Abstract
Background
Low density lipoprotein cholesterol (LDL-C) has been clearly associated with the risk of developing coronary heart disease (CHD). The best and most convenient method for determining LDL-C has come under increased scrutiny in recent years. We present comparisons of Friedewald’s calculated LDL-C (C-LDL-C) and direct LDL-C (D-LDL-C) using three different homogenous assays. This highlights differences between the two methods of LDL-C measurement, and how this affects the classification of samples into different LDL-C treatment goals as determined by NCEP ATP III guidelines thus potentially affecting treatment strategies.
Methods
Lipid profiles of a total of 2,208 clinic patients were retrieved from the Central Arkansas VA Healthcare System (CAVHS) clinical laboratory database. Samples studied were of one week period of time during the 3 periods studied, 2000 (period 1), 2002 (period 2) and 2005 (period 3). Different homogenous assays for D-LDL-C measurement were used for each of the 3 periods.
Results
There is a fundamental disagreement between D-LDL-C and C-LDL-C, even though Pearson’s correlation coefficients are 0.93, 0.97 and 0.98 for periods 1, 2 and 3 respectively. Using the model for period 1, when C-LDL-C is 70 mg/dl, the predicted D-LDL-C is 95 mg/dl (36% higher). The differences between C-LDL-C and predicted D-LDL-C progressively decrease at higher LDL-C cut points. In the assay used in period 3, there are 290 samples with D-LDL-C values between 100–130 mg/dl. Of these, only 182 samples show agreement with C-LDL-C values whereas 90 samples with a D-LDL-C in the 100–130 mg/dl range are in 70–100 mg/dl range using the C-LDL-C assay. While the kappa statistics suggests the LDL-C measures have relatively high levels of agreement, the significant generalized McNemar tests (p<0.01) provide additional evidence of disagreement between C-LDL-C and D-LDL-C during all the 3 periods.
Conclusion
Our results highlight D-LDL-C measurements using 3 different assays during 3 different time periods. In all assays there is substantial lack of agreement between D-LDL-C and C-LDL-C which in most cases resulted in higher D-LDL-C values than C-LDL-C. This leads to clinically significant misclassification of patient’s LDL-C to a different LDL-C treatment goal which would potentially result in more drug usage; thus exposing patients to more potential side effects and at a much greater cost with little evidence of benefit.
Keywords: direct LDL, calculated LDL, cholesterol
INTRODUCTION
Low density lipoprotein cholesterol (LDL-C) has been clearly associated with the risk of developing coronary heart disease (CHD)1, 2. The National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) guidelines uses LDL-C as a measure of CHD risk and lowering LDL-C as the primary goal of therapy3. The best and most convenient method for determining LDL-C has come under increased scrutiny in recent years. The NCEP ATP III guidelines recommend use of the Friedewald calculated LDL-C (C-LDL-C) for determination of LDL-C treatment goals for prevention of cardiovascular diseases; and the use of Center for Disease Control (CDC) reference method (beta-quantification technique) for continued standardization in clinical laboratories3. More and more clinical laboratories are using various direct LDL-C (D-LDL-C) assays because of their presumed advantage of not requiring a fasting sample and good correlation with beta-quantification techniques in patients with elevated triglyceride (TG) levels4, 5. A recent survey of the College of American Pathologists (CAP) providing accreditation to all types of clinical laboratories reported more than 2,200 laboratories using several different assays for D-LDL-C measurements, and more than 3,300 laboratories reporting C-LDL-C using the Friedewald calculation6. In Friedewald’s original paper, the C-LDL-C correlated extremely well with the ‘gold standard’ beta-quantification LDL-C results in patients with TG levels < 400 mg/dl7. The clinical trials upon which the ATP-III treatment goals for prevention of CHD and recommendations for intervention are based, use the C-LDL-C measurement8–14. Moreover, the following concerns still exist: (a) there remains an uncertainty as to what the various assays measuring D-LDL-C are actually measuring; (b) various D-LDL-C assays have not been adequately standardized; and (c) there remains a lack of association of non-fasting D-LDL-C with cardiovascular events5, 15, 16.
We present comparisons of C-LDL-C with the D-LDL-C using three different homogenous assays (described below). The three assays were used at different times at the Central Arkansas Veterans Healthcare System, Little Rock, Arkansas (CAVHS) clinical laboratory during routine clinical practice. Classification of samples based on D--LDL-C and corresponding C-LDL-C values are presented at common LDL-C treatment goals recommended by various leading organizations. Rather than only using simple correlations between the C-LDL-C and the various D-LDL-C assays, we have evaluated the degree of agreement between these assays. Excellent correlations do not necessarily indicate good agreement. This way of presenting the data highlights the reclassification of samples, if any, into a different category which would lead to a change in clinical decision making. This study also examines differences between C-LDL-C and D-LDL-C assays at various LDL-C levels and the effect of TG concentrations on the difference between the D-LDL-C and C-LDL-C measurements.
METHODS
Study samples
Lipid profiles (TC, HDL-C, TG, D-LDL-C) of clinic patients were retrieved from the Central Arkansas VA Healthcare system (CAVHS) clinical laboratory database. Lipid profiles of all patients performed during a one week period of time in each of the 3 years studied, 2000 (period 1), 2002 (period 2) and 2005 (period 3) were collected. Lipid profiles with TG levels < 400 mg/dl were included in the analyses. This comprised a total of 2,208 samples i.e. 464 samples for period 1, 807 for period 2 and 937 for period 3.
Characterization of plasma lipids and assays used
TC, HDL-C and TG were analyzed in the laboratory prior to 2003 using the “Flex” methods of the DADE automated Clinical Chemistry System and after 2003 using the Beckman SYNCHRON systems. Different homogenous assays for D-LDL-C measurement were used for the each of the periods 1, 2 and 3. In period 1 (2000)-Sigma EZ LDL Assay, period 2 (2002)-Polymedco Lipi+Plus Assay; and period 3 (2005) - Synchron LX system assays were used respectively. The methods provided by the manufacturers provide insufficient details to understand any fundamental differences between these 3 methods.
Statistical analysis
Several approaches were used to assess the agreement between C-LDL-C and D-LDL-C. First, standard linear regression methods were used to model D-LDL-C as a function of C-LDL-C for each period, separately. The intercept and slope estimates obtained from these models were tested for a difference from 0 and 1, respectively. These are the intercept and slope values one would expect if the two measures were in agreement. Secondly, the D-LDL-C and the C-LDL-C measures were tabulated based on common LDL-C treatment goals recommended by various leading organizations, i.e. 70 mg/dl (optional treatment goal), 100 mg/dl, 130 mg/dl and 160 mg/dl. Kappa statistics and generalized McNemar tests were used to assess and test for agreement. Briefly, kappa statistics are purported to be chance adjusted measures of agreement between two raters or methods whose rating scale is categorical. Larger kappa values are indicative of better agreement. Landis and Koch17 provide the following guidelines for interpreting the kappa statistic:
| Kappa | Interpretation |
|---|---|
| < 0 | No agreement |
| 0.0 – 0.20 | Slight agreement |
| 0.21 – 0.40 | Fair agreement |
| 0.41 – 0.60 | Moderate agreement |
| 0.61 – 0.80 | Substantial agreement |
| 0.81 – 1.00 | Almost perfect agreement |
Generalized McNemar tests, on the other hand, test whether the marginal distributions of two measures are similar, as one would expect if the measures agree. Finally, graphical methods were employed to examine the impact of TG on differences in D-LDL-C and C-LDL-C. Analyses were performed using SAS® version 9.2 (SAS Institute Inc., Cary, SC) and R version 2.9.2 (R Foundation for Statistical Computing, Vienna, Austria). P-values less than 0.01 were considered to be statistically significant.
RESULTS
The triglyceride means (and SDs) for periods 1–3 were 168 (84), 169 (79) and 154 (80) mg/dl, respectively. Period 3 was found to differ from Period 1 (p = 0.005) and Period 2 (p < 0.001); whereas Periods 1 and 3 were not significantly different (p > 0.98). Mean HDL-C was 43 (14) mg/dl for Period 1, 43 (13) mg/dl for Period 2 and 39 (12) mg/dl for Period 3. Again, Period 3 was found to differ from Periods 1 and 2 (p < 0.001 for both), while Periods 1 and 2 were not significantly different (p > 0.99).
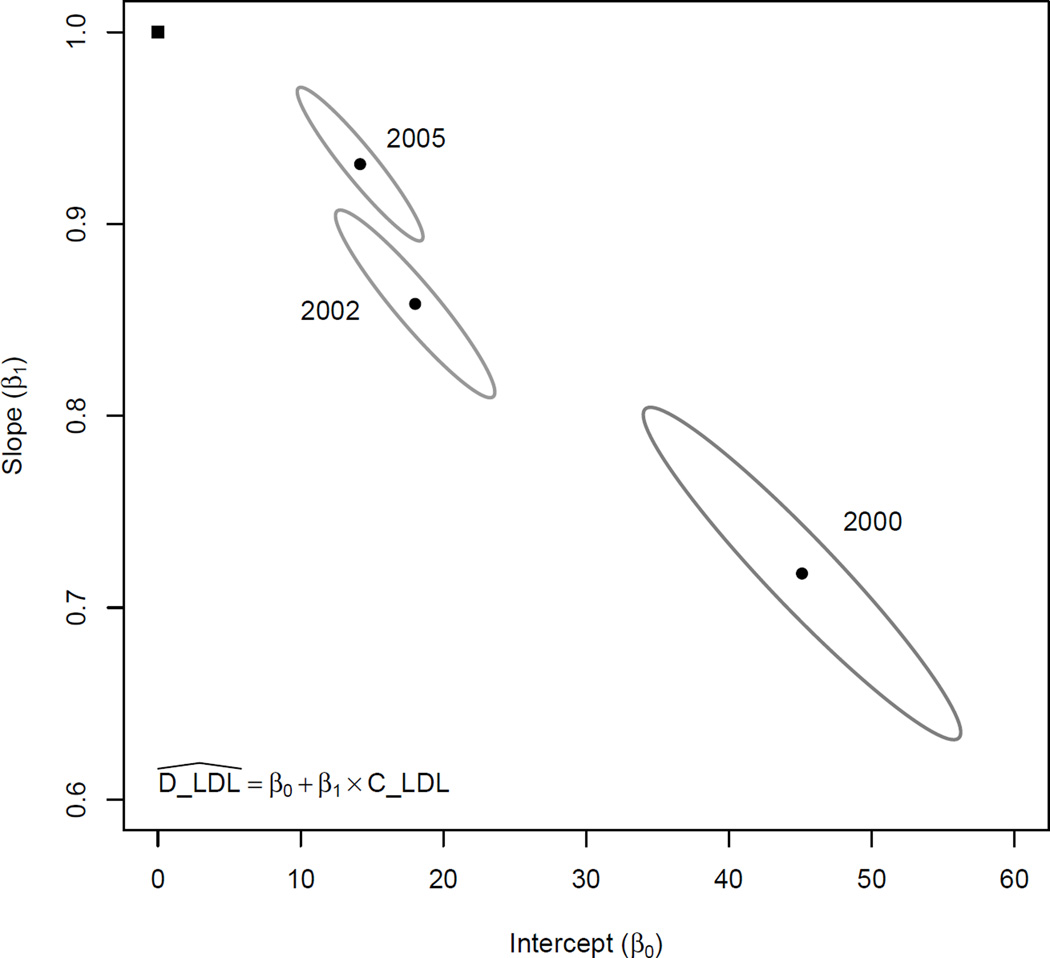
Regression analyses were performed fitting the D-LDL-C measures as a function of C-LDL-C. The regression parameters were then used to perform a test of agreement; namely, testing whether the intercept=0 and the slope=1 for each of the three assays used in different time periods. This data is presented in Figure 1, which presents joint 99.9% confidence ellipses of the intercept and slope estimates for each period. None of the regions contain the point corresponding to intercept=0 and slope=1, providing evidence that the C-LDL-C and D-LDL-C measures do not agree for any of the periods. Additionally, none of the confidence regions overlap, indicating the regression parameters differ by period. Pearson’s correlation coefficient between D-LDL-C and C-LDL-C were 0.93, 0.97 and 0.98 for periods 1, 2 and 3 respectively. These show subsequent better correlations between the two methods of LDL-C measurement over time. This demonstrates the important fact that high correlation does not imply agreement.
Figure 1.
Intercept and slope parameters estimated from linear regression models are shown for each time period (solid round symbols) with corresponding 99.99% confidence regions (indicated by the surrounding ellipses) A point representing the hypothetical regression parameters (β0=0 and β1=1) that one would expect if the two measures agreed is presented for comparison (solid square symbol). None of the 99.99% confidence regions contain this hypothetical point, providing evidence that D-LDL-C and C-LDL-C measures do not agree and that the extent of lack of agreement differs by time periods.
The regression analyses were subsequently used to predict D-LDL-C from C-LDL-C values at treatment goals commonly used for clinical decision making. These results are presented in Table 1. Using the assay for D-LDL-C measurement during period 1, at the 70 mg/dl optional treatment goal of C-LDL-C, the predicted D-LDL-C was 95 mg/dl (36% higher). The difference between C-LDL-C and predicted D-LDL-C progressively decreased at higher LDL-C treatment goals, and at the 160 mg/dl treatment goal of C-LDL-C, the D-LDL-C was 160 mg/dl as well. These trends were still present but much less pronounced in periods 2 and 3 using progressively newer assays for D-LDL-C measurement.
Table 1.
Predicted D-LDL-C from C-LDL-C values at treatment goals commonly used for clinical decision making. Values shown include those for all subjects with TG <400 and for subjects in the lower and higher TG ranges.
| Predicted D-LDL-C when C-LDL-C is | ||||||
|---|---|---|---|---|---|---|
| Period | TG levels | N | 70 mg/dl | 100 mg/dl | 130 mg/dl | 160 mg/dl |
| TG<400 | 464 | 95 | 117 | 138 | 160 | |
| 2000 | TG 300–399 | 41 | 116 | 137 | 158 | 179 |
| TG<100 | 114 | 90 | 109 | 127 | 146 | |
| TG<400 | 807 | 78 | 104 | 130 | 155 | |
| 2002 | TG 300–399 | 53 | 88 | 112 | 137 | 162 |
| TG <100 | 173 | 71 | 98 | 125 | 152 | |
| TG<400 | 937 | 79 | 107 | 135 | 163 | |
| 2005 | TG 300–399 | 61 | 91 | 114 | 138 | 161 |
| TG <100 | 287 | 73 | 103 | 132 | 162 | |
We further stratified the data based on various triglyceride levels (Table 1). With TG levels 300–399 mg/dl during period 1, at the 70 mg/dl optional treatment goal of C-LDL-C, the predicted D-LDL-C was 116 mg/dl. Even for periods 2 and 3 using newer assays, with TG levels 300–399 mg/dl, the greatest difference between predicted D-LDL-C and C-LDL-C were at the 70 mg/dl optional treatment goal, i.e. predicted D-LDL-C was 88 mg/dl in period 2 and 91 mg/dl in period 3. Progressively smaller differences between predicted D-LDL-C and C-LDL-C at each higher treatment goal were noticed in the subgroup of subjects with TG levels 300–399 mg/dl, until at the 160 mg/dl treatment goal of C-LDL-C, the C-LDL-C and predicted D-LDL-C were nearly identical, i.e. predicted D-LDL-C was 162 mg/dl in period 2 and 161 mg/dl in period 3.
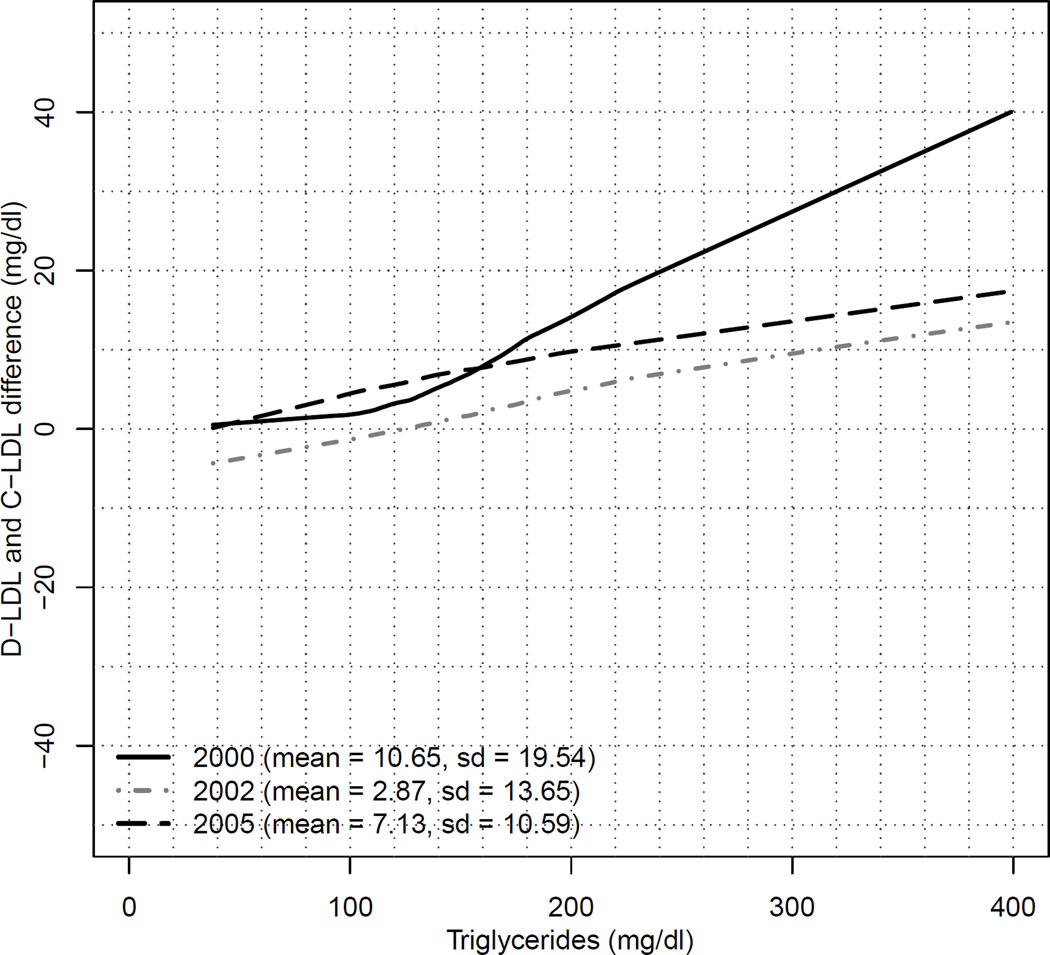
At the lower triglyceride levels (TG<100), the same trend was true in period 1, i.e. the greatest variation occurred at the 70 mg/dl optional treatment goal of C-LDL-C where predicted D-LDL-C was 90 mg/dl with decreasing differences at higher treatment goals of C-LDL-C 130 mg/dl where predicted D-LDL-C was 127 mg/dl. Results in period 2 and 3 for TG levels <100 mg/dl with the newer assays had much better correlations, with nearly identical results between C-LDL-C and predicted D-LDL-C in the newest 2005 assay at all 4 treatment goals of 70, 100, 130 and 160 mg/dl. The differences between D-LDL-C and C-LDL-C were also plotted against TG levels at each of the 3 time periods. This is presented in Figure 2. With all 3 assays for D-LDL-C, the graphs were up sloping, indicating that the difference between D-LDL-C and C-LDL-C rises steadily with increasing TG levels. The greatest effect of TG on the difference between D-LDL-C and C-LDL-C was observed in period 1, with D-LDL-C being almost 40 mg/dl higher than the C-LDL-C at a TG level of 400 mg/dl.
Figure 2.
Graph of D-LDL-C and C-LDL-C difference with corresponding triglyceride values for the periods 2000 (solid black line), 2002 (dashed gray line) and 2005 (dashed black line) using 3 different assays. In the parenthesis are shown the mean and the standard deviation of D-LDL-C and C-LDL-C difference for the 3 corresponding assay results. This Figure illustrates that differences between D-LDL-C and C-LDL-C increase as triglyceride levels increase, most striking in the 2000 time period.
Tables 2–4 present cross-tabulations of samples based on C-LDL and D-LDL measures at the clinically meaningful LDL treatment goals. Kappa statistics and generalized McNemar tests are presented for each period. While the kappa statistics suggests the LDL-C measures have moderate levels of agreement, the significant generalized McNemar tests (p<0.01) provide substantial evidence of disagreement. There were 151 samples with D-LDL-C values between 100–130 mg/dl measured by the assay used in 2000 (Table 3). Of these, only 96 samples show agreement with C-LDL-C values whereas 50 samples with a D-LDL-C between 100 and 130 mg/dl have a C-LDL-C in the 70–100 mg/dl range. Similarly, there are 290 samples with D-LDL-C values between 100–130 mg/dl measured in the assay used in 2005. Of these, only 182 samples show agreement with C-LDL-C values where as 90 samples with a D-LDL-C in the 100–130 mg/dl range are in 70–100 mg/dl range using the C-LDL-C assay. These trends are present throughout with all the 3 assays for D-LDL-C measurement showing that D-LDL-C measures overestimates LDL-C values as calculated by Friedewald equation, or C-LDL-C measures underestimate D-LDL-C.
Table 2.
Cross tabulation of year 2000 samples based on C-LDL-C and D-LDL-C at clinically meaningful LDL-C treatment goals. Numbers along the diagonal of the table (in bold) indicate where the measures agree. Numbers in the off-diagonal cells indicate some level of disagreement.
| C-LDL-C | D-LDL-C Treatment Goal (mg/dl) | |||||
|---|---|---|---|---|---|---|
| Treatment Goal (mg/dl) |
<70 | 70 – 100 | 100 – 130 | 130 – 160 | ≥ 160 | Total |
| <70 | 2 | 19 | 3 | 4 | 0 | 28 |
| 70 – 100 | 0 | 44 | 50 | 5 | 2 | 101 |
| 100 – 130 | 0 | 2 | 96 | 50 | 1 | 149 |
| 130 – 160 | 0 | 0 | 2 | 91 | 20 | 113 |
| ≥ 160 | 0 | 0 | 0 | 7 | 66 | 73 |
| Total | 2 | 65 | 151 | 157 | 89 | 464 |
Kappa Statistic: 0.5272 (0.4703, 0.5841)
Generalized McNemar Test: p < 0.001
Table 4.
Cross tabulation of year 2005 samples based on C-LDL-C and D-LDL-C at clinically meaningful LDL-C treatment goals. Numbers along the diagonal of the table (in bold) indicate where the measures agree. Numbers in the off-diagonal cells indicate some level of disagreement.
| C-LDL-C | D-LDL-C Treatment Goal (mg/dl) | |||||
|---|---|---|---|---|---|---|
| Treatment Goal (mg/dl) |
<70 | 70 – 100 | 100 – 130 | 130 – 160 | ≥ 160 | Total |
| <70 | 77 | 73 | 1 | 0 | 0 | 151 |
| 70 – 100 | 9 | 226 | 90 | 0 | 0 | 325 |
| 100 – 130 | 0 | 12 | 182 | 62 | 0 | 256 |
| 130 – 160 | 0 | 0 | 15 | 99 | 27 | 141 |
| ≥ 160 | 0 | 0 | 2 | 5 | 57 | 64 |
| Total | 86 | 311 | 290 | 166 | 84 | 937 |
Kappa Statistic: 0.5803 (0.5406, 0.6201)
Generalized McNemar Test: p < 0.001
Table 3.
Cross tabulation of year 2002 samples based on C-LDL-C and D-LDL-C at clinically meaningful LDL-C treatment goals. Numbers along the diagonal of the table (in bold) indicate where the measures agree. Numbers in the off-diagonal cells indicate some level of disagreement.
| C-LDL-C | D-LDL-C Treatment Goal (mg/dl) | |||||
|---|---|---|---|---|---|---|
| Treatment Goal (mg/dl) |
<70 | 70 – 100 | 100 – 130 | 130 – 160 | ≥ 160 | Total |
| <70 | 60 | 60 | 0 | 1 | 0 | 121 |
| 70 – 100 | 11 | 182 | 64 | 0 | 0 | 257 |
| 100 – 130 | 7 | 21 | 158 | 40 | 0 | 226 |
| 130 – 160 | 0 | 0 | 21 | 101 | 5 | 127 |
| ≥ 160 | 0 | 1 | 1 | 14 | 60 | 76 |
| Total | 78 | 264 | 244 | 156 | 65 | 807 |
Kappa Statistic: 0.5982 (0.5559, 0.6405)
Generalized McNemar Test: p < 0.001
DISCUSSION
Our results indicate that there exists significant variability between the two methods of LDL-C measurements. D-LDL-C was 36% higher than the Friedewald calculated LDL-C at 70 mg/dl optional treatment goal in period 1. These differences were much more pronounced at lower LDL-C values and progressively decreased at higher LDL-C values. Prior investigators have found that at lower LDL-C levels, D-LDL-C assays give higher values than C-LDL-C.; although a recent study found that D-LDL-C was consistently lower than C-LDL-C4, 16.
Our subsequent newer assays used in periods 2 and 3 had better correlation even at lower LDL-C values, but there still were increasing differences between the two methods of LDL-C measurement with rising TG levels. The correlation coefficients between D-LDL-C and C-LDL-C were nearing 1 with subsequent newer assays. However, using only correlation coefficients without taking into consideration the slope and intercept of the regression equations to conclude that a particular assay is better can be misleading as excellent correlation does not imply agreement.
We also evaluated the effect of TG concentrations on the difference between D-LDL-C and C-LDL-C measurements. D-LDL-C was observed to be as much as 40 mg/dl higher than C-LDL-C at higher TG levels during period 1 (Figure 2). This trend is present at all time periods at increasing TG levels (< 400 mg/dl). Previous reports have also indicated that various D-LDL-C assays showed a better correlation between D-LDL-C and C-LDL-C at lower TG levels, claiming that D-LDL-C assays better reflect true LDL-C4, 18. Although this has led some to conclude that the Friedewald C-LDL-C may underestimate the risk for CHD, one might equally conclude that the D-LDL-C overestimates CHD risk. In a recent study addressing this issue utilizing samples from the Women’s Health Study, Mora et al concluded that there was a lack of association of non-fasting D-LDL-C with cardiovascular disease raising questions regarding the clinical utility of a direct assay for LDL-C in non-fasting samples16. If in fact it is ultimately shown that C-LDL-C is the better indicator of CHD risk, then the usefulness of the D-LDL-C becomes limited in those very instances in which it is purported to be beneficial, i.e. moderate hypertriglyceridemia, and lack of need for a fasting sample. This information underscores the need for more in depth studies of D-LDL-C assays and their true relationship to CHD risk.
The ATPIII guidelines recommend using non-HDL cholesterol as a secondary treatment goal in persons with triglycerides in the range of 200–499 mg/dL. In persons with triglycerides in this range, one might propose using non-HDL cholesterol as the primary treatment goal which would circumvent the issue of difficulties with LDL-C measurements. Although non-HDL cholesterol may be an excellent secondary treatment goal in such patients, use of LDL-C as the primary treatment goal, even in patients with triglycerides in this range is recommended based on the large amount of clinical trial data, the vast majority of which was developed using the Friedewald calculated LDL-C.
The results shown in Tables 2–4 show that D-LDL-C is sufficiently higher than C-LDL-C to have clinically meaningful differences. For example, Table 4 shows that of 311 samples with D-LDL-C in the range 70–100 mg/dl, 73 of these samples have C-LDL-C measurements less than 70 mg/dl. Other investigators have similarly noted that subjects with LDL-C values in the lower range often show higher results using a D-LDL-C assay19. Use of the higher D-LDL-C values in clinical medicine when applied to the recommended LDL-C treatment goals will lead to misclassification of patient’s cardiovascular risk into a different treatment goal based on current NCEP guidelines. This will result in more drug usage, thus exposing patients to more potential side effects and at a much greater cost with little evidence of benefit.
Our results highlight significant lack of agreement between the Friedewald calculated LDL-C and three different assays for direct LDL-C measurement used in the CAVHS clinical laboratory during routine clinical practice. We would like to emphasize that the major clinical trials on which the NCEP ATPIII guidelines were based used the C-LDL-C 3. In addition, even since ATPIII, major clinical outcome lipid trials used C-LDL-C as a method of LDL-C measurement20–24. In view of this we would recommend continuing to use the Friedewald calculated LDL-C for routine clinical practice until direct LDL-C assays have been better standardized and there are more clinical trial outcome data using the D-LDL-C assay. With the inconsistencies in LDL-C measurement as shown above and the increasing use of D-LDL-C measurements in clinical practice, there is a need to reemphasize the 1995 recommendations by the NCEP working group on lipoprotein measurements 25.
ACKNOWLEDGMENT
We acknowledge the support of the Central Arkansas Veterans Healthcare System in this research project. The project described was also supported, in part, by Award Number 1UL1RR029884 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
References
- 1.Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomized placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Esteban-Salan M, Aguilar-Doreste JA, Arranz-Pena ML, et al. Multicentric evaluation of the homogeneous LDL-cholesterol plus assay: Comparison with beta-quantification and friedewald formula. Clin Biochem. 2008;41(16–17):1402–1409. doi: 10.1016/j.clinbiochem.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 5.Miller WG, Waymack PP, Anderson FP, et al. Performance of four homogeneous direct methods for LDL-cholesterol. Clin Chem. 2002;48(3):489–498. [PubMed] [Google Scholar]
- 6.College of American Patholigists 2009 C-B proficiency survey for Chemistry/Therapeutic drug monitoring [Google Scholar]
- 7.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 8.Sacks FM, Pfeffer MA, Moye' L, et al. Rationale and design of a secondary prevention trial of lowering normal plasma cholesterol levels after acute myocardial infarction: The cholesterol and recurrent events trial (CARE) Am J Cardiol. 1991;68(15):1436–1446. doi: 10.1016/0002-9149(91)90276-q. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Tonkin AM, Shepherd J, et al. Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: The prospective pravastatin pooling project. Circulation. 2000;102(16):1893–1900. doi: 10.1161/01.cir.102.16.1893. [DOI] [PubMed] [Google Scholar]
- 10.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. west of scotland coronary prevention study group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 11.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. the long-term intervention with pravastatin in ischaemic disease (LIPID) study group. N Engl J Med. 1998;339(19):1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 12.Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 13.The coronary primary prevention trial: Design and implementation: The lipid research clinics program. J Chronic Dis. 1979;32(9–10):609–631. doi: 10.1016/0021-9681(79)90092-4. [DOI] [PubMed] [Google Scholar]
- 14.Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: Results of AFCAPS/TexCAPS. air Force/Texas coronary atherosclerosis prevention study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 15.Lippi G, Montagnana M, Guidi GC. Direct LDL-cholesterol measurement: Not ready for the prime time? Int J Cardiol. 2009 doi: 10.1016/j.ijcard.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Mora S, Rifai N, Buring JE, et al. Comparison of LDL cholesterol concentrations by friedewald calculation and direct measurement in relation to cardiovascular events in 27,331 women. Clin Chem. 2009;55(5):888–894. doi: 10.1373/clinchem.2008.117929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 18.Jun KR, Park HI, Chun S, et al. Effects of total cholesterol and triglyceride on the percentage difference between the low-density lipoprotein cholesterol concentration measured directly and calculated using the friedewald formula. Clin Chem Lab Med. 2008;46(3):371–375. doi: 10.1515/CCLM.2008.064. [DOI] [PubMed] [Google Scholar]
- 19.Sibal L, Neely RD, Jones A, et al. Friedewald equation underestimates low-density lipoprotein cholesterol at low concentrations in young people with and without type 1 diabetes. Diabet Med. 2010;27(1):37–45. doi: 10.1111/j.1464-5491.2009.02888.x. [DOI] [PubMed] [Google Scholar]
- 20.Knopp RH, d'Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: The atorvastatin study for prevention of coronary heart disease endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29(7):1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 22.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350(15):1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 23.Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: The alliance study. J Am Coll Cardiol. 2004;44(9):1772–1779. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 24.de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: Phase Z of the A to Z trial. JAMA. 2004;292(11):1307–1316. doi: 10.1001/jama.292.11.1307. [DOI] [PubMed] [Google Scholar]
- 25.Bachorik PS, Ross JW. National Cholesterol Education Program recommendations for measurement of low-density lipoprotein cholesterol: Executive Summary. The National Cholesterol Education Program Working Group on Lipoprotein Measurement. Clin Chem. 1995 Oct;41(10):1414–1420. [PubMed] [Google Scholar]