Abstract
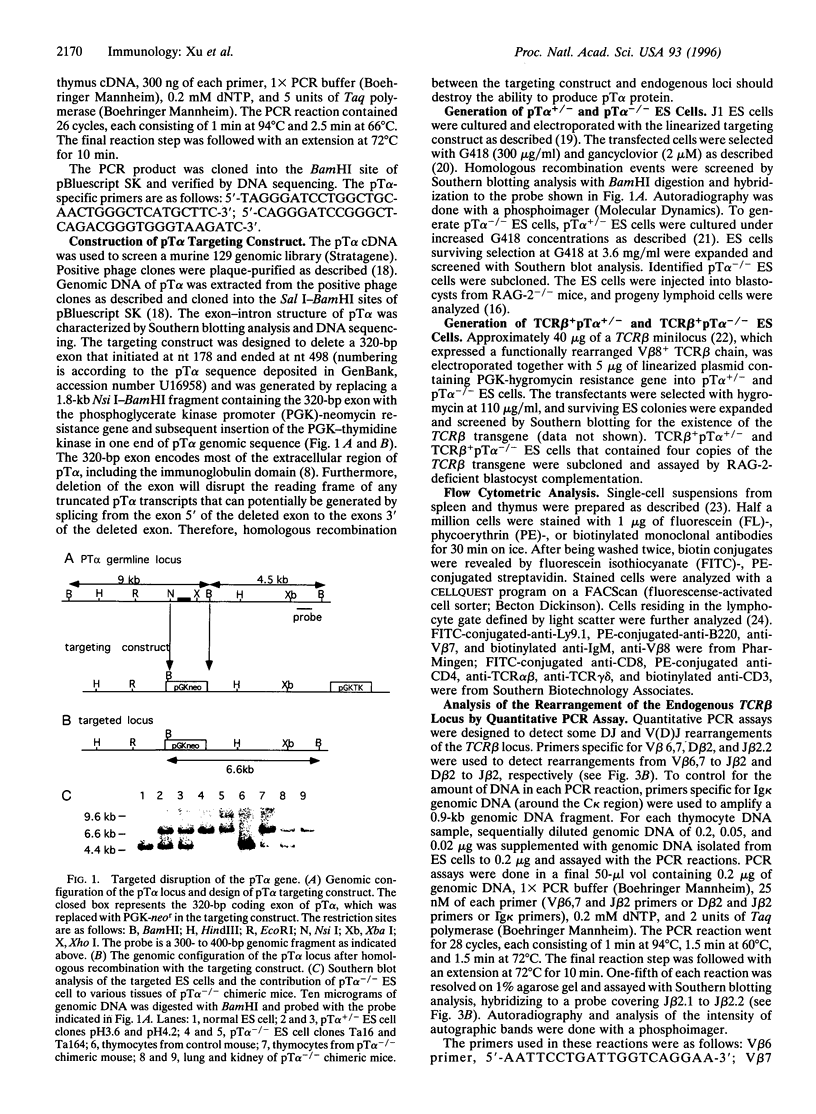
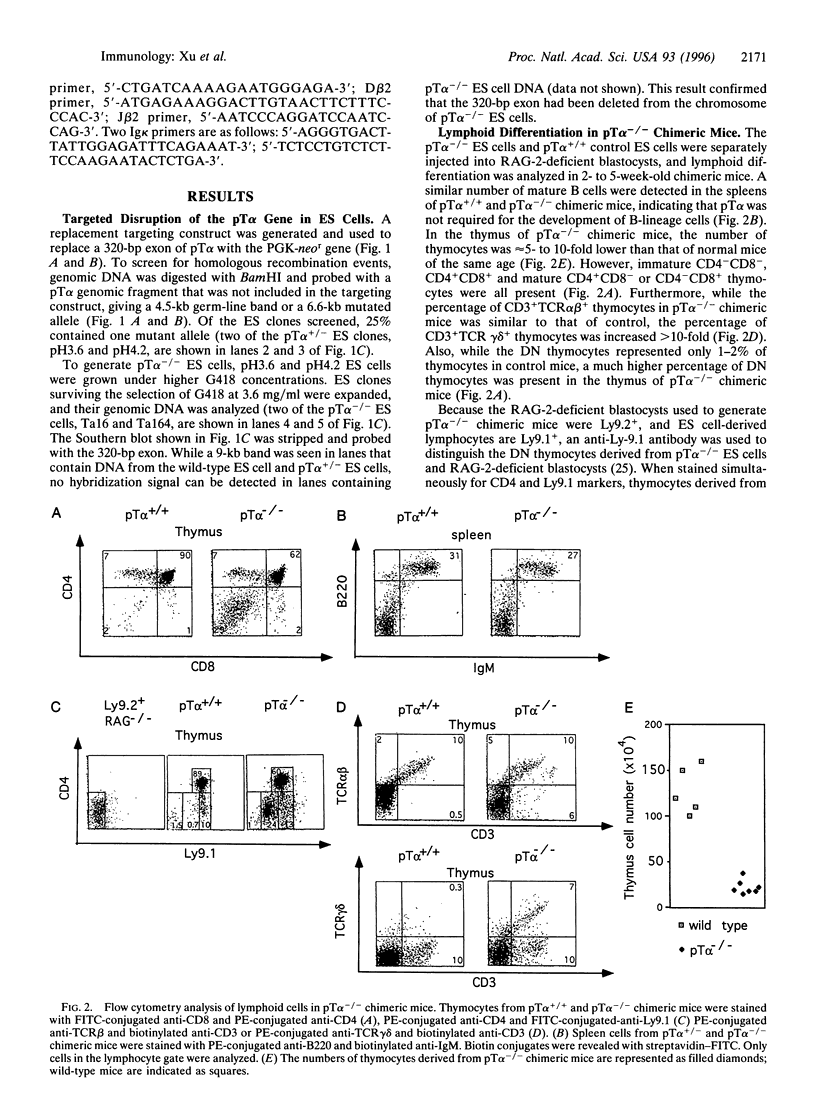
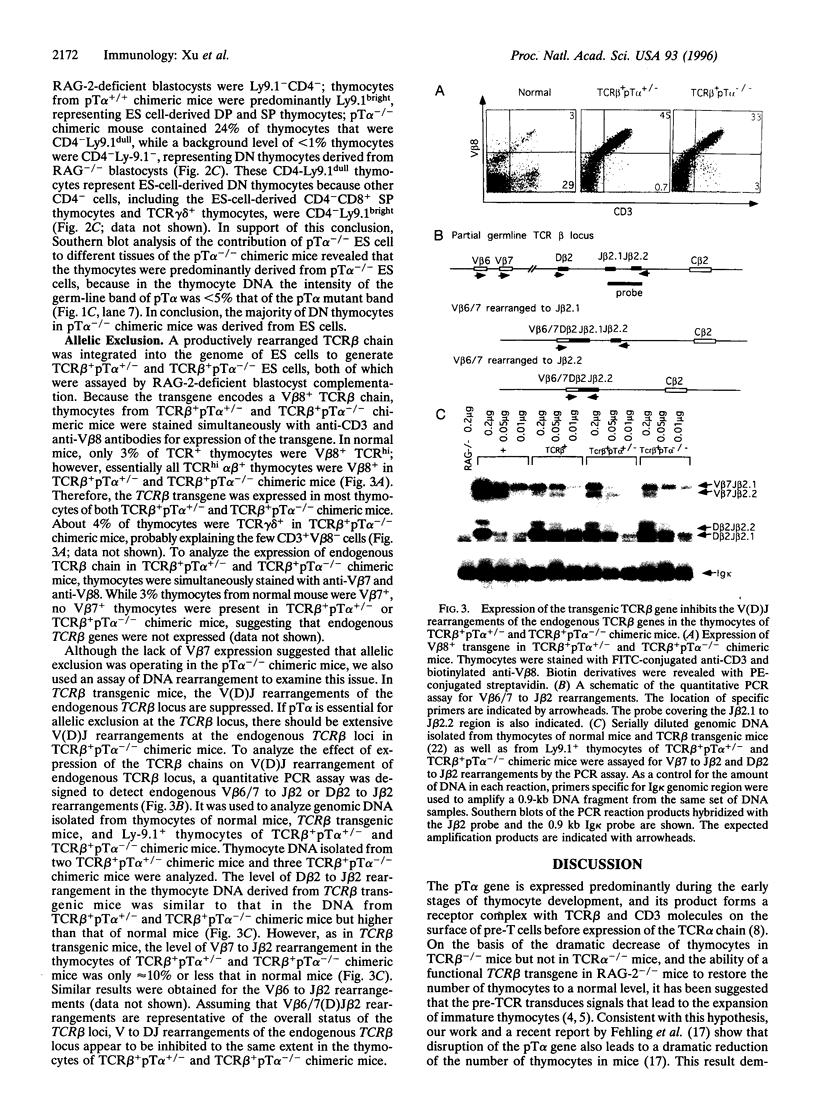
The pre-T-cell receptor, composed of the T-cell receptor (TCR) beta chain (TCRbeta), pre-Talpha (pTalpha) chain, and CD3 molecules, has been postulated to be a transducer of signals during the early stages of T-cell development. To examine the function of the transmembrane pTalpha chain during tbymocyte development, we generated pTalpha-/- embryonic stem cells and assayed their ability to differentiate into lymphoid cells in vivo after injection into recombination-activating gene (RAG)-2-deficient blastocysts. Thymocytes representing all stages of T-cell differentiation were detected in the thymus of pTalpha-/- chimeric mice, indicating that thymocyte development can occur without pTalpha. However, greatly reduced thymocyte numbers and substantially increased percentages of both CD4-CD8- thymocytes and TCRgammadelta+ thymocytes suggest that pTalpha plays a critical role in thymocyte expansion. To investigate the role of the pTalpha chain in allelic exclusion at the TCRbeta locus, a functionally rearranged TCRbeta minigene was introduced into pTalpha-/- and pTalpha+/- embryonic stem cells, which were subsequently assayed by RAG-2-deficient blastocyst complementation. In the absence of pTalpha, expression of the transgenic TCRbeta inhibited rearrangement of the endogenous TCRbeta locus to an extent similar to that seen in normal TCRbeta transgenic mice, suggesting that pTalpha may not be required for signaling allelic exclusion at the TCRbeta locus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen J., Lansford R., Stewart V., Young F., Alt F. W. RAG-2-deficient blastocyst complementation: an assay of gene function in lymphocyte development. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4528–4532. doi: 10.1073/pnas.90.10.4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley E. C., Petrie H. T., Shah L. M., Owen M. J., Hayday A. C. T cell receptor beta chain gene rearrangement and selection during thymocyte development in adult mice. Immunity. 1994 May;1(2):83–93. doi: 10.1016/1074-7613(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Fehling H. J., Krotkova A., Saint-Ruf C., von Boehmer H. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature. 1995 Jun 29;375(6534):795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- Förster I., Vieira P., Rajewsky K. Flow cytometric analysis of cell proliferation dynamics in the B cell compartment of the mouse. Int Immunol. 1989;1(4):321–331. doi: 10.1093/intimm/1.4.321. [DOI] [PubMed] [Google Scholar]
- Groettrup M., Baron A., Griffiths G., Palacios R., von Boehmer H. T cell receptor (TCR) beta chain homodimers on the surface of immature but not mature alpha, gamma, delta chain deficient T cell lines. EMBO J. 1992 Jul;11(7):2735–2745. doi: 10.1002/j.1460-2075.1992.tb05339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Rolink A., Shinkai Y., Young F., Alt F. W., Melchers F. The expression of Vpre-B/lambda 5 surrogate light chain in early bone marrow precursor B cells of normal and B cell-deficient mutant mice. Cell. 1994 Apr 8;77(1):133–143. doi: 10.1016/0092-8674(94)90241-0. [DOI] [PubMed] [Google Scholar]
- Kishi H., Borgulya P., Scott B., Karjalainen K., Traunecker A., Kaufman J., von Boehmer H. Surface expression of the beta T cell receptor (TCR) chain in the absence of other TCR or CD3 proteins on immature T cells. EMBO J. 1991 Jan;10(1):93–100. doi: 10.1002/j.1460-2075.1991.tb07924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Kudo A., Schaal S., Müller W., Melchers F., Rajewsky K. A critical role of lambda 5 protein in B cell development. Cell. 1992 May 29;69(5):823–831. doi: 10.1016/0092-8674(92)90293-l. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Rajewsky K. Targeted disruption of mu chain membrane exon causes loss of heavy-chain allelic exclusion. Nature. 1992 Mar 12;356(6365):154–156. doi: 10.1038/356154a0. [DOI] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Lin W. C., Desiderio S. Regulation of V(D)J recombination activator protein RAG-2 by phosphorylation. Science. 1993 May 14;260(5110):953–959. doi: 10.1126/science.8493533. [DOI] [PubMed] [Google Scholar]
- Malissen M., Gillet A., Ardouin L., Bouvier G., Trucy J., Ferrier P., Vivier E., Malissen B. Altered T cell development in mice with a targeted mutation of the CD3-epsilon gene. EMBO J. 1995 Oct 2;14(19):4641–4653. doi: 10.1002/j.1460-2075.1995.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Clarke A. R., Rudnicki M. A., Iacomini J., Itohara S., Lafaille J. J., Wang L., Ichikawa Y., Jaenisch R., Hooper M. L. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992 Nov 19;360(6401):225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- Mortensen R. M., Conner D. A., Chao S., Geisterfer-Lowrance A. A., Seidman J. G. Production of homozygous mutant ES cells with a single targeting construct. Mol Cell Biol. 1992 May;12(5):2391–2395. doi: 10.1128/mcb.12.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. M., Heimberger A. B., Loh D. Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990 Dec 21;250(4988):1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Saint-Ruf C., Ungewiss K., Groettrup M., Bruno L., Fehling H. J., von Boehmer H. Analysis and expression of a cloned pre-T cell receptor gene. Science. 1994 Nov 18;266(5188):1208–1212. doi: 10.1126/science.7973703. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Koyasu S., Nakayama K., Murphy K. M., Loh D. Y., Reinherz E. L., Alt F. W. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993 Feb 5;259(5096):822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Ma A., Cheng H. L., Alt F. W. CD3 epsilon and CD3 zeta cytoplasmic domains can independently generate signals for T cell development and function. Immunity. 1995 Apr;2(4):401–411. doi: 10.1016/1074-7613(95)90148-5. [DOI] [PubMed] [Google Scholar]
- Shortman K. Cellular aspects of early T-cell development. Curr Opin Immunol. 1992 Apr;4(2):140–146. doi: 10.1016/0952-7915(92)90003-w. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Dembić Z., Steinmetz M., von Boehmer H. Expression of T-cell antigen receptor genes during fetal development in the thymus. Nature. 1985 May 16;315(6016):232–233. doi: 10.1038/315232a0. [DOI] [PubMed] [Google Scholar]
- Storb U., Pinkert C., Arp B., Engler P., Gollahon K., Manz J., Brady W., Brinster R. L. Transgenic mice with mu and kappa genes encoding antiphosphorylcholine antibodies. J Exp Med. 1986 Aug 1;164(2):627–641. doi: 10.1084/jem.164.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembić Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988 Mar 25;52(6):831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- Zhang R., Alt F. W., Davidson L., Orkin S. H., Swat W. Defective signalling through the T- and B-cell antigen receptors in lymphoid cells lacking the vav proto-oncogene. Nature. 1995 Mar 30;374(6521):470–473. doi: 10.1038/374470a0. [DOI] [PubMed] [Google Scholar]





