Abstract
Objectives
Markers of prenatal hormone exposure have been associated with the development of eating disorder (ED) behaviors. Our aim was to determine whether 2D:4D ratio, a marker for in utero testosterone exposure, is associated with risk for ED in a large population-based cohort: the Avon Longitudinal Study of Parents and Children (ALSPAC).
Methods
This is the first study to investigate prenatal testosterone exposure in children at high-risk for ED, using 2D:4D as a marker. We compared children whose mothers reported a lifetime ED (anorexia, bulimia, or both; N = 446) to children whose mothers did not (n = 5,367).
Results
Daughters of women with lifetime bulimia nervosa (BN) had lower 2D:4D ratio (B: −0.01, 95% CI: −0.02 to −0.002, P = 0.02), indicating higher prenatal testosterone exposure, than daughters of mothers unaffected by ED. No differences were observed in the male children of women with an ED.
Conclusions
Findings suggest that children at high-risk for BN may be exposed to higher levels of testosterone in utero. Fetal exposure to androgen excess is thought to be causal in the development of polycystic ovary syndrome (PCOS), a disorder which is highly comorbid with binge eating and BN. Future research should investigate the potential role of testosterone exposure in utero as a risk factor for BN and binge eating. Am. J. Hum. Biol. 26:176–182, 2014. © 2013 Wiley Periodicals, Inc.
Eating disorders (ED) have a complex etiology that is poorly understood making intervention and treatment difficult. One striking feature of ED is the increased prevalence in females compared to males (American Psychiatric Association, 2013). Sociocultural influences have frequently been given as reasons for this gender difference (Field et al., 2001; Miller and Pumariega, 2001; Rolls 1991); however, evidence increasingly implicates biological causes such as differences in prenatal hormone exposure (Culbert et al., 2008,2009; Klump et al., 2001). Fetal exposure to testosterone has been shown to have a permanent effect on the development of sex-typed behaviors through the organization of sexually dimorphic neural circuits (Cohen-Bendahan et al., 2005; Lombardo et al., 2012b), and recent research has provided preliminary evidence that this hormonal effect on fetal development may also play a role in the development of ED behaviors (Culbert et al., 2008,2013; Klump et al., 2006; Quinton et al., 2011; Smith et al., 2010). Studies investigating this hypothesis have used two methods to indirectly measure prenatal testosterone exposure. The first method is based on evidence that females from opposite-sex twin pairs are exposed to higher levels of testosterone prenatally (produced by their male co-twins), than females from same-sex twin pairs. The prevalence of disordered traits is therefore compared across same-sex and opposite-sex twin pairs. Using this method within a general population sample, Culbert et al. (2008) found that same-sex female twins (assumed to be exposed to comparatively lower levels of prenatal testosterone), exhibited higher levels of disordered eating than opposite-sex female twins (assumed to be exposed to comparatively higher levels of prenatal testosterone) (Culbert et al., 2008). This finding implicates high prenatal testosterone exposure as a protective factor against the development of ED behaviors. However, despite other research groups attempting to replicate this finding using the same approach in much larger samples (Lydecker et al., 2012; Raevuori et al., 2008), it has only been replicated once by the same group of researchers (Culbert et al., 2013).
The second method of indirectly measuring prenatal testosterone exposure involves calculating the ratio of the second and fourth digits of the hand (2D:4D). 2D:4D has been found to correlate negatively with fetal testosterone/fetal estradiol ratio, (i.e. low 2D:4D is associated with high levels of fetal testosterone relative to fetal estradiol, and high 2D:4D is associated with low levels of fetal testosterone compared to estradiol) (Lutchmaya et al., 2004). Using this method, Klump et al. (2006) found that having a comparatively high 2D:4D ratio, indicating low prenatal testosterone exposure, was associated with increased ED behaviors in a community sample (Klump et al., 2006). A similar effect was observed in a community sample of males by Smith and Colleagues, who found that low 2D:4D (indicating high exposure to prenatal testosterone) was associated with less disordered eating and an increased drive for muscularity (Smith et al., 2010). Only one study to date has investigated the relationship between 2D:4D and ED diagnosis in a clinical sample of female participants, rather than investigating ED behaviors and cognitions in a community sample. In this study high 2D:4D was found to be associated with a diagnosis of bulimia nervosa (BN), while low 2D:4D was found to be associated with anorexia nervosa (AN). Healthy controls were found to lie in-between the ED groups (Quinton et al., 2011).
As is clear from the summary above, the relationship between indirect measures of prenatal testosterone exposure and ED is not a straightforward one. This may be due to differences in samples (i.e. clinical versus community), or perhaps differences resulting from whether ED behaviors or ED diagnoses are being investigated. Findings may also be limited by methodological differences in the measurement of prenatal testosterone, and the unreliability of proxy measurements. For example, though transfer of testosterone from male to female fetuses by means of amniotic fluid perfusion has been demonstrated in rats (Even et al., 1992; Ryan and Vandenbergh, 2002), there is no direct evidence of this occurring in humans (McIntyre 2006). In addition, many studies fail to find an increase of masculinized traits in females with a male co-twin (Tapp et al., 2011). However, direct measurement of prenatal testosterone exposure involves a risky and invasive procedure which is unethical to carry out without clinical motivations, making the use of indirect measurement necessary. Despite methodological differences and discrepant findings, overall evidence appears to suggest that high prenatal testosterone exposure (through indirect measurement) may be protective against the development of ED behaviors in later life, in community samples.
In this study we investigate prenatal testosterone exposure as a potential mechanism for the intergenerational transmission of risk for ED. The first-degree relatives of ED probands have been found to be at higher risk for developing ED in comparison to healthy and psychiatric controls (Ben-Dor et al., 2002; Holland et al., 1988; Strober et al., 2000), and heritability estimates of ED range between 50 and 80% (Bulik et al., 2006; Klump et al., 2009; Lear et al., 2007; Mazzeo et al., 2009). To understand the etiology of ED and develop effective intervention strategies, it is important to determine mechanisms by which this vulnerability or “risk” is inherited. Prenatal and in utero factors have previously been suggested as a possible mechanism for intergenerational risk transmission (Micali and Treasure, 2009), and evidence also suggests that prenatal testosterone exposure is genetically influenced. Twin studies report heritability of prenatal testosterone exposure, as measured by 2D:4D, to be approximately 66% in females (Gobrogge, 2008; Paul et al., 2006). In addition, fetal androgen receptor sensitivity has been found to be genetically determined via the X linked androgen receptor gene (AR), in which low CAG repeat length is associated with high androgen receptor sensitivity (Chamberlain et al., 1994). If there is indeed an association between low prenatal testosterone exposure and ED symptomatology, it is possible that prenatal exposure to testosterone may be an underlying trait or endophenotype for ED; meaning low exposure to testosterone prenatally should be observed at higher rates in the first-degree relatives of ED probands than in the general population. In other words, prenatal exposure to testosterone might be one of the in utero mechanisms to be relevant to the transmission of ED. One way to test this hypothesis is to investigate in utero testosterone exposure in children at high-risk for ED, due to being born to mothers with an ED. In the present study we used 2D:4D ratio as a proxy marker to investigate whether prenatal testosterone exposure differed in children at high-risk for ED, due to being born to women with an ED, in comparison to children not at high-risk. Given the higher prevalence of ED in females, we analyzed the 2D:4D ratio of girls and boys separately. Based on evidence to date, we predicted that maternal ED would be associated with children having comparatively higher 2D:4D ratio, indicating exposure to low levels of prenatal testosterone.
Methods
Sample
The Avon Longitudinal Study of Parents and Children (ALSPAC) is a population-based cohort of women who were enrolled during pregnancy (14,663), along with the child that they were pregnant with at the time. Following initial enrollment, data were obtained via postal questionnaire on 14,472 women. The children who were still alive at 1 year (n = 13,988) have been followed-up since, with data collected through questionnaires, as well as physical, behavioral, and psychological tests (Boyd et al., 2013; Fraser et al., 2012). Women were excluded from the current study if they had a multiple birth (199), or if they had not completed the questionnaire on psychological well being at 12 weeks gestation assessing exposure (2,019). Women who reported a history of other psychiatric disorders only (other than an ED) as part of their response to this questionnaire were also excluded from the current study (1,166) due to lack of information on the specific disorder making this a heterogeneous group. Women reporting other psychiatric disorders over the lifetime in addition to ED were included in the analysis. Children were eligible for this study if they had their 2D:4D measured at 11 years of age (7,108). Final sample size for analysis was dependent on data being available on both maternal lifetime ED and children's 2D:4D ratio, and consisted of 5,813 children (81.78% of those eligible); inclusive of 2,862 boys and 2,951 girls.
Exposure: Maternal lifetime ED
Mothers completed a questionnaire regarding their psychological well-being at 12 weeks gestation, which asked whether they had ever had AN and/or BN (i.e. an ED at any point up to pregnancy). From the 11,088 women who responded, 446 (4.02%) reported a history of lifetime ED: AN (n = 171, 1.54%), BN (n = 194, 1.75%), or both (n = 81, 0.73%). Self-report of ED has previously been shown to be reliable and has been validated using cognitive and behavioral characteristics in this sample (Micali et al., 2012b,2007). Evidence shows these groups to be distinct with regard to ED cognitions, BMI, and the frequency of compensatory behaviors; and the profile of women answering yes to both AN and BN (AN + BN) was found to resemble that of the binge/purge subtype of AN (AN-BP) (Micali et al., 2007). The use of this type of self-report as an indicator of ED diagnosis has been validated in a population sample based in the Netherlands, with self-reported lifetime AN showing a sensitivity of 100%, and a specificity of 96%, and self-reported lifetime BN showing a sensitivity of 94% and a specificity of 81% (Micali et al., 2012a). In addition, the prevalence of ED in this sample is consistent with lifetime prevalence of ED in the general population (Micali et al., 2012b). Evidence also suggests that self-report measures such as those used in this study are just as good a screener as more commonly used measures (Keski-Rahkonen et al., 2006).
Outcome: Children's 2D:4D ratio
At 11 years of age children had their hands photocopied, and the length of their second and forth digits were measured using digital calipers. 2D:4D ratio was calculated by dividing the length of the second digit by the length of the forth digit. A random sample of hands (left = 48 and right = 57) were measured in vivo to determine the validity of using photocopies to measure 2D:4D. Analysis showed that the correlation between measurements from photocopies and in vivo measurements was high for both hands (r > 0.97).
Data analysis
The 2D:4D ratio was found to be normally distributed. The association between mothers ED status and children's 2D:4D ratio was explored using linear regression. Maternal AN, maternal BN, and maternal AN and BN, were included as predictor variables and child 2D:4D ratio was included as the outcome variable. Initially a crude analysis was conducted (model 1), and in an adjusted model (model 2) a priori confounders (child gender and ethnicity), and additional potential confounders (parental social class and maternal age at delivery) were adjusted for by being included as additional predictors. A univariate ANOVA including gender as a moderator was run to directly test the interaction effect. Linear regression analyses were then run on males and females separately to investigate how the associations differed. All analyses were carried out on SPSS 21 and a two-tailed significance level of p < 0.05 was used. Due to the small number of outcomes (left and right 2D:4D ratio), the size of the sample, and the exploratory nature of the methodology, it was decided that the significance level would not be adjusted for multiple comparisons due to the likelihood of over-correction.
Attrition and missingness
Attrition (i.e. children who did not attend the measuring session), and missingness (children who did not have accurate 2D:4D measurements) was associated with socio-demographic factors. Participants had higher odds of being lost to attrition or missingness if children were male (OR: 1.18, 95% CI: 1.09–1.27, p = 0.0001), or of a lower social class (OR: 1.94, 95% CI: 1.75–2.16, p = 0.0001); and had lower odds if children were white (OR: 0.61, 95% CI:0.55–0.67, p = 0.0001) or mothers were older at delivery (OR: 0.92, 95% CI: 0.91–0.93, p = 0.0001). These variables were therefore included as confounders in the adjusted model.
Missing covariate data
Missing data on child ethnicity, social class, and maternal age at delivery was dealt with using multiple random imputation. All predictor and outcome variables were used as predictors in the imputation model, which was set for 10 imputations. Analyses were run on complete case and imputed datasets, and a comparison of results showed that differences were negligible. Only results based on imputed datasets are presented here as complete case analysis is thought to suffer from more chance variation, and multiple random imputation is assumed to correct any bias.
Procedure
The study was approved by the ALSPAC Law and Ethics Committee and Local Research Ethics Committees. Questionnaires and assessments were conducted after giving full information to participants and acquiring consent in accordance with the ALSPAC study design.
RESULTS
Sociodemographic characteristics of exposed and unexposed groups can be found in Table1. Female children had a mean 2D:4D of 0.97 (SD = 0.03) on both the left and right hands. As would be expected, male children had a slightly lower mean 2D:4D of 0.96 (SD = 0.03) on both hands, indicating higher prenatal testosterone exposure.
Table 1.
Comparison of sociodemographic factors across groups
| Unexposed | AN | BN | AN and BN | |
|---|---|---|---|---|
| Gender (male): n (%) | 2,750 (49.2) | 41 (53.2) | 49 (50) | 22 (45.8) |
| Ethnicity (white): n (%) | 5,217 (93.3) | 64 (83.1) | 89 (90.8) | 47 (97.9) |
| Social Class (nonmanual): n (%) | 4,549 (81.4) | 61 (79.2) | 78 (79.6) | 39 (81.3) |
| Age of Mother at Delivery: mean (SD)2 | 29.1 (4.5) | 31.4 (5.1) | 28.8 (4.4) | 30.4 (4.3) |
2D:4D ratio: All children
Crude regression analysis showed that the children of women with lifetime BN had a lower 2D:4D ratio on the right hand than the children of unexposed women (B: −0.01, 95% CI: −0.02 to −0.002, p = 0.02), indicative of higher prenatal testosterone exposure. This difference remained statistically significant in the adjusted model. No other statistically significant differences between exposed and unexposed groups were observed for either hand (see Table2). Results of the univariate ANOVA, run to test the interaction effect of gender, showed a significant interaction effect for both the right (F(0.17,22.78), p < 0.001) and the left (F(0.14,18.75), p < 0.001) hands.
Table 2.
Linear regression analysis of children's' 2D:4D ratio (boys and girls analyzed both together and separately): Comparison of exposed and unexposed groups
| Left hand |
Right hand |
||
|---|---|---|---|
| B (95% CI) | B (95% CI) | ||
| All children | |||
| Model 1 | AN | 0.000 (−0.007 to 0.007) | −0.004 (−0.012 to 0.003) |
| BN | −0.004 (−0.011 to 0.002) | −0.008 (−0.015 to −0.002)a | |
| AN + BN | 0.008 (−0.002 to 0.017) | 0.007 (−0.002 to 0.016) | |
| Model 2 | AN | 0.001 (−0.007 to 0.008) | −0.004 (−0.11 to 0.003) |
| BN | −0.004 (−0.011 to 0.002) | −0.008 (−0.014 to −0.002)a | |
| AN + BN | 0.007 (−0.002 to 0.017) | 0.006 (−0.003 to 0.015) | |
| Boys | |||
| Model 1 | AN | 0.003 (−0.007 to 0.013) | −0.003 (−0.013 to 0.007) |
| BN | −0.004 (−0.013 to 0.005) | −0.005 (−0.014 to 0.004) | |
| AN + BN | 0.003 (−0.011 to 0.016) | 0.006 (−0.007 to 0.019) | |
| Model 2 | AN | 0.003 (−0.007 to 0.013) | −0.003 (−0.013 to 0.006) |
| BN | −0.004 (−0.009 to 0.000) | −0.005 (−0.010 to 0.000) | |
| AN + BN | 0.003 (−0.004 to 0.010) | 0.006 (−0.007 to 0.019) | |
| Girls | |||
| Model 1 | AN | −0.002 (−0.013 to 0.009) | −0.005 (−0.015 to 0.006) |
| BN | −0.004 (−0.013 to 0.005) | −0.011 (−0.020 to −0.002)a | |
| AN + BN | 0.012 (−0.001 to 0.024) | 0.007 (−0.006 to 0.019) | |
| Model 2 | AN | −0.002 (−0.013 to 0.009) | −0.004 (−0.015 to 0.006) |
| BN | −0.004 (−0.009 to 0.000) | −0.011 (−0.020 to −0.002) | |
| AN + BN | 0.011 (−0.001 to 0.024) | 0.007 (−0.006 to 0.019) | |
<0.05.
Model 1, crude analysis. Model 2, adjusted for child gender, ethnicity, maternal age at delivery and social class.
2D:4D ratio: Boys
Analyses showed no differences in 2D:4D ratio between exposed and unexposed groups for either the left or the right hand (see Table2).
2D:4D ratio: Girls
The crude analysis showed that female children of women with lifetime BN had a lower 2D:4D ratio on the right hand than the children of unexposed women (B: −0.01, 95% CI: −0.02 to −0.002, p = 0.02), indicative of higher prenatal testosterone exposure. This difference remained statistically significant in the adjusted model. No other differences were observed for either hand between exposed and unexposed groups (see Table2).
DISCUSSION
Children of women with lifetime BN were found to have lower 2D:4D ratios on the right hand in comparison to unexposed children, suggestive of exposure to higher levels of testosterone in utero. Further analysis revealed that this effect was present in the female children of women with BN; whilst male children of women with BN showed no differences in comparison to boys whose mothers did not have an ED.
Previous studies focusing on the association between prenatal testosterone exposure and ED/ED behaviors have revealed mixed findings, in general suggesting that high prenatal testosterone is associated with low adult ED/ED behaviors. The majority of these studies focus not on ED diagnosis, but on the presence of subthreshold ED behaviors and cognitions in community samples. To our knowledge only one study has investigated the association between ED diagnosis and 2D:4D ratio, finding that lower mean 2D:4D was associated with AN, and higher mean 2D:4D was associated with BN, with controls lying in-between; however, this study is limited by its particularly small sample size (Quinton et al., 2011). We used a unique approach, examining 2D:4D ratio in children born to women with an ED, to investigate the association between prenatal exposure to testosterone and risk for ED. Our findings relating to children at high-risk contrast with previous research in that we found an association between high prenatal testosterone exposure and risk for ED; we explore possible explanations for this below.
Association between masculinizing hormone disorders and ED behaviors
It is known that circulating female and male hormones have an important effect on eating behaviors, for example estradiol inhibits food intake and decreases meal size, while testosterone stimulates food intake and increases the number of meals (Baker et al., 2012). Women with BN have been found to have higher levels of circulating testosterone in comparison to controls (Sundblad et al., 1994), with some evidence of testosterone antagonist flutamide causing a marked improvement in bulimic behaviors (Bergman and Eriksson, 1996). Moreover polycystic ovary syndrome (PCOS): a highly prevalent endocrine disorder characterized by hyperandrogenization and high testosterone (Goodarzi et al., 2011); has also been associated with altered eating behaviors and exposure to high levels of testosterone prenatally. Evidence from experimental animal studies and clinical observation has led to a developmental origin hypothesis for PCOS (Abbott et al., 2002) which suggests that fetal exposure to androgen excess, in part genetically determined, is a major contributor to its development (Goodarzi et al., 2011; Xita and Tsatsoulis, 2006). This intergenerational transmission is thought to occur via mechanisms influencing both androgen activity and increased androgen receptor sensitivity in utero (Abbott et al., 2002), influencing the differentiation of sexually dimorphic tissues during fetal development (Patterson et al., 1994). Women with PCOS have significantly higher androgen concentrations during pregnancy, and it is possible that this could increase androgen exposure in the fetus (Sir-Petermann et al., 2002; Xita and Tsatsoulis, 2006). This increase in fetal androgen activity may also be acting on fetal androgen receptors that are genetically determined to be more highly sensitive (Chamberlain et al., 1994; Urbanek et al., 1999). Genetic research has shown that 2D:4D ratio is not only associated with levels of prenatal testosterone, but also to fetal androgen receptor sensitivity (Manning et al., 2003). Androgen receptor sensitivity has been found to be genetically determined via the X linked androgen receptor gene (AR): in which low CAG repeat length is associated with high androgen receptor sensitivity (Chamberlain et al., 1994). As with the developmental origin hypothesis of PCOS, it is possible that variations in CAG repeat length of the AR gene and increased maternal androgen concentrations during pregnancy may contribute to risk for developing characteristics of bingeing-type ED (i.e. binge eating and weight gain) in the fetus through a process of prenatal androgenization during fetal brain organization. In addition, women with PCOS have increased prevalence of binge eating (Goodarzi et al., 2011), and there is evidence of an increased prevalence of PCOS in women with BN (Raphael et al., 1995), suggesting there may be some shared liability. The literature in this area is limited, but our findings suggest that more research could be fruitful. Our results also suggest that boys were exposed to similar levels of prenatal androgens irrespective of whether or not their mothers had ED. Future research could investigate whether this is because boys are exposed to high prenatal androgens as part of their normal development. Any additional contribution is proportionally much smaller which may mask the effect.
Evidence from animal studies
Animal studies allow for experimental manipulation of prenatal testosterone levels, and results show that increasing levels of prenatal testosterone in female rat fetuses leads to masculinized eating patterns in adulthood; including reduced meal number but increased meal size (Madrid et al., 1993; Wade, 1972), and increased weight (Demissie et al., 2008; Donohoe and Stevens, 1983). This effect is not observed when administering testosterone to male rodents prenatally (Wade, 1972), which is interesting as our findings only show significant associations in females when analyzing each gender individually. Our findings may represent a similar mechanism in that high prenatal testosterone may be associated with risk in human females for masculinized eating patterns, which at the extreme could present as binge eating behaviors. Previously, evidence from animal studies has been used to suggest that high prenatal testosterone, and consequently masculinized eating patterns, are protective against ED behaviors and cognitions; therefore, the higher exposure to testosterone in utero that males experience provides some explanation for the lower prevalence of ED in males. This is somewhat questionable as both our findings and the findings from animal studies suggest that differences in prenatal testosterone exposure do not have the same effect on males and females. In addition, evidence suggests that ED may present differently in males compared to females with the purpose of ED behaviors being to increase muscularity and decrease body fat (Weltzin et al., 2005), rather than to lose weight and become thin (Fernández-Aranda et al., 2009). Interestingly, the only study conducted with males using 2D:4D ratio indicated that high prenatal testosterone exposure was indeed associated with increased drive for muscularity, however potential gender differences in ED symptoms and psychopathology require further investigation before any conclusions can be reached regarding this.
High prenatal testosterone and traits associated with BN/binge eating disorder (BED)
Though research to date has not found a direct association between binge eating and high prenatal testosterone, several studies have found associations between prenatal testosterone and other traits associated with bingeing-type ED. High prenatal testosterone exposure (as measured by 2D:4D) has been associated with increased reward sensitivity (Lombardo et al., 2012a), increased impulsivity in women (but not men) (Lucas and Koff, 2010), high levels of aggression (Coyne et al., 2007), and poor social cognition (Williams et al., 2003); all traits that have previously been associated with bingeing-type ED (Cotrufo et al., 2000; Fischer et al., 2008; Harrison et al., 2010; Rothschild-Yakar et al., 2011). Poor social cognition is a particularly interesting in this context as it also a trait of autism spectrum disorder (ASD); another disorder that has been associated with high prenatal testosterone exposure when using 2D:4D as a proxy measure (Teatero and Netley, 2013). Given that research has found increasing evidence for an overlap of traits between ED and ASD (Baron-Cohen et al., 2013; Treasure, 2013; Zucker et al., 2007), it is possible that the low 2D:4D ratio observed in ED groups may be a marker of elevated autistic-like traits.
Strengths and limitations
Findings from this study should be considered by taking into account both strengths and limitations. Strengths of the study are the large sample size, the nature of the study (population-based), and the prospective data collection. Unfortunately a measure of maternal 2D:4D was unavailable, which meant that we were unable to directly compare the ratios of mothers and children. Due to the exploratory nature of the study, the minimal number of outcomes, and investigation of a specific outcome, significance levels were not adjusted for multiple comparisons to avoid over-correction; findings must be considered in light of this. A limitation of this research is that the use of 2D:4D ratio as a marker may not be as valid as direct assessment of prenatal androgen exposure (McIntyre, 2006); however, recent reviews suggest that 2D:4D ratio is in fact a useful and reliable proxy marker of prenatal testosterone exposure (Manning et al., 2002,2003; McIntyre, 2006), particularly for the right hand which has been shown to be a better indicator of prenatal androgenization (Hönekopp and Watson, 2010). There is also evidence indicating that measuring 2D:4D from photocopies (as has been done here) yields lower digit ratios than direct finger measurements (Manning et al., 2005), which suggests that direct measurement of finger length in this sample would have revealed bigger differences between children at high-risk and unexposed children. Measuring prenatal testosterone directly has associated economic costs and health risks, and therefore proxy measures such as 2D:4D are valuable. It is also worth noting that unlike the co-twin method, 2D:4D has been found to be a reliable marker of prenatal androgen receptor sensitivity, as well as prenatal testosterone activity, potentially implicating digit ratio as a more reliable marker of prenatal testosterone exposure (Manning et al., 2002,2003; McIntyre, 2006).
CONCLUSIONS
Children of women with lifetime BN were found to have lower 2D:4D ratio than unexposed children, indicating higher exposure to testosterone prenatally. Further research is clearly required, but our findings suggest that prenatal testosterone exposure may be one mechanism by which the intergenerational transmission of bingeing-type ED occurs (see Fig. 1). This is only a hypothesis, but is supported by evidence of high prenatal testosterone being causal to the development of PCOS (Goodarzi et al., 2011; Xita and Tsatsoulis, 2006) a disorder that shows high levels of bingeing and comorbidity with BN (Goodarzi et al., 2011; Raphael et al., 1995). Considering this, we feel that it is a hypothesis worthy of future exploration. Future research should aim to better understand biological mechanisms underlying the potential link between prenatal testosterone and risk for developing bulimic-type disorders.
Figure 1.
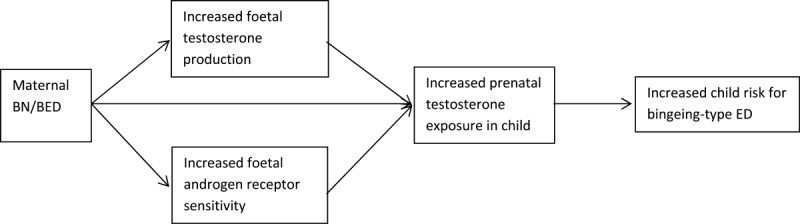
Risk model of prenatal testosterone exposure as mechanism for intergenerational transmission of bingeing.
Acknowledgments
The authors are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists, and nurses. This publication is the work of the authors and Radha Kothari, Joseph Gafton, Janet Treasure, and Nadia Micali will serve as guarantors for the contents of this article.
LITERATURE CITED
- Abbott D, Dumesic D, Franks S. Developmental origin of polycystic ovary syndrome-a hypothesis. J Endocrinol. 2002;174:1–5. doi: 10.1677/joe.0.1740001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. The diagnostic and statistical manual of mental disorders. Washington DC: American Psychiatric Association; 2013. [Google Scholar]
- Baker JH, Girdler SS, Bulik CM. The role of reproductive hormones in the development and maintenance of eating disorders. Expert Rev Obstet Gynecol. 2012;7:573–583. doi: 10.1586/eog.12.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Jaffa T, Davies S, Auyeung B, Allison C, Wheelwright S. Do girls with anorexia nervosa have elevated autistic traits? Mol Autism. 2013;4:24. doi: 10.1186/2040-2392-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dor DH, Laufer N, Apter A, Frisch A, Weizman A. Heritability, genetics and association findings in anorexia nervosa. Israel J Psychiatry Relat Sci. 2002;39:262–270. [PubMed] [Google Scholar]
- Bergman L, Eriksson E. Marked symptom reduction in two women with bulimia nervosa treated with the testosterone receptor antagonist flutamide. Acta Psychiatr Scand. 1996;94:137–139. doi: 10.1111/j.1600-0447.1996.tb09838.x. [DOI] [PubMed] [Google Scholar]
- Boyd A, Golding J, Macleod J, Lawlor DA, Fraser A, Henderson J, Molloy L, Ness A, Ring S, Davey Smith G. Cohort profile: the ‘children of the 90s’—the index offspring of the Avon longitudinal study of parents and children. Int J Epidemiol. 2013;42:111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- Chamberlain NL, Driver ED, Miesfeld RL. The length and location of CAG trinucleotide repeats in the androgen receptor N-terminal domain affect transactivation function. Nucleic Acids Res. 1994;22:3181–3186. doi: 10.1093/nar/22.15.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Bendahan CC, van de Beek C, Berenbaum SA. Prenatal sex hormone effects on child and adult sex-typed behavior: methods and findings. Neurosci Biobehav Rev. 2005;29:353–384. doi: 10.1016/j.neubiorev.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Cotrufo P, Monteleone P, d'Istria M, Fuschino A, Serino I, Maj M. Aggressive behavioral characteristics and endogenous hormones in women with bulimia nervosa. Neuropsychobiology. 2000;42:58–61. doi: 10.1159/000026673. [DOI] [PubMed] [Google Scholar]
- Coyne SM, Manning JT, Ringer L, Bailey L. Directional asymmetry (right–left differences) in digit ratio (2D:4D) predict indirect aggression in women. Pers Individ Differ. 2007;43:865–872. [Google Scholar]
- Culbert KM, Breedlove SM, Burt SA, Klump KL. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch Gen Psychiatry. 2008;65:329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbert KM, Breedlove SM, Sisk CL, Burt SA, Klump KL. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. J Abnorm Psychol. 2013;122:420–432. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demissie M, Lazic M, Foecking EM, Aird F, Dunaif A, Levine JE. Transient prenatal androgen exposure produces metabolic syndrome in adult female rats. Am J Physiol. 2008;295:E262–E268. doi: 10.1152/ajpendo.90208.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe TP, Stevens R. Effects of ovariectomy, estrogen treatment and CI-628 on food intake and body weight in female rats treated neonatally with gonadal hormones. Physiol Behav. 1983;31:325–329. doi: 10.1016/0031-9384(83)90196-8. [DOI] [PubMed] [Google Scholar]
- Even M, Dhar M, Vom Saal F. Transport of steroids between fetuses via amniotic fluid in relation to the intrauterine position phenomenon in rats. J Reprod Fertil. 1992;96:709–716. doi: 10.1530/jrf.0.0960709. [DOI] [PubMed] [Google Scholar]
- Fernández-Aranda F, Krug I, Jiménez-Murcia S, Granero R, Núñez A, Penelo E, Solano R, Treasure J. Male eating disorders and therapy: a controlled pilot study with one year follow-up. J Behav Ther Exp Psychiatry. 2009;40:479–486. doi: 10.1016/j.jbtep.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Field AE, Camargo CA, Taylor CB, Berkey CS, Roberts SB, Colditz GA. Peer, parent, and media influences on the development of weight concerns and frequent dieting among preadolescent and adolescent girls and boys. Pediatrics. 2001;107:54–60. doi: 10.1542/peds.107.1.54. [DOI] [PubMed] [Google Scholar]
- Fischer S, Smith GT, Cyders MA. Another look at impulsivity: a meta-analytic review comparing specific dispositions to rash action in their relationship to bulimic symptoms. Clin Psychol Rev. 2008;28:1413. doi: 10.1016/j.cpr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A, Macdonald-Wallis C, Tilling K, Boyd A, Golding J, Davey Smith G, Henderson J, Macleod J, Molloy L, Ness A, Ring S, Nelson SM, Lawlor DA. Cohort profile: the Avon longitudinal study of parents and children: ALSPAC mothers cohort. Int J Epidemiol. 2012;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge KL. Genetic and environmental influences on 2D: 4D finger length ratios: a study of monozygotic and dizygotic male and female twins. Arch Sex Behav. 2008;37:112. doi: 10.1007/s10508-007-9272-2. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- Harrison A, O'Brien N, Lopez C, Treasure J. Sensitivity to reward and punishment in eating disorders. Psychiatry Res. 2010;177:1–11. doi: 10.1016/j.psychres.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Holland AJ, Sicotte N, Treasure J. Anorexia nervosa: Evidence for a genetic basis. J Psychosom Res. 1988;32:561–571. doi: 10.1016/0022-3999(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Hönekopp J, Watson S. Meta-analysis of digit ratio 2D:4D shows greater sex difference in the right hand. Am J Human Biol. 2010;22:619–630. doi: 10.1002/ajhb.21054. [DOI] [PubMed] [Google Scholar]
- Keski-Rahkonen A, Sihvola E, Raevuori A, Kaukoranta J, Bulik CM, Hoek HW, Rissanen A, Kaprio J. Reliability of self-reported eating disorders: optimizing population screening. Int J Eat Disord. 2006;39:754–762. doi: 10.1002/eat.20277. [DOI] [PubMed] [Google Scholar]
- Klump KL, Gobrogge KL, Perkins PS, THORNE D, Sisk CL, Breedlove S. Preliminary evidence that gonadal hormones organize and activate disordered eating. Psychol Med. 2006;36:539–546. doi: 10.1017/S0033291705006653. [DOI] [PubMed] [Google Scholar]
- Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol Med. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Burt SA, McGue M, Iacono WG. Genetic and environmental influences on disordered eating: an adoption study. J Abnorm Psychol. 2009;118:797–805. doi: 10.1037/a0017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear S, Orit K, Apter A. Genetic influences in the development of eating disorders. In: Jaffa T, McDermott B, editors. Eating disorders in children and adolescents. New York: Cambridge University Press; 2007. pp. 70–81. [Google Scholar]
- Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Lai M-C, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. Fetal programming effects of testosterone on the reward system and behavioral approach tendencies in humans. Biol Psychiatry. 2012a;72:839–847. doi: 10.1016/j.biopsych.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 2012b;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Koff E. Delay discounting is associated with the 2D:4D ratio in women but not men. Pers Individ Differ. 2010;48:182–186. [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Lydecker JA, Pisetsky EM, Mitchell KS, Thornton LM, Kendler KS, Reichborn-Kjennerud T, Lichtenstein P, Bulik CM, Mazzeo SE. Association between co-twin sex and eating disorders in opposite sex twin pairs: evaluations in North American, Norwegian, and Swedish samples. J Psychosom Res. 2012;72:73–77. doi: 10.1016/j.jpsychores.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid J, Lopez-Bote C, Martín E. Effect of neonatal androgenization on the circadian rhythm of feeding behavior in rats. Physiol Behav. 1993;53:329–335. doi: 10.1016/0031-9384(93)90213-y. [DOI] [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Flanagan BF. The ratio of 2nd to 4th digit length: a proxy for transactivation activity of the androgen receptor gene? Med Hypotheses. 2002;59:334–336. doi: 10.1016/s0306-9877(02)00181-0. [DOI] [PubMed] [Google Scholar]
- Manning JT, Bundred PE, Newton DJ, Flanagan BF. The second to fourth digit ratio and variation in the androgen receptor gene. Evol Hum Behav. 2003;24:399–405. [Google Scholar]
- Manning JT, Fink B, Neave N, Caswell N. Photocopies yield lower digit ratios (2D:4D) than direct finger measurements. Arch Sex Behav. 2005;34:329–333. doi: 10.1007/s10508-005-3121-y. [DOI] [PubMed] [Google Scholar]
- Mazzeo SE, Mitchell KS, Bulik CM, Aggen SH, Kendler KS, Neale MC. A twin study of specific bulimia nervosa symptoms. Psychol Med. 2009;40:1203–1213. doi: 10.1017/S003329170999122X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre MH. The use of digit ratios as markers for perinatal androgen action. Reprod Biol Endocrinol. 2006;4:1–9. doi: 10.1186/1477-7827-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micali N, De Stavola B, dos-Santos-Silva I, Steenweg-de Graaff J, Jansen PW, Jaddoe VW, Hofman A, Verhulst FC, Steegers EA, Tiemeier H. Perinatal outcomes and gestational weight gain in women with eating disorders: a population-based cohort study. BJOG. 2012a;119:1493–1502. doi: 10.1111/j.1471-0528.2012.03467.x. [DOI] [PubMed] [Google Scholar]
- Micali N, Northstone K, Emmett P, Naumann U, Treasure JL. Nutritional intake and dietary patterns in pregnancy: a longitudinal study of women with lifetime eating disorders. Br J Nutr. 2012b;108:2093–2099. doi: 10.1017/S0007114512000256. [DOI] [PubMed] [Google Scholar]
- Micali N, Simonoff E, Treasure J. Risk of major adverse perinatal outcomes in women with eating disorders. Br J Psychiatry. 2007;190:255–259. doi: 10.1192/bjp.bp.106.020768. [DOI] [PubMed] [Google Scholar]
- Micali N, Treasure J. Biological effects of a maternal ED on pregnancy and foetal development: a review. Eur Eat Disord Rev. 2009;17:448–454. doi: 10.1002/erv.963. [DOI] [PubMed] [Google Scholar]
- Miller MN, Pumariega AJ. Culture and eating disorders: a historical and cross-cultural review. Psychiatry. 2001;64:93–110. doi: 10.1521/psyc.64.2.93.18621. [DOI] [PubMed] [Google Scholar]
- Patterson MN, McPhaul MJ, Hughes IA. 8 Androgen insensitivity syndrome. Baillières Clin Endocrinol Metab. 1994;8:379–404. doi: 10.1016/s0950-351x(05)80258-7. [DOI] [PubMed] [Google Scholar]
- Paul SN, Kato BS, Cherkas LF, Andrew T, Spector TD. Heritability of the second to fourth digit ratio (2d:4d): a twin study. Twin Res Hum Genet. 2006;9:215–219. doi: 10.1375/183242706776382491. [DOI] [PubMed] [Google Scholar]
- Quinton SJ, Smith AR, Joiner T. The 2nd to 4th digit ratio (2D: 4D) and eating disorder diagnosis in women. Pers Individ Differ. 2011;51:402–405. doi: 10.1016/j.paid.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raevuori A, Kaprio J, Hoek H, Sihvola E, Rissanen A, Keski-Rahkonen A. Anorexia and bulimia nervosa in same-sex and opposite-sex twins: lack of association with twin type in a nationwide study of Finnish twins. Am J Psychiatry. 2008;165:1604–1610. doi: 10.1176/appi.ajp.2008.08030362. [DOI] [PubMed] [Google Scholar]
- Raphael FJ, Rodin DA, Peattle A, Bano G, Kent A, Nussey SS, Lacey JH. Ovarian morphology and insulin sensitivity in women with bulimia nervosa. Clin Endocrinol. 1995;43:451–455. doi: 10.1111/j.1365-2265.1995.tb02617.x. [DOI] [PubMed] [Google Scholar]
- Rolls BJ. Gender differences in eating behavior and body weight regulation. Health Psychol. 1991;10:133. doi: 10.1037//0278-6133.10.2.133. [DOI] [PubMed] [Google Scholar]
- Rothschild-Yakar L, Eviatar Z, Shamia A, Gur E. Social cognition in eating disorders: encoding and representational processes in binging and purging patients. Eur Eat Disord Rev. 2011;19:75–84. doi: 10.1002/erv.1013. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Intrauterine position effects. Neurosci Biobehav Rev. 2002;26:665–678. doi: 10.1016/s0149-7634(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- Smith AR, Hawkeswood SE, Joiner TE. The measure of a man: associations between digit ratio and disordered eating in males. Int J Eat Disord. 2010;43:543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, Diamond J, Kaye W. Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. Am J Psychiatry. 2000;157:393–401. doi: 10.1176/appi.ajp.157.3.393. [DOI] [PubMed] [Google Scholar]
- Sundblad C, Bergman L, Eriksson E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr Scand. 1994;90:397–398. doi: 10.1111/j.1600-0447.1994.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Tapp AL, Maybery MT, Whitehouse AJ. Evaluating the twin testosterone transfer hypothesis: a review of the empirical evidence. Horm Behav. 2011;60:713–722. doi: 10.1016/j.yhbeh.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Teatero ML, Netley C. A critical review of the research on the extreme male brain theory and digit ratio (2D: 4D) J Autism Dev Disord. 2013:1–13. doi: 10.1007/s10803-013-1819-6. [DOI] [PubMed] [Google Scholar]
- Treasure J. Coherence and other autistic spectrum traits and eating disorders: building from mechanism to treatment. The Birgit Olsson lecture. Nordic J Psychiatry. 2013;67:38–42. doi: 10.3109/08039488.2012.674554. [DOI] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF, Spielman RS, Dunaif A. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc Natl Acad Sci USA. 1999;96:8573–8578. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Weltzin TE, Weisensel N, Franczyk D, Burnett K, Klitz C, Bean P. Eating disorders in men: update. J Mens Health Gender. 2005;2:186–193. [Google Scholar]
- Williams JHG, Greenhalgh KD, Manning JT. Second to fourth finger ratio and possible precursors of developmental psychopathology in preschool children. Early Hum Dev. 2003;72:57–65. doi: 10.1016/s0378-3782(03)00012-4. [DOI] [PubMed] [Google Scholar]
- Xita N, Tsatsoulis A. Fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. J Clin Endocrinol Metab. 2006;91:1660–1666. doi: 10.1210/jc.2005-2757. [DOI] [PubMed] [Google Scholar]
- Zucker NL, Losh M, Bulik CM, LaBar KS, Piven J, Pelphrey KA. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychol Bull. 2007;133:976–1006. doi: 10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]


