmore JA, Liu J, Gao DY, Critser JK. Determination of optimal cryoprotectants and procedures for their addition and removal from human spermatozoa. Hum Reprod. 1997;12:112–118. doi: 10.1093/humrep/12.1.112. [DOI] [PubMed] [Google Scholar]
- 28.Gordeeva OF, Manuilova ES, Grivennikov IA, Smirnova IuA, Krasnikova NIu, Zinov’eva RD, Khrushchov NG. Expression of regulatory genes Oct-4, Pax-6, Prox-1, Ptx-2 at the initial stages of differentiation of embryonic stem cells in vitro. Ontogenez. 2003;34:174–182. [PubMed] [Google Scholar]
- 29.Ha SY, Jee BC, Suh CS, Kim HS, Oh SK, Kim SH, Moon SY. Cryopreservation of human embryonic stem cells without the use of a programmable freezer. Hum Reprod. 2005;20:1779–1785. doi: 10.1093/humrep/deh854. [DOI] [PubMed] [Google Scholar]
- 30.Heng BC, Kuleshova LL, Bested SM, Liu H, Cao T. The cryopreservation of human embryonic stem cells. Biotechnol Appl Biochem. 2005;41:97–104. doi: 10.1042/BA20040161. [DOI] [PubMed] [Google Scholar]
- 31.Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Cao T. Kinetics of cell death of frozen-thawed human embryonic stem cell colonies is reversibly slowed down by exposure to low temperature. Zygote. 2006;14:341–348. doi: 10.1017/S0967199406003893. [DOI] [PubMed] [Google Scholar]
- 32.Heng BC, Ye CP, Liu H, Toh WS, Rufaihah AJ, Yang Z, Bay BH, Ouyang HW, Lee EH, Cao T. Loss of viability during freeze-thaw of intact and adherent human embryonic stem cells with conventional slow-cooling protocols is predominantly due to apoptosis rather than cellular necrosis. J Biomed Sci. 2006;13:433–445. doi: 10.1007/s11373-005-9051-9. [DOI] [PubMed] [Google Scholar]
- 33.Introduction to Human Embryonic Stem Cell Culture Methods. 2003 cited; Available from: http://www.wicell.org/forresearchers/index.jsp?catid=12&subcatid=20.
- 34.Ji L, de Pablo JJ, Palecek SP. Cryopreservation of adherent human embryonic stem cells. Biotechnol Bioeng. 2004;88:299–312. doi: 10.1002/bit.20243. [DOI] [PubMed] [Google Scholar]
- 35.Kashuba Benson CM, Benson JD, Critser JK. An improved cryopreservation method for a mouse embryonic stem cell line. Cryobiology. 2008;56:120–130. doi: 10.1016/j.cryobiol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katkov II. A two-parameter model of cell membrane permeability for multisolute systems. Cryobiology. 2000;40:64–83. doi: 10.1006/cryo.1999.2226. [DOI] [PubMed] [Google Scholar]
- 37.Katkov II. Challenge from the simple: some caveats in linearization of the Boyle-van’t Hoff and Arrhenius plots. Cryobiology. 2008;57:142–149. doi: 10.1016/j.cryobiol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochim Biophys Acta. 1958;1000:413–430. [PubMed] [Google Scholar]
- 39.Kim SJ, Park JH, Lee JE, Kim JM, Lee JB, Moon SY, Roh SI, Kim CG, Yoon H. Effects of type IV collagen and laminin on the cryopreservation of human embryonic stem cells. Stem Cells. 2004;22:950–61. doi: 10.1634/stemcells.22-6-950. [DOI] [PubMed] [Google Scholar]
- 40.Li T, Mai Q, Gao J, Zhou C. Cryopreservation of human embryonic stem cells with a new bulk vitrification method. Biol Reprod. 2010;82:848–53. doi: 10.1095/biolreprod.109.080713. [DOI] [PubMed] [Google Scholar]
- 41.Lovelock J. The haemolysis of human red blood-cells by freezing and thawing. Biochim Biophys Acta. 1953;10:414–26. doi: 10.1016/0006-3002(53)90273-x. [DOI] [PubMed] [Google Scholar]
- 42.Mazur P. Kinetics Of Water Loss From Cells At Subzero Temperatures And The Likelihood Of Intracellular Freezing. J Gen Physiol. 1963;47:347–69. doi: 10.1085/jgp.47.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazur P, Leibo SP, Chu EH. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res. 1972;71:345–355. doi: 10.1016/0014-4827(72)90303-5. [DOI] [PubMed] [Google Scholar]
- 44.McGann LE, Turner AR, Turc JM. Microcomputer interface for rapid measurements of average volume using an electronic particle counter. Med Biol Eng Comput. 1982;20:117–120. doi: 10.1007/BF02441862. [DOI] [PubMed] [Google Scholar]
- 45.Miszta-Lane H, Gill P, Mirbolooki M, Lakey JRT. Effect of slow freezing versus vitrification on the recovery of mouse embryonic stem cells. Cell Pres Tech. 2007;5:16–24. [Google Scholar]
- 46.Nagy A, et al., editors. Preparation of ES Cells for Injection, in Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press: Cold Spring Harbor; New York: 2003. p. 469. [Google Scholar]
- 47.Nobel PS. The Boyle-Van’t Hoff relation. Journal of Theoretical Biology. 1969;23:375–379. doi: 10.1016/0022-5193(69)90025-3. [DOI] [PubMed] [Google Scholar]
- 48.Phelps MJ, Benson JD, Liu J, Willoughby CE, Gilmore JA, Critser JK. Effects of Percoll separation, cryoprotective agents, and temperature on plasma membrane permeability characteristics of murine spermatozoa and their relevance to cryopreservation. Biol Reprod. 1999;61:1031–1041. doi: 10.1095/biolreprod61.4.1031. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez-Solis R, Ryder E, Houghton R, White JK, Bottomley J. Large-scale mouse knockouts and phenotypes. Wiley Interdiscip Rev Syst Biol Med. 2012;4:547–563. doi: 10.1002/wsbm.1183. [DOI] [PubMed] [Google Scholar]
- 50.Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Hum Reprod. 2001;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- 51.Richards M, Fong CY, Tan S, Chan WK, Bongso A. An efficient and safe xeno-free cryopreservation method for the storage of human embryonic stem cells. Stem Cells. 2004;22:779–789. doi: 10.1634/stemcells.22-5-779. [DOI] [PubMed] [Google Scholar]
- 52.Schenke-Layland K, Angelis E, Rhodes KE, Heydarkhan-Hagvall S, Mikkola HK, Maclellan WR. Collagen IV induces trophoectoderm differentiation of mouse embryonic stem cells. Stem Cells. 2007;25:1529–1538. doi: 10.1634/stemcells.2006-0729. [DOI] [PubMed] [Google Scholar]
- 53.Schofield PN, Hoehndorf R, Gkoutos GV. Mouse genetic and phenotypic resources for human genetics. Hum Mutat. 2012;33:826–836. doi: 10.1002/humu.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Udy GB, Evans MJ. Microplate DNA preparation, PCR screening and cell freezing for gene targeting in embryonic stem cells. Biotechniques. 1994;17:887–94. [PubMed] [Google Scholar]
- 55.Ure JM, Fiering S, Smith AG. A rapid and efficient method for freezing and recovering clones of embryonic stem cells. Trends Genet. 1992;8:6. doi: 10.1016/0168-9525(92)90004-n. [DOI] [PubMed] [Google Scholar]
- 56.Ware CB, Nelson AM, Blau CA. Controlled-rate freezing of human ES cells. Biotechniques. 2005;38:879–883. doi: 10.2144/05386ST01. [DOI] [PubMed] [Google Scholar]
- 57.Weinstein AM, Stephenson JL. Electrolyte transport across a simple epithelium. steady-state and transient analysis. Biophys J. 1979;27:165–86. doi: 10.1016/S0006-3495(79)85209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology. 2004;48:146–156. doi: 10.1016/j.cryobiol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Woods EJ, Liu J, Derrow CW, Smith FO, Williams DA, Critser JK. Osmometric and permeability characteristics of human placental/umbilical cord blood CD34+ cells and their application to cryopreservation. J Hematother Stem Cell Res. 2000;9:161–173. doi: 10.1089/152581600319379. [DOI] [PubMed] [Google Scholar]
- 60.Woods EJ, Zieger MA, Lakey JR, Liu J, Critser JK. Osmotic characteristics of isolated human and canine pancreatic islets. Cryobiology. 1997;35:106–13. doi: 10.1006/cryo.1997.2029. [DOI] [PubMed] [Google Scholar]
- 61.Wu CF, Tsung HC, Zhang WJ, Wang Y, Lu JH, Tang ZY, Kuang YP, Jin W, Cui L, Liu W, Cao YL. Improved cryopreservation of human embryonic stem cells with trehalose. Reprod Biomed Online. 2005;11:733–739. doi: 10.1016/s1472-6483(10)61692-6. [DOI] [PubMed] [Google Scholar]
- 62.Zieger MA, Woods EJ, Lakey JR, Liu J, Critser JK. Osmotic tolerance limits of canine pancreatic islets. Cell Transplant. 1999;8:277–284. doi: 10.1177/096368979900800308. [DOI] [PubMed] [Google Scholar]


