Abstract
Mutations in the Cu/Zn Superoxide Dismutase (SOD1) gene cause an inherited form of ALS with upper and lower motor neuron loss. The mechanism underlying mutant SOD1-mediated motor neuron degeneration remains unclear. While defects in mitochondrial dynamics contribute to neurodegeneration, including ALS, previous reports remain conflicted. Here, we report an improved technique to isolate, transfect, and culture rat spinal cord motor neurons. Using this improved system, we demonstrate that mutant SOD1G93A triggers a significant decrease in mitochondrial length and an accumulation of round fragmented mitochondria. The increase of fragmented mitochondria coincides with an arrest in both anterograde and retrograde axonal transport and increased cell death. In addition, mutant SOD1G93A induces a reduction in neurite length and branching that is accompanied with an abnormal accumulation of round mitochondria in growth cones. Furthermore, restoration of the mitochondrial fission and fusion balance by dominant-negative dynamin-related protein 1 (DRP1) expression rescues the mutant SOD1G93A-induced defects in mitochondrial morphology, dynamics, and cell viability. Interestingly, both SIRT3 and PGC-1α protect against mitochondrial fragmentation and neuronal cell death by mutant SOD1G93A. This data suggests that impairment in mitochondrial dynamics participates in ALS and restoring this defect might provide protection against mutant SOD1G93A-induced neuronal injury.
Keywords: Mitochondrial dynamics, axonal trafficking, dominant-negative DRP1, real-time imaging, astrocyte, motor neuron, SIRT3, ALS, PGC-1α
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by degeneration of upper and lower motor neurons (Boillee et al., 2006; Bruijn et al., 2004; Gurney et al., 1994; Wood-Allum and Shaw, 2010). The vast majority of ALS cases are sporadic, whereas only approximately 10% have a familial mode of inheritance (Rosen, 1993). Previous work has focused on mutations in the SOD1 gene, which encodes Cu/Zn superoxide dismutase. This highly expressed and predominantly cytoplasmic enzyme catalyzes the conversion of superoxide to hydrogen peroxide and oxygen. However, mutant SOD1 causes motor neuron death through a toxic gain-of-function mechanism as opposed to a simple loss-of-function in superoxide scavenging activity (Boillee et al., 2006; Bruijn et al., 2004; Knott and Bossy-Wetzel, 2007; Magrane et al., 2009).
While the mechanisms of mutant SOD1-mediated motor neuron death remain unclear, multiple observations indicate that mitochondrial dysfunction plays a key role. First, abnormal mitochondrial morphology and ultrastructure with cristae vacuolization have been observed in mutant SOD1 mice (Kong and Xu, 1998; Wong et al., 1995) and ALS patient samples (Sasaki and Iwata, 1996). In addition, mutant SOD1 binds preferentially to mitochondria, impairs respiration, decreases the Ca2+ buffering capacity, blocks protein import, and induces apoptosis through Bcl-2 inhibition (Damiano et al., 2006; Israelson et al., 2010; Mattiazzi et al., 2002; Pedrini et al., 2010). Furthermore, substantial evidence points to a role for secondary glutamate-mediated excitotoxicity (Boillee et al., 2006; Shaw and Ince, 1997).
An important attribute of mitochondria is their ability to divide and fuse and be transported across long motor neuron axons. These processes, collectively known as mitochondrial dynamics, are critical for neuronal energy production, synaptic function, and cell survival. Proper transport and positioning of mitochondria at synapses of the neuromuscular junction are believed to play an important role in motor neuron function (Dadon-Nachum et al., 2011). Impaired mitochondrial dynamics has been proposed to trigger axonal degeneration and is consistent with the “dying back” hypothesis of neuronal projections as an initiating event in ALS pathogenesis (Dadon-Nachum et al., 2011).
Fast axonal transport of mitochondria is mediated by the kinesin family and the dynactin/dynein motor complex. The fact that mutations in KIF5A, KIF1Bβ, and Dynactin-1 cause motor neuron degeneration further underscores the importance of this process in motor neuron functionality (Puls et al., 2003; Reid et al., 2002; Zhao et al., 2001). In addition, impairment of mitochondrial fission and fusion balance has been linked to transport defects and neurodegeneration (Chan, 2006; Knott et al., 2008). Dynamin-related protein 1 (DRP1) mediates mitochondrial fission while mitofusin 1 (MFN1), mitofusin 2 (MFN2), and optic atrophy 1 (OPA1) mediate fusion (Hoppins et al., 2007; Legros et al., 2002; Meeusen et al., 2006; Song et al., 2009). Also, MFN2 mutations cause Charcot-Marie-Tooth type-2A (CMT-2A), a neurodegenerative disorder characterized by motor neuron degeneration (Zuchner et al., 2004). These observations suggest that there is a connection between the mitochondrial fission and fusion machinery and the molecular motors that mediate axonal trafficking. Finally, excessive mitochondrial fission is induced by a wide variety of neurotoxins, i.e. nitrosative/oxidative stress, and is functionally implicated in neuronal injury and cell death (Barsoum et al., 2006; Liot et al., 2009; Yuan et al., 2007).
Recently, several studies suggested possible defects in mitochondrial dynamics in models of ALS (De Vos et al., 2007; Magrane et al., 2009; Magrane et al., 2012). The first report identified a selective decrease in anterograde mitochondrial transport in "pure" motor neuron and cortical neuronal cultures isolated from mutant SOD1 transgenic rats (De Vos et al., 2007). To visualize mitochondria, the authors used MitoTracker Red, a fluorescent mitochondrial membrane potential sensitive dye, which is not incorporated into mitochondria that lack a membrane potential. Therefore it stains predominantly mitochondria with intact membrane potential, perhaps limiting the interpretation of the results. Additionally, this fluorescent probe tends to lack specificity to label only mitochondria, and has the potential to be neurotoxic. The second report demonstrated that a decrease in both anterograde and retrograde transport increased mitochondrial fragmentation and cell death in NSC-34 cells expressing mutant SOD1 targeted to the mitochondrial intermembrane space (Magrane et al., 2009). However, this study was done only in a cell line and not primary motor neurons. The most recent report implicating defective mitochondrial dynamics in ALS was performed using “pure” motor neuron cultures, lacking astrocytic support (Magrane et al., 2012). The study identified mitochondrial defects in anterograde transport, but not retrograde transport. Although mitochondria were visualized by transfecting MitoDendra, which has no reported toxicity, potential problems might be found in the lack of astrocytic support.
To reconcile the apparent contradicting observations and to improve the experimental models, we co-expressed DsRed2-Mito, a red fluorescent protein which exhibits no toxicity in primary neurons, to visualize mitochondria. Additionally, we co-cultured motor neurons on top of a spinal cord astrocyte monolayer. This key modification makes this experimental system of greater physiological relevance compared to cell lines and "pure" neuronal culture models.
Caloric restriction delays aging and age-related diseases including neurodegeneration (Anderson et al., 2009; Colman et al., 2009; Qin et al., 2006; Sohal and Weindruch, 1996; Someya et al., 2007; Weindruch and Walford, 1982). In recent years the sirtuins, a group of NAD+-dependent deacetylases, have been found to mediate the protective effects of caloric restriction (Cohen et al., 2004; Donmez and Guarente, 2010; Finkel et al., 2009; Haigis and Sinclair, 2010; Howitz et al., 2003; Lin et al., 2000; Someya et al., 2010). SIRT3, the majority of which resides in the mitochondria, plays an important role in the cellular ROS defense and mitochondrial energy metabolism, and provides protection against aging-related hearing loss in vivo and excitotoxic insults in cultured neurons in vitro (Bell and Guarente, 2011; Qiu et al., 2010; Shi et al., 2005; Someya et al., 2010; Tao et al., 2010). Moreover, SIRT3 has been shown to be regulated by PGC-1α which stimulates mitochondrial biogenesis and is associated with ROS suppression and neuroprotection (Giralt et al., 2011; Kong et al., 2010; St-Pierre et al., 2006; Wu et al., 1999). Whether SIRT3 can restore defects in mitochondrial dynamics and protect against mutant SOD1G93A has never been tested.
Here, we report that mutant SOD1G93A causes mitochondrial fragmentation and inhibits both anterograde and retrograde mitochondrial transport in primary spinal cord motor neurons co-cultured with spinal cord astrocytes. In addition, we demonstrate that mutant SOD1G93A-mediated mitochondrial changes are associated with impaired motor neuron development and reduced dendritic sprouting. Furthermore, restoring the fission and fusion balance with dominant-negative DRP1K38A expression rescued the neurons from SOD1G93A-induced mitochondrial fragmentation and cell death. In addition, SIRT3 and PGC-1α expression prevented mitochondrial fragmentation and cell death by mutant SOD1G93A. In summary, our data provides an updated model of the effects of mutant SOD1G93A on mitochondrial morphology and transport in motor neurons.
EXPERIMENTAL PROCEDURES
Reagents and plasmids
The pDsRed2-Mito vector was obtained from Clontech. The pcDNA3.1-SOD1WT and -SOD1G93A vectors were obtained from Dr. Alvaro G. Estevez (University of Central Florida, Orlando, FL, USA). The pcDNA3-DRP1K38A vector was from Dr. Alexander M. van der Bliek (David Geffen School of Medicine at UCLA, Los Angeles, CA, USA). The pcDNA3.1-SIRT3-HA vector was provided by Dr. Eric M. Verdin (Gladstone Institute of Virology and Immunology at UCSF, San Francisco, CA, USA). The pcDNA4-myc-PGC-1α (Addgene plasmid 10974) was obtained from Dr. Toren Finkel (Center for Molecular Medicine, NIH, Bethesda, MD, USA) through Addgene. The pβ–actin-Map2c/EGFP was from Dr. Doll (Novartis Institute for Biomedical Research, Basel, Switzerland). All plasmids were purified using the Endotoxin-free Marligen Maxiprep Kit (Diagnostic technology, Belrose, Australia). The detergents, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate (CHAPS), were purchased from Omega Scientific (Tarzana, CA, USA) and G-biosciences (Maryland Heights, MO, USA), respectively. Poly-L-lysine, BSA, DNase I, Opti-prep, Phenol Red, Penicillin-streptomycin (Pen/Strep), nicotinamide (NAM), N-ethylmaleimide (NEM), trichostatin A (TSA), cytosine arabinoside, formaldehyde, glutamine, MgCl2, pyruvate and F-12 HAM were all obtained from Sigma-Aldrich (St. Louis, MO, USA). Phenol-free Dulbecco's Modified Eagle Medium (DMEM) with high glucose and bovine calf serum (BCS) were obtained from Thermo Scientific (Rockford, IL, USA). Trypsin (2.5%, 10×), L-15, GlutaMAX and Earle’s Balanced Salt Solution (EBSS) were purchased from Gibco (Carlsbad, CA, USA). Lipofectamine 2000, Hoechst 33342, and neurobasal medium were purchased from Invitrogen (Carlsbad, CA, USA). Protease inhibitor cocktails were purchased from Roche (Indianapolis, IN, USA). EDTA was purchased from Calbiochem (Darmstadt, Germany). Dithiothreitol (DTT) was purchased from GE Healthcare (Piscataway, NJ, USA). Alexa 488-conjugated goat anti-mouse secondary antibodies were from Molecular Probes (Grand Island, NY, USA). Amaxa Nucleofector kit for rat neurons was purchased from Lonza (Basel, Switzerland). Mouse monoclonal anti-rat p75 antibody MC-192 and mouse monoclonal anti neurofilament H non-phosphorylated (SMI-32) was from Abcam (Cambridge, MA, USA). Rat anti-mouse IgG1 microbeads and MACS separation columns were obtained from Miltenyi Biotec (Auburn, CA, USA). SIRT2/3 Fluorimetric Drug Discovery Kit was purchased from Enzo Life Sciences (Farmingdale, NY, USA).
Mice and Rats
SOD1G93A transgenic mice (B6.Cg-Tg (SOD1-G93A) 1Gur/J) were purchased from Jackson Laboratory. Timed-pregnant Sprague Dawley rats were purchased from Charles River. All experiments were approved by the Institutional Animal Care and Use Committee of University of Central Florida College of Medicine.
Astrocyte-motor neuron co-cultures
Astrocytes were prepared from E18 rat embryos. Spinal cords were isolated and then incubated for 40 min at 37°C in 0.025% Trypsin in EBSS agitating frequently. Tissues were transferred into a new tube with 1 ml L15 supplemented with 0.4% BSA and 0.1mg/ml DNase I and triturated about 10 times using P1000 pipette. The fragments were allowed to settle for 2 min and trituration was repeated until cells were well dissociated. Cells were then spun down through 2 ml 4% BSA cushion which was disposed onto the bottom of the tube at 470 rpm and room temperature for 5 min using centrifuge with swing-out rotor (Eppendorf 5810R). The cell pellet was resuspended in DMEM medium supplemented with 10% FBS, Pen/Strep, and 15 mM HEPES pH 7.4 and cultured at 37°C, 5% CO2 for one week. After reaching confluence, the astrocyte cultures were placed on a shaking platform and 300 rpm shaking was applied to eliminate microglia for 24 h at 37°C. Supernatant with floating cells was removed and repeat shaking for another 24 h in the fresh medium. After removing the supernatant, the last shaking was applied with 10 µM cytosine arabinoside in the medium for 48 h. The remaining cells comprised of astrocytes were trypsinized and plated into the poly-L-lysine (1mg/ml) coated Lab-Tek II (#1.5 German coverglass) 8-chamber slides (Thermo Fisher Scientific, Pittsburgh, PA, USA) at the density of 2×104 cells/cm2 in phenol-free DMEM medium supplemented with 10% BCS, 2 mM glutamine, 25 mM HEPES pH 7.4, 10% F-12 HAM, and Pen/Strep. Motor neurons were seeded on top after 5 days in grown in culture.
Motor neurons were prepared from one litter of E15 rat embryos. Spinal cords were isolated, separated into two 15 ml polystyrene tubes, and then incubated for 10 min at 37°C in 1 ml 0.025% Trypsin (2.5%, 10×) in EBSS under frequent agitation. The fragments of the spinal cords were transferred into a new 15 ml tube with 1 ml L-15 supplemented with 0.4% BSA and 0.1mg/ml DNase I. The tissues were then dissociated using a p1000 blue tip by triturating for 8 times and allowed to settle for 2 min at room temperature. The supernatant was then centrifuged at 470 g at room temperature for 5 min on top of a 2 ml 4% BSA cushion in L-15. After removing the supernatant, cell pellet was resuspended in Amaxa Nucleofector buffer (for rat neurons) and 4 µg DNA was added, followed by electroporation using the electroporator device (Amaxa) and G13 program (Basel, Switzerland). The transfected cells were then diluted in 4 ml L-15 and layered on top of 2 ml solution containing 12% OptiPrep, 88% L-15, and 0.4% Phenol Red followed by gradient centrifugation using centrifuge with swing-out rotor (Eppendorf 5810R) for 15 min at 830 g, room temperature. Motor neurons, located at the interface between the clear and red phases, were collected into 1 ml using a P1000 pipette, and pelleted by centrifugation at 470 g at room temperature for 5 min through a 2ml 4% BSA cushion. After removing the supernatant, cell pellet was resuspended in 98 µl PBS with 0.5% BSA. Two microliter mouse monoclonal anti-rat p75 MC-192 antibodies were added to the motor neurons and incubated for 10 min at 4 °C. Cells were washed with 10 ml 0.5% BSA in PBS followed by centrifugation for 5 min at 470 g, room temperature through a 2 ml 4% BSA cushion. Pellet was resuspended in 80 µl 0.5% BSA in PBS and 20 µl rat anti-mouse IgG1 microbeads were added to the motor neuron-p75 antibody mix. After 15 min incubation at 4 °C, cells were washed with 10 ml 0.5% BSA in PBS followed by centrifugation for 5 min at 470 g, room temperature through a 2 ml 4% BSA cushion. Cell pellet was resuspended in 500 ml 0.5% BSA in PBS and applied onto the MACS separation columns which were already placed in the mini MACS magnet and pre-washed with 500 µl 0.5% BSA in PBS. The columns were rinsed 3 times with 500 µl 0.5% BSA in PBS and then removed from the magnet. One microliter 0.5% BSA in PBS was applied onto the column and the positive fraction was collected as purified motor neuron fraction. The purified motor neurons were seeded on top of an astrocyte monolayer in 50% conditioned medium from the astrocyte culture and 50% fresh neuronal medium composed of DMEM medium supplemented with 10% BCS, 2 mM glutamine, 25 mM HEPES pH 7.4, 10% F-12 HAM, and Pen/Strep. Analysis was performed at 2 DIV.
Primary cortical neuronal culture preparation and transfection
Cortical neuronal cultures were prepared from embryonic day E18 rat embryos in neuronal medium (2% Neuronal Supplement 21, 1× Pen/Strep, 2 mM GlutaMAX in neurobasal medium) (Barsoum et al., 2006). Neuronal Supplement 21 was prepared according to Chen et al (Chen et al., 2008). Cells were placed on poly-L-lysine (1mg/ml) coated coverslips at 40,000 cells per well in 24-well plates. Transfection was performed on 5 DIV using Lipofectamine 2000.
Live cell imaging
Time-lapse imaging was performed using an Axiovert Zeiss 100M inverted fluorescence microscope equipped with a Plan-Apochromat 63×1.4 NA oil objective, a DG-4/Lambda 10-2 combo Xe-arc illumination unit (Sutter), and a Sensicam QE cooled CCD camera (PCO AG, Germany) and controlled by MetaMorph 7.5 software (Molecular Devices). Motor neurons were grown on Lab-Tek II 8-chamber slides (#1.5 German coverglass) in DMEM medium supplemented with 10% BCS, 2 mM glutamine, 25 mM HEPES pH 7.4, 10% F-12 HAM, and Pen/Strep without phenol red. Time-lapse images were acquired in a controlled environment using Axiovert Incubator XL 100/135 (37°C, humidified 5% CO2). To visualize DsRed2-Mito, the excitation filter was S555/28× (Chroma) and the emission filter was S617/73m (Chroma). To visualize EGFP, the excitation filter was S490/20× (Chroma) and the emission filter was S528/38m (Chroma). 3D images were acquired with the Multi-Dimensional Acquisition module in MetaMorph 7.5. For the mitochondrial movement experiments, one neurite (~ 100 µm in length) was selected for 10 neurons per group at 2 DIV (Song et al., 2011). The mitochondrial movement was recorded for 5 min at 5 second intervals (5 planes, 1 µm step size). The kymographs were generated of 25 µm widthby average projections of all planes without background subtraction using MetaMorph 7.5. The measurements of mitochondrial velocity and moving distance were exported to Microsoft Excel for further analysis.
Imaging of fixed cultures
Two days after transfection, the cortical neurons were fixed with 3.7% formaldehyde and 5% sucrose in PBS pH 7.4 for 15 min at 37 °C. Three-dimensional images were acquired (2×2 binning, 0.2 µm step size, 20–25 z-planes) with the Multi-Dimensional Acquisition module in Metamorph 7.5. Images were surface rendered using Metamorph 4D-viewer and exported as TIFF files. Mitochondrial numbers and length were measured as previously described (Song et al., 2008).
Immunocytochemistry of motor neurons and cell death
Two days after transfection, neuronal cultures were fixed with 3.7% formaldehyde and 5% sucrose in PBS pH 7.4 for 15 min at 37 °C. After fixation the cultures were permeabilized with 0.1% Triton X-100 in PBS, pH 7.4, for 10 min at room temperature. Unspecific binding was blocked with 3% BSA, 3% FBS in PBS, pH 7.4, for 1 h at room temperature. Mouse monoclonal anti-SMI-32 antibodies (Abcam) were used for motor neuron staining at 1:5000 dilution in blocking solution and were incubated overnight at 4°C. After washing three times with PBS, the cultures were incubated with Alexa 488-conjugated goat anti-mouse secondary antibodies (Molecular Probes) at 1:200 dilution in blocking solution (1 h, room temperature). After washing three times with PBS, nuclei were stained using Hoechst 33342 (1:10,000 in PBS) at room temperature for 10 min. Neuronal cell death was scored by microscopy. Neurons with reduced soma size, retracted processes, fragmented mitochondria, and condensed nuclei were scored as dead.
Cell death scoring was performed on triplicate coverslips from three independent experiments (Barsoum et al., 2006; Song et al., 2011).
Deacetylase activity assay
Spinal cord tissues from control littermates or SOD1G93A transgenic mice (5–7 months old) were lysed with buffer containing 50mM HEPES pH 7.4, 2mM MgCl2, 1mM EDTA, 2% CHAPS, 2mM DTT, 10mM NAM, 1uM TSA, 10mM NEM and protease inhibitor cocktails (Roche). Eight micrograms of protein was used to perform the duplicate measurements of deacetylase activity assay using the SIRT2/3 Fluorimetric Drug Discovery Kit (Enzo Life Sciences). The data were collected using 2104 EnVision Multi-label Plate Reader (PerkinElmer) with the excitation at 355 nm and the emission at 435 nm.
Statistics
Data from populations or velocity of mitochondria are represented as mean ± S.E.M. Student’s t-test was used to compare groups. Statistical analyses were performed using Microsoft Excel.
RESULTS
Mutant SOD1G93A triggers mitochondrial fragmentation and neuronal cell death in spinal cord motor neurons
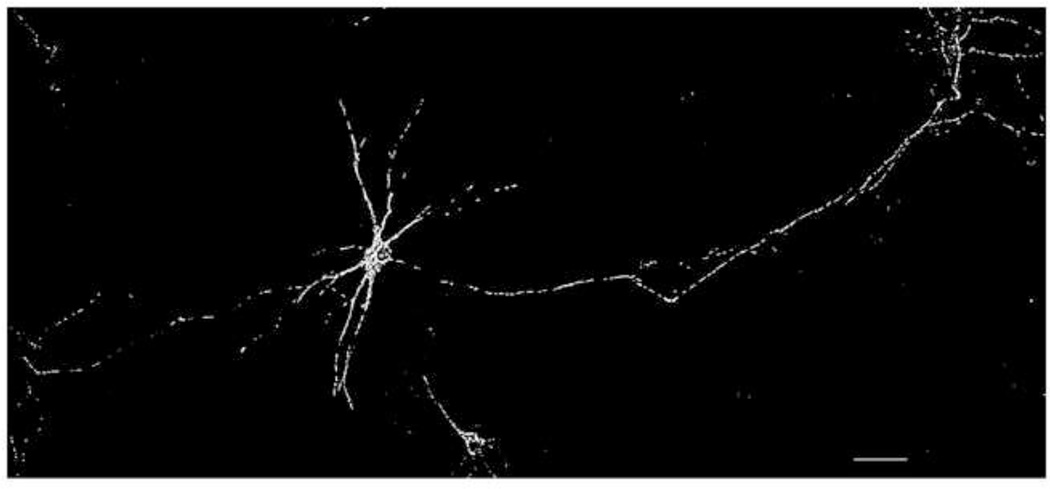
To investigate the effects of mutant SOD1G93A on mitochondrial morphology, we isolated rat spinal cord motor neurons using an improved technique. We were able to obtain motor neuron cultures with smooth neurites (Fig. 1). The neurites of 7 DIV motor neurons exhibited a length of several hundred microns, affirming that the motor neurons were well differentiated and in good condition.
Figure 1.
A representative fluorescence wide-field microscopic image of one 7 DIV rat motor neuron co-cultured with astrocytes. The motor neuron was transfected with DsRed2-Mito to visualize mitochondria. Scale bar: 100µm.
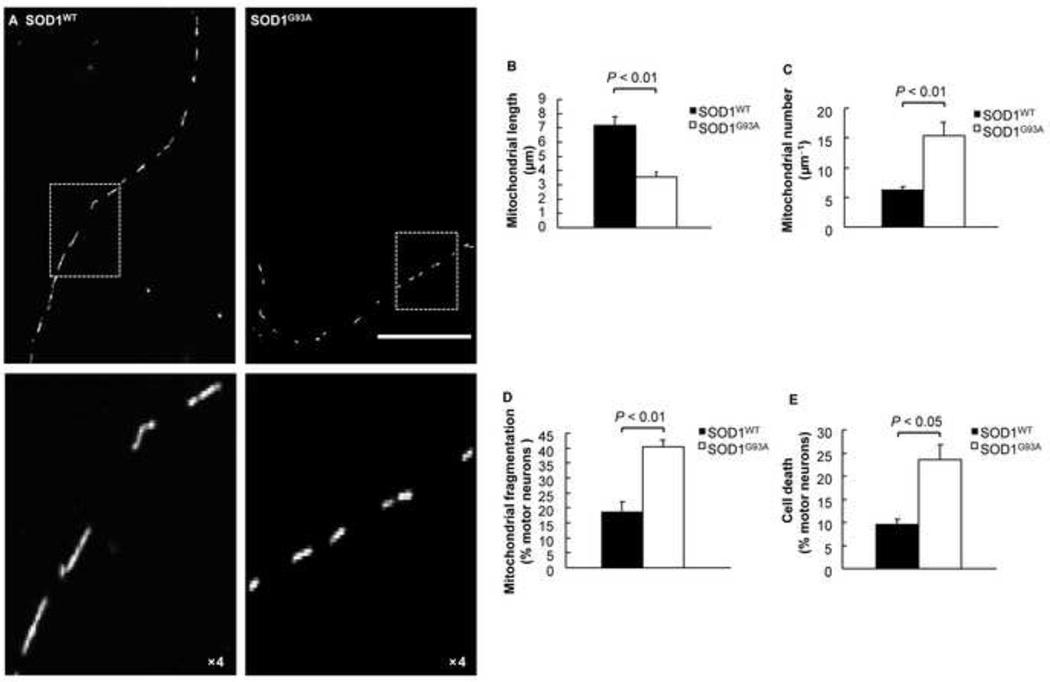
Mitochondria of SOD1WT motor neurons exhibited normal elongated morphology. By contrast, mutant SOD1G93A motor neurons revealed mitochondria of smaller size and more round shape (Fig. 2A). The reduced size may be due to either an increase in mitochondrial fission or an inhibition of mitochondrial fusion. Further analysis showed that the mean mitochondrial length was 7.1 µm for SOD1WT motor neurons, while it was only 3.9 µm for SOD1G93A motor neurons (Fig. 2B). The increased fission to fusion events led to higher mitochondrial number in the neurites of SOD1G93A motor neurons than that of SOD1WT (Fig. 2C). To further quantify the reduced mitochondrial length, we scored individual motor neurons. The percentage of neurons exhibiting short, round mitochondria was significantly greater (~40%) for SOD1G93A motor neurons compared to SOD1WT neurons (~18%) (Fig. 2D). In addition, the change in mitochondrial morphology correlated with elevated motor neuron cell death (Fig. 2E). In summary, these results indicate that mutant SOD1G93A triggers an increase in round mitochondria in spinal cord motor neurons, suggestive of increased mitochondrial fission and inhibition of fusion.
Figure 2.
Mitochondrial fragmentation occurs in mutant SOD1G93A motor neurons. A) Fluorescence micrographs (top panels) and close up views (bottom panels) of mitochondria in 2 DIV motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A co-cultured with astrocytes. Scale bar: 50µm. B) Mean length of mitochondria in motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A (n = 10). C) Number of mitochondria in 100 µm neurites of motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A (n = 10). D) Mitochondrial fragmentation of motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A in mixed cultures. E) Cell death of 2 DIV motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A. P value: two-tail paired Student’s t-test.
Mutant SOD1G93A triggers a decrease in bi-directional axonal transport of mitochondria
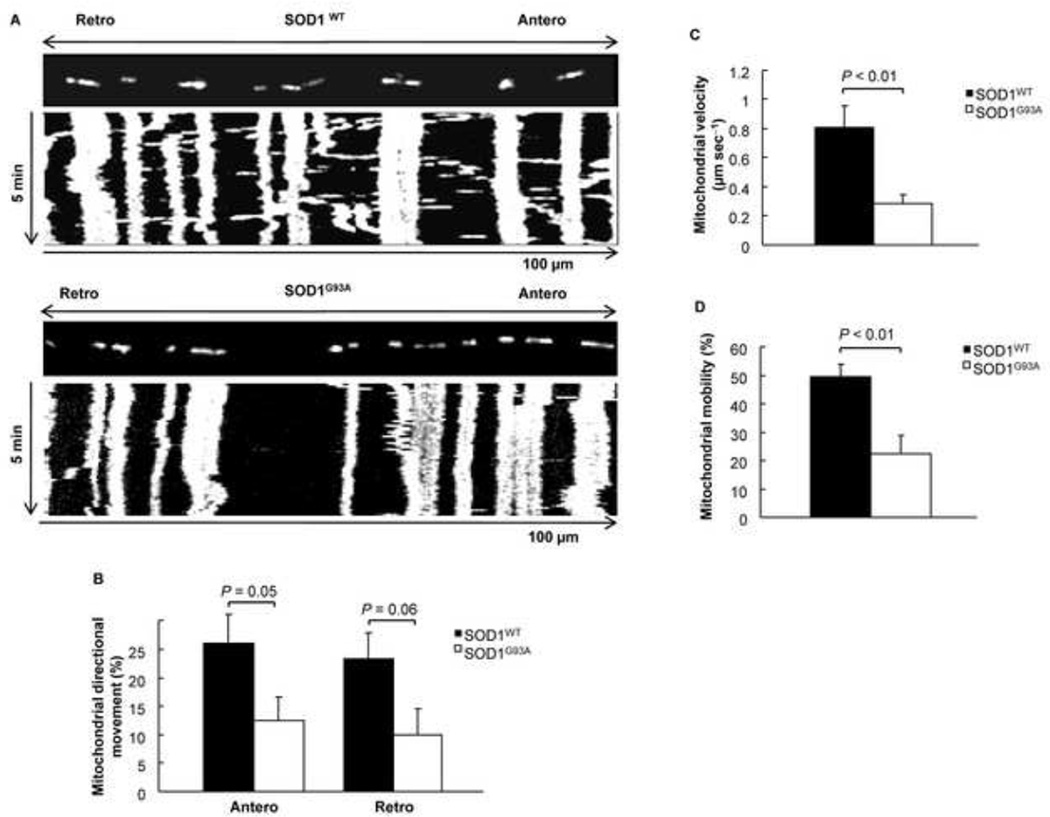
Defects in transport of mitochondria have been previously reported in mutant SOD1G93A expressing “pure” motor neuron cultures and the motor neuron-like cell line NSC-34 (De Vos et al., 2007; Magrane et al., 2009). However, the results were partially conflicting. To investigate how changes in SOD1G93A-induced mitochondrial fragmentation affect mitochondrial axonal transport in motor neuron-astrocyte co-culture system, we used fast acquisition fluorescence time-lapse imaging to measure the directional (anterograde and retrograde) movement of mitochondria over a period of 5 minutes. A representative kymograph of motor neurons expressing SOD1WT reveals both vertical and horizontal lines, indicating that mitochondria were mobile and exhibited substantial movement in both anterograde and retrograde directions (Fig. 3A, top panel, and Supplemental Movie 1). By contrast, a kymograph for mutant SOD1G93A motor neurons displayed mostly vertical lines, indicating very little mitochondrial movement and reduced axonal transport (Fig. 3A, bottom panel, and Supplemental Movie 2). Quantitative analysis indicated that the mitochondrial anterograde movement was significantly decreased from 26.29% in SOD1WT motor neurons to 12.56% in SOD1G93A motor neurons (Fig. 3B). Similarly, retrograde transport was decreased from 23.09% in SOD1WT motor neurons to 9.95% in SOD1G93A motor neurons (Fig. 3B). Further analysis demonstrated that the velocity of mitochondrial transport was decreased from 0.81 µm sec–1 in SOD1WT motor neurons to 0.28 µm sec–1 in SOD1G93A motor neurons (Fig. 3C). Moreover, the mitochondrial mobility was also decreased by more than 50% (Fig. 3D). These results demonstrate that SOD1G93A triggers bi-directional mitochondrial axonal transport defects in motor neurons co-cultured with astrocytes. The changes in mitochondrial morphology may provide an explanation for the defects in axonal transport (Misko et al., 2010; Varadi et al., 2004).
Figure 3.
Mutant SOD1 G93A triggers a decrease in axonal anterograde and retrograde transport of mitochondria in 2 DIV motor neurons. A) Kymographs of motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A (Supplement movie 1 and 2). B) Mitochondrial directional movement in motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A. C) Mean velocity of mitochondria in the axons of motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A. D) Mitochondrial mobility in motor neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A. P value: two-tail paired Student’s t-test, n = 10.
Mutant SOD1G93A triggers abnormal neuronal differentiation
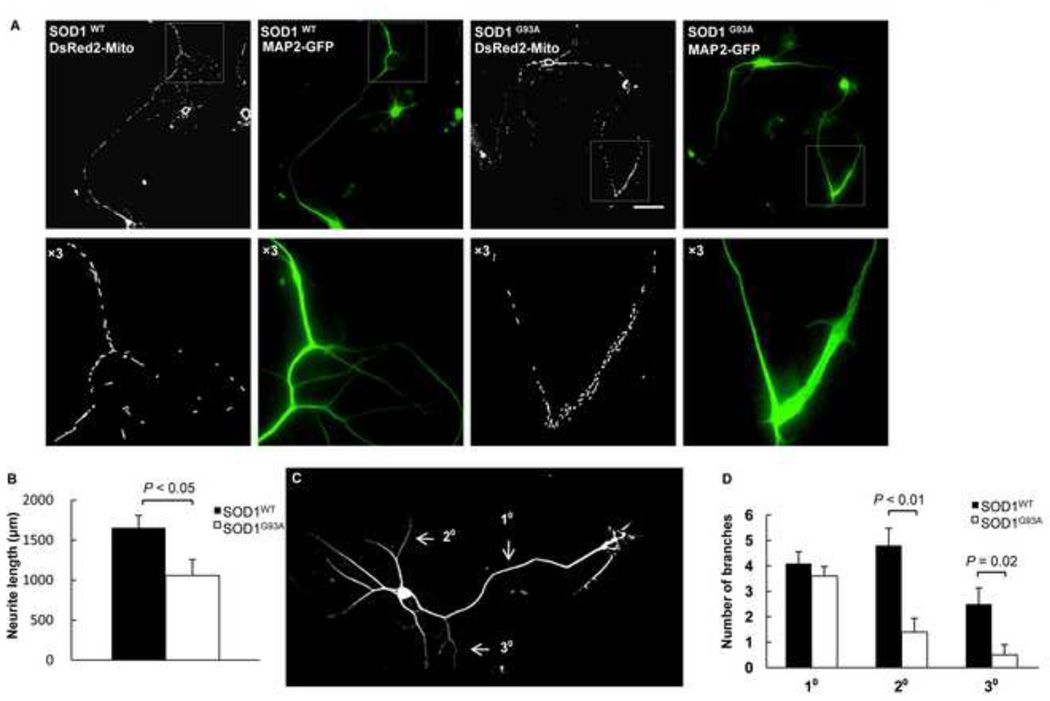
To investigate whether the defects in transport of mitochondria impact neuronal differentiation, we expressed SOD1WT or SOD1G93A plus DsRed2-Mito and MAP2-EGFP in spinal cord motor neurons. In SOD1WT motor neurons, mitochondria were elongated and evenly distributed along neurites (Fig. 4A, left). However, in motor neurons expressing mutant SOD1G93A, more fragmented mitochondria were present and accumulated in growth cones (Fig. 4A, right). Furthermore, the average neurite length of 1655 µm for control motor neurons (n = 10) was significantly decreased to 1057 µm in the presence of mutant SOD1G93A (n = 10) (Fig. 4B). To further characterize the apparent developmental defect caused by SOD1G93A, we quantified the degree of neurite branches. The neuronal extensions were grouped into first, second, and third arbor as illustrated (Fig. 4C). Both second and third arbors were significantly decreased in SOD1G93A motor neurons (Fig. 4D). Thus, the SOD1G93A-induced abnormal mitochondrial morphology is accompanied by altered neuronal differentiation.
Figure 4.
Mutant SOD1 G93A triggers defective neurite branching and abnormal mitochondrial accumulation in growth cones. A) Representative 3D fluorescence wide-field microscopic images and close up views below of motor neurons expressing DsRed2-Mito, MAP2-EGFP, and either SOD1WT or SOD1G93A. Scale bar: 50 µm. B) The total neurite length of motor neurons expressing DsRed2-Mito, MAP2-EGFP, and either SOD1WT or SOD1G93A. C) The scheme for the neurite branching quantification. D) The number of branches of motor neurons expressing DsRed2-Mito, MAP2-EGFP, and either SOD1WT or SOD1G93A. P value: two-tail paired Student’s t-test, n = 10.
Restoring mitochondrial fusion with dominant-negative DRP1K38A rescues neuronal cell death
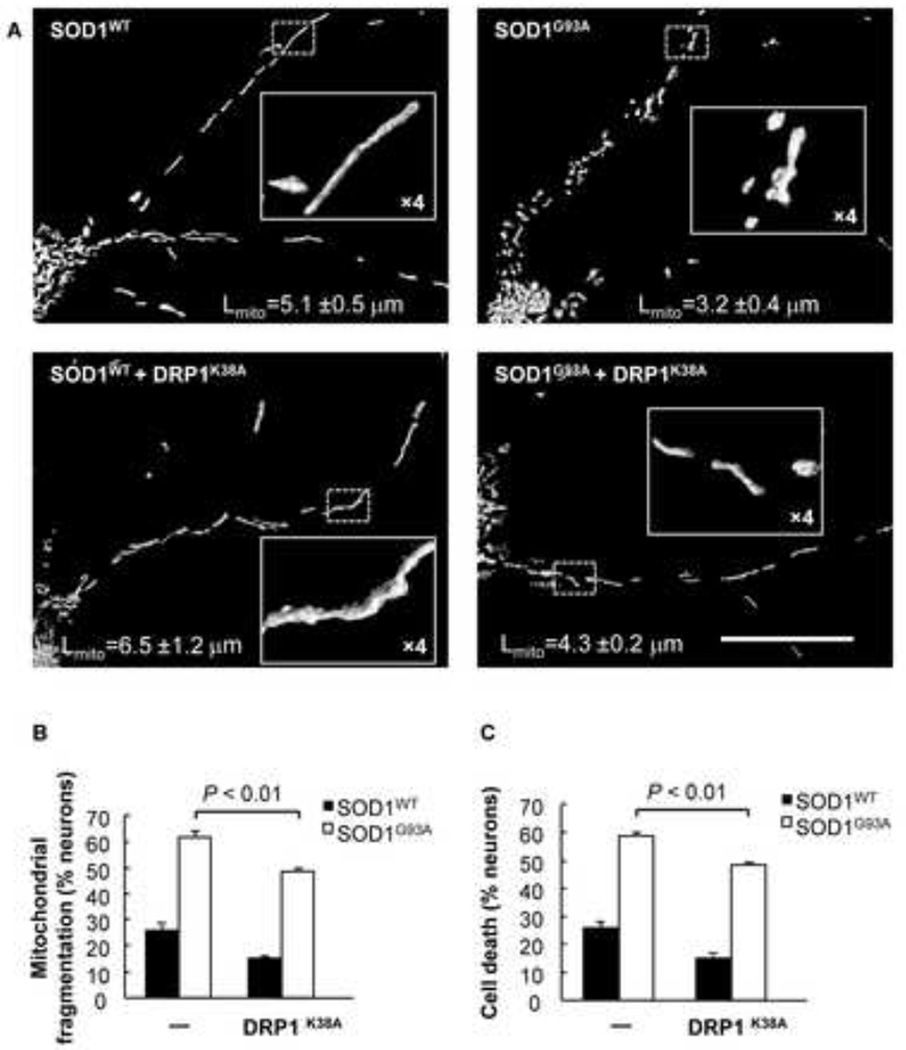
The dominant-negative DRP1K38A mutant exhibits no GTPase activity and therefore inhibits mitochondrial fission (Smirnova et al., 2001; Smirnova et al., 1998; van der Bliek et al., 1993). To test whether blocking excessive mitochondrial fission by DRP1K38A could rescue SOD1G93A-induced mitochondrial fragmentation and neuronal cell death, we co-transfected cortical neurons with SOD1WT or SOD1G93A and DsRed2-Mito, either alone or in combination with DRP1K38A. SOD1WT neurons co-expressing DRP1K38A demonstrated a moderate increase in mitochondrial length (~6.5 µm) (Fig. 5A, bottom left) compared to SOD1WT neurons (~5.1 µm) (Fig. 5A, top left). Expression of both SOD1G93A and DRP1K38A led to an increase in mitochondrial length (~4.3 µm) (Fig. 5A, bottom right) when compared with neurons expressing SOD1G93A alone (Fig. 5A, top right). Furthermore, SOD1WT neurons exhibited ~25.8% mitochondrial fragmentation, which is expected for healthy neurons (Song et al., 2011), while the percentage of mitochondrial fragmentation was decreased to 14.9% when DRP1K38A was present (Fig. 5B). SOD1G93A neurons exhibited 61% mitochondrial fragmentation, which was significantly decreased to 48% when DRP1K38A was co-expressed (Fig. 5B). The reduction in mitochondrial fragmentation coincided with improved neuronal survival (Fig. 5C). In summary, these results indicate that the inhibition of mitochondrial fission by DRP1K38A is able to rescue neurons from the mitochondrial fragmentation and cell death caused by SOD1G93A.
Figure 5.
Inhibiting mitochondrial fission with the GTPase-defective DRP1K38A mutant rescues mitochondrial fragmentation and neuronal cell death by mutant SOD1G93A. A) Fluorescence micrographs and close up views of cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A at day 2 post transfection. Scale bar, 50 µm. Mitochondrial length (Lmito) is indicated as mean ± S.E.M. (n = 10). B) Mitochondrial fragmentation of cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. C) Cell death of cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. P value: two-tail paired Student’s t-test.
Dominant-negative DRP1K38A rescues neurons from trafficking defects
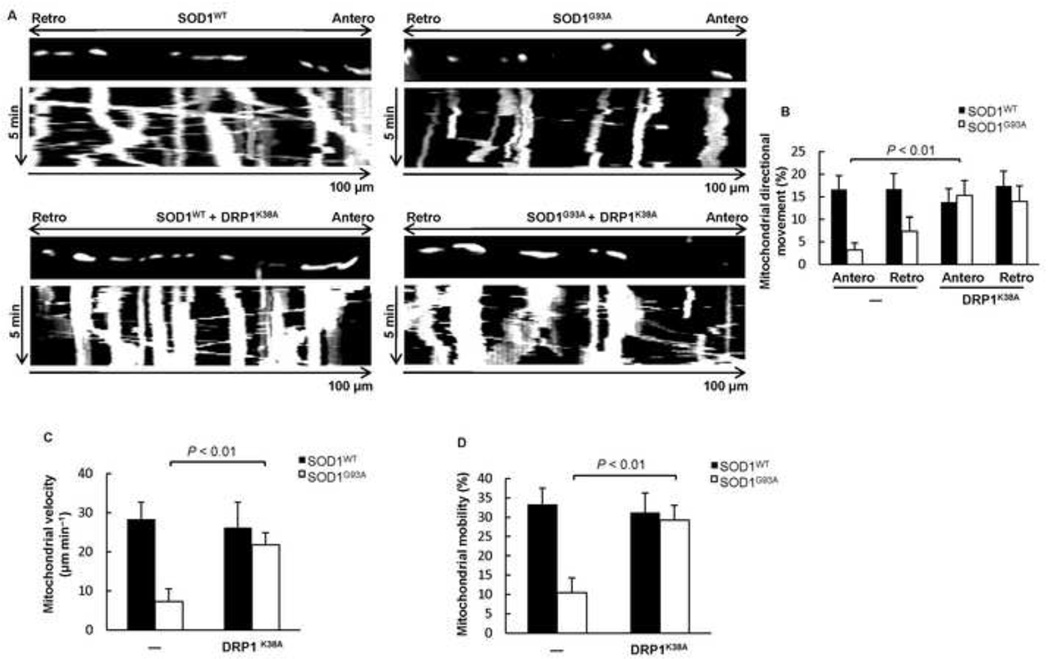
We showed above that mitochondrial fragmentation correlates with defects in mitochondrial transport and increased neuronal cell death in mutant SOD1G93A spinal cord motor neurons (Figs. 2, 3). To test whether restoration of balanced mitochondrial fission and fusion could also rescue the defects in directional mitochondrial axonal transport, we co-expressed SOD1WT or SOD1G93A with DsRed2-Mito, either alone or in combination with the dominant-negative DRP1K38A mutant in cortical neurons. SOD1WT neurons had the expected bi-directional mitochondrial transport typical for healthy neurons (Fig. 6A) and in agreement with previous studies (De Vos et al., 2007; Magrane et al., 2012), while SOD1G93A neurons exhibited defects in both anterograde and retrograde mitochondrial transport (Fig. 6A). Mitochondrial transport was restored in SOD1G93A neurons expressing DRP1K38A (Fig. 6A, B). In addition, both mitochondrial velocity (Fig. 6C) and motility (Fig. 6D) were rescued in SOD1G93A neurons expressing DRP1K38A. Collectively, our data shows that directional mitochondrial transport, mean velocity, and overall mobility are all regained by lowering DRP1 activity.
Figure 6.
DRP1K38A rescues neurons from mitochondrial trafficking defects. A) Kymographs of mitochondrial transport in cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. B) Mitochondrial directional movement in cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. C) Mitochondrial velocity in cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. D) Mitochondrial mobility in cortical neurons expressing DsRed2-Mito and either SOD1WT or SOD1G93A alone, or in combination with DRP1K38A. P value: two-tail paired Student’s t-test, n = 10.
SIRT3 and PGC-1α rescue SOD1G93A-induced defects in mitochondrial dynamics
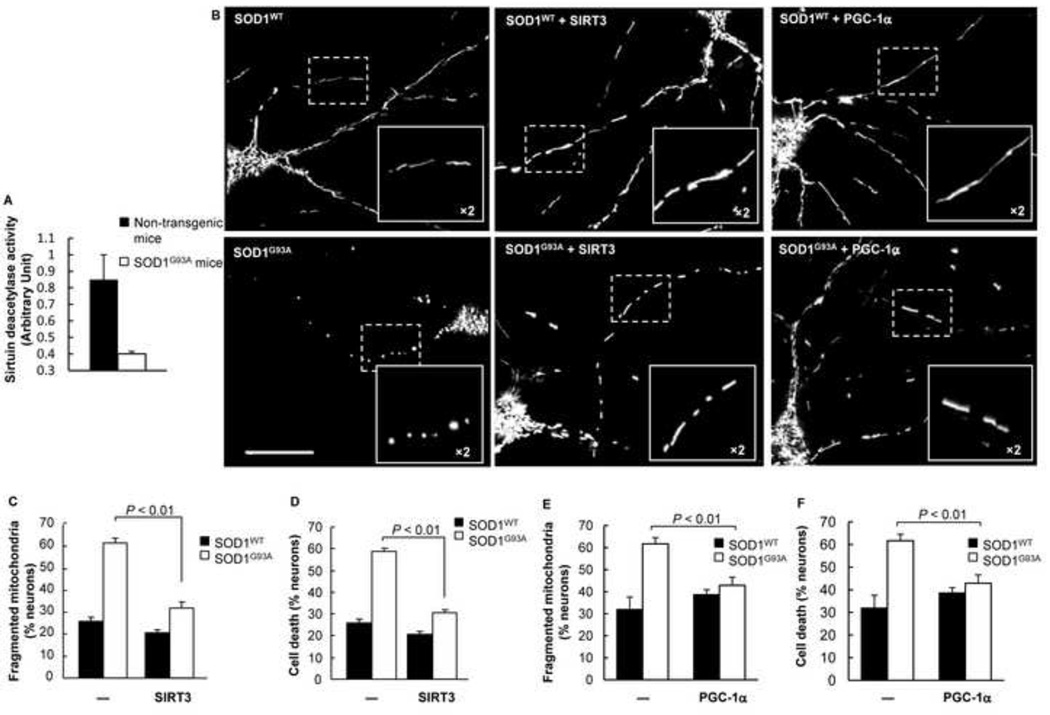
Nitrosative stress causes mitochondrial fragmentation and neuronal cell death (Barsoum et al., 2006). Recently, a number of reports have emphasized the significance of SIRT3 in ROS regulation and mitochondrial energy metabolism (Hirschey et al., 2010; Hirschey et al., 2011; Qiu et al., 2010; Someya et al., 2010; Tao et al., 2010; Verdin et al., 2010). Although SIRT3 protein was not down-regulated, we detected decreased sirtuin deacetylase activity in spinal cord lysates from ALS mice compared to the non-transgenic littermates (Fig. 7A). To investigate whether SIRT3 could restore the mitochondrial fission and fusion balance in SOD1G93A neurons, we evaluated the mitochondrial morphology after exogenous SIRT3 expression. As hypothesized, mitochondria fragmented in SOD1G93A neurons, while displaying elongated morphology when SIRT3 was also expressed (Fig. 7B). Quantitative analysis indicated that mutant SOD1G93A-induced mitochondrial fragmentation was significantly reduced with SIRT3 co-expression (Fig. 7C). Similarly, SIRT3 expression protected neurons from SOD1G93A-induced cell death (Fig. 7D). Thus, our data suggests that SIRT3 provides neuroprotection against SOD1G93A-induced toxicity by promoting mitochondrial dynamics.
Figure 7.
Both SIRT3 and PGC-1α rescue SOD1G93A-induced mitochondrial fragmentation. A) Sirtuin deacetylase activity of spinal cord tissue lysates isolated from non-transgenic and transgenic mutant SOD1G93A mice. B) Fluorescence micrographs and close up views of mitochondria in cortical neurons expressing DsRed2-Mito and SOD1WT or SOD1G93A alone, or in combination with SIRT3 or PGC-1α. Scale bar: 50µm. C) Mitochondrial fragmentation of cortical neurons expressing DsRed2-Mito and SOD1WT or SOD1G93A alone, or in combination with SIRT3. D) Cell death of cortical neurons expressing DsRed2-Mito and SOD1WT or SOD1G93A alone, or in combination with SIRT3. E) Mitochondrial fragmentation of cortical neurons expressing DsRed2-Mito and SOD1WT or SOD1G93A alone or in combination with PGC-1α. F) Cell death of cortical neurons expressing DsRed2-Mito and SOD1WT or SOD1G93A, alone or in combination with PGC-1α. P value: two-tail paired Student’s t-test.
PGC-1α, a key regulator of mitochondrial biogenesis, is a transcriptional co-activator for a number of nuclear genes that encode mitochondrial proteins which are imported into mitochondria, including SIRT3 (Giralt et al., 2011; Kong et al., 2010; St-Pierre et al., 2006; Wu et al., 1999). Thus, whether or not dynamic defects of mitochondria induced by mutant SOD1G93A can be restored by PGC-1α remains unclear. To test this idea, we co-expressed PGC-1α with wild-type or mutant SOD1G93A. Similar to SIRT3, PGC-1α was able to decrease mitochondrial fragmentation in mutant SOD1G93A neurons (Fig. 7E), which correlated with improved cell survival (Fig. 7F). Collectively, our data suggests that both SIRT3 and PGC-1α have positive effects on maintaining the equilibrium of mitochondrial fission and fusion, required for cell viability.
DISCUSSION
Mitochondrial dynamics including fission, fusion, and transport are crucial for the maintenance of bioenergetic function and prevention of apoptosis (Chan, 2006; Detmer and Chan, 2007; Knott et al., 2008). Neurons are a cell-type with high energy demands, especially at the nodes of Ranvier and synaptic terminals, where mitochondria need to be transported often across long distances in order to provide ATP and maintain Ca2+ homeostasis. A defective mitochondrial fission and fusion balance affects mitochondrial transport, which in turn can lead to synaptic dysfunction and neurodegeneration (Knott et al., 2008). Consequently, mitochondrial dynamics might be of higher importance to neurons as opposed to other cell types, and defects in this process might play a role in the pathogenesis of Alzheimer’s Disease (AD), Huntington’s Disease (HD), Parkinson’s Disease (PD), and ALS (Detmer and Chan, 2007; Knott et al., 2008; Magrane and Manfredi, 2009).
Mitochondrial dysfunction occurs in both ALS patients and animal models of ALS (Damiano et al., 2006; Mattiazzi et al., 2002; Wiedemann et al., 2002). Altered mitochondrial morphology has been reported in ALS patients, mice, and cell culture models (Kong and Xu, 1998; Magrane et al., 2009; Sasaki and Iwata, 2007). Thus, abnormal mitochondrial dynamics may play a causal role in the pathogenesis of ALS.
DRP1 mediates mitochondrial fission and its overexpression increases vulnerability to mitochondrial fragmentation and neuronal cell death (Barsoum et al., 2006). In addition, our recent work (Song et al., 2011) revealed that DRP1 is activated in HD, while lowering DRP1 activity by expressing dominant-negative DRP1K38A mutant restores mitochondrial dynamics and in turn rescues neuronal cell death. In this current work, we show that defective axonal mitochondrial dynamics is present in motor neurons expressing mutant SOD1G93A (Figs. 2–4). We also demonstrate that DRP1K38A expression restores mitochondrial dynamics (Fig. 6). Similarly, neuronal cell death is inhibited by lowering DRP1 activity (Fig. 5). Therefore, aberrant mitochondrial dynamics might play a causal role in ALS-linked motor neuron degeneration. Recently, Magrane et al. showed impaired mitochondrial fusion using photo-switchable fluorescent MitoDendra in "pure" motor neuron cultures, which correlated with fewer mitochondria at synapses and synaptic loss (Magrane et al., 2012). Fission and fusion are not independent events and impact each other. Defects in mitochondrial fusion may result from increased fission rates, which is consistent with our observations. This provides the basis for future studies using animal models of ALS to investigate if lowing DRP1 activity might be able to mediate neuroprotection in vivo.
One important consideration is that motor neurons are among the most difficult neuronal types to culture in vitro. Recently published work shows abnormal mitochondrial dynamics in ALS models in vitro using either “pure” motor neurons, “pure” cortical neurons, or motor neuron-like NSC34 cells (De Vos et al., 2007; Magrane et al., 2009; Magrane et al., 2012). However, the reported defects in mitochondrial movement are in part inconsistent, which may be due to different experimental models. It is our experience that "pure" motor neuron cultures, without astroglial support, are stressed and therefore require ample amounts of neurotrophic factors in the culture medium for their cell survival. This artificial excess of neurotrophins may obfuscate the physiological response of motor neurons, resulting in conflicting experimental findings. The characteristics of motor neurons make their analyses a laborious and technically challenging task. Here, we report an improved motor neuron-astrocyte co-culture system. Astrocytes, as supportive cells, are important for motor neuron development and function (Sidoryk-Wegrzynowicz et al., 2011; Walz, 2002). In our system, medium supplementation with neurotrophins is not required for the motor neuron survival in vitro, providing an advantage. Thus, our motor neuron-astrocyte co-culture system might represent an improved experimental model for motor neuron studies, one which may mimic more closely the in vivo situation. We grew an astrocyte mono-layer and seeded the DsRed2-Mito electroporated purified motor neurons on top of the established mono-layer (Fig. 1). The neurites of these motor neurons not only grew up to several hundred microns in length, but also connected to their target of either an astrocyte or another motor neuron. Previous studies have shown that disease progression in ALS might not be cell-autonomous (Beers et al., 2006; Harraz et al., 2008; Nagai et al., 2007). There are also several studies indicating that mutant SOD1 astrocytes have significant effects on motor neuron degeneration in models of ALS (Nagai et al., 2007; Yamanaka et al., 2008). Therefore, we believe it is better not to omit astrocytes in any study of motor neurons. Future investigations will test the effects of mutant SOD1G93A astrocytes on the mitochondrial dynamics of wild-type motor neurons.
Nitrosative and oxidative stress trigger mitochondrial fragmentation (Barsoum et al., 2006; Liot et al., 2009). Of note, oxidative damage is also implicated in ALS pathogenesis (Mattiazzi et al., 2002). Recently, Bosco et al found that purified oxidized SOD1WT from sporadic ALS patients tissues can lead to defects in fast axonal transport (Bosco et al., 2010). These findings not only suggest an SOD1-dependent mechanism in sporadic ALS, but also highlight the critical role of nitrosative and oxidative stress. Remarkably, although oxidized SOD1WT contributes to ALS pathogenesis, overexpressing of SOD1WT alone does not necessarily cause motor neuron cell death per se either in culture systems or in mice (Tu et al., 1996), but exacerbates the toxicity of mutant SOD1 by a mechanism not entirely understood (Prudencio et al., 2010; Sahawneh et al., 2010). Aging or overactivation of NMDA receptors may increase the levels of oxidized SOD1WT. In this report, neurons expressing SOD1WT show the expected mitochondrial morphology and dynamics of healthy neurons (Figs. 2–7), which is consistent with previous reports (De Vos et al., 2007; Magrane et al., 2012).
SIRT3 lowers ROS by directly deacetylating SOD2 and IDH2 and increases their enzymatic activities (Qiu et al., 2010; Someya et al., 2010; Tao et al., 2010). Thus, it is possible that SIRT3 maintains mitochondrial dynamics by keeping oxidative stress in check. A decrease in SIRT3 activity might increase ROS, which in turn could lead to mitochondrial fragmentation and neuronal cell death. Here, we report a reduction in sirtuin deacetylase activity using spinal cord extracts of mutant SOD1G93A transgenic mice (Fig. 7A). We cannot exclude the possibility that the activities of SIRT1 or other sirtuins may have been decreased as the substrate may not be specific. In addition, previous studies have revealed that SIRT1 up-regulates SIRT3 transcription through PGC-1α (Giralt et al., 2011; Kong et al., 2010; Rodgers et al., 2005). Thus, a decrease in SIRT1 may also lower SIRT3.
SIRT3 overexpression protects against mutant SOD1G93A-induced mitochondrial fragmentation and neuronal cell death (Fig. 7). In agreement, SIRT3 protects against apoptosis linked to aging in mice and excitotoxic insults in cultured neurons (Hafner et al., 2010; Kim et al., 2011; Someya et al., 2010). The exact mechanism underlying SIRT3-mediated protection against mutant SOD1G93A-induced toxicity remains elusive. Another potential mechanism may involve cyclophilin D, a component of the mitochondrial permeability transition pore. Cyclophilin D reduction delays motor neuron cell death and extends the lifespan of SOD1G93A mice (Martin et al., 2009). Interestingly, cyclophilin D is a SIRT3 substrate. Deacetylation by SIRT3 inhibits cyclophilin D function and prevents mitochondrial permeability transition and age-related cardiac hypertrophy (Hafner et al., 2010). Irrespective of the underlying mechanism, SIRT3 may represent a novel therapeutic target to combat ALS.
PGC-1α plays a central role in mitochondrial biogenesis and promotes the expression of SIRT3 (Giralt et al., 2011; Kong et al., 2010; Palacios et al., 2009; Shi et al., 2005; St-Pierre et al., 2006). Moreover, PGC-1α also directly regulates mitochondrial dynamics by increasing MFN2 transcription and mitochondrial fusion (Misko et al., 2010; Soriano et al., 2006). We show that similar to SIRT3, PGC-1α is able to restore mitochondrial dynamics and cell viability of mutant SOD1G93A neurons (Fig. 7).
In summary, we generated a novel astrocyte-motor neuron co-culture system, which provides an improved model for studies of mutant SOD1G93A-induced toxicity in vitro. Moreover, we showed that defects in the mitochondrial fission and fusion equilibrium play a functional role in mutant SOD1G93A-induced mitochondrial trafficking defects and neuronal cell death. Although the precise underlying mechanisms require further investigation, our study suggests that agents that DRP1, SIRT3 or PGC-1α may represent new opportunities to slow motor neuron neurodegeneration linked to ALS.
Supplementary Material
Highlights.
Defective mitochondrial dynamics in ALS
Dominant-negative DRP1K38A rescues mitochondrial fragmentation and cell death
SIRT3 and PGC-1α restore mitochondrial fission and fusion in culture models of ALS.
ACKNOWLEDGMENTS
We thank S. Lubitz, V. DeAssis, and C. Eldon for technical assistance; and M. Kaliszewski for manuscript editing. We would also like to thank Dr. A. Estevez for the advice on the purification of motor neurons. This research was supported by NIH grants R01EY016164 and R01NS055193 (EBW).
Abbreviations
- ALS
amyotrophic lateral sclerosis
- BCS
bovine calf serum
- BSA
bovine serum albumin
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
- CMT
Charcot-Marie-Tooth
- DIV
days in vitro
- DMEM
Dulbecco's Modified Eagle Medium
- DRP1
dynamin-related protein 1
- DTT
dithiothreitol
- EBSS
Earle's Balanced Salt Solution
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IDH
isocitrate dehydrogenase
- MFN
mitofusin
- NAD+
nicotinamide adenine dinucleotide
- NAM
nicotinamide
- NEM
N-ethylmaleimide
- OPA1
optic atrophy 1
- PBS
phosphate buffered saline
- Pen/Strep
penicillin-streptomycin
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- ROS
reactive oxygen species
- SIRT
silent information regulator two
- SMI-32
neurofilament H non-phosphorylated
- SOD1
Cu/Zn superoxide dismutase
- TSA
trichostatin A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors declare a financial or any other conflict of interest.
REFERENCES
- Anderson RM, et al. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37:47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum MJ, et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers DR, et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011;42:561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boillee S, et al. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Bosco DA, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijn LI, et al. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125:1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Methods. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, et al. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadon-Nachum M, et al. The"dying-back" phenomenon of motor neurons in ALS. J Mol Neurosci. 2011;43:470–477. doi: 10.1007/s12031-010-9467-1. [DOI] [PubMed] [Google Scholar]
- Damiano M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–1361. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- De Vos KJ, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum Mol Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Donmez G, Guarente L. Aging and disease: connections to sirtuins. Aging Cell. 2010;9:285–290. doi: 10.1111/j.1474-9726.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- Finkel T, et al. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem. 2011;286:16958–16966. doi: 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hafner AV, et al. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA. Mammalian sirtuins: biological insights and disease relevance. Annu Rev Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz MM, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, et al. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Howitz KT, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Israelson A, et al. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 2010;67:575–587. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kim SH, et al. Neuronal Sirt3 protects against excitotoxic injury in mouse cortical neuron culture. PLoS One. 2011;6:e14731. doi: 10.1371/journal.pone.0014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Bossy-Wetzel E. ALS: astrocytes take center stage, but must they share the spotlight? Cell Death Differ. 2007;14:1985–1988. doi: 10.1038/sj.cdd.4402241. [DOI] [PubMed] [Google Scholar]
- Knott AB, et al. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Xu Z. Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1. J Neurosci. 1998;18:3241–3250. doi: 10.1523/JNEUROSCI.18-09-03241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Jr, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Legros F, et al. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol Biol Cell. 2002;13:4343–4354. doi: 10.1091/mbc.E02-06-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, et al. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Liot G, et al. Complex II inhibition by 3-NP causes mitochondrial fragmentation and neuronal cell death via an NMDA- and ROS-dependent pathway. Cell Death Differ. 2009;16:899–909. doi: 10.1038/cdd.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, et al. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–4564. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, Manfredi G. Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis. Antioxid Redox Signal. 2009;11:1615–1626. doi: 10.1089/ars.2009.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane J, et al. Mitochondrial Dynamics and Bioenergetic Dysfunction Is Associated with Synaptic Alterations in Mutant SOD1 Motor Neurons. J Neurosci. 2012;32:229–242. doi: 10.1523/JNEUROSCI.1233-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, et al. The mitochondrial permeability transition pore in motor neurons: involvement in the pathobiology of ALS mice. Exp Neurol. 2009;218:333–346. doi: 10.1016/j.expneurol.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–29633. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- Meeusen S, et al. Mitochondrial inner-membrane fusion and crista maintenance requires the dynamin-related GTPase Mgm1. Cell. 2006;127:383–395. doi: 10.1016/j.cell.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Misko A, et al. Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J Neurosci. 2010;30:4232–4240. doi: 10.1523/JNEUROSCI.6248-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios OM, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrini S, et al. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19:2974–2986. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, et al. An examination of wild-type SOD1 in modulating the toxicity and aggregation of ALS-associated mutant SOD1. Hum Mol Genet. 2010;19:4774–4789. doi: 10.1093/hmg/ddq408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls I, et al. Mutant dynactin in motor neuron disease. Nat Genet. 2003;33:455–456. doi: 10.1038/ng1123. [DOI] [PubMed] [Google Scholar]
- Qin W, et al. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Qiu X, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Reid E, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10) Am J Hum Genet. 2002;71:1189–1194. doi: 10.1086/344210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;364:362. doi: 10.1038/364362c0. [DOI] [PubMed] [Google Scholar]
- Sahawneh MA, et al. Cu,Zn-superoxide dismutase increases toxicity of mutant and zinc-deficient superoxide dismutase by enhancing protein stability. J Biol Chem. 2010;285:33885–33897. doi: 10.1074/jbc.M110.118901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Ultrastructural study of synapses in the anterior horn neurons of patients with amyotrophic lateral sclerosis. Neurosci Lett. 1996;204:53–56. doi: 10.1016/0304-3940(96)12314-4. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Iwata M. Mitochondrial alterations in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2007;66:10–16. doi: 10.1097/nen.0b013e31802c396b. [DOI] [PubMed] [Google Scholar]
- Shaw PJ, Ince PG. Glutamate, excitotoxicity and amyotrophic lateral sclerosis. J Neurol. 1997;244(Suppl 2):S3–S14. doi: 10.1007/BF03160574. [DOI] [PubMed] [Google Scholar]
- Shi T, et al. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005;280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- Sidoryk-Wegrzynowicz M, et al. Role of astrocytes in brain function and disease. Toxicol Pathol. 2011;39:115–123. doi: 10.1177/0192623310385254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, et al. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, et al. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, et al. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007;28:1613–1622. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Someya S, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, et al. Assessing mitochondrial morphology and dynamics using fluorescence wide-field microscopy and 3D image processing. Methods. 2008;46:295–303. doi: 10.1016/j.ymeth.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17:377–382. doi: 10.1038/nm.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, et al. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol Biol Cell. 2009;20:3525–3532. doi: 10.1091/mbc.E09-03-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano FX, et al. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Tao R, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu PH, et al. Transgenic mice carrying a human mutant superoxide dismutase transgene develop neuronal cytoskeletal pathology resembling human amyotrophic lateral sclerosis lesions. Proc Natl Acad Sci U S A. 1996;93:3155–3160. doi: 10.1073/pnas.93.7.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek AM, et al. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, et al. Mutations in FUS an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A, et al. Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J Cell Sci. 2004;117:4389–4400. doi: 10.1242/jcs.01299. [DOI] [PubMed] [Google Scholar]
- Verdin E, et al. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–675. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz W. Contemporary neuroscience. Totowa, N.J: Humana Press; 2002. The neuronal environment brain homeostasis in health and disease. [Google Scholar]
- Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- Wiedemann FR, et al. Mitochondrial DNA and respiratory chain function in spinal cords of ALS patients. J Neurochem. 2002;80:616–625. doi: 10.1046/j.0022-3042.2001.00731.x. [DOI] [PubMed] [Google Scholar]
- Wong PC, et al. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
- Wood-Allum C, Shaw PJ. Motor neurone disease: a practical update on diagnosis and management. Clin Med. 2010;10:252–258. doi: 10.7861/clinmedicine.10-3-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, et al. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H, et al. Mitochondrial fission is an upstream and required event for bax foci formation in response to nitric oxide in cortical neurons. Cell Death Differ. 2007;14:462–471. doi: 10.1038/sj.cdd.4402046. [DOI] [PubMed] [Google Scholar]
- Zhao C, et al. Charcot-Marie-Tooth disease type 2A caused by mutation in a microtubule motor KIF1Bbeta. Cell. 2001;105:587–597. doi: 10.1016/s0092-8674(01)00363-4. [DOI] [PubMed] [Google Scholar]
- Zuchner S, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.