Abstract
The squid-vibrio symbiosis is an experimental system being studied as a model of the chronic colonization of animal epithelia by bacterial partners. One principal question being asked with this model is: what is the role of the immune system in the dynamics of the onset and maintenance of the symbiotic state? This review focuses upon results of research to date, which have demonstrated that both cell-mediated and cell-free components of the innate immune system are involved in these processes.
Keywords: Euprymna, Vibrio fischeri, hemocytes, MAMP, PRR, symbiosis, complement
1. Introduction
The principal function of the immune system is to mediate interactions of the host with the microbiotic world. For many years, considerations of this function focused upon how the immune system responds to microbial pathogens (1). However, recent studies of a wide variety of organisms have demonstrated that many, if not most, animal and plant species have associations with microbial partners that are essential for host health. Unlike pathogenic interactions, such associations are usually persistent, both over the life of an individual and between generations, and are restricted to a coevolved set of microbes. These new findings require that we incorporate into our views of the immune system the role that mutualistic symbionts have played in evolutionary selection on immune system form and function.
Symbiotic associations vary widely across the animal kingdom. Microbial partners can occur as intracellular or extracellular, in monospecific populations or in complex multispecies consortia. The characterization of essential conserved mechanisms by which animals interface with their microbial populations and communities should be well informed by comparative studies, in much the same way that developmental biology has used a wide variety of vertebrate and invertebrate models to define conserved and divergent mechanisms of embryogenesis. One of the goals of current research in animal-microbe interactions is the development of experimental models that will shed light on the processes underlying their dynamics (2).
Perhaps the most common type of symbiosis is the colonization of the apical surfaces of animal epithelia by bacteria. Usually in vertebrates, these interactions involve highly complex communities. Understanding the 'conversation' among the partners in these speciose situations can be likened to trying to unravel the dynamics of interactions among participants at a large scientific conference. To reveal the essence of cell-cell conversation between host and symbiont, a community of biologists has been studying the binary association between the Hawaiian sepiolid squid Euprymna scolopes and its luminous vibrio partner Vibrio fischeri 3, 4). This light-organ symbiosis is horizontally transmitted, i.e., it is acquired each generation, like most extracellular bacterial partnerships with host epithelia. The squid host is colonized within hours of hatching into the environment and maintains its symbiosis throughout life. This review provides an overview of what has been learned about the role of the host's immune system in the processes of initiation, development and maintenance of this symbiotic association.
2. Basic processes bearing on immune function
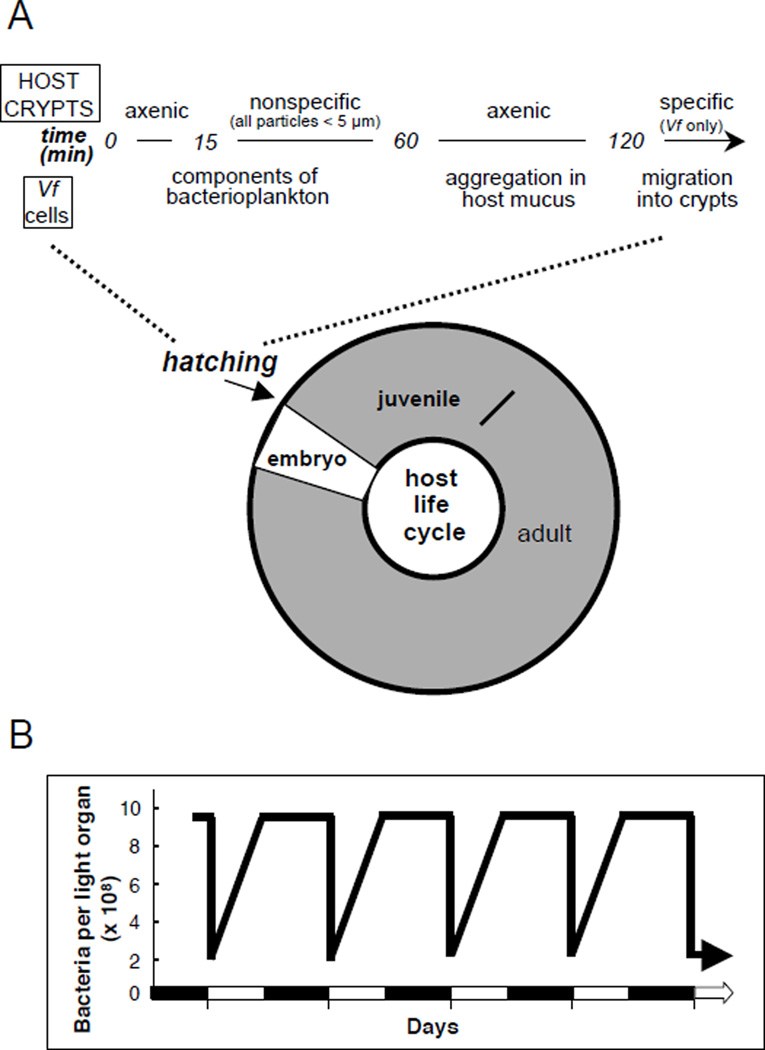
During embryogenesis the squid host develops a set of tissues poised for colonization (5). These tissues consist of a juvenile-specific superficial ciliated epithelium, the activity of which potentiates inoculation by V. fischeri, and a series of deep invaginated epithelium-lined crypt spaces, the site of eventual symbiont colonization (Fig. 1). The superficial epithelium consists of a single layer of epithelial cells supported by a blood sinus. The crypts connect to the surface of the organ by long ciliated ducts. Within minutes of hatching, the nascent symbiotic tissues begin to respond to the surrounding bacteria-rich seawater (Fig. 2A). Peptidoglycan (PGN), a microbe-associated molecular pattern (MAMP) that is shed by the bacterioplankton into the seawater, induces the host to shed copious amounts of mucus from the superficial epithelium of the juvenile light organ (6, 7). The symbiont cells, which represent less than 0.1% of the million bacterial cells in the seawater, are then harvested in the mucus. Whereas all Gram-negative bacteria will adhere to this mucus matrix, eventually V. fischeri dominates (8), resulting in an aggregated biofilm. The symbiont cells increase in numbers in the mucus due to harvesting from the water, not growth within the mucus. After a period of 2–3 hours of gathering, the symbiont population migrates through the pores on the surface, travels up the ducts, and takes up residence in the crypt spaces.
Fig. 1.
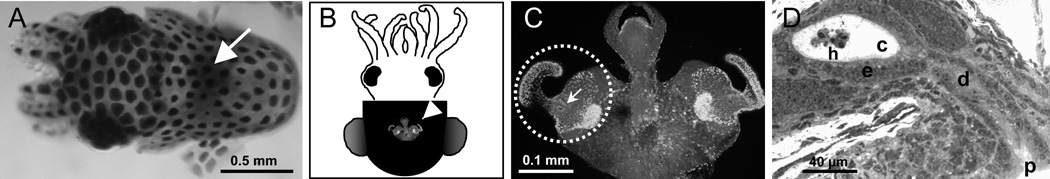
The nascent symbiotic tissues of the host. A. The light organ of the juvenile squid, which will become colonized by the symbiont, can be seen through the translucent dorsal mantle as a dark region in the center of the mantle cavity (white arrow). B. A ventral view of the light organ (white arrowhead) reveals the juvenile-specific morphology. C. A higher magnification of the organ shows the elaborate ciliated fields on each lateral surface (dotted circle, one circumscribed field). The pores where the symbionts will enter host tissues occur in the center of each field (white arrow). D. A histological section through the organ reveals the path through which the symbionts will invade host tissues. After aggregating in host-shed mucus, the symbionts will migrate into a pore (p), through ducts (d) and into the crypts spaces (e), where they interact with the crypt epithelium (e) and a population of hemocytes (h).
Fig. 2.
The dynamics of the V. fischeri populations during initiation and maintenance of the symbiosis. A. Whereas the host tissues are devoid of symbionts during embryogenesis (white portion of the host life cycle), they colonize within minutes and are present persistently (grey portion). In the minutes following hatching, the symbiont specificity of the crypts spaces is resolved. B. The symbiont population in the host organ is controlled by a daily venting of the symbionts into the surrounding environment.
In the absence of V. fischeri, no other bacteria permanently colonize the crypts; thus, mechanisms to promote symbiont colonization and deter other microbes must be operating. Analyses of the early events involved with this process (Fig. 2A) have been made possible by the ability to view the transport of fluorescently labeled materials from the surrounding seawater into the crypts by confocal microscopy (7). Such studies revealed that the crypts, which begin as axenic or sterile, are then permissive to entry. Any type of bacterial cell, as well as latex beads, can be visualized in the crypt spaces during this period. However, nothing, including V. fischeri cells, persist during this phase, and eventually all such early entrants into host tissues are eliminated by an unknown mechanism. The crypts then enter a short restrictive phase, where nothing appears to enter. This phase is coincident with the aggregation of V. fischeri on the surface of the organ. Following the eventual initial entry of symbiont cells into the crypts, all evidence suggests that the crypts are from then on exclusive to the symbiont.
In response to crypt colonization by V. fischeri cells, the host undergoes a morphogenetic program that results in the loss of the superficial ciliated field on the light organ, i.e., those tissues that have potentiated the colonization by the symbiont (9). These epithelial cells undergo apoptosis (10) and are sloughed into the mantle (body) cavity of the squid. The loss of this field takes about 4 days, but the process is irreversibly triggered by the symbionts at around 12 h following first exposure to environmental V. fischeri 11). The bacterial symbionts also induce other cellular changes in host light organ tissues, including constriction of the ducts (12), a swelling of the crypt epithelial cells (13), mucus shedding into the crypt spaces (7), and an increase in the density of the microvilli on the apical surfaces of the crypt cells (14), all of which are triggered between 12 and 24 h following first exposure to the symbionts.
Also beginning at twelve hours is a daily rhythm on the symbiosis (15–17) (Fig. 2B). Each day, in response to the light cue of dawn, much of the crypt contents are vented into the surrounding seawater. This material includes 90–95% of the symbiont population, the protein-rich matrix that surrounds the symbionts, and host hemocytes, the squid's macrophage-like blood cells, which are routinely found in the crypt spaces (see section 3 below). Following this expulsion event, the 5% of the symbiont population remaining grows to repopulate the organ. This rhythm continues throughout the life of the host.
3. Cellular immunity characters - hemocytes through the life of the symbiosis
E. scolopes, like other cephalopod species, seems to have a single blood cell type, or hemocyte. Hemocyte function appears to be principally immune, as these cells do not carry the blood pigment hemocyanin, which occurs as an extracellular protein in the hemolymph. Studies of hemocytes in the squid-vibrio symbiosis have demonstrated that they are involved in all stages of the association.
3.1 Hemocytes in early development of the symbiosis -
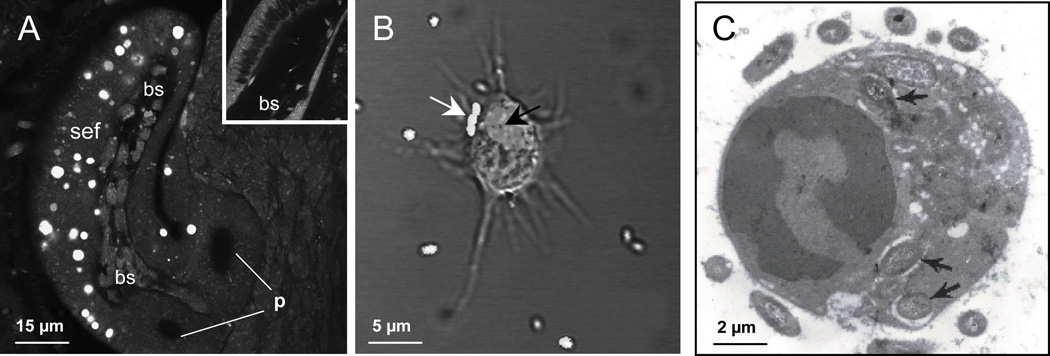
Hemocyte trafficking into the superficial epithelium of the light organ is the first symbiont-specific phenotype of the host that has been observed (18). Within two hours of exposure to V. fischeri cells, i.e., while the symbionts are aggregating outside the light organ in mucus and before they enter host tissues, hemocytes have begun to infiltrate the blood sinuses of the superficial epithelium. The response is specific to the microbial partner, as this phenotype is only present in host animals exposed to unfiltered seawater in which V. fischeri is present, i.e., unfiltered water without V. fischeri contains around a million bacterial cells to which the host does not respond. Studies of early postembryonic migration of hemocytes into the light organ have demonstrated that it occurs in response to the peptidoglycan monomer, or TCT ('tracheal cytotoxin', so called as it is the causative agent of the destruction of the airway epithelia in whooping cough caused by the bacterial pathogen Bordetella pertussis). TCT, presented as a pharmacological agent, is capable of mimicking the full hemocyte response induced by exposure to the symbiont. The trafficking of hemocytes into the superficial epithelium continues to occur such that, within hours, the blood sinuses are filled with these cells (Fig. 3).
Fig. 3.
Host hemocytes as key cellular components of the symbiosis. A. Hemocytes migrate into each blood sinus (bs) of superficial epithelial fields (sef) of the juvenile organ in response to interactions with symbiont MAMPs. (p, two of the three pores of one lateral surface). B/C. Hemocytes phagocytose bacteria that they encounter. B. A confocal/differential-interference-contrast image shows extracellular bacteria on the surface of the hemocyte (white arrow) and phagocytosed cells (black arrow). C. A transmission electron micrograph localizes bacterial cells (black arrows) to the cytoplasm of the hemocyte.
The function of the hemocytes in the superficial epithelium is not well defined at this time. Initially, it was thought that these cells migrate into the sinuses to phagocytose epithelial cells undergoing apoptosis. However, we have no evidence that the hemocytes have this function; as mentioned above, most, if not all, of the cells undergoing apoptosis are sloughed into the mantle cavity. However, some characteristics of these hemocytes suggest that they participate in developmental regression of these ciliated fields. Specifically, whereas hemocytes will continue to traffic into these blood sinuses even if the light organ is cured before the irreversible signal for morphogenesis, that signal appears to induce a change in the hemocyte gene expression (19). In earlier studies of the symbiosis, a dramatic increase in genes encoding subunits of the proteasome (20), a complex that mediates protein degradation, occurs in symbiotic animals. Studies with inhibitors of proteasome activity demonstrated that this increase in proteasome-subunit gene expression is accompanied by changes in the activity of this complex. Further, in animals cured of symbionts before the 12-h irreversible morphogenetic signal, the hemocytes in the blood sinuses do not show this transcriptional change (19). Thus, a change in hemocyte behavior occurs concomitantly with symbiont induction of host development. However, whether they are directly involved in signaling apoptosis, involved in wound healing that might be critical as cells are sloughed, or have some other function remains to be determined.
Hemocytes are also observed in the crypt spaces of juvenile light organs (Fig. 1D), although generally at very low numbers (19). Whereas an increase in the numbers of hemocytes in the superficial epithelium occurs upon first exposure to V. fischeri, significant symbiosis-associated increase in crypt hemocyte numbers is only seen after 36 h, suggesting that the symbionts in the crypts are 'invisible' to cell-mediated immunity of the host in these early hours. When present in recently colonized juvenile hosts, these hemocytes have been observed to have bacteria within their vacuoles (17) Nyholm and McFall-Ngai, 1998).
3.2 Host hemocytes in the mature symbiosis
Hemocytes occur in the crypt spaces of adult animals, but unlike those in the juvenile crypts, they have not been observed to contain bacterial cells. The function of hemocytes in the crypt spaces remains obscure, but behavior of the circulating hemocytes suggests that communication occurs between these spaces and the general circulatory system of the animal. This suggestion arose from studies of isolated hemocytes of adult animals (21). These cells can be studied ex vivo in mature E. scolopes; they are isolated from anesthetized adult animals by drawing hemolymph from the cephalic artery. The hemolymph contains approximately 5000 cells/microliter, and up to 50 – 100 microliters can be removed in a single bleed without harming the host.
Experiments with hemocytes of adults have indicated that these cells 'learn' to discriminate between the symbiont cells and the cells of other bacteria (21). The crypts of the adult can be cleared or cured of symbionts by exposing the host animals to antibiotics. Hemocytes isolated from colonized adults bind V. fischeri at very low numbers, whereas the symbiont binds in significantly higher numbers to hemocytes isolated from cured animals. The hemocytes do not change their behavior toward other closely related bacterial species as a result of curing. These data provide evidence that the hemocytes become adapted to the presence of the specific symbiont within the light organ crypts resulting in a type of “tolerance” towards V. fischeri. Such a response occurs to both cultured symbiont cells, as well as cells freshly isolated from the animal crypts. This finding demonstrates that the V. fischeri cells themselves need not be adapted to the crypt environment for the hemocytes to respond in this way, i.e., any related feature on the symbiont cell is likely to be constitutively produced. Analyses with strains of the symbiont defective in the production of the protein OmpU, an outer membrane porin, demonstrated that these mutant cells lose the ability to resist binding to host hemocytes. Co-incubation with wild type V. fischeri resulted in rescuing the OmpU mutant, suggesting that V. fischeri may secrete some component(s) that inhibits hemocyte adhesion. The comparative biochemistry and molecular biology of hemocytes from colonized and cured animals to determine characters that underly this specificity, as well as the precise nature of the ompU defect, are currently areas of active investigation.
4. Molecules/pathways of the immune system in the symbiosis
As an invertebrate, E. scolopes does not have an immune system with the clonal selection capability of the adaptive immune system of vertebrates. However, in our studies of their symbiosis with V. fischeri, we have identified many of the genes and proteins that are associated with the innate immune system. These discoveries indicate that such molecules are involved in the dynamics of a mutualistic association, i.e., the 'language' of pathogenesis is also used to mediate host-microbe interactions in a beneficial relationship.
4.1 The complement system
Analysis of the EST database created from the juvenile squid's light organs revealed a set of proteins expressed in these tissues that have sequence similarity to members of the complement system (Table 1). Components of all three complement pathways have been identified in the database. Thus far, only the C3 component has been studied in any depth in E. scolopes 22). The encoded protein has all of the molecular features defined as required for C3 function. Immunocytochemistry localized the C3 protein to the apical surfaces of epithelial cells.
Table 1.
cDNAs in the EST database encoding proteins with similarity to components of the complement cascade.
| Complement Protein | Derived Squid Protein Length (aa) |
Human Protein Size (aa) / % Similarity to Squid Protein |
Function in Vertebrates |
|---|---|---|---|
| C3a (C,L,A)b | 1696 | 1663 / 45 | Central component of all three pathways |
| C1qc (C) | 233 | 245 / 43 | Binds to antibody-antigen complex and pathogen surfaces |
| C1qBP (C) | 249 | 282 / 59 | C1q binding protein |
| C1r (C) | 156 | 705 / 55 | Cleaves C1s to an active protease |
| C1s (C) | 236 | 688 / 40 | Cleaves C4 and C2 |
| MASP (L) | 259 | 686–729 / 47 | Activating enzyme |
| Factor D (A) | 260 | 246 / 42 | Activating enzyme (serine protease) |
| Factor H (A) | 391 | 1231 / 40 | Protects host cells from activated complement |
| Factor I (A) | 243 | 583 / 47 | Protease that degrades complement components |
| C4BP (C, L) | 398 | 597 / 33 | C4 binding protein |
| CR2 (NA) | 508 | 1033–1092 / 41 | Complement receptor of B cells |
From Castillo et al., 2009.
Component of the classical (C), lectin (L), and/or alternative (A) complement pathway; NA, not applicable.
Sequences available on the Sanger Institute Database (www.sanger.ac.uk/DataSearch)
Some of the complement-encoding genes identified in the squid EST database have rarely, if ever, been shown to have homologs in the invertebrates. However, as more sequence data become available for invertebrate species, it is likely that similar molecules will be found widely represented across the animal kingdom. For example, a C3 has recently been reported in another mollusk, a bivalve (23), and a gene encoding a protein with similarity to C1q was identified in a recent study of the leech Hirudo medicinalis 24). Interestingly, the C1q protein localizes to neurons in the leech and appears to be involved in CNS repair. The finding of these putative homologs in E. scolopes provides a rich horizon for the analysis of how complement pathways are regulated in mutualistic animal-bacterial interactions.
4.2 MAMPs, PRRs, and associated pathways
The interactions of MAMPs, pattern-recognition receptors (PRRs) and the induction of their associated pathways, such as the NF-kappaB pathway, have been well studied by the immunology community in recent years. A number of studies of the squid-vibrio system have implicated MAMPs in the dynamics of the symbiosis. As mentioned above, a response to PGN in the surrounding seawater induces the mucus shedding that enables enrichment of the symbiont, and the PGN monomer TCT, which is exported by V. fischeri, induces hemocyte trafficking into the blood sinuses of the superficial epithelium of the light organ (18). These events take place while the symbiont is outside of host tissues. Once in the tissues, symbiont MAMPs induce the developmental loss of the superficial epithelium. Specifically, exposure to the lipid A component of LPS causes these cells to enter early-stage apoptosis, and TCT works synergistically with LPS to drive the cells into late stage apoptosis. Together, these molecules when added pharmacologically can induce nearly the entire morphogenetic program. Studies with mutants of V. fischeri defective in light production have suggested that symbiont luminescence also participates in producing the full morphogenetic program (19). One conundrum that has not yet been solved is how MAMPs and light production, which are presented in the crypts, reach the target tissue on the surface.
Studies of host receptors and triggered pathways may provide clues about the function of MAMPs in the symbiosis. We have identified several putative receptors, including four peptidoglycan-recognition proteins (PGRPs) (25), three lipopolysaccharide-binding proteins (LBPs; unpublished data), and one Toll-like receptor (TLR) (25). These molecules are under active investigation. Thus far, we have focused on the EsPGRPs (E. scolopes PGRPs). Analysis of the derived amino acid sequences of the full-length cDNAs predicted that EsPGRP1 is intracellular, EsPGRP2 is secreted, EsPGRP3 is a GPI (glycophosphatidylinositol)-anchored protein, and EsPGRP4 is an integral membrane protein (25). Immunocytochemistry of EsPGRP1 revealed that it is intranuclear in epithelial cells throughout the body (26). In response to either symbionts or TCT and LPS, EsPGRP1 is lost from only the nuclei of the superficial epithelium of the light organ; this loss occurs prior to their entry into late-stage apoptosis. This study was the first to show MAMP-PRR interactions as a driver of apoptosis.
The only other PRR that has been studied thus far is EsLBP1. A microarray study of early development of the host light organ demonstrated that the genes encoding two PRRs, EsPGRP1 and EsLBP1 were upregulated at 18 h into the symbiosis (27). Why EsPGRP1 gene transcription is increased concomitant with a decrease in the above-described ICC labeling will require further investigation. In contrast, the upregulation of EsLBP1 was correlated with a pronounced symbiosis-induced secretion of EsLBP1 protein into the crypt spaces (27).
Genomic and transcriptomic data, i.e., the light-organ EST database and microarrays, have implicated the NF-kappaB pathway in responses of the host to interactions with the symbiont cells (27). However, more research must be performed before we understand how this pathway behaves under the conditions of this particular mutualistic symbiosis.
4.1 Toxic oxygen-derived products/ toxic nitrogen oxides
Early studies of the symbiosis demonstrated that one of the principal mRNA species in the light organ encodes a protein with high sequence similarity to vertebrate myeloperoxidase (28). This protein of the innate immune response converts hydrogen peroxide, produced by respiratory burst activity, to hypochlorous acid, a potent microbicide. High levels of message encoding the squid halide peroxidase as well as the protein itself occur in tissues of the body that interact with bacteria, specifically the light organ, accessory nidamental gland (ANG), and the gills (29, 30). The ANG is a component of the reproductive system of the females that contains a dense consortium of bacteria; it is hypothesized that these bacteria and/or their products are incorporated into the egg capsule and that their activity prevents fouling of the eggs by microbes in the environment. When bacteria invade the blood stream of squid, they are cleared by the gills. Thus, this protein is involved in controlling both potential pathogens and mutualistic symbionts in E. scolopes.
Nitric oxide (NO), which at high concentrations is bacteriostatic or bacteriocidal, is also abundant in the squid light organ (31). In juveniles, NO and NOS, the enzyme that catalyzes the reaction resulting in NO production, occurs at very high levels in the superficial epithelium and in the ducts of the organ. Vesicles of NO occur in mucus secreted from the superficial epithelium, and inhibition of NOS or scavenging of NO causes the formation of hyperaggregates of V. fischeri. These data suggest that NO functions in some way to structure or limit the aggregating population of V. fischeri cells. Provision in the mucus may also serve to induce the gathering symbionts to turn on genes associated with resistance to NO stress, a stress that they will encounter as they pass through the ducts on their way to the crypt spaces. Colonization of the crypts by the symbiont results in a dramatic turndown in nitric oxide synthase, as well as in the product NO itself. This finding was of interest, as NOS/NO production is typically turned up as part of the innate immune response to pathogens.
5. Concluding remarks and future challenges
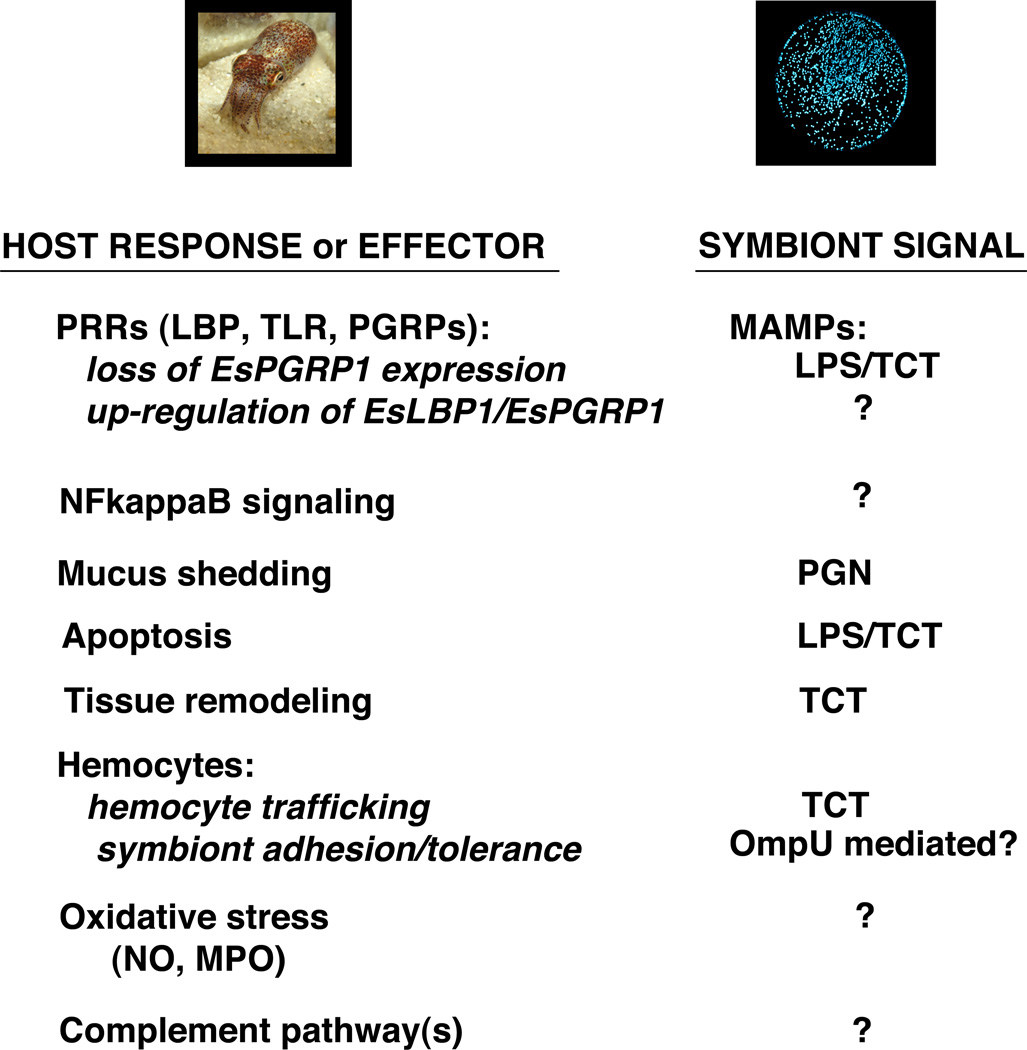
Over the past twenty years, the squid/vibrio association has served as a model for understanding the effects of beneficial bacteria on animal development. Much progress has been made in understanding the “language” of this highly specific and co-evolved symbiosis, and all indications are that interactions between V. fischeri and the innate immune system of the host heavily influence specificity and maintenance throughout the life of the association (Fig. 4).
Fig. 4.
Interpreting the “language” of the squid/vibrio symbiosis. Studies over the past twenty years have identified a number of host responses and effector mechanisms that are either induced or repressed by colonization of host tissues by V. fischeri. For many of these, the symbiont signal that induces these host effects can be linked to MAMPs such as LPS, PGN, or TCT. However, the molecular mechanisms behind some of these processes have yet to be characterized.
Many questions remain to be answered: Does the hemocyte response to V. fischeri change during host development and maturation of the association, and, if so, do these changes contribute to immune tolerance of the symbiont? What are the major hemocyte receptors and down-stream signaling pathways that are active in response to V. fischeri binding? How do the components of the complement pathway, recently described in E. scolopes, function in the context of the symbiosis? How does expression of host PRRs change during time and space in the association? What other components of the host immune system are involved in the recognition and maintenance of the symbiont?
Translating a “language”, however, requires understanding the basis of both languages. Describing the molecular mechanisms by which the symbiont influences the host’s innate immune system will be critical to interpreting the conversation. Much progress has been made in understanding how V. fischeri MAMPs (i.e., LPS, PGN and TCT) contribute to the initiation and establishment of the association and host tissue morphogenesis, but the underlying molecular mechanisms by which these processes occur (i.e., specific binding to host receptors and down stream effector mechanisms) remain largely uncharacterized. Also critical to interpreting the conversation will be understanding the symbiont factors that contribute to host immune tolerance and how this “education” may influence the fidelity of this binary relationship.
Finally, the ability of components of the innate immune system to distinguish between closely-related bacterial species in the absence of adaptive immunity suggests the existence of specificity mechanisms that are more complex then the currently described MAMP/PRR models. Recent findings in other systems suggest that highly specific and polymorphic alternate forms of immune recognition have evolved in animals (32–37). The squid/vibrio system is poised to serve as a model association for understanding novel contributions of innate immunity to host/microbe recognition and specificity.
Acknowledgements
This research was funded by NIH RO1-AI50661 to MMN, NSF IOS 0841507 to MMN and EG Ruby, NIH RR R01-12294 to EG Ruby, the WM Keck Foundation to MMN and EG Ruby, and The University of Connecticut Research Foundation to SVN.
Abbreviations
- HPO
halide peroxidase
- LBP
lipopolysaccharide-binding protein
- LPS
lipopolysaccharide
- MAMP
microbe-associated molecular pattern
- NF-kappaB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGN
peptidoglycan
- PGRP
peptidoglycan-recognition protein
- PRR
pattern-recognition receptor
Contributor Information
Margaret McFall-Ngai, Email: mjmcfallngai@wisc.edu.
Spencer V. Nyholm, Email: spencer.nyholm@uconn.edu.
Maria G. Castillo, Email: mcastill@nmsu.edu.
References
- 1.McFall-Ngai M. Are biologists in 'future shock'? Symbiosis integrates biology across domains. Nat Rev Microbiol. 2008;6:789–792. doi: 10.1038/nrmicro1982. [DOI] [PubMed] [Google Scholar]
- 2.Ruby EG. Symbiotic conversations are revealed under genetic interrogation. Nat Rev Microbiol. 2008;6:752–762. doi: 10.1038/nrmicro1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyholm SV, McFall-Ngai MJ. The winnowing: establishing the squid-vibrio symbiosis. Nat Rev Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 4.Visick KL, Ruby EG. Vibrio fischeri and its host: it takes two to tango. Curr Opin Microbiol. 2006;9:632–638. doi: 10.1016/j.mib.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Montgomery MK, McFall-Ngai MJ. Embryonic development of the light organ of the sepiolid squid Euprymna scolopes. Biol Bull. 1993;184:296–308. doi: 10.2307/1542448. [DOI] [PubMed] [Google Scholar]
- 6.Nyholm SV, Stabb EV, Ruby EG, McFall-Ngai MJ. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc Natl Acad Sci USA. 2000;97:10231–10235. doi: 10.1073/pnas.97.18.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nyholm SV, Deplancke B, Gaskins HR, Apicella MA, McFall-Ngai MJ. Roles of Vibrio fischeri and nonsymbiotic bacteria in the dynamics of mucus secretion during symbiont colonization of the Euprymna scolopes light organ. Appl Environ Microbiol. 2002;68:5113–5122. doi: 10.1128/AEM.68.10.5113-5122.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nyholm SV, McFall-Ngai MJ. Dominance of Vibrio fischeri in secreted mucus outside the light organ of Euprymna scolopes : the first site of symbiont specificity. Appl Environ Microbiol. 2003;69:3932–3937. doi: 10.1128/AEM.69.7.3932-3937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery MK, McFall-Ngai M. Bacterial symbionts induce host organ morphogenesis during early postembryonic development of the squid Euprymna scolopes. Development. 1994;120:1719–1729. doi: 10.1242/dev.120.7.1719. [DOI] [PubMed] [Google Scholar]
- 10.Foster JS, McFall-Ngai MJ. Induction of apoptosis by cooperative bacteria in the morphogenesis of host epithelial tissues. Dev Genes Evol. 1998;208:295–303. doi: 10.1007/s004270050185. [DOI] [PubMed] [Google Scholar]
- 11.Doino JA, McFall-Ngai MJ. Transient exposure to competent bacteria initiates symbiosis-specific squid light organ morphogenesis. Biol Bull. 1995;189:347–355. doi: 10.2307/1542152. [DOI] [PubMed] [Google Scholar]
- 12.Kimbell JR, McFall-Ngai MJ. Symbiont-induced changes in host actin during the onset of a beneficial animal-bacterial association. Appl Environ Microbiol. 2004;70:1434–1441. doi: 10.1128/AEM.70.3.1434-1441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J Bacteriol. 2000;182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamarcq LH, McFall-Ngai MJ. Induction of a gradual, reversible morphogenesis of its host's epithelial brush border by Vibrio fischeri. Infect Immun. 1998;66:777–785. doi: 10.1128/iai.66.2.777-785.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boettcher KJ, Ruby EG, McFall-Ngai MJ. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J Comp Physiol. 1996;179:65–73. [Google Scholar]
- 16.Graf J, Ruby EG. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc Natl Acad Sci USA. 1998;95:1818–1822. doi: 10.1073/pnas.95.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nyholm SV, McFall-Ngai MJ. Sampling the light-organ microenvironment of Euprymna scolopes: description of a population of host cells in association with the bacterial symbiont Vibrio fischeri. Biol Bull. 1998;195:89–97. doi: 10.2307/1542815. [DOI] [PubMed] [Google Scholar]
- 18.Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- 19.Koropatnick TA, Kimbell JR, McFall-Ngai MJ. Responses of host hemocytes during the initiation of the squid-vibrio symbiosis. Biol Bull. 2007;212:29–39. doi: 10.2307/25066578. (2007) [DOI] [PubMed] [Google Scholar]
- 20.Kimbell JR, Koropatnick TA, McFall-Ngai MJ. Evidence for the participation of the proteasome in symbiont-induced tissue morphogenesis. Biol Bull. 2006;211:1–6. doi: 10.2307/4134572. [DOI] [PubMed] [Google Scholar]
- 21.Nyholm SV, Stewart JJ, Ruby EG, McFall-Ngai MJ. Recognition between symbiotic Vibrio fischeri and the haemocytes of Euprymna scolopes. Environ Microbiol. 2009;11:483–493. doi: 10.1111/j.1462-2920.2008.01788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castillo MG, Goodson MS, McFall-Ngai M. Identification and molecular characterization of a complement C3 molecule in a lophotrochozoan, the Hawaiian bobtail squid Euprymna scolopes. Dev Comp Immunol. 2009;33:69–76. doi: 10.1016/j.dci.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prado-Alvarez M, Rotllant J, Gestal C, Novoa B, Figueras A. Characterization of a C3 and a factor B-like in the carpet-shell clam, Ruditapes decussatus. Fish shellfish Immunol. 2009;26:305–315. doi: 10.1016/j.fsi.2008.11.015. (2009) [DOI] [PubMed] [Google Scholar]
- 24.Tahtouh M, Croq F, Viziol J, Sautiere PE, Van Camp C, Salzet M, Daha MR, Pestel J, Lefebvre C. Evidence for a novel chemotactic C1q domain-containing factor in the leech nerve cord. Mol Immunol. 2009;46:523–531. doi: 10.1016/j.molimm.2008.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Goodson MS, Kojadinovic M, Troll JV, Scheetz TE, Casavant TL, Soares MB, McFall-Ngai MJ. Identifying components of the NF-kappaB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl Environ Microbiol. 2005;71:6934–6946. doi: 10.1128/AEM.71.11.6934-6946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodson MS, Crookes-Goodson WJ, Kimbell JR, McFall-Ngai MJ. Characterization and role of p53 family members in the symbiont-induced morphogenesis of the Euprymna scolopes light organ. Biol Bull. 2006;211:7–17. doi: 10.2307/4134573. (2006) [DOI] [PubMed] [Google Scholar]
- 27.Chun CK, Troll JV, Koroleva I, Brown B, Manzella L, Snir, et al. Effects of colonization, luminescence, and autoinducer on host transcription during development of the squid-vibrio association. Proc Natl Acad Sci USA. 2008;105:11323–11328. doi: 10.1073/pnas.0802369105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomarev SI, Zinovieva RD, Weis VM, Chepelinsky AB, Piatigorsky J, McFall-Ngai MJ. Abundant mRNAs in the squid light organ encode proteins with a high similarity to mammalian peroxidases. Gene. 1993;132:219–226. doi: 10.1016/0378-1119(93)90199-d. [DOI] [PubMed] [Google Scholar]
- 29.Weis VM, Small AL, McFall-Ngai MJ. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna-Vibrio mutualism. Proc Natl Acad Sci USA. 1996;93:13683–13688. doi: 10.1073/pnas.93.24.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small AL, McFall-Ngai MJ. Halide peroxidase in tissues that interact with bacteria in the host squid Euprymna scolopes. J Cellul Biochem. 1999;72:445–457. [PubMed] [Google Scholar]
- 31.Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. NO means 'yes' in the squid-vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell Microbiol. 2004;6:1139–1151. doi: 10.1111/j.1462-5822.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang SM, Adema CM, Kepler TB, Loker ES. Diversification of Ig superfamily genes in an invertebrate. Science. 2004;305:251–254. doi: 10.1126/science.1088069. [DOI] [PubMed] [Google Scholar]
- 33.Pancer Z, Amemiya CT, Ehrhardt GR, Ceitlin J, Gartland GL, Cooper MD. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature. 2004;430:174–180. doi: 10.1038/nature02740. [DOI] [PubMed] [Google Scholar]
- 34.De Tomaso AW, Nyholm SV, Palmeri KJ, Ishizuka KJ, Ludington WB, Mitchel K, Weissman IL. Isolation and characterization of a protochordate histocompatibility locus. Nature. 2005;438:454–459. doi: 10.1038/nature04150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nyholm SV, Passegue E, Ludington WB, Voskoboynik A, Mitchel K, Weissman IL, De Tomaso AW. fester, A candidate allorecognition receptor from a primitive chordate. Immunity. 2006;25:163–173. doi: 10.1016/j.immuni.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Ayres JS, Schneider DS. A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol. 2008;6:2764–2773. doi: 10.1371/journal.pbio.0060305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anyanful A, Easley KA, Benian GM, Kalman D. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe. 2009;5:450–462. doi: 10.1016/j.chom.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]