Abstract
The avascular lens of the eye is covered anteriorly by an epithelium containing nucleated, metabolically active cells. This epithelium contains the first lens cells to encounter noxious external stimuli and cells that can develop compensatory or protective responses. Lens epithelial cells express the gap junction proteins, connexin43 (Cx43) and connexin50 (Cx50). Cx43 and Cx50 form gap junction channels and hemichannels with different properties. Although they may form heteromeric hemichannels, Cx43 and Cx50 probably do not form heterotypic channels in the lens. Cx50 channels make their greatest contribution to intercellular communication during the early postnatal period; subsequently, Cx43 becomes the predominant connexin supporting intercellular communication. Although epithelial Cx43 appears dispensable for lens development, Cx50 is critical for epithelial cell proliferation and differentiation. Cx43 and Cx50 hemichannels and gap junction channels are regulated by multiple different agents and likely contribute to both normal lens physiology and to pathology.
Keywords: Connexin43, Connexin50, cataract, lens
1. Introduction
The lens is a transparent organ whose main function is to transmit light and focus it on the retina. It is suspended between the aqueous humor and the vitreous. The cells of the lens communicate through an extensive network of gap junctions that are critical for cell homeostasis and maintenance of transparency, since the lens has no direct blood supply.
The lens contains two cell types: epithelial cells that constitute a single layer along the anterior surface and fiber cells that form the bulk of the organ. These two cell types originate during embryogenesis from the lens vesicle when cells in the posterior region elongate to form the primary fibers. Afterwards, epithelial cells near the lens equator differentiate into fiber cells. Epithelial-to-fiber cell differentiation involves cell elongation and loss of nuclei and organelles and occurs throughout the lifespan of the organism. Connexin46 (Cx46) and connexin50 (Cx50) are the two most abundant gap junction proteins in lens fiber cells [1;2]. These two connexins co-localize at gap junction plaques and can form mixed hexamers [1;3]. Substantial attention has been paid to Cx46 and Cx50, since cataracts develop in people or animals with mutations of these genes and in “knock-out” mice. These connexins and their roles in the lens have been recently reviewed [4].
This review will focus on the role of connexins for epithelial cell function. These cells are critically important for the lens, since they contain most of its metabolic, synthetic and active transport machinery [5]. Moreover, since fiber cells lose their nuclei, the epithelial cells are the only lens cells capable of proliferation. Thus, the division of these cells directly contributes to lens growth. Proliferation and differentiation of lens epithelial cells are influenced by various growth factors (including FGFs, BMPs, and TGF) and signaling cascades (including MAPK/ERK and Wnt/Fz) (reviewed by [6]).
2. Connexins expressed in lens epithelial cells
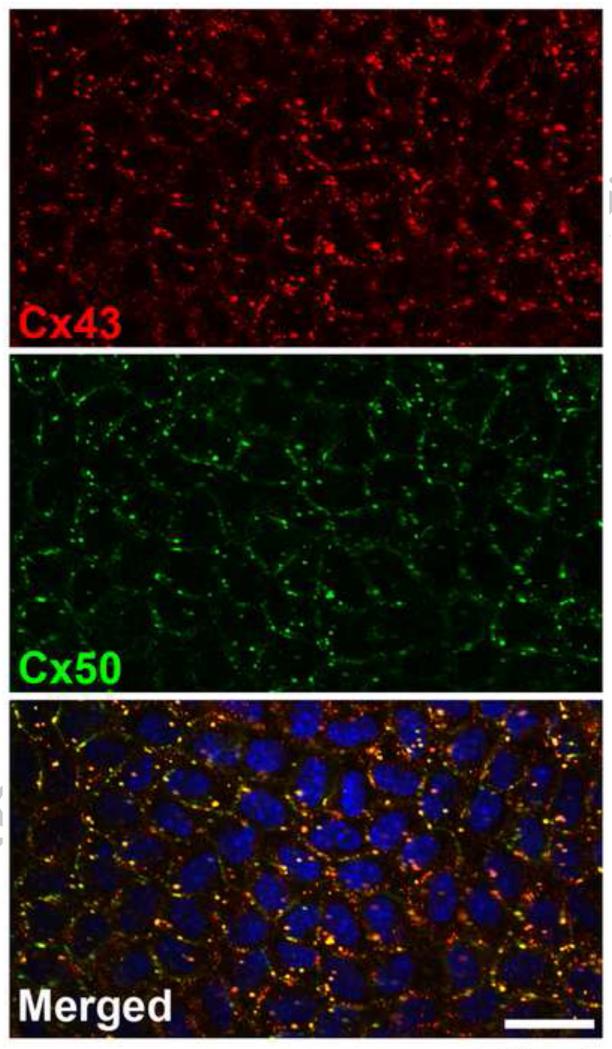
The gap junctions between epithelial cells are morphologically and physiologically distinct from those between fiber cells. Electron micrographs have shown that epithelial gap junctions contain tightly grouped connexons (with a near crystalline organization similar to junctions between cardiac myocytes or hepatocytes) while those between fiber cells are more randomly dispersed [7;8]. While the basis for the “crystalline” appearance of gap junctions is poorly understood (despite multiple electron microscopy studies), this difference suggested that epithelial and fiber cell gap junctions might have different protein components and might have some different physiological properties [8]. Epithelial cell gap junctions are differentially regulated from those between fiber cells; for instance, they are more sensitive to closure in response to cytoplasmic acidification [8]. Two connexins are extensively expressed by epithelial cells, Cx43 and Cx50. Immunofluorescence studies show that the distributions of Cx43 and Cx50 are substantially overlapping, with some gap junction plaques containing both connexins and others containing only Cx43 or Cx50 (Fig. 1 and [9]).
Figure 1.
Immunofluorescence of Cx43 and Cx50 in the epithelium. Confocal images showing the distribution of Cx50 (green) and Cx43 (red) in a flat mount of the epithelium removed from the lens of a 1.9 month old C3H mouse. These images illustrate the variations in relative proportions of Cx43 and Cx50 and their co-localization. In some areas, cells have an increased proportion of Cx43 whereas in other areas, cells show an increased proportion of Cx50 punctate staining. While some of the cells show a high degree of co-localization between the two connexins, others show a more uniform punctate staining with some co-localization between Cx43 and Cx50. Bar, xx μm
3. Cx43 and Cx50 channels
3a. Gap junction channels
The properties of Cx43 and Cx50 channels in epithelial cells can be extrapolated from studies of these connexins performed in exogenous expression systems. Both Cx43 and Cx50 form functional homomeric/homotypic gap junction channels (i.e., two hemichannels composed of the same connexin isoform docked to each other), but these channels differ in some properties including voltage gating, single channel conductance and permeability. Cx50 channels are more sensitive to transjunctional voltage than Cx43 channels. The single channel conductance of the main state of Cx50 channels is about 220 pS [10] whereas that of Cx43 channels is about 100 pS [11;12]. Cx43 channels exhibit similar permeabilities to both cations and anions [13], but Cx50 channels are more permeable to cations than anions [10]. While both Cx43 and Cx50 make gap junction channels that are permeable to glutathione, the permeability of Cx43 channels to glutathione is greater [14;15]. These channels may have different relative size selectivities, since Cx43 channels are more permeable to some larger gap junction tracers (including Lucifer yellow and Alexa594) than ones formed of the chicken Cx50 ortholog [16]. The differences in voltage gating and permeability between gap junction channels formed of Cx43 and Cx50 are influenced by differences in N-terminal amino acids between these connexins [16].
It is not entirely clear whether Cx43 and Cx50 can combine to form functional channels containing both connexins. Gap junction channels containing two different connexins can be heterotypic (formed by the docking of hemichannels composed of different connexins) or heteromeric (formed by the mixing of two different connexins within a hemichannel). Cx43 and Cx50 do not form heterotypic channels in Xenopus oocyte pairs [17]. However, they may form heteromeric channels, since Xenopus oocytes co-injected with Cx43 and Cx50 cRNAs have lower junctional conductances than ones injected with either cRNA alone [18]. Moreover, some Cx50 mutants (Cx50P88S and Cx50S50P) do not localize to gap junction plaques when expressed by themselves, but they do so when transfected into cells that endogenously express Cx43 or when they are co-expressed with Cx43 [18;19]; this “rescue” of mutant protein trafficking by wild type Cx43 suggests that they may interact and form heteromeric connexons.
3b. Cx43 and Cx50 hemichannels
Both Cx43 and Cx50 can form functional hemichannels. They have primarily been studied in non-lens cells or in exogenous expression systems where hemichannel opening is induced by incubation in extracellular solutions containing very low concentrations of divalent cations.
Cx43 hemichannels have unitary conductances of ~220 pS (about twice the conductance of a single Cx43 intercellular channel) [20]. In addition to opening by exposure to low concentrations of extracellular divalent cations, Cx43 hemichannels open in response to metabolic inhibition, some cytokines, and oxidative stress [21]. Opening of Cx43 hemichannels is modulated by intracellular pH concentration and the phosphorylation status of the protein. Cx43 hemichannels are permeable to a variety of common dye tracers (like Lucifer yellow, ethidium, DAPI and propidium) and can allow the release of cytoplasmic small molecules (including ATP, glutamate, NAD+, glutathione, PGE2, and ascorbate) [22;23].
The electrophysiological properties and regulation of Cx50 hemichannels have been extensively characterized. Cx50 hemichannels open in response to reduction of extracellular calcium and transmembrane depolarization; they are closed by extracellular acidification [24]. When expressed in Xenopus oocytes, Cx50 forms inwardly rectifying, high conductance (470 pS) single hemichannels [25]. In HeLa cells, the single channel conductance of the main state of Cx50 channels is 352 pS [26]. Hemichannels formed of Cx50 are also sensitive to extracellular monovalent cations. Replacement of extracellular Na+ with K+ (or other monovalent cations) potentiates Cx50 hemichannel current; apparently, K+ reduces the ability of divalent cations like Ca2+ to close Cx50 hemichannels [27].
3c. Pharmacology
Some of the relatively non-selective gap junction channel “blockers”, like octanol, heptanol, flufenamic acid, and glycyrrhetinic acid derivatives inhibit both Cx43 and Cx50 homomeric/homotypic channels. However, Cx43 and Cx50 channels differ in some pharmacological properties. Cx50 gap junction channels are inhibited by quinine (IC50 73 μM), mefloquine (IC50 ~1.1 μM) and several of their analogs [28;29]. Although Cx43 channels are also inhibited by these drugs, much higher concentrations are required [29].
Cx50 channels are also more sensitive to 2-aminoethoxydiphenyl borate (2-APB) than Cx43 channels (IC50, 3.7 μM for Cx50 vs. 51.6 μM for Cx43) [30]. The effect of 2-APB on Cx50 gap junction channel function is due to a decrease in open probability without changes in voltage-dependent gating or single channel conductance of the main state [30].
Cx50 channels (expressed in transiently transfected N2A cells) are inhibited by the triarylmethanes, clotrimazole (IC50, 5 μM), T122 (N-[(2-methoxyphenyl) diphenylmethyl]-1,3-thiazol-2-amine) (IC50, 1.2 μM) and T136 (N-[(2-iodophenyl)diphenylmethyl]-1,3-thiazol-2-amine) (IC50, 2.4 μM) [31]. Cx43 channels are not significantly inhibited by these compounds, even at 10 μM, a concentration that almost completely inhibits Cx50 junctional currents [31].
Cx43 and Cx50 hemichannels are inhibited by similar concentrations of the non-selective blockers that inhibit intercellular Cx43 and Cx50 channels (including carbenoxolone, α-glycyrrhetinic acid, flufenamic acid, alkanols). Similar to their intercellular channels, Cx50 hemichannels are blocked by quinine analogs. Studies of the quinine derivative, N-benzylquininium, suggest that it inhibits Cx50 hemichannels by reducing their opening probability [32]. In contrast, Cx43 hemichannels open in response to high concentrations of quinine (≥50 mM), since it induces ATP release, Ca2+ oscillations and limited Ca2+ waves (that are partially ATP-dependent) in Cx43-expressing C6 glioma cells [33]. (These effects were not observed in untransfected C6 cells.) Cx50 hemichannels are blocked by diethyl pyrocarbonate [24].
3c. Regulation by kinases
Both Cx43 and Cx50 are phosphoproteins, and are modified by multiple Ser/Thr kinases. Cx43 is also subject to tyrosine phosphorylation (by Src kinase). Phosphorylation of connexins has been implicated in the regulation of several steps of their life cycle including trafficking, targeting to the plasma membrane, assembly into gap junction plaques, channel gating, internalization and degradation.
The roles of Cx43 phosphorylation by various kinases (and their stimulation by signal transduction pathways) have been elucidated in different cell types and have been extensively reviewed [34;35]. Cx43 is phosphorylated by PKC on Ser368. Phosphorylation at this site modulates the single channel conductance and permeability of Cx43 gap junction channels [36;37] and abolishes the permeability of reconstituted Cx43 hemichannels to Lucifer yellow and sucrose [38]. In single Xenopus laevis oocytes expressing Cx43, treatment with PKC inhibitors increases the uptake of 5(6) carboxyfluorescein [39].
Although less extensively studied, Cx50 channels are also regulated by kinases relevant to lens epithelial cells. Indeed, studies performed in cultures of chicken lens epithelial cells implicated FGF signaling through the ERK pathway in the regulation of Cx50 gap junctions, because treatment of these cultures with FGF increased gap junctional coupling [40]. In agreement with this observation, Cx50-mediated junctional conductance was significantly increased when a constitutively active form of MEK1 was expressed together with Cx50 in Xenopus oocytes [41]. Junctional conductance was also increased by FGF treatment of Xenopus oocytes that had been co-injected with Cx50 and FGF receptor cRNAs, likely by activation of MAPK signaling [41].
4. Properties of connexin channels in lens epithelia
The difference in pharmacology between Cx43 and Cx50 channels has been used to evaluate the contribution of each of these connexins to gap junction coupling in lens epithelial cells. Initial studies concluded that most of the gap junctions between lens epithelial cells from adult animals had properties corresponding to Cx43 channels [42]. Cx43 is also primarily responsible for the gap junction-mediated communication in epithelial cell sheets from 1-2 week-old mice, since the intercellular transfer of Neurobiotin and Lucifer yellow was reduced by deleting Cx43 expression, but unaffected by deleting Cx50 [18]. However, gap junctional currents in lens epithelial cells isolated from post-natal day 6 mice were reduced by ~60% by the triarylmethanes, T122 and T136, which do not significantly inhibit Cx43 channels [31]. Similarly, in pairs of dissociated epithelial cells from 3-day-old mice quinine treatment reduced junctional conductance by 60-70%, although the extent of this effect decreased with age [43]. Taken together, these findings suggest that Cx50 makes a major contribution to epithelial coupling during the first few post-natal days, but it decreases at later ages. This Cx50 coupling temporally correlates with the postnatal proliferative peak in mouse lens epithelium (that peaks at postnatal days 2-3) [43].
Lens epithelial cells express various kinases including ERK1 and ERK2 [44] and several isoforms of PKC [45]. Intercellular communication among epithelial cells is decreased following treatment with phorbol esters, which activate PKC [46-48]. Experiments performed in different systems have implicated various mechanisms for this effect including targeting of Cx43 for proteasomal degradation, changes in Cx43 antigenicity, decreased levels of Cx50 mRNA, disappearance of Cx50 from gap junction plaques, and subcellular redistribution of Cx43 [47;49-52]. The effects on Cx43 may not be direct consequences of Cx43 phosphorylation, since phosphorylation of Cx43 at the PKC target site (Ser368) was not detectable in embryonic or adult lenses using site-specific antibodies [53].
5. Functions of connexin channels in lens epithelia
As noted, various protein kinases that are activated in response to growth factors can alter connexin phosphorylation and modulate epithelial cell communication. Epithelial gap junctions can also influence or mediate the effects of growth factors on epithelial cell proliferation and differentiation.
Although Cx43 is expressed early during lens development [54;55], various mouse studies suggest an uncertain importance of Cx43 for lens epithelial cells. Although Cx43-null mice die neonatally, their lenses are similar in size to those of wild type animals [56]. The gross development of the lens appears normal for at least 2 weeks after birth in nestin-Cre/Cx43flox/flox mice, which have a conditional deletion of Cx43 in nervous system and several ocular tissues [57]. Moreover, MLR10-CreCx43flox/flox mice that have a lens-specific deletion of Cx43 have clear lenses that appear normal through at least 6 months of age [18]. Although counter-intuitive, these results suggest that expression of Cx43 is not crucial for the development of the lens or the proliferation of epithelial cells.
In contrast, studies of mice with homologous deletion of Cx50, clearly demonstrate its importance in lens epithelial cells. Cx50-null mice develop small lenses due to reduced proliferation of lens epithelial cells during the first postnatal week [43;58]. This effect appears specific for Cx50, since its replacement with Cx46 only partially restores the increased mitosis that normally occurs on post-natal days 2 and 3 [58]. These authors concluded that Cx50 influences this neonatal mitotic burst independent of MAPK signaling, because they detected no significant differences in the levels of pERK between wild type, Cx50KO and Cx46KI animals [58].
However, the MAPK/ERK signaling pathway does have a significant influence on lens epithelial cells and their connexins. ERK2 has a major role in proliferation in the germinative (peripheral) zone, and both ERK1 and ERK2 influence proliferation of epithelial cells in the central region [44]. FGF treatment or ERK activation increases intercellular coupling (attributed to Cx50) in cultured chicken embryo lens cells [40]. However, in vivo expression of a constitutively active form of MEK1 (an upstream activator of MAPK/ERK) in the mouse lens results in several defects including macrophthalmia, cataracts and lens rupture [59]. It also significantly increases the contribution of Cx50 to lens epithelial cell coupling, which correlates with an increase in mitosis at post-natal day 5 [41].
The abundance and function of epithelial cell connexins may influence the differentiation of lens epithelial cells. Expression of the chicken Cx50 ortholog (including a non-functional mutant) or a chicken Cx46 chimera containing the Cx50 carboxyl terminus promotes differentiation of chicken lens cells in culture [60;61]. The mechanism for this “non-channel”-induced effect of Cx50 has not been clarified, but it has been suggested that it involves interactions of its carboxyl terminus with other cellular proteins to modulate intracellular signaling critical for lens cell differentiation. Cultures derived from dissociated chick embryo lens epithelial cells may mimic events occurring at the lens equator where FGFs influence epithelial cell differentiation [62]. Although FGF increases gap junction coupling and induces synthesis of several fiber cell-specific proteins in these cultures, these processes are not directly linked, since treatment with gap junction blockers did not affect the markers for fiber cell differentiation [63]. However, studies of mice with the non-functional Cx50D47A mutation show that loss of Cx50 function may disrupt completion of the fiber cell differentiation program.
6. Pathologic abnormalities of lens epithelial connexins
Alterations in epithelial cells can contribute to lens pathologies (including reduced growth and development of cataracts). Therefore, studies of these cells can help elucidate their initial changes, pathways and mechanisms.
In many cases, the development of opacities in the lens (cataracts) has been attributed to the damage to crystallins and other lens proteins. Studies of epithelial cells have shown that such damage can be caused by ultraviolet irradiation or generation of reactive oxygen species [64-67]. Lens gap junction channels allow the permeation of molecules that facilitate maintenance of a reducing environment (like cysteine, ascorbate, and glutathione), which are taken up from the aqueous humor or generated in epithelial cells; their intercellular movement would be disrupted by abnormalities of lens epithelial connexins and might perturb the redox state of the lens. The levels of reduced glutathione are reduced in cataractous lenses [68], and depletion of glutathione causes damage to epithelial and fiber cells in neonatal mice [69;70]. In other cell types, some toxic agents (like CCl4 and paraquat) that induce free radical generation cause cellular uncoupling [71;72]. However, cells may also develop compensatory responses to increase gap junctional communication and restore homeostasis. For example, 7-ketocholesterol is a cholesterol oxide that is increased in human cataracts. Treatment of primary cultures of lens epithelial cells with 7-ketocholesterol; this functional change is accompanied by an increase in the phosphorylated forms of Cx43, the insolubility of Cx43 in 1% Triton X-100, and the abundance of Cx43 at the plasma membrane [73].
Although deletion of Cx43 in the lens does not cause pathologies (see above) and Cx43 mutations have not been linked to lens abnormalities, mutations of Cx50 can lead to both reduced lens size and cataracts. Several lines of mice expressing Cx50 mutants (including Cx50R205G, Cx50S50P, Cx50V64A, and Cx50D47A) have smaller lenses than wild type animals [9;74-76]. The severity of the size reductions and the difference in size between wild type, heterozygous and homozygous mouse lenses depends on the particular Cx50 mutation. Analogy to the decreased mitosis in homozygous Cx50-null animals suggests the hypothesis that expression of these Cx50 mutants decreases proliferation of lens epithelial cells and consequently reduces lens growth.
Cx50 mutants that cause cataracts may also affect Cx43 and epithelial gap junction function. In Cx50S50P mice, the mutant Cx50 may interact with and affect the wild type Cx43, since the epithelia from these mice have decreased and less organized Cx43 gap junction plaques, reduced levels of Cx43 and reduced intercellular communication [18]. In the epithelia of mice expressing Cx50D47A, Cx50 immunoreactivity shows an increase in cytoplasmic staining and a decrease in co-localization with Cx43; these changes are more pronounced in homozygous than in heterozygous animals [9].
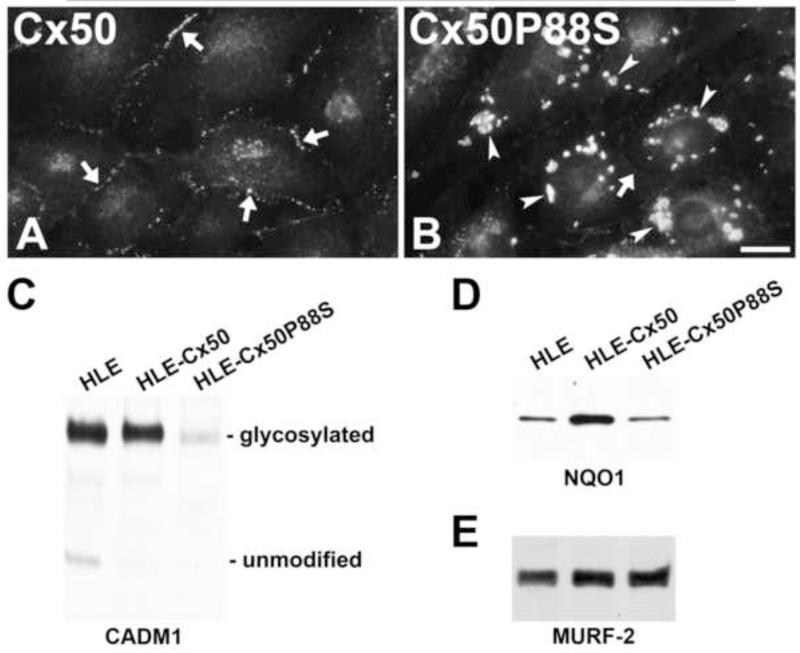
The possible interaction of Cx50 and Cx43 in lens epithelia led us to explore immortalized human lens epithelial cells (HLE) as a potential model to study the consequences of alterations of lens epithelial connexins. There are various different HLE lines, and we chose to use SRAA01/04 cells [77]. Although these cells do not produce high levels of all lens-specific proteins, they express Cx43 and low levels of Cx50 and have been used to investigate the responses of lens cells to various insults like UV irradiation or oxidative stresses [78-80]. We stably transfected the HLE cells with wild type human Cx50 (HLE-Cx50) or the cataract-linked mutant, Cx50P88S (HLE-Cx50P88S). As expected, wild type Cx50 localized along appositional membranes in a distribution consistent with that expected for a gap junction protein. In contrast, Cx50P88S formed large cytoplasmic inclusions similar to the autophagosome-associated/ER-derived structures that we have seen when this protein is expressed in other cell types [19;81;82] (Fig. 2A). Expression of these two forms of Cx50 led to changes in levels of other proteins (Fig. 2B). Expression of wild type Cx50 did not alter the levels of the cell adhesion molecule, CADM1 (also known as SynCam), as compared with the levels found in untransfected HLE cells, but expression of Cx50P88S dramatically reduced its levels. Levels of the quinone reductase 1, NQO1, were increased in HLE-Cx50 cells, but unaffected in HLE-Cx50P88S cells. Intracellular proteolysis and components of the ubiquitin/proteasomal pathway have been implicated in the responses of lens epithelial cells to various stresses [83;84]. Therefore, we examined the E3 ubiquitin ligase, MURF2 which was abundant in these cells; however, its levels were unaffected by transfection with either wild type or mutant Cx50. These results imply that wild type and mutant connexins differentially alter some (but not all) lens epithelial cell proteins and that stably transfected HLE cells may prove a reasonable system for studying such changes. Because CADM1 is expressed in epithelial and cortical fiber cells [85], the decrease in of CADM1 levels suggests that the cataract-associated connexin mutant may affect cell adhesion between epithelial cells or cortical fiber cells in the lens in viv.
Figure 2.
Photomicrographs show the distribution of immunoreactive Cx50 in HLE-Cx50 (A) and HLE-Cx50P88S (B). Arrows indicate staining at appositional membranes consistent with the expected distribution of gap junctions; this staining is prominent in cells expressing Cx50, but not in those expressing Cx50P88S. Arrowheads indicate cytoplasmic accumulations seen only in cells expressing Cx50P88S. C-E. Immunoblots show levels of unmodified and glycosylated CADM1 (C), NQO1 (D) and MURF2 (E) in total homogenates of untransfected HLE cells or cells transfected with Cx50 or Cx50P88S. Levels of CADM1 were decreased in HLE-Cx50P88S compared to untransfected and HLE-Cx50 cells and levels of NQO1 were increased in HLE-Cx50 compared to untransfected and HLE-Cx50P88S cells. Levels of MURF2 were similar between HLE-Cx50 and HLE-Cx50P88S.
Connexin hemichannels may be involved in disease or age-related cataracts that result from the cumulative effects of various insults on lens components, since some of these stresses (like metabolic inhibition or reactive oxygen species) also provoke opening of hemichannels [21;86]. Although openings of connexin hemichannels (likely composed of Cx46) have been observed in isolated lens fiber cells [87], it is likely that hemichannels rarely open under normal conditions in lens epithelial cells. Cx43 and Cx50 hemichannels are mostly closed in the presence of physiological concentrations of extracellular Ca2+ [86;88]. In astrocytes, pathological opening of Cx43 hemichannels has been linked to S-nitrosylation of the protein [89]. However, the lens may be protected from the deleterious consequences of such hemichannel opening, because this effect could be antagonized by high concentrations of reduced glutathione (like the ones present in the lens). Some of the deleterious “hemichannel-like” events that occur in the lens may also be due to contributions of other (non-connexin) proteins, since other proteins (including pannexins) expressed in lens epithelial cells form relatively non-selective, large pore channels [90;91].
7. Perspectives
The importance of connexins for the lens is certain, based on the abnormalities observed in mice with homologous deletion of Cx46 or Cx50 and in people or rodents expressing mutant forms of these proteins. At least some of the cellular and functional abnormalities of these connexin mutants are manifested in the lens epithelial cells. Because of their superficial location, the epithelial cells are the first lens cells exposed to many insults that contribute to cataract formation. Because they are nucleated and metabolically active, they are the best equipped lens cells to defend and protect the rest of the organ from these noxious stimuli. Therefore, epithelial cells have been studied by many investigators interested in the pathogenesis of age- and disease-related cataracts. Although underexplored, it is likely that the gap junction channels and hemichannels formed by connexins in the lens epithelium influence these processes. Indeed, connexin channels may assist with tissue protection but they may also contribute to injury.
Acknowledgments
Supported by NIH grants RO1EY08368 (to ECB), 2T35D062719 (training support of PO), and UL1TR000430 (core subsidy to ECB). The SRA01/04 human lens epithelial cells were a kind gift from Drs. Frank Giblin and Venkat Reddy of Oakland University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Paul DL, Ebihara L, Takemoto LJ, Swenson KI, Goodenough DA. Connexin46, a novel lens gap junction protein, induces voltage- gated currents in nonjunctional plasma membrane of Xenopus oocytes. J. Cell Biol. 1991;115:1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].White TW, Bruzzone R, Goodenough DA, Paul DL. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol. Biol. Cell. 1992;3:711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jiang JX, Goodenough DA. Heteromeric connexons in lens gap junction channels. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1287–1291. doi: 10.1073/pnas.93.3.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Beyer EC, Ebihara L, Berthoud VM. Connexin mutants and cataracts. Front. Pharmacol. 2013:4. doi: 10.3389/fphar.2013.00043. doi: 10.3389/fphar.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mathias RT, Rae JL. The lens: local transport and global transparency. Exp. Eye Res. 2004;78:689–698. doi: 10.1016/j.exer.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [6].Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev. Biol. 2005;280:1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- [7].Rae JL, Kuszak JR. The electrical coupling of epithelium and fibers in the frog lens. Exp. Eye Res. 1983;36:317–326. doi: 10.1016/0014-4835(83)90114-8. [DOI] [PubMed] [Google Scholar]
- [8].Miller TM, Goodenough DA. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J. Cell Biol. 1986;102:194–199. doi: 10.1083/jcb.102.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Berthoud VM, Minogue PJ, Yu H, Schroeder R, Snabb JI, Beyer E. Connexin50D47A decreases levels of fiber cell connexins and impairs lens fiber cell differentiation. Invest. Ophthalmol. Vis. Sci. 2013;54:7614–7622. doi: 10.1167/iovs.13-13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Srinivas M, Costa M, Gao Y, Fort A, Fishman GI, Spray DC. Voltage dependence of macroscopic and unitary currents of gap junction channels formed by mouse connexin50 expressed in rat neuroblastoma cells. J. Physiol (Lond) 1999;517:673–689. doi: 10.1111/j.1469-7793.1999.0673s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fishman GI, Moreno AP, Spray DC, Leinwand LA. Functional analysis of human cardiac gap junction channel mutants. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Veenstra RD, Wang HZ, Westphale EM, Beyer EC. Multiple connexinsconfer distinct regulatory and conductance properties of gap junctions in developing heart. Circ. Res. 1992;71:1277–1283. doi: 10.1161/01.res.71.5.1277. [DOI] [PubMed] [Google Scholar]
- [13].Veenstra RD, Wang HZ, Beblo DA, Chilton MG, Harris AL, Beyer EC, Brink P. Selectivity of connexin-specific gap junctions does not correlate with channel conductance. Circ. Res. 1995;77:1156–1165. doi: 10.1161/01.res.77.6.1156. [DOI] [PubMed] [Google Scholar]
- [14].Goldberg GS, Lampe PD, Nicholson BJ. Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat. Cell Biol. 1999;1:457–459. doi: 10.1038/15693. [DOI] [PubMed] [Google Scholar]
- [15].Srinivas M, Rubinos C. Permeability of lens gap junctions to glutathione; XX Biennial Meeting international Society for Eye Research; 2012. 0113. Abstract. [Google Scholar]
- [16].Dong L, Liu X, Li H, Vertel BM, Ebihara L. Role of the N-terminus in permeability of chicken connexin45.6 gap junctional channels. J. Physiol. 2006;576:787–799. doi: 10.1113/jphysiol.2006.113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].White TW, Paul DL, Goodenough DA, Bruzzone R. Functional analysis of selective interactions among rodent connexins. Mol. Biol. Cell. 1995;6:459–470. doi: 10.1091/mbc.6.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].De Rosa AM, Mese G, Li L, Sellitto C, Brink PR, Gong X, White TW. The cataract causing Cx50-S50P mutant inhibits Cx43 and intercellular communication in the lens epithelium. Exp. Cell Res. 2009;315:1063–1075. doi: 10.1016/j.yexcr.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berthoud VM, Minogue PJ, Guo J, Williamson EK, Xu X, Ebihara L, Beyer EC. Loss of function and impaired degradation of a cataract-associated mutant connexin50. Eur. J. Cell Biol. 2003;82:209–221. doi: 10.1078/0171-9335-00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Contreras JE, Sànchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MVL, Sàez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc. Natl. Acad. Sci. U. S. A. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- [23].Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beahm DL, Hall JE. Hemichannel and junctional properties of connexin 50. Biophys. J. 2002;82:2016–2031. doi: 10.1016/S0006-3495(02)75550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Srinivas M, Kronengold J, Bukauskas FF, Bargiello TA, Verselis VK. Correlative studies of gating in Cx46 and Cx50 hemichannels and gap junction channels. Biophys. J. 2005;88:1725–1739. doi: 10.1529/biophysj.104.054023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Valiunas V, Weingart R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflügers Arch. 2000;440:366–379. doi: 10.1007/s004240000294. [DOI] [PubMed] [Google Scholar]
- [27].Srinivas M, Calderon DP, Kronengold J, Verselis VK. Regulation of connexin hemichannels by monovalent cations. J. Gen. Physiol. 2006;127:67–75. doi: 10.1085/jgp.200509397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bai D, del Corsso C, Srinivas M, Spray DC. Block of specific gap junction channel subtypes by 2-aminoethoxydiphenyl borate (2-APB) J. Pharmacol. Exp. Ther. 2006;319:1452–1458. doi: 10.1124/jpet.106.112045. [DOI] [PubMed] [Google Scholar]
- [31].Bodendiek SB, Rubinos C, Trelles MP, Coleman N, Jenkins DP, Wulff H, Srinivas M. Triarylmethanes, a new class of Cx50 inhibitors. Front. Pharmacol. 2012;3:106. doi: 10.3389/fphar.2012.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rubinos C, Sanchez HA, Verselis VK, Srinivas M. Mechanism of inhibition of connexin channels by the quinine derivative N-benzylquininium. J. Gen. Physiol. 2012;139:69–82. doi: 10.1085/jgp.201110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in castrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- [34].Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int. J. Biochem. Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem. J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J. Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ek-Vitorin JF, King TJ, Heyman NS, Lampe PD, Burt JM. Selectivity of connexin 43 channels Is regulated through protein kinase C-dependent phosphorylation. Circ. Res. 2006;98:1498–1505. doi: 10.1161/01.RES.0000227572.45891.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bao X, Reuss L, Altenberg GA. Regulation of purified and reconstituted connexin 43 hemichannels by protein kinase C-mediated phosphorylation of Serine 368. J. Biol. Chem. 2004;279:20058–20066. doi: 10.1074/jbc.M311137200. [DOI] [PubMed] [Google Scholar]
- [39].Bao X, Altenberg GA, Reuss L. Mechanism of regulation of the gap junction protein connexin 43 by protein kinase C-mediated phosphorylation. Am. J. Physiol. Cell Physiol. 2004;286:C647–C654. doi: 10.1152/ajpcell.00295.2003. [DOI] [PubMed] [Google Scholar]
- [40].Le AC, Musil LS. A novel role for FGF and extracellular signal-regulated kinase in gap junction-mediated intercellular communication in the lens. J. Cell Biol. 2001;154:197–216. doi: 10.1083/jcb.200101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Shakespeare TI, Sellitto C, Li L, Rubinos C, Gong X, Srinivas M, White TW. Interaction between Connexin50 and mitogen-activated protein kinase signaling in lens homeostasis. Mol. Biol. Cell. 2009;20:2582–2592. doi: 10.1091/mbc.E08-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Donaldson PJ, Roos M, Evans C, Beyer E, Kistler J. Electrical properties of mammalian lens epithelial gap junction channels. Invest. Ophthalmol. Vis. Sci. 1994;35:3422–3428. [PubMed] [Google Scholar]
- [43].White TW, Gao Y, Li L, Sellitto C, Srinivas M. Optimal lens epithelial cell proliferation is dependent on the connexin isoform providing gap junctional coupling. Invest. Ophthalmol. Vis. Sci. 2007;48:5630–5637. doi: 10.1167/iovs.06-1540. [DOI] [PubMed] [Google Scholar]
- [44].Upadhya D, Ogata M, Reneker LW. MAPK1 is required for establishing the pattern of cell proliferation and for cell survival during lens development. Development. 2013;140:1573–1582. doi: 10.1242/dev.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Berthoud VM, Westphale EM, Grigoryeva A, Beyer EC. PKC isoenzymes in the chicken lens and TPA-induced effects on intercellular communication. Invest Ophthalmol. Vis. Sci. 2000;41:850–858. [PubMed] [Google Scholar]
- [46].Reynhout JK, Lampe PD, Johnson RG. An activator of protein kinase C inhibits gap junction communication between cultured bovine lens cells. Exp. Cell Res. 1992;198:337–342. doi: 10.1016/0014-4827(92)90388-o. [DOI] [PubMed] [Google Scholar]
- [47].TenBroek EM, Louis CF, Johnson R. The differential effects of 12-O-tetradecanoylphorbol-13-acetate on the gap junctions and connexins of the developing mammalian lens. Dev. Biol. 1997;191:88–102. doi: 10.1006/dbio.1997.8703. [DOI] [PubMed] [Google Scholar]
- [48].Long AC, Colitz CM, Bomser JA. Regulation of gap junction intercellular communication in primary canine lens epithelial cells: role of protein kinase C. Curr Eye Res. 2007;32:223–231. doi: 10.1080/02713680601186714. [DOI] [PubMed] [Google Scholar]
- [49].Wagner LM, Saleh SM, Boyle DJ, Takemoto DJ. Effect of protein kinase Cγ on gap junction disassembly in lens epithelial cells and retinal cells in culture. Mol. Vis. 2002;8:59–66. [PubMed] [Google Scholar]
- [50].Girao H, Pereira P. Phosphorylation of connexin 43 acts as a stimuli for proteasome-dependent degradation of the protein in lens epithelial cells. Mol. Vis. 2003;9:24–30. [PubMed] [Google Scholar]
- [51].Lin D, Boyle DL, Takemoto DJ. IGF-I-induced phosphorylation of connexin 43 by PKCgamma: regulation of gap junctions in rabbit lens epithelial cells. Invest Ophthalmol. Vis. Sci. 2003;44:1160–1168. doi: 10.1167/iovs.02-0737. [DOI] [PubMed] [Google Scholar]
- [52].Nguyen TA, Boyle DL, Wagner LM, Shinohara T, Takemoto DJ. LEDGF activation of PKC gamma and gap junction disassembly in lens epithelial cells. Exp. Eye Res. 2003;76:565–572. doi: 10.1016/s0014-4835(03)00049-6. [DOI] [PubMed] [Google Scholar]
- [53].King TJ, Lampe PD. Temporal regulation of connexin phosphorylation in embryonic and adult tissues. Biochim. Biophys. Acta. 2005;1719:24–35. doi: 10.1016/j.bbamem.2005.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J. Membr. Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- [55].Yancey SB, Biswal S, Revel JP. Spatial and temporal patterns of distribution of the gap junction protein connexin43 during mouse gastrulation and organogenesis. Development. 1992;114:203–212. doi: 10.1242/dev.114.1.203. [DOI] [PubMed] [Google Scholar]
- [56].White TW, Sellitto C, Paul DL, Goodenough DA. Prenatal lens development in connexin43 and connexin50 double knockout mice. Invest. Ophthalmol. Vis. Sci. 2001;42:2916–2923. [PubMed] [Google Scholar]
- [57].Calera MR, Wang Z, Sanchez-Olea R, Paul DL, Civan MM, Goodenough DA. Depression of intraocular pressure following inactivation of connexin43 in the nonpigmented epithelium of the ciliary body. Invest Ophthalmol. Vis. Sci. 2009;50:2185–2193. doi: 10.1167/iovs.08-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Sellitto C, Li L, White TW. Connexin50 is essential for normal postnatal lens cell proliferation. Invest. Ophthalmol. Vis. Sci. 2004;45:3196–3202. doi: 10.1167/iovs.04-0194. [DOI] [PubMed] [Google Scholar]
- [59].Gong X, Wang X, Han J, Niesman I, Huang Q, Horwitz J. Development of cataractous macrophthalmia in mice expressing an active MEK1 in the lens. Invest Ophthalmol. Vis. Sci. 2001;42:539–548. [PubMed] [Google Scholar]
- [60].Gu S, Yu XS, Yin X, Jiang JX. Stimulation of lens cell differentiation by gap junction protein connexin 45.6. Invest Ophthalmol. Vis. Sci. 2003;44:2103–2111. doi: 10.1167/iovs.02-1045. [DOI] [PubMed] [Google Scholar]
- [61].Banks EA, Yu XS, Shi Q, Jiang JX. Promotion of lens epithelial-fiber differentiation by the C-terminus of connexin 45.6 a role independent of gap junction communication. J Cell Sci. 2007;120:3602–3612. doi: 10.1242/jcs.000935. [DOI] [PubMed] [Google Scholar]
- [62].Musil LS. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. J. Membr. Biol. 2012;245:357–368. doi: 10.1007/s00232-012-9458-y. [DOI] [PubMed] [Google Scholar]
- [63].Le AC, Musil LS. Normal differentiation of cultured lens cells after inhibition of gap junction-mediated intercellular communication. Dev. Biol. 1998;204:80–96. doi: 10.1006/dbio.1998.9030. [DOI] [PubMed] [Google Scholar]
- [64].Andley UP, Walsh A, Kochevar IE, Reddan JR. Effect of ultraviolet-B radiation on protein synthesis in cultured lens epithelial cells. Curr Eye Res. 1990;9:1099–1106. doi: 10.3109/02713689008997583. [DOI] [PubMed] [Google Scholar]
- [65].Giblin FJ, Reddan JR, Schrimscher L, Dziedzic DC, Reddy VN. The relative roles of the glutathione redox cycle and catalase in the detoxification of H2O2 by cultured rabbit lens epithelial cells. Exp. Eye Res. 1990;50:795–804. doi: 10.1016/0014-4835(90)90130-m. [DOI] [PubMed] [Google Scholar]
- [66].Hightower KR, Reddan JR, Dziedzic DC. Susceptibility of lens epithelial membrane SH groups to hydrogen peroxide. Invest Ophthalmol. Vis. Sci. 1989;30:569–574. [PubMed] [Google Scholar]
- [67].Hightower KR, Reddan JR, McCready JP, Dziedzic DC. Lens epithelium: a primary target of UVB irradiation. Exp. Eye Res. 1994;59:557–564. doi: 10.1006/exer.1994.1141. [DOI] [PubMed] [Google Scholar]
- [68].Spector A. Review: Oxidative stress and disease. J Ocul. Pharmacol. Ther. 2000;16:193–201. doi: 10.1089/jop.2000.16.193. [DOI] [PubMed] [Google Scholar]
- [69].Martensson J, Steinherz R, Jain A, Meister A. Glutathione ester prevents buthionine sulfoximine-induced cataracts and lens epithelial cell damage. Proc Natl Acad Sci U S A. 1989;86:8727–8731. doi: 10.1073/pnas.86.22.8727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Laver NM, Robison WG, Jr., Calvin HI, Fu SC. Early epithelial lesions in cataracts of GSH-depleted mouse pups. Exp. Eye Res. 1993;57:493–498. doi: 10.1006/exer.1993.1151. [DOI] [PubMed] [Google Scholar]
- [71].Saez JC, Bennett MV, Spray DC. Carbon tetrachloride at hepatotoxic levels blocks reversibly gap junctions between rat hepatocytes. Science. 1987;236:967–969. doi: 10.1126/science.3576214. [DOI] [PubMed] [Google Scholar]
- [72].Ruch RJ, Klaunig JE. Inhibition of mouse hepatocyte intercellular communication by paraquat-generated oxygen free radicals. Toxicol. Appl. Pharmacol. 1988;94:427–436. doi: 10.1016/0041-008x(88)90283-9. [DOI] [PubMed] [Google Scholar]
- [73].Girao H, Catarino S, Pereira P. 7-Ketocholesterol modulates intercellular communication through gap-junction in bovine lens epithelial cells. Cell Commun. Signal. 2004;2:2. doi: 10.1186/1478-811X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Graw J, Loster J, Soewarto D, Fuchs H, Meyer B, Reis A, Wolf E, Balling R, De Angelis MH. Characterization of a mutation in the lens-specific MP70 encoding gene of the mouse leading to a dominant cataract. Exp. Eye Res. 2001;73:867–876. doi: 10.1006/exer.2001.1096. [DOI] [PubMed] [Google Scholar]
- [75].Xia CH, Cheung D, De Rosa AM, Chang B, Lo WK, White TW, Gong X. Knock-in of α3 connexin prevents severe cataracts caused by an α8 point mutation. J. Cell Sci. 2006;119:2138–2144. doi: 10.1242/jcs.02940. [DOI] [PubMed] [Google Scholar]
- [76].Xia CH, Chang B, De Rosa AM, Cheng C, White TW, Gong X. Cataracts and microphthalmia caused by a gja8 mutation in extracellular loop 2. PLoS. ONE. 2012;7:e52894. doi: 10.1371/journal.pone.0052894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Ibaraki N, Chen SC, Lin LR, Okamoto H, Pipas JM, Reddy VN. Human lens epithelial cell line. Exp. Eye Res. 1998;67:577–585. doi: 10.1006/exer.1998.0551. [DOI] [PubMed] [Google Scholar]
- [78].Goswami S, Sheets NL, Zavadil J, Chauhan BK, Bottinger EP, Reddy VN, Kantorow M, Cvekl A. Spectrum and range of oxidative stress responses of human lens epithelial cells to H2O2 insult. Invest Ophthalmol. Vis. Sci. 2003;44:2084–2093. doi: 10.1167/iovs.02-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Reddy VN, Kasahara E, Hiraoka M, Lin LR, Ho YS. Effects of variation in superoxide dismutases (SOD) on oxidative stress and apoptosis in lens epithelium. Exp. Eye Res. 2004;79:859–868. doi: 10.1016/j.exer.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [80].Osada H, Yoshitake Y, Ikeda T, Ishigaki Y, Takata T, Tomosugi N, Sasaki H, Yonekura H. Ultraviolet B-induced expression of amphiregulin and growth differentiation factor 15 in human lens epithelial cells. Mol. Vis. 2011;17:159–169. [PMC free article] [PubMed] [Google Scholar]
- [81].Lichtenstein A, Gaietta GM, Deerinck TJ, Crum J, Sosinsky GE, Beyer EC, Berthoud VM. The cytoplasmic accumulations of the cataract-associated mutant, Connexin50P88S, are long-lived and form in the endoplasmic reticulum. Exp. Eye Res. 2009;88:600–609. doi: 10.1016/j.exer.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Lichtenstein A, Minogue PJ, Beyer EC, Berthoud VM. Autophagy: a pathway that contributes to connexin degradation. J. Cell Sci. 2011;124:910–920. doi: 10.1242/jcs.073072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Taylor A, Berger JJ, Reddan J, Zuliani A. Effects of aging in vitro on intracellular proteolysis in cultured rabbit lens epithelial cells in the presence and absence of serum. In Vitro Cell Dev. Biol. 1991;27A:287–292. doi: 10.1007/BF02630905. [DOI] [PubMed] [Google Scholar]
- [84].Shang F, Deng G, Obin M, Wu CC, Gong X, Smith D, Laursen RA, Andley UP, Reddan JR, Taylor A. Ubiquitin-activating enzyme (E1) isoforms in lens epithelial cells: origin of translation, E2 specificity and cellular localization determined with novel site-specific antibodies. Exp. Eye Res. 2001;73:827–836. doi: 10.1006/exer.2001.1091. [DOI] [PubMed] [Google Scholar]
- [85].De Maria A, Shi Y, Luo X, Van Der Weyden L, Bassnett S. Cadm1 expression and function in the mouse lens. Invest Ophthalmol. Vis. Sci. 2011;52:2293–2299. doi: 10.1167/iovs.10-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Retamal MA, Schalper KA, Shoji KF, Bennett MV, Saez JC. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8322–8327. doi: 10.1073/pnas.0702456104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Ebihara L, Tong JJ, Vertel B, White TW, Chen TL. Properties of connexin 46 hemichannels in dissociated lens fiber cells. Invest. Ophthalmol. Vis. Sci. 2010;52:882–889. doi: 10.1167/iovs.10-6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zampighi GA, Loo DD, Kreman M, Eskandari S, Wright EM. Functional and morphological correlates of connexin50 expressed in Xenopus laevis oocytes. J. Gen. Physiol. 1999;113:507–524. doi: 10.1085/jgp.113.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dvoriantchikova G, Ivanov D, Pestova A, Shestopalov V. Molecular characterization of pannexins in the lens. Mol. Vis. 2006;12:1417–1426. [PubMed] [Google Scholar]
- [91].Scemes E. Nature of plasmalemmal functional “hemichannels”. Biochim. Biophys Acta. 2011;1818:1880–1883. doi: 10.1016/j.bbamem.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]