Summary
Presenilins (PSs) are catalytic components of the γ-secretase complexes that promote the ε-cleavage of cell surface proteins producing cytosolic peptides shown to function in cell signaling and gene expression. In addition, secretase cleavages at γ-sites of APP substrates produce the Aβ peptides found in all people. Aggregation of Aβ peptides form the amyloid fibrils found in amyloid plaques of AD patients and aged individuals.
A common hypothesis suggests that Alzheimer's disease (AD) is caused by aggregated Aβ peptides but treatments with either inhibitors of Aβ production or anti-Aβ antibodies showed no therapeutic value. Importantly, recent evidence (Marambaud et al., 2003) shows that PS FAD mutations cause a loss of γ-secretase cleavage function at ε site of substrates manifested by decreased production of cytosolic peptides and accumulation of transmembrane γ-secretase substrates. These data support a hypothesis that PS FAD mutations promote neurotoxicity by inhibiting the γ-secretase-catalyzed ε cleavage of substrates thus reducing cell signaling while causing accumulation of membrane-bound cytotoxic peptides. Similar mechanisms may be involved in toxicities observed in clinical trials of γ-secretase inhibitors. A model of allelic interference may explain the dominant negative transmission of neurotoxic loss of function in FAD neurodegeneration.
Keywords: Allelic interference, PS and BDNF neuroprotection, excitotoxicity, FAD mutations, epsilon cleavage of γ-secretase
Introduction
Despite intense research efforts, the cause of the accelerated neuronal degeneration that causes Alzheimer's disease (AD) remains unclear. Most AD cases are sporadic affecting people over 65 or 70 years old although a small fraction of cases (<%) are caused by genetic mutations and classified as familial AD (FAD). FAD usually occurs at younger ages and follows a more aggressive clinical course than sporadic AD (SAD). Presently, age and apolipoprotein allele E4 are the most important risk factors for SAD. In contrast, FAD is caused by specific genetic changes in the genes of the amyloid precursor protein (APP), presenilin1 (PS1), and PS2. In addition to its involvement in FAD APP is the precursor of the Aβ peptides that aggregate to form the amyloid plaques (APs) used to define the disorder. PS proteins (PSs) are functional components of the γ-secretase proteolytic system that produces the Aβ peptides which aggregate in vivo to form insoluble amyloid fibrils that precipitate in the brain as APs. Aβ peptides are derived from the amyloidogenic processing of APP through the proteolyric activities of β (beta) and γ (gamma) secretases while in the non-amyloidogenic processing APP is cleaved by α (alpha) secretase within the amyloid sequence preventing production of amyloidogenic peptides. Amyloidogenic processing involves γ-secreatse cleavages at γ sites of the transmembrane sequence of APP while γ-secreatse cleavages at the ε (epsilon) site of substrates produce intracellular peptides that contain the cytoplasmic sequence of substrates. Recent work shows that a large number of cell surface proteins, including APP, are cleaved at ε sites producing intracellular peptides (termed CTF2s) similar to AICD. A number of these peptides have been shown to function in cell signaling and gene expression (reviewed in 1). Interestingly, distinct PS/γ-secretase complexes process specific substrates. Thus, APP is processed by both PS1- and PS2-containing γ-secretase complexes, whereas N-cadherin and efnB are processed only by PS1-containing complexes (1).
Despite extensive research in the last two decades there is no agreement on the proposed neurotoxicity of APs and it remains unclear whether these structures are the main causative agents of AD (reviewed in 2). A more recent hypothesis is that soluble oligomers of Aβ are the neurotoxic agents. Indeed, evidence suggests that such oligomers may interfere with synaptic function in vitro or memory function in experimental animal models (3). It is important to note however, that these models are often based on overexpression of exogenous APP, an artificial condition that does not apply to AD where there is no evidence for APP overxpression (2). Furthermore, abnormalities in animal models based on protein overexpression may result from non-specific toxicities caused by the overexpressed polypeptides, a problem that should be addressed using appropriate controls. Additional complications of overexpression mouse models arise from the fact that APP is metabolized to a large number of derivatives some of which, such as membrane-bound C-terminal fragments, may be cytotoxic (1, 2).
FAD mutations cause a loss of γ-secretase cleavage function at ε sites of substrates
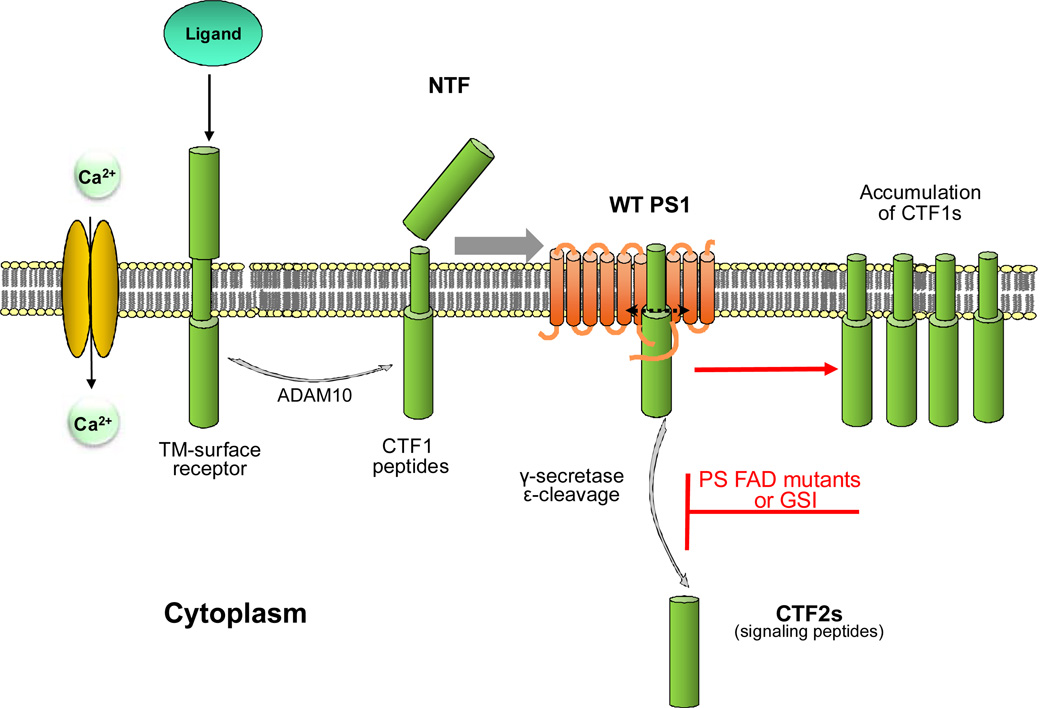
In 2003, Marambaud et al reported that PS1 FAD mutations cause a loss of γ-secretase cleavage activity at the ε-site of N-cadherin (4). This observation was replicated in other protein systems including efnB2, EphB2 and APP where it was shown that PS FAD mutants inhibit production of CTF2 peptides (5, 6, 7). These data support the hypothesis that PS FAD mutations may contribute to neurotoxicity by inhibiting production of peptides with useful biological functions (4, 8). Transmembrane CTF1 peptides, the substrates of γ-secretase, derive from the extracellular cleavage of cell surface proteins and reduction of the γ-secretase-dependent ε cleavage results in the accumulation of CTF1s and reduction of CTF2 peptides (4, 6, see also Fig. 1). Additional work reveals that increased levels of CTF1s, including those derived from APP and netrin, are cytotoxic (for review see 2). Thus, accumulation of unprocessed transmembrane CTF1 peptides caused by inhibition of γ-secretase may contribute to the neurodegeneration associated with FAD mutations in vivo. Perhaps membrane accumulation of these peptides interferes with the mobility of transmemberane proteins such as receptors in the plane of the membrane with toxic consequences. Thus, PS FAD mutations may promote neurotoxicity by reducing production of biologically active cytosolic CTF2 peptides while also promoting accumulation of toxic CTF1 fragments. Both result from loss of γ-secretase activity at ε site of substrates suggesting that increasing γ-secretase cleavage activity may be of therapeutic interest in FAD (1). Interestingly, inhibition of Aβ by γ-secretase inhibitors (GSI) has been associated with toxicity in clinical trials (9), an observation consistent with reduced production of CTF2 peptides and increased accumulation of membrane-bound CTF1 fragments expected from inhibition of γ-secretase activity. Thus, by inhibiting the ε cleavage of γ-secretase, PS FAD mutations and GSIs may cause neurototoxicity by similar mechanisms; decreasing CTF2 peptides active in cell signaling while increasing accumulation of cytotoxic CTF1 fragments. These data suggest that an important biological function of the γ-secretase system in vivo is the removal of potentially toxic transmembrane fragments resulting from extracellular cleavages, usually by ADAM (A Disintegrin And Metalloproteinase) and BACE, of cell surface proteins.
Figure 1.
Extracellular cleavage of cell surface proteins by MP ADAM10 produces TM CTF1 peptides that can be processed at ε sites by γ-secretase to produce cytosolic CTF2 peptides. The ADAM10 cleavage may be constitutive but also activated by ligand binding to receptors or calcium influx through NMDAR (2). Inhibition of the γ-secretase-dependent ε cleavage of CTF1s by either dominant heterozygous FAD mutations (see text) or GSIs may cause cytotoxicity by decreasing functional CTF2 peptides and promoting accumulation of unprocessed CTF1 substrates of γ-secretase. MP: metalloproteinase; TM: transmembrane; CTF: carboxy-terminal fragments of protein; NTF: N-terminal fragment of protein; GSI: γ secretase inhibitors; WT: wild type
The ability of BDNF and efnB to protect cortical neurons from toxic insults depends on PS1
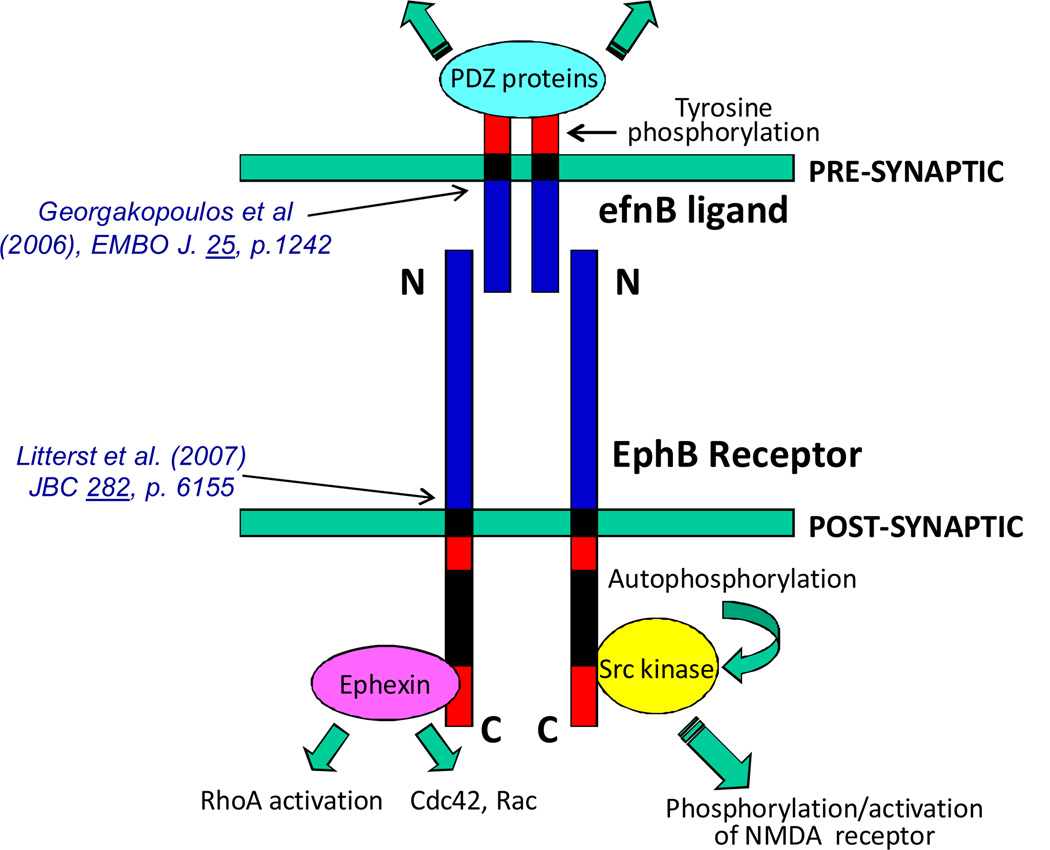
Neuronal exposure to toxic insults, such as excitotoxicity and oxidative stress, is believed to play crucial roles in neurodegenerative disorders of the CNS including AD and Parkinson's disease (PD) (10). Recent data shows that PS1 functionally interacts with EphB2 (6), a member of the EphB family of tyrosine kinase receptors that play pivotal roles in neuronal development and cell function. Interestingly, efnB ligands also act as receptors upon binding to EphB proteins and transmit information to the efnB-expressing cell. Thus, formation of the efnB-EphB complex on neuronal surfaces acts as a bidirectional system that transmits information into both, the efnB and EphB expressing cells (Fig. 2). It has been reported that ligand-activated EphB2 interacts with the NMDA receptor (NMDAR) regulating neuronal physiology and memory-related functions (11). Following ligand-receptor binding, the PS1/γ-secretase system participates in the processing of both EphB2 and efnB (5, 6) indicating that PS1 plays pivotal roles in the processing of both members of the bidirectional efnB-EphB2 ligand-receptor system (Fig. 2).
Figure 2.
Bidirectional synaptic signaling through binding of efnB to EphB. Indicated references are to publications that describe the MP/γ-secretase processing of the corresponding proteins. A limited number of factors that bind the cytoplasmic sequence of EphB and efnB are indicated. Green arrows indicate flow of signaling cascades in pre- and post-synaptic cells.
We reported that activation of EphB2 receptors by efnB protects primary cortical neuronal cultures from glutamate-induced excitotoxicity and from oxidative stress and that these functions of the efnB-EphB system depend on PS1. We also showed that PS1 is required for the neuroprotective activity of brain neurotrophins such as brain-derived neurotrophic factor (BDNF), the ligand of TrkB receptor (12). Specifically, the ability of BDNF to protect cortical neurons from glutamate-induced death depends on PS1. It is noteworthy that loss of one allele of PS1 resulted in severe reduction of the neuroprotective function of both BDNF and efnB. Thus, neuronal protection by either BDNF or efnB against toxic insults requires the presence of both WT alleles of PS1. Furthermore, we found that the function of PS1 in neuroprotection is independent of γ-secretase (12). In summary, our data suggest that neurons may rely on a number of neuroprotective ligand-receptor systems to survive toxic insults and that the function of at least some of these neuroprotective systems, such as BDNF-TrkB and efn-EphB2 depends on PS1. Chronic loss of the neuroprotective activity of PS1 against toxic insults (such as excitotoxicity and oxidative stress) in vivo would be expected to decrease the survival probability of neurons leading to increased death rates and dementia.
Our data show that inactivation of even one PS1 allele results in significant reduction of the BDNF-dependent neuroprotection against excitotoxicity (12). The requirement of both WT PS1 alleles for efficient neuroprotection in vitro seems analogous to familial frontotemporal dementia (FTD), a disease transmitted in an autosomal dominant pattern, where loss or inactivation of one progranulin (PGRN) allele by a genetic mutation (haploinsufficiency) results in increased neurodegeneration (13). There is now evidence that PS1 FAD mutations cause loss of PS1 functions, including loss of γ-secretase cleavage at ε sites, and that this loss of function is involved in FAD (4, 14). Unlike FTD mutations however which cause loss of function by inactivating one PGRN allele and reducing the amounts of cellular protein, no haploinsufficiency FAD mutants have been detected that reduce the levels of PS protein. Combined with evidence that PS forms dimmers (1, 15) and the dominant-negative pattern of FAD transmission, these observations support the hypothesis that in addition to losing functional activity, the protein products of the PS FAD mutant alleles may interfere with and inhibit the function of the WT PS protein. This model of "allelic interference in FAD" (2) is analogous to dominant-negative mutations in hereditary FXI deficiency where mutations in one allele not only impair the function of the mutant FXI homodimers but also inhibit the function of the wild-type FXI by forming heterodimers with it (16). A similar mechanism operating in FAD mutations would result in only 25% of active PS in the cell, an amount insufficient to support BDNF-dependent neuroprotection as indicated by data that even a less dramatic reduction of 50% of PS1 results in reduced neuroprotection by this neurotrophin (12). Importantly, the "allelic interference" model predicts that a 50% reduction of PS (caused by haploinsufficiency-inducing mutations) would be less toxic to neurons than a heterozygous PS FAD mutant that causes allelic interference, an outcome consistent with the absence of haploinsufficiency mutants in FAD. The allelic interference mechanism of heterozygous FAD mutations would also result in stronger inhibition of γ-secretase and increased amounts of membrane-bound cytotoxic CTF1 fragments (see above and Fig. 1) compared to simple inactivation of one allele.
Acknowledgements
supported by NIH grants R37AG017926 and R01-NS47229
References
- 1.Barthet G, Georgakopoulos A, Robakis NK. Cellular mechanisms of γ-secretase substrate selection, processing and toxicity. Progress in Neurobiology. 2012;98:166–175. doi: 10.1016/j.pneurobio.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robakis NK. Mechanisms of AD neurodegeneration may be independent of Abeta and its derivatives. Neurobiol Aging. 2011;32:372–379. doi: 10.1016/j.neurobiolaging.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 4.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, Robakis NK. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Georgakopoulos A, Litterst C, Ghersi E, Baki L, Xu C, Serban G, Robakis NK. Metalloproteinase/Presenilin1 processing of ephrinB regulates EphB-induced Src phosphorylation and signaling. EMBO J. 2006;25:1242–1252. doi: 10.1038/sj.emboj.7601031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/gamma-secretase-processing pathways of EphB2 receptor. J. Biol. Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiley JC, Hudson M, Kanning KC, Schecterson LC, Bothwell M. Familial Alzheimer's disease mutations inhibit gamma-secretase-mediated liberation of beta-amyloid precursor protein carboxy-terminal fragment. J. Neurochem. 2005;94:1189–1201. doi: 10.1111/j.1471-4159.2005.03266.x. [DOI] [PubMed] [Google Scholar]
- 8.Fortini ME. Neurobiology: double trouble for neurons. Nature. 2003;425:565–566. doi: 10.1038/425565a. [DOI] [PubMed] [Google Scholar]
- 9.Cummings J. What can be inferred from the interruption of the semagacestat trial for treatment of Alzheimer's disease? Biol Psychiatry. 2010;68:876–878. doi: 10.1016/j.biopsych.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Mattson MP. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. NeuroMolecular Medicine. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 11.Pasquale EB. Eph-ephrin bidirectional signaling in physiology and disease. Cell. 2008;133:38–52. doi: 10.1016/j.cell.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Barthet G, Dunys J, Shao Z, Xuan Z, Ren Y, Xu J, Arbez N, Mauger G, Bruban J, Georgakopoulos A, Shioi J, Robakis NK. Presenilin mediates neuroprotective functions of ephrinB and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiology of Aging. 2013;34:499–510. doi: 10.1016/j.neurobiolaging.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M, Spillantini MG. Frontotemporal lobar degeneration through loss of progranulin function. Brain. 2006;129(Pt 11):2808–2810. doi: 10.1093/brain/awl291. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer's disease: evidence for a loss-of-function pathogenic mechanism. PNAS. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, Tomita T, Iwatsubo T, Moore CL, Goate A, Wolfe MS, Shearman M, Kopan R. A presenilin dimer at the core of the gamma-secretase enzyme: insights from parallel analysis of Notch 1 and APP proteolysis. PNAS. 2003;100:13075–13080. doi: 10.1073/pnas.1735338100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kravtsov DV, Wu W, Meijers JC, et al. Dominant factor XI deficiency caused by mutations in the factor XI catalytic domain. Blood. 2004;104:128–134. doi: 10.1182/blood-2003-10-3530. [DOI] [PubMed] [Google Scholar]