Abstract
Ketamine produces rapid antidepressant effects in treatment-resistant depression (TRD), but the magnitude of response varies considerably between individual patients. Brain-derived neurotrophic factor (BDNF) has been investigated as a biomarker of treatment response in depression and has been implicated in the mechanism of action of ketamine. We evaluated plasma BDNF and associations with symptoms in 22 patients with TRD enrolled in a randomized controlled trial of ketamine compared to an anaesthetic control (midazolam). Ketamine significantly increased plasma BDNF levels in responders compared to non-responders 240 min post-infusion, and Montgomery–Åsberg Depression Rating Scale (MADRS) scores were negatively correlated with BDNF (r=–0.701, p=0.008). Plasma BDNF levels at 240 min post-infusion were highly negatively associated with MADRS scores at 240 min (r=–0.897, p=.002), 24 h (r=–0.791, p=0.038), 48 h (r=–0.944, p=0.001) and 72 h (r=–0.977, p=0.010). No associations with BDNF were found for patients receiving midazolam. These data support plasma BDNF as a peripheral biomarker relevant to ketamine antidepressant response.
Keywords: BDNF, biomarker, depression, ketamine
Introduction
Recent evidence implicates glutamatergic dysregulation in the pathophysiology of mood disorders (Yuksel and Ongur, 2010). Consistent with these findings, a series of clinical reports have demonstrated that the glutamate N-methyl-d-aspartate (NMDA) receptor antagonist ketamine has rapid antidepressant efficacy (Mathew et al., 2012). A single subanaesthetic dose of ketamine produces quick and enduring antidepressant effects in patients with treatment-resistant depression (TRD) refractory to conventional antidepressant therapies (Zarate et al., 2006; Murrough et al., 2013).
The neurobiological mechanisms underlying ketamine's antidepressant effects are complex and not fully understood. Preclinical studies indicate that NMDA receptor blockade leads to upregulation in AMPA receptor expression and subsequent activation of the mammalian target of rapamycin (mTOR) intracellular cascade that is required for ketamine's antidepressant action (Li et al., 2010). In particular, rapid and transient up-regulation of the neuroplasticity marker brain derived neurotrophic factor (BDNF) is implicated as a critical component of the antidepressant mechanism of ketamine (Autry and Monteggia, 2012; Duman et al., 2012). BDNF is a neurotrophin important in facilitating and supporting certain neuronal populations during development and mediating synaptic plasticity associated with learning and memory (Poo, 2001).
Numerous studies implicate BDNF in mood disorders. Peripheral BDNF levels are decreased in patients with MDD (major depressive disorder) and this deficit is reversed in those who respond to antidepressant therapy (Lim et al., 2008; Kurita et al., 2012). Interestingly, upon successful pharmacotherapy, remitted patients with MDD show elevated BDNF levels that are negatively correlated with Montgomery–Åsberg Depression Rating Scale (MADRS) scores (Kurita et al., 2012).
Ketamine produces rapid antidepressant effects in the majority of patients with TRD, although the magnitude and persistence of response is unpredictable and varies (Zarate et al., 2006; Murrough et al., 2013). Determining the optimal sampling schedule for BDNF is also complicated by the temporal effects of ketamine's activity. Responders to ketamine, however, showed increased peripheral BDNF levels at approximately 230–240 min after dosing, which were associated with concomitant decreases in MADRS scores (Machado-Vieira et al., 2009; Cornwell et al., 2012; Duncan et al., 2013). At this same time point, patients with TRD with rapid and robust improvements in depression symptoms also exhibited increased stimulus-evoked somato-sensory cortical responses, a marker of synaptic plasticity, whereas non-responders did not (Cornwell et al., 2012). This time point also corresponds to the elimination half-life of ketamine's major active metabolite, norketamine, while the parent drug's elimination half-life is approximately 2 h (White et al., 1985). Taken together, these data suggest that ~240 min after receiving ketamine may be a critical window period to distinguish responders and nonresponders to ketamine therapy in patients with TRD.
The present study examined the relationship between plasma BDNF levels and depression severity obtained during the largest randomized controlled trial of ketamine in TRD to date (Murrough et al., 2013). In this trial, the benzodiazepine anaesthetic midazolam served as a pharmacological control. Our primary aim was to determine plasma BDNF levels (baseline and 240 min) in this TRD sample and evaluate the relationship between clinical outcomes and plasma BDNF. We also explored whether BDNF levels were predictive of ketamine's persistence of antidepressant benefit in responders. To evaluate the specificity of findings for ketamine, we also examined plasma BDNF in patients randomized to midazolam.
Methods
The present study was conducted as part of a two-site randomized, double-blind clinical trial of ketamine compared to midazolam in patients with TRD (see ClinicalTrials.gov Identifier: NCT00768430 for an overview of methods and primary clinical outcomes (Murrough et al., 2013)). Briefly, patients aged 21–80 with TRD (defined as inadequate response to at least three antidepressant trials at an adequate dose and duration) in a current major depressive episode were randomized to a single 40 min intravenous (IV) infusion of ketamine 0.5 mg/kg or midazolam 0.045 mg/kg in a 2:1 randomization scheme. Midazolam was selected as an anaesthetic control condition for ketamine based on similar pharmacokinetics and time course of transient psychoactive effects (Kanto, 1985). Major exclusion criteria included a lifetime history of a psychotic illness or bipolar disorder, alcohol or substance abuse/dependence in the previous 2 yr, unstable medical illness, or use of contra-indicated medications. Notably, concomitant treatment with an antidepressant or any other psychotropic medication (except a stable dose of a non-benzodiazepine hypnotic) was exclusionary. The Institutional Review Boards at both participating sites approved the study (Baylor College of Medicine and Mount Sinai School of Medicine). After complete description of the study to the subjects, written informed consent was obtained.
Depression severity was assessed at baseline (prior to study drug administration) using the MADRS (Montgomery and Åsberg, 1979), and was repeated at 240 min, 24 h, 48 h, 72 h and 7 d following infusion. A study responder was defined as a 50% or greater reduction in MADRS score compared to baseline at 7 d post-infusion.
Blood samples were obtained at baseline and 240 min after infusion of study drug. Whole blood samples were collected in vacutaner tubes containing EDTA then centrifuged at 3000 r/min for 15 min. Plasma supernatant was then transferred to a new sterile microfuge tube and sample stored at –80 °C until processed for BDNF. Samples were processed within 1 h of being collected to decrease variability (Fujimura et al., 2002; Zuccato et al., 2011). BDNF concentrations were quantitatively determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions (DuoSet ELISA Development Kit R&D Systems, Minneapolis, USA). Samples were diluted 1:20 in sample diluent buffer, then aliquotted onto 96 well plates coated with a monoclonal antibody raised against BDNF. BDNF standards (human) and plasma samples were assayed in duplicate. Plates were incubated then washed with buffer (1XPBS). BDNF conjugated to horseradish peroxidase was then added. Following additional washing, substrate solution followed by a stop solution was added to halt the reaction. Absorbance was determined at 450 nm using a Multiskan FC plate reader (Thermo Fischer Scientific Inc., USA), with the correction wavelength set at 540 nm. A standard curve was constructed by plotting the mean absorbance for each standard against BDNF concentration. The data was then linearized by plotting the log of the BDNF concentration vs. the log of the O.D. and the best fit line was determined by regression analysis. BDNF concentrations generated in duplicate were averaged to give a value in ng/ml after correcting for sample dilution factor and subtraction of background (blank from standard curve). Standard curves were included on each individual plate and sample concentrations calculated using the specific curve generated to each plate. All assays were conducted under blinded conditions.
Statistical analyses
Data analyses were performed using SigmaStat 12.0 (SYSTAT Software Inc., USA). Normality (Shapiro–Wilk) and equal variance tests were performed prior to analyses to ensure statistical assumptions were met. Group differences in patient demographics and plasma BDNF levels at baseline and 240 min were compared using t-tests. MADRS scores between groups (responders and non-responders to ketamine) were assessed using a two-way analysis of variance (ANOVA) with group (responder vs. non-responder) and time (baseline, 240 min, 24 h, 48 h, 72 h, and 7 d) as factors. Significant main effects were followed with pair-wise multiple comparison procedures (Student–Newman–Keuls method). Correlation analysis (Pearson's) was initially used to determine relationships between MADRS scores and plasma BDNF at the 240 min time point. Baseline BDNF levels, body mass index (BMI), gender, and age were predicted to influence post-infusion BDNF levels. Therefore, these factors, in addition to BDNF levels at 240 min, were included in a multiple regression analysis model to assess their ability to predict the dependent variables, MADRS scores at 240 min, 24 h, 48 h, 72 h and 7 d following IV ketamine. Significance was set at p<0.05 and all data are presented as mean±s.e..
Results
Samples were collected from 22 patients (15 patients received ketamine, seven patients received midazolam). Demographic and clinical features of patients that received ketamine or midazolam, including age (48.53±3.30 and 42.71±4.85 yr), age of onset of illness (23.00±2.80 and 21.29±4.78 yr), duration of illness (21.80±3.82 and 20.00±5.96 yr), number of antidepressant treatment failures (5.87±0.58 and 4.86±0.40), and baseline MADRS scores (32.33±1.21 and 31.71±2.17) did not differ between treatment groups (p>0.05).
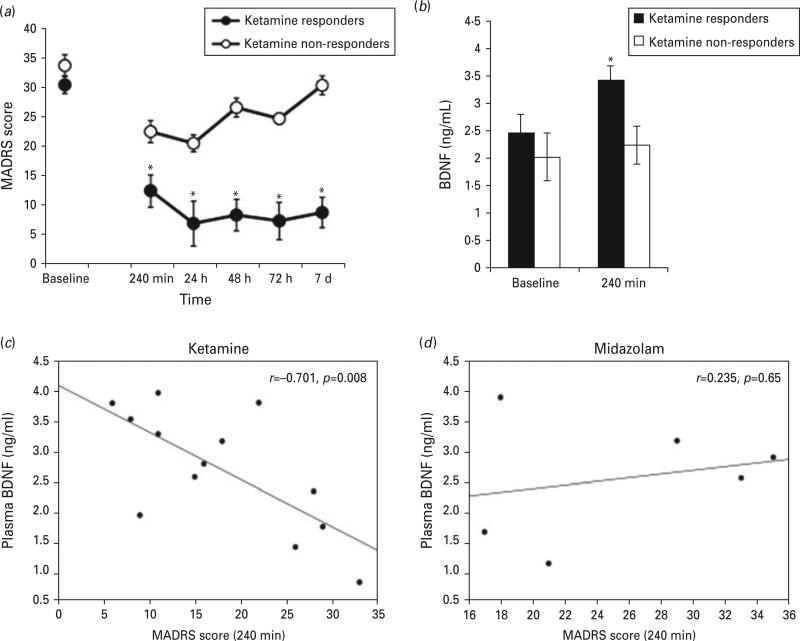
We were unable to obtain plasma samples and MADRS scores from three patients at all time points, so the data analysis reflects these missing data points. Seven patients receiving ketamine met response criteria at Day 7, whereas two patients receiving midazolam met response criteria. A two-way ANOVA comparing MADRS scores over time in ketamine-responders and nonresponders revealed significant group (F1,86=104.253, p<0.001) and time (F5,86=15.412, p<0.001) main effects and a group-by-time interaction (F5,86=3.93, p=0.003). Multiple comparisons showed significant differences in MADRS scores at all time points except baseline (p<0.05) (Fig. 1(a)). As shown in Fig.1(b), ketamine responders had greater BDNF levels than ketamine nonresponders at 240 min post-infusion (t(11)=2.450, p=0.03). BDNF levels did not significantly differ between midazolam responders and non-responders (t(4)=0.727, p=0.50).
Fig. 1.
Plasma BDNF levels and depression outcomes. Benefit of ketamine was greater in responders (N=7) compared to nonresponders (N=8) as revealed by significantly lower Montgomery–Åsberg Depression Rating Scale (MADRS) scores over time (a, *p<0.05). Plasma BDNF levels were significantly increased (b, *p=0.032) at 240 min in responders following ketamine. Relationship between plasma BDNF levels and MADRS scores in patients that received ketamine (c, N=15) and midazolam (d, N=7). Plasma BDNF levels were highly correlated with MADRS scores at 240 min in patients that received ketamine but not midazolam. Responders represent patients with a greater than or equal to a 50% reduction MADRS score 7 d post-ketamine infusion compared to baseline.
Associations between MADRS scores and plasma BDNF levels at 240 min post-infusion in patients receiving ketamine or midazolam are presented in Fig. 1(c, d), respectively. There was a highly significant negative correlation between MADRS scores and plasma BDNF in patients receiving ketamine (F1,12=10.646, p=0.008) but not midazolam (F1,5=0.235, p=0.653).
A multiple linear regression analysis model was employed that included plasma BDNF (baseline and 240 min), age, BMI, and gender. As shown in Table 1, after controlling for multiple factors, BDNF at 240 min remained a highly significant predictor of MADRS scores at 240 min (t=–4.808, p=0.002, β=–0.997), 24 h (t=–2.556, p=0.038, β=–0.732), 48 h (t=–5.662, p=0.001, β=–0.941), 72 h (t=–4.777, p=0.010, β=–0.660), but not 7 d (t=–1.910, p=0.098, β=–0.673).
Table 1.
Multiple linear regression Montgomery–Åsberg Depression Rating Scale scores and plasma BDNF
| 240 min | 24 h | 48 h | 72 h | 7 d | |
|---|---|---|---|---|---|
| BDNF 240 min | r = –0.897, p = 0.002 | r = –0.791, p = 0.038 | r = –0.944, p = 0.001 | r = –0.777, p = 0.023 | r = –0.659, p = 0.098 |
Discussion
A number of significant findings were observed in this study. First, we found that ketamine, but not midazolam, increased plasma BDNF levels at 240 min post-infusion in responders compared to non-responders. Second, plasma BDNF levels at 240 min were negatively correlated with MADRS scores at that same time point in patients receiving ketamine but not midazolam. Finally, BDNF levels at 240 min were highly predictive of MADRS scores up to 72 h post-ketamine infusion. These results provide support for the hypothesis that early changes in plasma BDNF are associated with clinical outcomes for patients receiving ketamine therapy for TRD.
Our findings reinforce the idea that the 240 min time point may represent a critical window during which BDNF levels convey clinically relevant information. The importance of this time point is corroborated by recent studies (aan het Rot et al., 2010; Cornwell et al., 2012; Duncan et al., 2013) but not all (Machado-Vieira et al., 2009). Although similar protocols and methods were employed, Machado-Vieira et al. (2009) found neither correlations at any time point between plasma BDNF and MADRS scores, nor elevated BDNF levels at 240 min between responders and non-responders to ketamine. Factors responsible for these divergent findings from the present study are unknown.
The observed rapid reduction in MADRS scores 240 min post-ketamine infusion replicates findings from numerous previous studies (Zarate et al., 2006; Machado-Vieira et al., 2009; Cornwell et al., 2012; Duncan et al., 2013). However, to the best of our knowledge, we are the first to describe a highly significant negative correlation between MADRS scores and plasma BDNF at 240 min post-ketamine infusion in patients with a durable response for at least 7 d post-infusion. This finding is potentially consonant with a report that showed plasma BDNF levels were negatively correlated with MADRS scores in patients responding to conventional therapy (Kurita et al., 2012). Another novel finding was that BDNF levels at the 240 min time point were highly predictive of MADRS scores up to 72 h after a single IV ketamine infusion. The uncharacteristically high correlations found between plasma BDNF levels and MADRS scores over time remained significant even after controlling for factors known to influence BDNF (baseline BDNF, age, gender and BMI). Future studies employing larger samples sizes are needed to confirm this finding.
For this study, we focused on responders who showed clear evidence of persistence of benefit. Patients must have met MADRS response criteria on Day 7 following a single ketamine infusion to be designated study responders (Murrough et al., 2013). Moreover, the Day 7 assessments were conducted in outpatients, since patients were discharged from the research facility 24 h following infusion. That patients continued to benefit from ketamine outside the research unit suggests a durable response in a select group of patients. Our finding that early ketamine-induced increases in plasma BDNF levels were greater in responders compared to non-responders may reflect biological mechanisms associated with durability of response. Future studies are needed to prospectively assess BDNF levels at multiple time points (up to 7 d) and whether levels may predict clinical outcome to ketamine.
Several limitations should be considered when interpreting these results. Although the primary findings appear to be statistically robust, they must be considered preliminary until replicated in a larger patient population. Second, we focused analyses on plasma rather than serum BDNF, because of concerns regarding stability and reliability of using serum as an assay medium for BDNF (Fujimura et al., 2002; Zuccato et al., 2011). While BDNF can cross the blood–brain barrier, and plasma levels correlate with cerebrospinal fluid levels in certain disease states, we did not directly measure brain BDNF nor additional key neurobiological mediators of ketamine response, such as mTOR (Poduslo and Curran, 1996; Pillai et al., 2010; Denk et al., 2011; Yang et al., 2013). Overall, however, these findings suggest that plasma BDNF levels obtained approximately 240 min after a single ketamine infusion are highly associated with, and powerfully predict, depression outcomes in patients with TRD.
Acknowledgments
This work was supported by National Institutes of Health (NIH)/National Institute of Mental Health (NIMH) grant RO1MH081870 (SJM), UL1TR000067 from the NIH National Center for Advancing Translational Sciences, Department of Veterans Affairs, and a NARSAD Independent Investigator Award (SJM). This work was supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX. Dr Murrough is supported by a Career Development Award from NIH/NIMH (1K23MH094707-01).
In the previous 36 months Dr Murrough has received research support from Evotec, Janssen Pharmaceuticals and Avanir. Dr Iosifescu has received research funding through Icahn School of Medicine at Mount Sinai from AstraZeneca, Brainsway, Euthymics, Neosync, and Roche; he has received consulting fees from CNS Response, Otsuka, and Servier. Dr Charney has been named as an inventor on a pending use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr Charney and Icahn School of Medicine at Mount Sinai could benefit financially. Dr Mathew has been named as an inventor on a use patent of ketamine for the treatment of depression. Dr Mathew has relinquished his claim to any royalties and will not benefit financially if ketamine were approved for this use. Dr Mathew has received consulting fees or research support from Allergan, AstraZeneca, Bristol-Myers Squibb, Cephalon, Inc., Corcept, Johnson & Johnson, Naurex, Noven, Roche Pharmaceuticals, and Takeda.
Footnotes
Statement of Interest
All other authors declare no conflict of interest.
References
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psych. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell BR, Salvadore G, Furey M, Marquardt CA, Brutsche NE, Grillon C, Zarate CA., Jr Synaptic potentiation is critical for rapid antidepressant response to ketamine in treatment-resistant major depression. Biol Psych. 2012;72:555–561. doi: 10.1016/j.biopsych.2012.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk MC, Rewerts C, Holsboer F, Erhardt-Lehmann A, Turck CW. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of rapamycin activation. Am J Psychiat. 2011;168:751–752. doi: 10.1176/appi.ajp.2011.11010128. [DOI] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacol. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, Yuan P, Brutsche N, Manji HK, Tononi G, Zarate CA. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharm (CINP) 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura H, Altar CA, Chen R, Nakamura T, Nakahashi T, Kambayashi J, Sun B, Tandon NN. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemostasis. 2002;87:728–734. [PubMed] [Google Scholar]
- Kanto JH. Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy. 1985;5:138–155. doi: 10.1002/j.1875-9114.1985.tb03411.x. [DOI] [PubMed] [Google Scholar]
- Kurita M, Nishino S, Kato M, Numata Y, Sato T. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS ONE. 2012;7:e39212. doi: 10.1371/journal.pone.0039212. doi:10.1371/journal.pone.0039212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science (NY) 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, Zarate CA., Jr Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70:1662–1666. doi: 10.4088/JCP.08m04659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, Ostermeyer B, Murrough JW. Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs. 2012;26:189–204. doi: 10.2165/11599770-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Brit J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu D, Chang L, Al Jurdi R, Green C, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site, randomized controlled trial. Am J Psychiatry. doi. 2013 doi: 10.1176/appi.ajp.2013.13030392. 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Kale A, Joshi S, Naphade N, Raju MS, Nasrallah H, Mahadik SP. Decreased BDNF levels in CSF of drug-naive first-episode psychotic subjects: correlation with plasma BDNF and psychopathology. Int J Neuropsychopharm (CINP) 2010;13:535–539. doi: 10.1017/S1461145709991015. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- White PF, Schuttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Brit J Anaesth. 1985;57:197–203. doi: 10.1093/bja/57.2.197. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou ZQ, Gao ZQ, Shi JY, Yang JJ. Acute increases in plasma Mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psychiatry. 2013;73:e35–e36. doi: 10.1016/j.biopsych.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Vitali B, Tarditi A, Mariotti C, Valenza M, Lahiri N, Wild EJ, Sassone J, Ciammola A, Bachoud-Levi AC, Tabrizi SJ, Di Donato S, Cattaneo E. Brain-derived neurotrophic factor in patients with Huntington's disease. PLoS ONE. 2011;6:e22966. doi: 10.1371/journal.pone.0022966. [DOI] [PMC free article] [PubMed] [Google Scholar]