Abstract
Aims
Although most children with type 1 diabetes don’t achieve optimal glycemic control, no systematic method exists to identify and address self-management barriers. This study develops and validates PRISM (Problem Recognition in Illness Self-Management), a survey-based tool for efficiently identifying self-management barriers experienced by children/adolescents with diabetes and their parents.
Methods
Adolescents 13 years and older and parents of children 8 years and older visiting for routine diabetes management (n=425) were surveyed about self-management barriers. HbA1c was abstracted from the electronic health record. To develop PRISM, exploratory and confirmatory factor analyses were used. To assess validity, the association of PRISM scores with HbA1c was examined using linear regression.
Results
Factor analyses of adolescent and parent data yielded well-fitting models of self-management barriers, reflecting the following domains: 1) Understanding and Organizing Care, 2) Regimen Pain and Bother, 3) Denial of Disease and Consequences, and 4) Healthcare Team, 5) Family, or 6) Peer Interactions. All models exhibited good fit, with X2 ratios<2.21, root mean square errors of approximation<0.09, Confirmatory Fit Indices and Tucker-Lewis Indices both >0.92, and weighted root mean square residuals<1.71. Greater PRISM barrier scores were significantly associated with higher HbA1cs.
Conclusions
Our findings suggest at least six different domains exist within self-management barriers, nearly all of which are significantly related to HbA1c. PRISM could be used in clinical practice to identify each child and family’s unique self-management barriers, allowing existing self-management resources to be tailored to the family’s barriers, ultimately improving effectiveness of such services.
Keywords: Type 1 Diabetes Mellitus, Factor Analysis, Self-Care, Adherence, Patient-Centered Care
Introduction
The majority of children with type 1 diabetes are unable to adequately adhere to their self-management regimen [1, 2], resulting in about 20% of children with poor glycemic control [3] and over 50% with sub-optimal glycemic control [4]. Children unable to achieve glycemic control can face devastating complications, seriously affecting duration and quality of life, as well as family dynamics and finances [2, 5–7]. Further, sub-optimal glycemic control in childhood predicts sub-optimal control in adulthood [8]. A recent publication concluded, ‘The high percentage of US youth with HbA1c levels above the target value (…) indicates an urgent need for effective treatment strategies to improve metabolic status in youth with diabetes (p.668) [3].’
While efficacious strategies to improve glycemic control are available, no single strategy addresses self-management barriers adequately for every child or family. Many existing strategies to promote diabetes self-management, such as the ADA self-management curriculum or motivational interviewing, have small to moderate effects on adherence or HbA1c [9–12]. Larger effects are often achieved by multi-component interventions that incorporate both behavioral and educational strategies [9, 11, 13]. Yet these strategies can be resource intensive with their delivery averaging 9 sessions over 7 months [11]. For example, Behavioral Family Systems Therapy, a well-designed psychological intervention helping adolescents with diabetes work with their families to achieve better control, was delivered as 12 sessions over a 6 month period [14]. Only 27% of eligible families agreed to enroll in the study, despite $200 incentives to participate. Thus, with families for whom adherence is already problematic, completing lengthy, intensive interventions may not be feasible.
In keeping with recommendations for family-centered care [15], attention to the unique barriers experienced by the child/adolescent and their parents could improve effectiveness of strategies to improve self-management. Understanding and addressing self-management barriers for both parents and children/adolescents is essential, given the critical role families play in optimizing diabetes outcomes as well as the developmental changes occurring through childhood and adolescence[16]. Accepted conceptual frameworks, such as the Theory of Planned Behavior, suggest numerous barriers that children and families may face [17]. Adolescent perspectives on self-management barriers have been assessed for asthma and type 1 diabetes, with the latter focused specifically on psychosocial barriers such as stress and stigma [18, 19]. Scores on these surveys were associated with disease control, suggesting instrument validity. In addition, parent perspectives on diabetes self-management barriers suggest potential commonalities across barriers as experienced by adolescents and their parents [20].
Tailoring interventions to a family’s unique self-management barriers may be more effective and efficient for families and for healthcare providers, rather than offering single-component interventions that may not address a family’s own self-management barriers or multi-component interventions that are not convenient or feasible. Ultimately, a tool to identify self-management barriers could facilitate tailoring of available self-management resources to these identified barriers. To that end, this research develops and validates PRISM (Problem Recognition in Illness Self-Management), a survey-based tool to efficiently identify the self-management barriers experienced by children with diabetes and their parents. We use factor analyses to develop and validate the PRISM tool’s structure. To assess concurrent validity, we use linear regression to examine associations between PRISM scores and HbA1c.
Subjects and procedures
During the same 5-month period of two consecutive years, all parents accompanying children 8 years and older for routine diabetes management visits were asked to complete a survey including demographics, disease or regimen factors, and potential barriers to diabetes self-management. This 5-month period was implemented to ensure adequate power for the study. We wished to represent the barriers to self-management as experienced by three different types of participants (parents of adolescents (13–17 years), the adolescents, and parents of children (8–12 years)). Thus, based on standard approaches for estimating sample size requirements for factor analyses, we required about 100–150 subjects in each respondent group [21, 22]. The ~500 families of children with type 1 diabetes at our center are typically asked to visit every 3 months. By extending our recruitment period 2 months beyond the 3-month interval expected between visits, we were able to recruit families who may not rigorously attend quarterly visits while minimizing effort expended on re-contacting families who had already been approached. Adolescents at least 13 years of age also completed their own survey. The vast majority of families approached (93%) agreed to participate, resulting in a sample of 425 children/adolescents recruited from this large, mid-western children’s hospital’s pediatric diabetes clinic. We excluded non-English speaking families due to lack of valid survey measures in these populations.
Ethical considerations
The study received Institutional Review Board approval from the University of Wisconsin prior to undertaking data collection. As is standard practice, all potential subjects were initially approached by a member of their healthcare team, who ascertained their willingness to learn about the study. For those who were interested, research assistants trained in ethical treatment of human subjects and consenting practices in pediatrics then explained the study in lay language and provided written study materials. In keeping with institutional policies regarding consent, all parents and children ages 15–17 years gave informed consent, while children ages 8–14 years provided assent.
Materials and Methods
Development of diabetes self-management item pool
To ensure inclusion of items broadly representing theorized self-management barriers, we used a recommended approach of using concepts from more than one accepted conceptual model of health behavior [17, 23, 24]. Specifically, we retained the structure of the Theory of Planned Behavior (TPB) because of its success in explaining variability in adherence and its strong linkage between intention to adhere and actual adherence. The TBP includes measurable constructs such as subjective norms [25]. Subjective norms reflect the person’s determination of whether their interactions with important referent individuals (e.g., peers, families, or healthcare providers) support their diabetes self-management behaviors. To this structure, we added the concepts of perceived barriers and perceived benefits of self-management from the Health Belief Model [26, 27]. For example, perceived barriers are beliefs about the tangible and psychological costs of self-management (e.g., the belief that following the diabetes self-management regimen takes a lot of time and work).
Two authors (EDC and KAF) reviewed the literature on diabetes self-management barriers [18, 28–31] and an existing validated survey of adolescents’ asthma self-management barriers [18], to develop a comprehensive item pool representing known pediatric chronic disease self-management barriers. Based on review of the literature about known challenges to self-management in pediatric diabetes, items reflecting family interactions as an important barrier to self-management were drawn from validated surveys [32]. These items reflected concepts such as listening to the youth’s ideas about their diabetes, understanding how the child feels about his/her diabetes, or becoming angry when the child doesn’t take care of his/her diabetes. To capture varying experiences and perspectives across parents of adolescents, adolescents, and parents of children, we revised item wording to reflect each participant’s perspective. For example, the first person language appropriate for the adolescent’s perspective was adapted to “my child” in the parent surveys. We pilot tested the items with families of children with diabetes, gaining parent and adolescent feedback on clarity and completeness. Based on their feedback, definitions were provided for some terms (e.g., “regimen”) and items were iteratively revised, but no items were dropped or added. Responses on the 32 items were reported using a 5-point scale to quantify agreement with experiencing the barrier (1=strongly disagree; 5=strongly agree). Positively worded items were reverse-scored.
Other measures
In addition, surveys also assessed participant characteristics and disease or regimen factors that have known or hypothesized relationships to glycemic control or self-management barriers [7, 33–41]. Demographics included parent/child ages (continuous) and genders, race/ethnicity (white, non-Hispanic vs all other), and parent education (standard categories). Disease and regimen factors included years since diagnosis, child health status (excellent or good vs all others), insulin pump use (yes/no) and comorbid chronic illnesses (e.g., attention deficit/hyperactivity disorder, learning disorders, or developmental delay). The surveyed chronic conditions were selected based on their frequency in populations in general pediatrics or children with type 1 diabetes.
Glycemic control, measured by HbA1c, was abstracted from the medical record by one author (KAF) and two trained abstractors. Coinciding with each quarterly clinic visit, a blood sample for hemoglobin HbA1c is either assessed by point of care HbA1c or sent to clinical laboratories. Prior research suggests HbA1c values from these two methods are comparable [42]. For each participant, we used the HbA1c value obtained closest to the date of the survey completion. We excluded HbA1c values for 10% of respondents whose sample was obtained more than 2 weeks before or after the date of survey completion. The inter-abstractor reliability for HbA1c values was “near perfect” (intraclass correlation coefficient=0.90). The values were considered continuous for modeling purposes.
Analyses
We used means with standard deviations (sd) and proportions to describe participants. In developing PRISM, we implemented the recommended practice of confirming the PRISM structure across two independent datasets, using one dataset to develop the measure and another dataset to confirm the measure [43]. To achieve this, we separated our data into three samples (Figure 1) for use in our three analyses. To develop the PRISM barrier domains and their items, we used Year 2 responses from those participants who contributed data in both study years (n=248). To confirm the PRISM model structure on an independent sample, we used data from those who participated in only one of the two survey years (n=177, confirmatory sample). Lastly, to relate PRISM barrier scores to HbA1c, we used responses from all Year 1 participants (n=297) for whom the HbA1c available from medical record abstraction was within 2 weeks before or after the PRISM survey completion.
Figure 1.
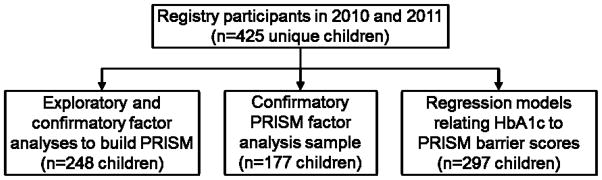
Origin of three analysis datasets
We used an iterative exploratory factor analysis (EFA) and confirmatory factor analysis (CFA) process to develop the PRISM structure [44]. Because we wished to represent the barriers to self-management as experienced by three different types of participants (parents of adolescents (13–17 years), the adolescents, and parents of children (8–12 years)), responses from these participant types were modeled separately. Eigenvalues were used to determine the number of self-management barrier domains to be considered. Items that cross-loaded in the EFA were forced to be orthogonal, based on conceptual frameworks and factor loadings. Model fit was assessed using standard cut points on recommended criteria (χ2/degrees of freedom (df), root mean square error of approximation (RMSEA), comparative fit index (CFI), Tucker-Lewis Index (TLI), and weighted root mean square residual (WRMR) [45]. Based on results of factor analyses, items were permitted to load on different factors for each of the participant types. For example, the item reflecting parent anger when the child makes a slip in self-management loaded on the “Denial of Disease and Consequences” domain for parents of adolescents and loaded on the “Family Interactions” domain for the adolescents.
Once well-fitting PRISM models for our three participant types were developed, CFA was performed to confirm PRISM model structures by constraining the measurement models to be similar across our two independent datasets [43]. Construct validity for models was assessed using standard model fit criteria as described previously [45]. Discriminant validity was assessed with average variance extracted.
To assess concurrent validity, we performed linear regressions to examine the association of PRISM scores with HbA1c, using data from Year 1 participants. Results are reported as beta coefficients and 95% confidence intervals representing the increase in HbA1c associated with a 1-unit increase in the PRISM domain barrier score. We regarded a two-tailed p<0.05 as significant for all analyses.
Results
Participant characteristics
Table 1 provides participant characteristics for our three samples: 1) the factor analysis sample to construct PRISM (n=248), 2) the independent, confirmatory sample (n=177), and 3) the regression analysis sample to relate PRISM barrier scores to HbA1c (n=297). In general, most children were non-Hispanic, White, and in good to excellent health, with slightly less than one-half having at least one other chronic illness. The mean HbA1c was comparable across the samples, ranging from 8.3% (67 mmol/mol) to 8.5% (69 mmol/mol) respectively. About half of the children were using an insulin pump, and the mean time since diabetes diagnosis was 5–6 years. Parents who accompanied children to the visits were predominantly mothers and had a wide range of educational attainment.
Table 1.
Family characteristics*
| Factor analysis sample (n=248)
|
Confirmatory PRISM factor analysis sample (n=177)
|
Regression analysis sample (n=297)
|
|
|---|---|---|---|
| Child characteristics | |||
| Child age, years (mean(sd)) | 13.2 (2.7) | 13.4 (2.8) | 13.1 (2.7) |
| Female child, % (n) | 51.6% (128) | 50.3% (89) | 56.6% (168) |
| Non-Hispanic, White, % (n) | 93.5% (232) | 88.7% (157) | 90.2% (268) |
| Child health excellent to good, % (n) | 95.6% (237) | 92.7% (164) | 93.6% (278) |
| Other chronic disease, % (n) | 41.5% (103) | 47.5% (84) | 44.2% (126) |
| HbA1c | |||
| NGSP,† % (mean(sd)) | 8.3% (1.4) | 8.5% (1.6) | 8.4% (1.4) |
| IFCC,‡ mmol/mol (mean(sd)) | 67 (15.3) | 69 (17.5) | 68 (15.3) |
| Insulin pump use, % (n) | 54.8% (136) | 45.8% (81) | 54.2% (161) |
| Years since diagnosis (mean(sd)) | 5.9 (3.4) | 6.0 (4.2) | 5.5 (3.6) |
| Parent characteristics | |||
| Parent age, years (mean(sd)) | 43.7(6.0) | 43.8 (7.6) | 43.3 (6.5) |
| Mother accompanied, % (n) | 79.0% (196) | 73.5% (130) | 77.1% (229) |
| Parent education, % (n) | |||
| High school or less | 21.4% (53) | 17.0% (30) | 21.2% (63) |
| Some college | 35.1% (87) | 36.2% (64) | 34.0% (101) |
| Bachelor’s degree or more | 42.7% (106) | 46.3% (82) | 43.1% (128) |
Values may not add to 100% due to rounding
National Glycohemoglobin Standardization Program
International Federation of Clinical Chemistry
Identifying PRISM Self-Management Barrier Domains
For parents of adolescents, analyses suggested a 6-factor solution (eigenvalues of 10.9, 4.0, 2.5, 1.5, 1.3, and 1.3), including 1) Understanding and Organizing Care, 2) Regimen Pain and Bother, 3) Denial of Disease and Consequences, and 4) Healthcare Team, 5) Family, or 6) Peer Interactions. For adolescents, analyses yielded a 5-factor solution (eigenvalues of 10.0, 4.1, 2.4, 2.1, and 1.7), including all factors in the parent model except Denial (Factor 2). For parents of children, analyses yielded a 4-factor solution (eigenvalues of 10.0, 5.3, 2.1, and 1.9), including all factors in the parent of adolescent model except Family and Peer Interactions (Factors 5 and 6). Items and factor loadings are available in Table 2.
Table 2.
Unstandardized and standardized factor loadings for PRISM barrier items for parents and adolescents
| Parent of adolescent (n=135) | Adolescent (n=149) | Parent of child (n=113) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unstd† (s.e.) | Std‡ | Unstd† (s.e.) | Std‡ | Unstd† (s.e.) | Std‡ | |
|
| ||||||
| Factor 1. Understanding/Organizing Care | ||||||
| It’s hard for my child and I to stay organized enough to keep track of medications or other things related to his/her illness. | 1.11 (0.06) | 0.69 | 1.27 (0.14) | 0.80 | 1.06 (0.05) | 0.89 |
| My child and I have trouble understanding what the doctors tell us to do for his/her regimen. | 1.22 (0.08) | 0.62 | 1.37 (0.15) | 0.87 | --- | --- |
| It’s hard for my child and I to plan things out carefully, so sometimes we don’t get around to following his/her regimen. | 1.10 (0.08) | 0.65 | 1.22 (0.13) | 0.77 | 1.03 (0.06) | 0.86 |
| When there are changes to his/her regimen, my child and I sometimes get confused. | 1.10 (0.07) | 0.80 | 0.89 (0.13) | 0.56 | 1.00 (0.00) | 0.84 |
| Sometimes my child and I can’t remember everything we’re supposed to do about his/her illness. | 1.00 (0.00) | 0.79 | 1.00 (0.00) | 0.63 | 0.83(0.06) | 0.69 |
| When my child feels anxious or nervous about something, it’s hard for him/her to follow his/her regimen. | 1.04 (0.07) | 0.58 | --- | --- | 1.04 (0.04) | 0.88 |
| Factor 2. Regimen Pain and Bother | ||||||
| Following his/her regimen causes my child physical pain and discomfort. | 1.07 (0.08) | 0.82 | 1.14 (0.06) | 0.93 | 0.95 (0.09) | 0.73 |
| My child’s regimen has side effects that he/she really doesn’t like. | 1.13 (0.08) | 0.87 | 1.00 (0.00) | 0.82 | 1.05 (0.09) | 0.81 |
| My child feels that his/her regimen takes a lot of time and work. | 0.92 (0.08) | 0.70 | 0.81 (0.07) | 0.66 | 1.07 (0.08) | 0.82 |
| My child’s regimen causes changes to his/her body that he/she really doesn’t like. | 1.00 (0.00) | 0.76 | 1.04 (0.06) | 0.84 | 1.00 (0.00) | 0.77 |
| Factor 3. Denial | ||||||
| My child feels that nothing bad would happen to him/her if he/she didn’t follow his/her regimen. | 1.00 (0.00) | 0.82 | --- | --- | --- | --- |
| My child refuses to give up time with his/her friends to take care of his/her illness. | 0.81 (0.09) | 0.67 | --- | --- | --- | --- |
| My child tries to forget that he/she has an illness. | 0.82 (0.08) | 0.68 | --- | --- | --- | --- |
| I get angry when my child makes a slip in taking care of his/her diabetes (he/she doesn’t take care of his/her diabetes). | 0.67 (0.09) | 0.55 | --- | --- | 0.83 (0.08) | 0.71 |
| My child thinks, “None of my friends have to deal with this, why do I?” | --- | --- | --- | --- | 0.83 (0.08) | 0.71 |
| My child feels that our family doesn’t understand what it’s like to live with his/her illness. | --- | --- | --- | --- | 1.00 (0.00) | 0.86 |
| Factor 4. Healthcare Team Interactions | ||||||
| My child and I don’t always trust the doctors and nurses. | 1.13 (0.09) | 0.88 | 1.00 (0.00) | 0.82 | 0.91 (0.08) | 0.81 |
| The doctors treat my child like a little kid who can’t take care of him/herself. | 1.01 (0.07) | 0.79 | 1.03 (0.06) | 0.85 | 0.89 (0.08) | 0.80 |
| The doctors are too busy or rushed to talk with my child and I about his/her illness and regimen. | 1.00 (0.00) | 0.78 | 0.91 (0.06) | 0.75 | 1.00 (0.00) | 0.83 |
| The doctors don’t seem to understand how much my child’s regimen gets in the way of important things in his/her life. | 0.91 (0.83) | 0.71 | --- | --- | --- | --- |
| My child feels that his/her doctors are friendly and easy to talk to. | --- | --- | 1.06 (0.06) | 0.87 | --- | --- |
| The doctors do a good job of explaining things to my child and me. | --- | --- | 0.98 (0.07) | 0.81 | --- | --- |
| Factor 5. Family Interactions | ||||||
| My child feels that our family doesn’t understand what it’s like to live with his/her illness. | 0.96 (0.06) | 0.87 | 1.00 (0.00) | 0.76 | --- | --- |
| Our family gives my child a lot of support to help him/her follow his/her regimen. | 1.00 (0.00) | 0.90 | 1.25 (0.10) | 0.94 | --- | --- |
| My child has someone in our family to talk to about his/her diabetes. | 0.82 (0.05) | 0.74 | --- | --- | --- | --- |
| I listen to my child’s ideas about taking care of his/her diabetes. | 0.82 (0.08) | 0.74 | --- | --- | --- | --- |
| I understand how my child feels about having diabetes. | 0.30 (0.09) | 0.27 | 1.01 (0.08) | 0.77 | --- | --- |
| I get angry when my child makes a slip in taking care of his/her diabetes (he/she doesn’t take care of his/her diabetes). | --- | --- | 0.62 (0.09) | 0.47 | --- | --- |
| Factor 6. Peer Interactions | ||||||
| My child doesn’t want his/her friends to know about his/her illness. | 1.00 (0.00) | 0.75 | 1.00 (0.00) | 0.81 | --- | --- |
| My child thinks, “None of my friends have to deal with this, why do I?” | 0.71 (0.10) | 0.53 | 0.84 (0.09) | 0.68 | --- | --- |
| My child doesn’t mind if his/her friends bring up his/her illness or ask him/her questions about it. | --- | --- | 1.01 (0.08) | 0.82 | --- | --- |
Unstandardized factor loadings are on the original item scales, reflecting the extent to which the domain covaries with the item.
Standardized factor loadings reflect the extent to which the domain is correlated with the item.
For factor 1, Understanding and Organizing Care, and factor 2, Regimen Pain and Bother, items loaded similarly across the three participant types. Specifically, Understanding and Organizing Care consisted of six items for parents of adolescents, five of which loaded for both adolescents and parents of children. These items reflected knowledge of and ability to follow the self-management regimen. Items for factor 2, Regimen Pain and Bother, reflecting the undesirable effects of following the self-management regimen, were the same four items across participant types. Factor 3, Denial, consisted of three items for parents of adolescents and four items for parents of children. For parents of adolescents, these items focused around the adolescent’s desire to forget s/he has an illness, ignore diabetes consequences, and not put time into managing the disease. For parents of children, these items focused on the child’s negative perceptions of how other unaffected family members’ and friends’ respond to them and their disease. For factor 4, Healthcare Team Interactions, four items loaded for parents of adolescents, three items for parents of children, and five items for adolescents. The three items common across all participant types reflect trust in the providers, age-appropriate treatment of the child or adolescent, and believing one has adequate time with the provider. For parents of adolescents, this factor also contained a fourth item reflecting the healthcare provider’s understanding of the regimen’s effect on important aspects of the adolescents’ lives. For adolescents, two additional items characterizing the healthcare providers’ verbal communication skills loaded on to the Healthcare Team Interactions factor. Family Interactions, factor 5, consisted of four items for adolescents and five for their parents; three of these items were common to both groups. Factor 6, Peer Interactions, was represented by two items for parents of adolescents and three for adolescents, addressing how the adolescents deal with diabetes in their interactions with peers. Overall, four items did not load on any factor and were dropped.
Construct validity
CFA indicated good model fit for the final PRISM structures for all three participant types. In addition, CFA confirmed this model fit on the independent confirmatory sample of respondents, with good model fit (Table 3). All models exhibited good fit, with X2 ratios<2.21, root mean square errors of approximation<0.09, Confirmatory Fit Indices and Tucker-Lewis Indices both >0.92, and weighted root mean square residuals<1.71. Tau equivalence testing suggested unity weighting for the items in each domain, indicating appropriateness for constructing average PRISM barrier domain scores for participants.
Table 3.
Confirmatory factor analysis model fit criteria for parent of adolescent, adolescent, and parent of child PRISM models
| Criterion
|
Parent of adolescent 13–17 years
|
Adolescent 13–17 years
|
Parent of child 8–12 years
|
|||
|---|---|---|---|---|---|---|
| Factor Analysis Sample (n=135)
|
Confirmatory Sample (n=94)
|
Factor Analysis Sample (n=149)
|
Confirmatory Sample (n=92)
|
Factor Analysis Sample (n=113)
|
Confirmatory Sample (n=83)
|
|
| χ2/df | 1.88 | 1.79 | 2.21 | 1.92 | 1.78 | 1.66 |
| Root mean square error of approximation | 0.08 | 0.08 | 0.09 | 0.09 | 0.08 | 0.08 |
| Confirmatory fit index | 0.93 | 0.91 | 0.94 | 0.93 | 0.97 | 0.96 |
| Tucker-Lewis index | 0.92 | 0.91 | 0.93 | 0.93 | 0.96 | 0.96 |
| Weighted root mean residual | 1.06 | 1.71 | 1.00 | 1.66 | 0.90 | 1.29 |
Discriminant validity
Average variances extracted (AVE) suggested discriminant validity for nearly all PRISM domains as modeled for each of our three participant types. Specifically, for parents of adolescents, discriminant validity was demonstrated for all domains except Peer Interactions (AVE=0.42), with Denial and with Family Interactions. Similarly, for adolescents, discriminant validity for all domains was demonstrated except for Understanding and Organizing Care (AVE=0.54) with Regimen Pain and Bother and for Family Interactions (AVE=0.57) with Healthcare Team Interactions. For parents of children, all PRISM domains displayed discriminant validity.
Concurrent validity
HbA1c values were significantly related to PRISM barrier scores for nearly all barriers across all three participant types (Table 4). Specifically, for parents of adolescents, four of the six PRISM barriers were significantly related to HbA1c, with a 1-unit increase in PRISM barrier score associated with 0.46% (5 mmol/mol) to 0.76% (8 mmol/mol) increases in HbA1c. Similarly for adolescents, four of the five PRISM barriers were significantly related to HbA1c, with a 1-unit increase in PRISM barrier score associated with a 0.34% (4 mmol/mol) to 0.89% (10 mmol/mol) increase in HbA1c. Lastly, for parents of children, all 4 PRISM barriers were related to HbA1c, with a 1-unit increase in PRISM barrier score associated with a 0.25% (3 mmol/mol) to 0.51% (6 mmol/mol) increase in HbA1c.
Table 4.
Regression coefficients and 95% confidence interals (CI) for association of PRISM* barrier scores with hemoglobin HbA1c percentages by participant type
| Barrier
|
Parent of adolescent (n=146)
|
Adolescent (n= 144)
|
Parent of child (n=139)
|
|||
|---|---|---|---|---|---|---|
| β†
|
95% CI
|
β†
|
95% CI
|
β†
|
95% CI
|
|
| Understanding/Organizing Care | 0.76 | (0.36, 1.16) | 0.55 | (0.21, 0.88) | 0.44 | (0.16, 0.72) |
| Motivation | ||||||
| Regimen Pain and Bother | 0.06 | (−0.29,0.40) | 0.46 | (0.10, 0.83) | 0.29 | (0.05, 0.53) |
| Denial | 0.52 | (0.19, 0.85) | --- | --- | 0.25 | (0.06, 0.44) |
| Healthcare Team Interaction | 0.58 | (0.12, 1.03) | 0.89 | (0.36, 1.42) | 0.51 | (0.16, 0.80) |
| Family Interaction | 0.46 | (0.13, 0.78) | 0.34 | (0.03, 0.65) | --- | --- |
| Peer Interaction | 0.29 | (−0.03, 0.62) | 0.20 | (−0.11, 0.51) | --- | --- |
Problem Recognition in Illness Self-Management
Beta indicates the mean change in HbA1c percentage resulting from a 1-unit increase in the PRISM barrier
Discussion
Our findings suggest PRISM is a reliable and valid tool for identifying self-management barriers among adolescents, their parents, and parents of children with type 1 diabetes. PRISM administration in routine diabetes visits has strong potential to support the healthcare system’s ability to address self-management barriers in a family-centered manner using existing, efficacious self-management resources. Family-centered care incorporates the child and family’s input as to the education and support they need to participate in care [15]. PRISM would encourage this input by efficiently identifying an individual child or family’s unique self-management barriers on a short survey during routine diabetes management visits. Our findings suggest at least 6 domains exist within parent and adolescent self-management barriers: 1) Understanding and Organizing Care, 2) Regimen Pain and Bother, 3) Denial of Disease and Consequences, and 4) Healthcare Team, 5) Family, or 6) Peer Interactions.
As a whole, our findings suggest PRISM’s content, construct, concurrent, and discriminant validity. The barriers identified by adolescents, their parents, and parents of children correspond to those of accepted conceptual models of health behavior, including the Health Beliefs Model and the Theory of Planned Behavior [17, 23]. Specifically, we found domains consistent with motivation to self-manage (the regimen’s pain or bother and denial of the disease or its consequences) and self-efficacy around understanding and organizing self-care, as well as the influences of subjective norms as defined by peers, families or healthcare team members. Further, the barrier domains suggested by our analyses correspond to those articulated by children and adolescents with chronic disease and their parents [18, 19]. Lastly, PRISM scores for all three participant types predicted glycemic control, as measured by HbA1c.
As expected based on the stages of child development, the barrier domains experienced differ by participant perspective. Specifically, adolescence is characterized by increasing importance of peer relationships and attempts to separate from parents to assert independence [46]. Thus, not surprisingly, adolescents and their parents provided responses reflecting barriers around peer or family interactions, while parents of children with diabetes did not. In addition, adolescence is often characterized by an inability to recognize one’s own mortality and the consequences of one’s actions [46]. Not surprisingly, adolescents did not endorse denial of diabetes and its consequences as a barrier to their diabetes care, but parents did.
Use of PRISM has several potential benefits for healthcare delivery systems. Most importantly, PRISM identifies barriers for which healthcare systems may already have efficacious interventions to meet the specific needs of the patient and family. The necessary providers for these interventions may already be integrated into today’s multidisciplinary diabetes care delivery. For example, if PRISM identified the barrier “Understanding and Organizing Care,” many multi-disciplinary pediatric diabetes clinics would have a diabetes educator available at the clinic visit. Further, access to these services is greatly enhanced when the patient and family can be served in coordination with the routine diabetes self-management visit. Group services in conjunction with or in lieu of routine diabetes self-management visits have been shown to improve self-management in adult populations with type 2 diabetes and for adolescent patients with type 1 diabetes [47, 48]. This arrangement also facilitates dialogue between members of the care team. In addition, PRISM would allow a healthcare organization to obtain data about the types of self-management support that would be most beneficial to the families they serve. This data could be instrumental in planning budgets and making staffing decisions. PRISM results may also illuminate areas where a new resource may need to be developed, either de novo or through adaptation of existing resources.
As with all observational research, limitations must be considered. Although our work relies on data from a single institution, our population is similar with regard to demographics and disease factors to that of a nationally-representative survey of children with diabetes [3]. However, the limited number of minority respondents and fathers prohibited exploration of the model’s fit for these specific populations. The self-reported nature of our data could, through social desirability bias, lead to underreporting of self-management barriers, resulting in our underestimating the prevalence of the barrier domains. While all models of health behavior have intrinsic strengths and weaknesses [49], other models could also be considered for conceptualizing the types of self-management barriers experienced by children with type 1 diabetes and their families. As recommended, our work was grounded in a blend of two well-accepted models [17, 23, 24], but future work could expand this model to consider additional self-management barriers suggested in other models such as the Health Action Process Approach. While our results address many aspects of reliability and validity for the PRISM, future work could examine additional aspects such as the variability in PRISM scores over time and how change in PRISM scores is related to fluctuations in glycemic control. Future analyses could also relate PRISM barriers to other relevant outcomes such as quality of life, as HbA1c may not always be strongly related to self-management [50]. In addition, given the large number of factors that can potentially influence self-management, PRISM may represent only the first step in comprehensively characterizing self-management barriers. Lastly, our analyses did not account for clustering of response by physician. This likely reduces variability in responses and could overestimate model fit.
In summary, PRISM offers a promising brief survey to identify the unique self-management barriers faced by children with diabetes and their families. Identifying these barriers can facilitate tailoring of self-management resources to meet these needs, potentially resulting in greater effectiveness and efficiency in achieving glycemic control. Future work will further examine the validity of the tool across gender and socioeconomic status, its potential relationship to quality of life, and how its use in clinical settings can influence outcomes such as glycemic control.
Acknowledgments
The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021. Funding for this study was also provided by the University of Wisconsin Graduate School and the School of Medicine and Public Health’s Department of Pediatrics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funders. The authors wish to thank the University of Wisconsin Children’s Diabetes Center staff and patients.
Abbreviations
- PRISM
Problem Recognition in Illness Self-Management
- sd
standard deviations
- EFA
exploratory factor analysis
- CFA
confirmatory factor analysis
- df
degrees of freedom
- RMSEA
root mean square error of approximation
- CFI
comparative fit index
- TLI
Tucker-Lewis Index
- WRMR
weighted root mean square residual
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemanek KL, Kamps J, Chung NB. Empirically supported treatments in pediatric psychology: regimen adherence. J Pediatr Psychol. 2001;26:253–275. doi: 10.1093/jpepsy/26.5.253. [DOI] [PubMed] [Google Scholar]
- 2.Weinger K, O’Donnell KA, Ritholz MD. Adolescent views of diabetes-related parent conflict and support: a focus group analysis. J Adolesc Health. 2001;29:330–336. doi: 10.1016/s1054-139x(01)00270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petitti DB, Klingensmith GJ, Bell RA, Andrews JS, Dabelea D, Imperatore G, et al. Glycemic control in youth with diabetes: The SEARCH for Diabetes in Youth Study. J Pediatr. 2009;155:668–672. doi: 10.1016/j.jpeds.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein J, Klingensmith G, Copeland K, Plotnick L, Kaufman F, Laffel L, et al. Care of children and adolescents with type 1 diabetes: a statement of the American Diabetes Association. Diabetes Care. 2005;28:186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- 5.Nansel TR, Weisberg-Benchell J, Wysocki T, Laffel L, Anderson B. Quality of life in children with type 1 diabetes: a comparison of general and diabetes-specific measures and support for a unitary diabetes quality-of-life construct. Diabet Med. 2008;25:1316–1323. doi: 10.1111/j.1464-5491.2008.02574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laffel LM, Connell A, Vangsness L, Goebel-Fabbri A, Mansfield A, Anderson BJ. General quality of life in youth with type 1 diabetes: relationship to patient management and diabetes-specific family conflict. Diabetes Care. 2003;26:3067–3073. doi: 10.2337/diacare.26.11.3067. [DOI] [PubMed] [Google Scholar]
- 7.Naughton MJ, Ruggiero AM, Lawrence JM, Imperatore G, Klingensmith GJ, Waitzfelder B, et al. Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for Diabetes in Youth Study. Arch Pediatr Adolesc Med. 2008;162:649–657. doi: 10.1001/archpedi.162.7.649. [DOI] [PubMed] [Google Scholar]
- 8.Ellis DA, Frey MA, Naar-King S, Templin T, Cunningham P, Cakan N. Use of multisystemic therapy to improve regimen adherence among adolescents with type 1 diabetes in chronic poor metabolic control: a randomized controlled trial. Diabetes Care. 2005;28:1604–1610. doi: 10.2337/diacare.28.7.1604. [DOI] [PubMed] [Google Scholar]
- 9.Hampson SE, Skinner TC, Hart J, Storey L, Gage H, Foxcroft D, et al. Effects of educational and psychosocial interventions for adolescents with diabetes mellitus: A systematic review. Health Technol Assess. 2001;5:1–79. doi: 10.3310/hta5100. [DOI] [PubMed] [Google Scholar]
- 10.Winkley K, Landau S, Eisler I, Ismail K. Psychological interventions to improve glycaemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomised controlled trials. BMJ. 2006;333:65–68A. doi: 10.1136/bmj.38874.652569.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33:590–611. doi: 10.1093/jpepsy/jsm128. [DOI] [PubMed] [Google Scholar]
- 12.Viner RM, Christie D, Taylor V, Hey S. Motivational/solution-focused intervention improves HbA(1c) in adolescents with type 1 diabetes: a pilot study. Diabet Med. 2003;20:739–742. doi: 10.1046/j.1464-5491.2003.00995.x. [DOI] [PubMed] [Google Scholar]
- 13.Knight KM, Dornan T, Bundy C. The diabetes educator: trying hard, but must concentrate more on behaviour. Diabet Med. 2006;23:485–501. doi: 10.1111/j.1464-5491.2005.01802.x. [DOI] [PubMed] [Google Scholar]
- 14.Wysocki T, Harris MA, Buckloh LM, Mertlich D, Lochrie AS, Taylor A, et al. Effects of behavioral family systems therapy for diabetes on adolescents’ family relationships, treatment adherence, and metabolic control. J Pediatr Psychol. 2006;31:928–938. doi: 10.1093/jpepsy/jsj098. [DOI] [PubMed] [Google Scholar]
- 15.Eichner JM, Betts JM, Chitkara MB, Jewell JA, Lye PS, Mirkinson LJ, et al. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129:394–404. doi: 10.1542/peds.2011-3084. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PD. Teenagers with Type 1 Diabetes: A Curriculum for Adolescents and Families. American Diabetes Association; Alexandria, VA: 2000. [Google Scholar]
- 17.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 18.Logan D, Zelikovsky N, Labay L, Spergel J. The Illness Management Survey: identifying adolescents’ perceptions of barriers to adherence. J Pediatr Psychol. 2003;28:383–392. doi: 10.1093/jpepsy/jsg028. [DOI] [PubMed] [Google Scholar]
- 19.Mulvaney SA, Hood KK, Schlundt DG, Osborn CY, Johnson KB, Rothman RL, et al. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract. 2011;94:77–83. doi: 10.1016/j.diabres.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ginsburg KR, Howe CJ, Jawad AF, Buzby M, Ayala JM, Tuttle A, et al. Parents’ perceptions of factors that affect successful diabetes management for their children. Pediatrics. 2005;116:1095–1104. doi: 10.1542/peds.2004-1981. [DOI] [PubMed] [Google Scholar]
- 21.MacCallum RC, Browne MW, Sugawara HM. Power analysis and determination of sample size for covariance structure modeling. Psychol Methods. 1996;1:130–149. [Google Scholar]
- 22.Green SB, Lissitz RW, Mulaik SA. Limitations of coefficient alpha as an index of test unidimensionality. Educ Psychol Meas. 1977;37:827–838. [Google Scholar]
- 23.Rosenstock IM, Strecher VJ, Becker MH. Social learning theory and the health belief model. Health Educ Q. 1988;15:175–183. doi: 10.1177/109019818801500203. [DOI] [PubMed] [Google Scholar]
- 24.Bosworth H, Oddone E, Weinberger M. Patient Treatment Adherence; Concepts, Interventions, and Measurement. Taylor & Francis, Inc; Philadelphia, PA: 2005. [Google Scholar]
- 25.Fishbein M, Ajzen I, Albarracin D, Hornik RC. Prediction and change of health behavior: applying the reasoned action approach. Lawrence Erlbaum Associates; Mahwah, N.J: 2007. Prediction and change of health behavior: a reasoned action approach; pp. 3–21. [Google Scholar]
- 26.Rosenstock IM. The health belief model and preventive health behavior. Health Educ Monogr. 1974:354–386. [Google Scholar]
- 27.Becker MH. The health belief model and personal health behavior. Health Educ Monogr. 1974;2:324–473. [Google Scholar]
- 28.Lewin AB, Heidgerken AD, Geffken GR, Williams LB, Storch EA, Gelfand KM, et al. The relation between family factors and metabolic control: the role of diabetes adherence. J Pediatr Psychol. 2006;31:174–183. doi: 10.1093/jpepsy/jsj004. [DOI] [PubMed] [Google Scholar]
- 29.Bearman KJ, La Greca AM. Assessing friend support of adolescents’ diabetes care: the diabetes social support questionnaire-friends version. J Pediatr Psychol. 2002;27:417–428. doi: 10.1093/jpepsy/27.5.417. [DOI] [PubMed] [Google Scholar]
- 30.Kyngas H, Hentinen M, Barlow JH. Adolescents’ perceptions of physicians, nurses, parents and friends: help or hindrance in compliance with diabetes self-care? J Adv Nurs. 1998;27:760–769. doi: 10.1046/j.1365-2648.1998.00608.x. [DOI] [PubMed] [Google Scholar]
- 31.Lancaster BM, Pfeffer B, McElligott M, Ferguson AT, Miller M, Wallace D, et al. Assessing treatment barriers in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2010;90:243–249. doi: 10.1016/j.diabres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 32.McKelvey J, Waller DA, North AJ, Marks JF, Schreiner B, Travis LB, et al. Reliability and validity of the Diabetes Family Behavior Scale (DFBS) Diabetes Educ. 1993;19:125–132. doi: 10.1177/014572179301900206. [DOI] [PubMed] [Google Scholar]
- 33.Nardi L, Zucchini S, D’Alberton F, Salardi S, Maltoni G, Bisacchi N, et al. Quality of life, psychological adjustment and metabolic control in youths with type 1 diabetes: a study with self- and parent-report questionnaires. Pediatr Diabetes. 2008;9:496–503. doi: 10.1111/j.1399-5448.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 34.Kalyva E, Malakonaki E, Eiser C, Mamoulakis D. Health-related quality of life (HRQoL) of children with type 1 diabetes mellitus (T1DM): self and parental perceptions. Pediatr Diabetes. 2011;12:34–40. doi: 10.1111/j.1399-5448.2010.00653.x. [DOI] [PubMed] [Google Scholar]
- 35.Matziou V, Tsoumakas K, Vlahioti E, Chrysicopoulou L, Galanis P, Petsios K, et al. Factors influencing the quality of life of young patients with diabetes. J Diabetes. 2011;3:82–90. doi: 10.1111/j.1753-0407.2010.00106.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang JT, Wiebe DJ, White PC. Developmental trajectories of metabolic control among White, Black, and Hispanic youth with type 1 diabetes. J Pediatr. 2011;159:571–576. doi: 10.1016/j.jpeds.2011.03.053. [DOI] [PubMed] [Google Scholar]
- 37.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delamater AM, Patino-Fernandez AM, Smith KE, Bubb J. Measurement of diabetes stress in older children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2013;14:50–56. doi: 10.1111/j.1399-5448.2012.00894.x. [DOI] [PubMed] [Google Scholar]
- 39.Nuboer R, Borsboom GJ, Zoethout JA, Koot HM, Bruining J. Effects of insulin pump vs. injection treatment on quality of life and impact of disease in children with type 1 diabetes mellitus in a randomized, prospective comparison. Pediatr Diabetes. 2008;9:291–296. doi: 10.1111/j.1399-5448.2008.00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Muller-Godeffroy E, Treichel S, Wagner VM. Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus--a large-scale multicentre pilot study. Diabet Med. 2009;26:493–501. doi: 10.1111/j.1464-5491.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 41.Barnard KD, Lloyd CE, Skinner TC. Systematic literature review: quality of life associated with insulin pump use in Type 1 diabetes. Diabet Med. 2007;24:607–617. doi: 10.1111/j.1464-5491.2007.02120.x. [DOI] [PubMed] [Google Scholar]
- 42.Tamborlane WV, Kollman C, Steffes MW, Ruedy KJ, Dongyuan X, Beck RW, et al. Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes. 2005;6:13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- 43.Cudeck R, Browne MW. Cross-Validation of Covariance-Structures. Multivariate Behavioral Research. 1983;18:147–168. doi: 10.1207/s15327906mbr1802_2. [DOI] [PubMed] [Google Scholar]
- 44.Kroonenberg PM, Lewis C. Methodological issues in the search for a factor model: exploration through confirmation. J Educ Stat. 1982;7:69–89. [Google Scholar]
- 45.Hu L, Bentler PM. Cutoff criteria for fit indexes in structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 46.Hazen E, Schlozman S, Beresin E. Adolescent psychological development: a review. Pediatr Rev. 2008;29:161–167. doi: 10.1542/pir.29-5-161. [DOI] [PubMed] [Google Scholar]
- 47.Deakin TA, McShane CE, Cade JE, Williams R. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database of Systematic Review. 2009:1. doi: 10.1002/14651858.CD003417.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Graue M, Wentzel-Larsen T, Hanestad BR, Sovik O. Health-related quality of life and metabolic control in adolescents with diabetes: the role of parental care, control, and involvement. J Pediatr Nurs. 2005;20:373–382. doi: 10.1016/j.pedn.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Schwarzer R. Self-efficacy: Thought control of action. Hemisphere; Washington, DC: 1992. Self-efficacy in the adoption and maintenance of health behaviors: Theoretical appraches and a new model; pp. 217–243. [Google Scholar]
- 50.Hood KK, Peterson CM, Rohan JM, Drotar D. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124:1171–1179. doi: 10.1542/peds.2009-0207. [DOI] [PubMed] [Google Scholar]


