Abstract
Angiotensin (Ang) II exaggerates cerebral injury in ischemic damage. Angiotensin-converting enzyme type 2 (ACE2) converts Ang II into Ang (1–7) and thus, may protect against the effects of Ang II. We hypothesized that neuronal ACE2 over-expression decreases ischemic stroke in mice with Ang II overproduction. Human renin and angiotensinogen double transgenic (RA) mice and RA mice with neuronal over-expression of ACE2 (SARA) were used for the study. The mean arterial pressure (MAP) was calculated from telemetry-recorded blood pressure (BP). SARA mice were infused peripherally with Norepinephrine to “clamp” the BP, or intracerebroventricularly-infused with a Mas receptor antagonist (A-779). Middle cerebral artery occlusion (MCAO) surgery was performed to induce permanent focal ischemic stroke. Cerebral blood flow (CBF) and neurological function were determined. Two days after surgery, brain samples were collected for various analyses. Results showed: 1) When compared to chronically hypertensive RA mice, SARA mice had lower basal MAP, less MCAO-induced infarct volume, and increased CBF, neurological function and cerebral microvascular density in the peri-infarct area; 2) These changes in SARA mice were not altered after MAP “clamping”, but partially reversed by brain infusion of A-779; 3) Ang (1–7)/Ang II ratio, angiogenic factors, endothelial nitric oxide synthase (eNOS) expression and nitric oxide production were increased, whereas, NADPH oxidase subunits and reactive oxygen species were decreased in the brain of SARA mice. ACE2 protects brain from ischemic injury via the regulation of NADPH oxidase/eNOS pathways by changing Ang (1–7)/Ang II ratio, independently of MAP changes.
Keywords: Angiotensin-converting enzyme type 2, angiotensin II, ischemic stroke, blood pressure, oxidative stress
1. Introduction
Ischemic stroke, the third leading cause of death in the United States, is known as a severe complication of hypertension and arteriosclerosis. There are limited avenues for preventing and treating ischemic stroke. Contribution of the Angiotensin converting enzyme (ACE)/angiotensin (Ang) II/AT1 receptor (AT1R) pathway, the main axis of the renin-angiotensin system (RAS), is well accepted in the pathophysiology of ischemic stroke (Chrysant 2005, Saavedra et al. 2006). Overproduction of Ang II induces enlarged brain ischemic injury (Chen et al. 2009). ACE inhibitors and AT1R blockers have been shown to protect the brain from ischemic injury in both animal studies (Nishimura et al. 2000, Iwanami et al. 2010) and clinical trials (Dahlof et al. 2002, Kjeldsen et al. 2005). The protective effect may relate to blood pressure (BP) control and improvement of cerebral blood supply (Nishimura et al. 2000, Iwanami et al. 2010). However, there is limited information regarding BP-independent effects of Ang II on ischemic stroke. On the other hand, a new arm of the RAS, ACE2/Ang (1–7)/Mas receptor (MasR), has been shown to counteract the effects of ACE/Ang II/AT1R axis (Guo et al. 2008). ACE2 cleaves Ang II into the vasodilatory peptide Ang (1–7), which in turn, binds the MasR to exert opposite properties to Ang II (Guo et al. 2008). An earlier study showed that central administration of Ang (1–7) stimulates nitric oxide (NO) release and upregulates endothelial nitric oxide synthase (eNOS) expression in ischemic tissues following focal cerebral ischemia/reperfusion in rats (Zhang et al. 2008). Another report further demonstrated that the beneficial effects of central administration of Ang (1–7) or pharmacological activation of ACE2 in endothelin-1-induced ischemic stroke, which can be reversed by A-779, a Mas receptor antagonist (Mecca et al. 2011). Recent evidence demonstrates that ACE2 has vascular protective effects by counteracting the deleterious actions of Ang II on BP regulation and vascular function (Silva et al. 2005, Chen et al. 2011). Therefore, ACE2 holds a great potential for developing new avenues to treat vascular diseases. However, whether the ACE2/Ang (1–7)/MasR axis exhibits protective effects on ischemic stroke by counteracting the ACE/Ang II/AT1R axis and what are the underlying mechanisms remains to be determined.
The diverse actions of ACE/Ang II/AT1R axis on vascular and cerebral damage are mediated by coupling to NADPH oxidase (Nox) which generates reactive oxygen species (ROS). Nox-derived ROS play a key role in Ang II-induced endothelial damage (Doughan et al. 2008), neuronal apoptosis and cerebral inflammation (Yamamoto et al. 2008). Moreover, Ang II-induced endothelial dysfunction is associated with the reduction of NO bioavailability and decreased NO production, which also plays an important role in cerebrovascular disease (Sierra et al. 2011). Recent evidence show that ACE2 has an anti-atherosclerotic effect by down-regulating Ang II-mediated ROS production (Doughan et al. 2008). Although there is much evidence for Nox/ROS and eNOS/NO as downstream pathways of the ACE/Ang II/AT1R axis, there is still a paucity of information regarding how these pathways are modulated by the ACE2/Ang (1–7)/MasR arm in ischemic stroke.
We previously showed enlarged ischemic injury in the human renin and angiotensinogen double transgenic (RA) mice (Chen et al. 2009). In this study, we examined the counteracting effect of ACE2/Ang (1–7)/MasR axis to ACE/Ang II/AT1R axis on ischemic stroke by over-expressing ACE2 in the neurons of RA mice. The BP independent and downstream (Nox/ROS, eNOS/NO and cytokines) mechanisms were investigated.
2. Materials and methods
2.1. Experimental animals
RA mice were produced using colonies maintained in our laboratory and genotyped as previously described (Iida et al. 2005). RA mice were bred with syn-hACE2 transgenic mice with neuronspecific ACE2 over-expression to generate a triple transgenic model (SARA) (Xia et al. 2009). Male SARA and RA mice aged 6 to 10 weeks were used for all experiments in this study. Mice were fed with standard chow and water ad libitum. All experimental procedures were approved by the Wright State University Laboratory Animal Care and Use Committee and were in accordance with the Guide for the Care and Use of Laboratory Animals issued by the National Institutes of Health.
2.2. Experimental protocols for Experiment 1
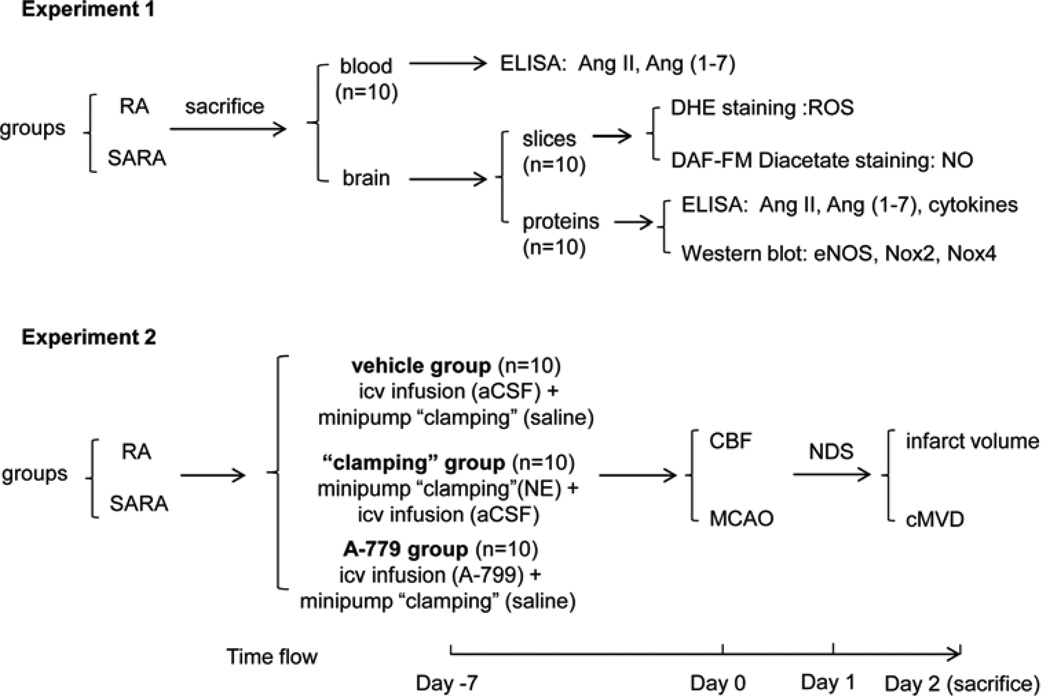
The brain and blood samples from healthy RA and SARA mice were obtained (Fig. 1). The blood samples were used for measuring the level of Ang II and Ang (1–7) (2.3.). Brain tissues were used for immunohistochemical staining of ROS/NO production (2.5.), and for isolating total protein or blood vessels and brain parenchyma fractions for ELISA (2.4. and 2.6.) and western blot analyses (2.7.).
Fig. 1.
Experimental procedure and time flow chart. DHE: dihydroethidium; ROS: reactive oxygen species; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; Nox: NADPH oxidase; aCSF: artificial cerebrospinal fluid; NE: norepinephrine; CBF: cerebral blood flow; cMVD: cerebral microvascular density; icv: intracerebrolventricular; NDS: neurologic deficit scores.
2.3. Measurements of plasma concentration of Ang II and Ang (1–7)
The blood samples were taken from the left ventricle of heart the blood samples were taken from the left ventricle of heart in a chilled blood collection tubes with a cocktail of protease inhibitors (25 mmol/L EDTA, 0.44 mmol/L o-phenylenediamine, and 0.12 mmol/L pepstatin A) as previously reported (Iyer et al. 1998). After immediate centrifuge, plasma samples were quickly transferred into a pre-cooled tube, and put into the dry ice container, then stored in −80°C. The levels of Ang II and Ang (1–7) in the plasma were determined by commercial ELISA kits (Enzo Life Sciences, Farmingdale, NY) within 3 days. The 96-well plates were placed on a microplate reader (Packard; Meriden, CT) to measure absorbance at 450 nm.
2.4. Measurements of brain concentration of angiogenic cytokines
After collection of blood, the brain tissues were immediately dissected and the protein was extracted in lysis buffer. After centrifuge at 10,000 for 10 min, the protein was transferred to a new tube on dry ice and stored in −80°C. The levels of angiogenic cytokines in the total brain protein was determined by angiogenesis ELISA assay kit (Signosis, Sunnyvale, CA). The cytokines included in this kit are vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), interleukin-6 (IL-6) and interferon gamma (IFN-γ), tumor necrosis factor α (TNF-α), Insulin-like growth factor 1 (IGF-1), basic fibroblast growth factor (FGF-β), and Leptin.
2.5. Measurement of ROS production and NO generation
After collection of blood from heart, the brains collected from RA and SARA mice were immediately frozen in Tissue-Tek OCT embedding medium, cryostat-sectioned (20 µm) directly onto chilled microscope slides, and dried without fixation. The sections were sequentially put into six separate wells of a 6-well plate containing 2 ml of phosphate-buffered saline (PBS). For ROS measurement, brain sections were reacted with dihydroethidium (DHE, 2 µmol/L) in dark for 10 min at room temperature (RT) (Yamamoto et al. 2008). For NO determination, brain slices were incubated for 1 hr at 37°C in DMEM (Mediatech, Manassas, VA) containing 10 µM dye 4-amino-5-methylamino-2’,7’-difluorofluorescein diacetate (DAF-FM Diacetate) (Invitrogen, Carlsbad, CA) (Kojima et al. 2001). After incubation, sections were washed twice for 3 min with phosphate buffer saline (PBS). Following the final wash, sections were coversliped and imaged using a fluorescence microscope (Leica TCS SP2). Fluorescence extent of random chosen microscopy fields located in cortex and stratum areas was quantified using Image J. Totally five random microscopy fields in each and slice and six slices of each brain were averaged to present the data of each mouse. The data were expressed as percentile of fluorescence level in the sections of RA mice.
2.6. Isolation of cerebral vessel and brain parenchyma
In the other set of experiment, the cerebral blood vessel and brain parenchyma were separately isolated from the brains of RA and SARA mice according to previous methods (Williams et al. 1988, Williams and Chung 2006). Briefly, The brains were removed free of meninges, dissected into small pieces and homogenized in 0.4 M sucrose solution containing 0.1 mM CaCl2, 0.4 mM MES buffer, 0.1 mM ATP, 1% BSA, and 20,000 IU sodium heparin at 4°C. The protease inhibitors (0.5 µg/ml aprotinin, 0.5 µg/ml leupeptin, and 0.7 µg/ml pepstatin) and 0.1 mM EDTA were added. The homogenate was subsequently passed through nylon mesh sieve (125 µm). After centrifuging at 200×g for 5 min, the pellet was resuspended in 18% Detran solution and centrifuged at 10000×g for 10 min. The pellet was resuspended in Ca2+-Mg2+ free Hank balance salt solution (HBSS) and pass through nylon mesh filters (75 µm). The microvasculature retained on the filter was obtained by rinsing the filter with HBSS. The resulting blood vessel and brain parenchyma fractions were used for determining brain Ang II/Ang (1–7) level by ELISA kit (see 2.3.) and gene expression by western blot analyses (see below).
2.7. Western blot analysis
Proteins from blood vessel and vessel-depleted brain parenchyma fractions were isolated with lysis buffer (Roche Diagnostic, San Francisco, CA). The proteins (50 µg) were subjected to SDS-PAGE electrophoresis and transferred onto nitrocellulose membranes. The primary antibodies used were eNOS (1:1000, Abcam, Cambridge, MA), Nox2 (1:1000, Abcam) and Nox4 (1:250, Abcam). The second antibody used was horseradish peroxidase conjugated IgG (1:40000, Jackson Lab). β-actin (1:4000, Sigma, MO) was used to normalize protein loading.
2.8. Experimental design for Experiment 2
The time flow is shown in Fig. 1. All animals were implanted with telemetric probes for BP recording. Meanwhile, animals were randomly divided into three groups: vehicle, “clamping” and A-779. The mice in both vehicle and “clamping” groups were infused with artificial cerebrospinal fluid (aCSF), the mice in “clamping” group were infused with norepinephrine (NE) via an osmotic minipump, and the mice in A-779 group were infused with A-779 by intracerebroventricular (icv) and ‘mock’ clamped by infused with saline via a minipump. Seven days after icv infusion, mice were subjected to middle cerebral artery occlusion (MCAO) surgery for inducing permanent focal ischemic stroke. The neurological deficit scores (NDS) were evaluated 24 hr after MCAO surgery. Two days after MCAO, mice were euthanized and the brain samples were collected for infarct volume and cerebral microvascular density (cMVD) analyses.
2.9. BP recording and “clamping”
A radiotelemetry system (TA11PA-C10, Data Science International) was used for recording arterial pressure as we described previously (Chen et al. 2009, Chen et al. 2006). The telemetric probe was implanted in the left carotid artery. BP was continuously recorded for 24 hr before and after MCAO to calculate the mean arterial pressure (MAP). We recorded the BP for 10 minutes (sample rate 500 hz) once a hour for 24 hrs. The BP was started to record at the same time for each mouse. To exclude the benefits of the ACE2 BP-lowering effect on ischemic stroke, NE (5.6 mg/kg/day, minipump) was used to “clamp” the BP of the SARA mice to a similar level as the RA group (Cassis et al. 2009).
2.10. Intracerebroventricular infusion
Mice were instrumented with a mouse brain infusion kit (ALZET, infusion rate, 0.25 µl/hr) for chronic icv infusion of aCSF or the MasR antagonist (A-779, 200 ng/kg/min, Bachem) for 7 days. The dose for A-779 was chosen based on a previous study (Xiao et al. 2011). The flow moderator of the minipump was connected with a polyethylene catheter to a metal cannula (30-gauge) to allow for long-term icv infusion. The minipump was placed in a sterile isotonic saline solution overnight at 37°C to initiate their operation at a constant pumping rate and to minimize the possibility of clot formation in the catheter. Implantation of infusion kit was performed in a stereotaxic apparatus. The skull was exposed by a midline sagittal incision through the scalp and a subcutaneous pocket was prepared at the back of the mouse. The minipump was placed into the pocket, and the metal cannula was inserted through the skull into the brain (1.0 mm lateral and caudal 0.6 mm to the bregma, 3 mm in depth) to reach the ventricle.
2.11. MCAO surgery
Mice were subjected to MCAO by an intraluminal filament according to our previous reports (Chen et al. 2009, Chen et al. 2011). Briefly, mice were anaesthetized with 2.5% isoflurane inhalation, and body temperature was maintained in the range of 37.0 ± 0.5°C with a heating pad throughout the procedure. The right common carotid artery, external carotid artery (ECA) and internal carotid artery (ICA) were isolated through a ventral midline incision. A 2.0-cm-length of monofilament nylon suture (size, 7-0), with its tip rounded by heating near a flame, was inserted from the right ECA into the lumen of ICA, then advanced until resistance was felt (0.8–1.0 cm from the bifurcation). The filament remained there until the mice were sacrificed. Pain and discomfort were minimized by an initial injection of Buprenorphine (0.1 mg/kg, sc) and Carperofen (5 mg/kg, sc) followed with another Carperofen injection every 24 hrs.
2.12. Cerebral blood flow measurement
The relative CBF in the peri-infarct area was determined immediately before and after MCAO surgery (Girouard et al. 2008, Chen et al. 2013). Briefly, mouse was anesthetized with 2.5% isoflurane and placed on a stereotaxic apparatus. Before MCAO surgery, the volumes of CBF at the site (core: 2 mm posterior, 6 mm lateral to bregma; peri-infarct: 2 mm posterior and 3 mm lateral to bregma) representing the blood flow supplied by the MCA were sequentially determined for 5 minute using a laser Doppler flowmeter (PF2B, Perimed, Sweden) for measuring the basal CBF. Immediately after MCAO surgery, the CBF was recorded at the same site (peri-infarct area) for another 5 minutes. The averaged volume over 5 min was used to represent CBF for each site. The relative CBF was calculated using the formula: relative CBF= CBF after MCAO/ basal CBF ×100%. The person who performed CBF measurements was unaware of the information of animal grouping.
2.13. Functional evaluation of neurological deficits
The NDS were evaluated 24 hr after MCAO surgery by using the 5-point scale method as previously described (Chen et al. 2013). The five points are: 0, normal motor function; 1, flexion of contralateral torso and forelimb upon lifting the whole animal by the tail; 2, circling to the contralateral side but normal posture at rest; 3, leaning to the contralateral side at rest; 4, no spontaneous motor activity. The neurologic behavior of mice was scored by an investigator who was unaware of animal grouping.
2.14. Measurement of infarct volume
Mice were sacrificed 2 days after MCAO surgery. The brains were immediately collected and fixed in 4% paraformaldehyde (PFA) overnight and in 4% PFA plus 30% sucrose for 3 days. The brains were then stored at −80°C. For measurement, each frozen brain was cryostat-sectioned into coronal sections (20 µm) and sequentially put into a 6-well plate containing 2 ml/well of phosphate-buffered saline (PBS). Totally four representative slices (from rostral to caudal) with similar locations in each brain were selected to stain with Fluoro-Jade (Histo-chem, Jefferson, AR) to reveal the infarct area. To compensate for brain edema, the percentage of the infarct area to the total brain slice area was calculated by using Image J software (NIH) as we previously described (Chen et al. 2013, Chen et al. 2011). In all experiments involving image analysis, the individual(s) taking the photomicrographs and conducting the analysis was blinded to the treatment grouping.
2.15. Measurement of cerebral microvascular density
The measurement of cMVDs in the contralateral and the ipsilateral (peri-infarct area) were achieved by immunofluorescence staining (Chen et al. 2013, Chen et al. 2011). Briefly, brain sections were reacted with primary antibody (CD31, 1:50 in 1% donkey serum, BD Biosciences, San Jose, CA) and second antibody (Alexa Fluor 594-conjuated donkey anti-rat IgG, 1:200, Molecular Probes, Invitrogen, Carlsbad, CA). Five images in the non-ischemic cortex or peri-infarct regions, determined by staining the adjacent sections with Fluoro-Jade as described above, were taken from each section under a confocal microscope (Leica TCS SP2, Wetzlar, Germany). Quantification of vessel density was obtained from these pictures using Image J software (NIH). The mean volume of cMVD from six sequential brain sections of individual mouse was calculated and expressed as numbers/mm2.
2.16. Statistical analysis
All data, excepting neurologic deficit scores, are presented as mean ± SE. The neurologic deficit scores were expressed as median (range). The neurological deficit scores among different groups were compared by the Kruskal–Wallis test. When the Kruskal–Wallis test showed a significant difference, the Mann–Whitney U-tests were applied. For the remaining experiments, comparisons for two groups were performed by the student’s t test. Multiple comparisons were analyzed by one- or two-way ANOVA. For all tests, a P-value <0.05 was considered significant.
3. Results
3.1. Neuronal over-expression of ACE2 decreases brain Ang II/Ang (1–7) ratio
The levels of Ang II and Ang (1–7) in plasma, brain parenchyma and cerebral vessels of RA and SARA mice are presented in Table 1. The level of Ang II was decreased and the level of Ang (1–7) was increased in the brain parenchyma of SARA mice (P<0.01). There were no changes in the plasma and cerebral vessels. These data indicates that the beneficial effects of ACE2 over-expression might be attributed to the increased brain Ang (1–7) and decreased Ang II level.
Table 1.
Ang II/Ang (1–7) balance in RA and SARA mouse
| RA | SARA | |
|---|---|---|
| Plasma (pg/ml) | ||
| Ang II | 225 ± 43 | 209 ± 41 |
| Ang (1–7) | 128 ± 33 | 107 ± 33 |
| Ang II/Ang (1–7) | 1.75 ± 0.26 | 1.95 ± 0.24 |
| Brain parenchyma (fmol/mg) | ||
| Ang II | 110 ± 22 | 89 ± 15** |
| Ang (1–7) | 79 ± 20 | 110 ± 28** |
| Ang II/Ang (1–7) | 1.32 ± 0.10 | 0.81 ± 0.10** |
| Cerebral vessel (fmol/mg) | ||
| Ang II | 58 ± 15 | 55 ± 16 |
| Ang (1–7) | 40 ± 11 | 42 ± 18 |
| Ang II/Ang (1–7) | 1.45 ± 0.10 | 1.22 ± 0.11 |
Data are means ± SE.
P< 0.05,
P< 0.01, compared with RA. n=10/group.
Ang II: Angiotensin II, Ang (1–7): Angiotensin (1–7).
3.2. Neuronal over-expression of ACE2 increases the brain level of angiogenic factors
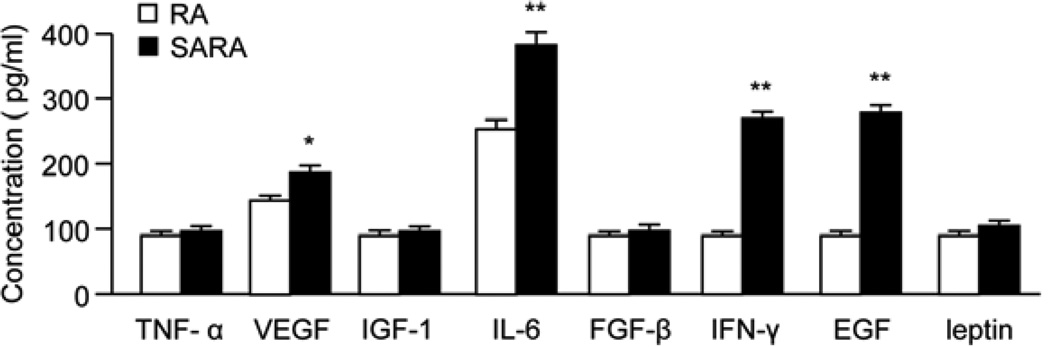
The ELISA assay revealed that the levels of VEGF, EGF, IL-6 and IFN-γ in the total brain protein of SARA mice were higher than in RA mice (P<0.05 or 0.01; Fig. 2).
Fig. 2.
Brain angiogenic cytokines in RA and SARA mice. The level of angiogenic factors was determined by ELISA kit. The levels of angiogenic factors are increased in SARA mice. *P<0.05, **P<0.01 vs. RA, n=10/group.
3.3. Neuronal over-expression of ACE2 changes the Nox/eNOS expression and NO/ROS levels
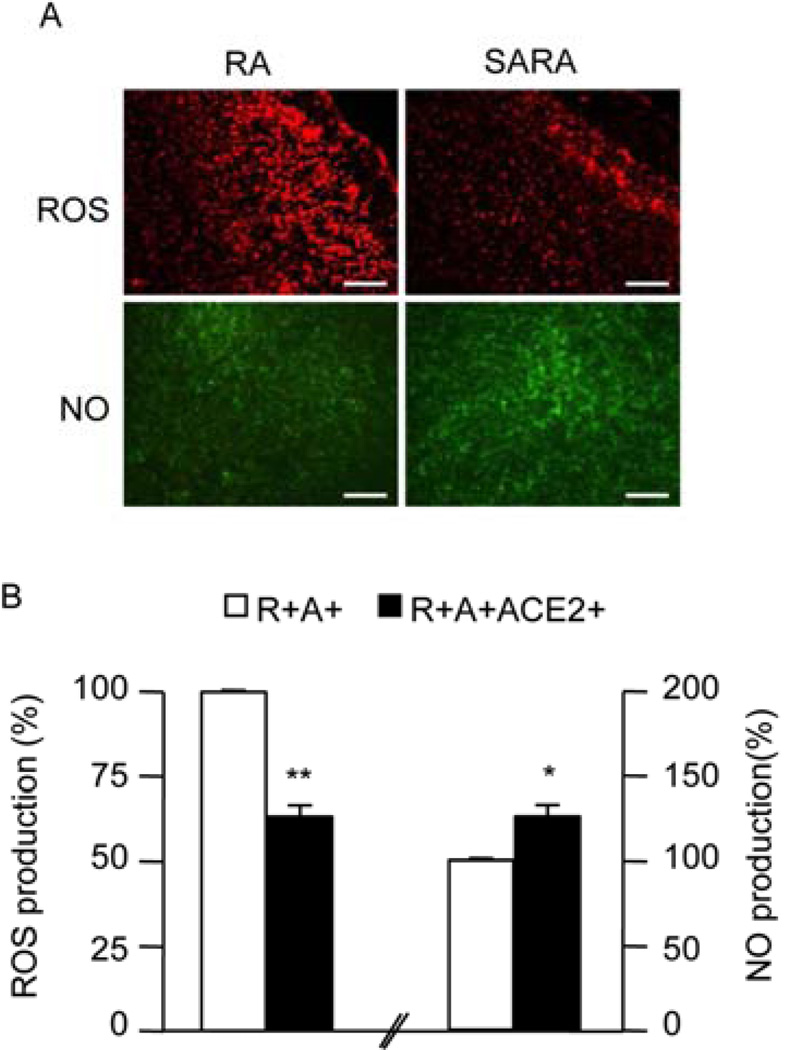
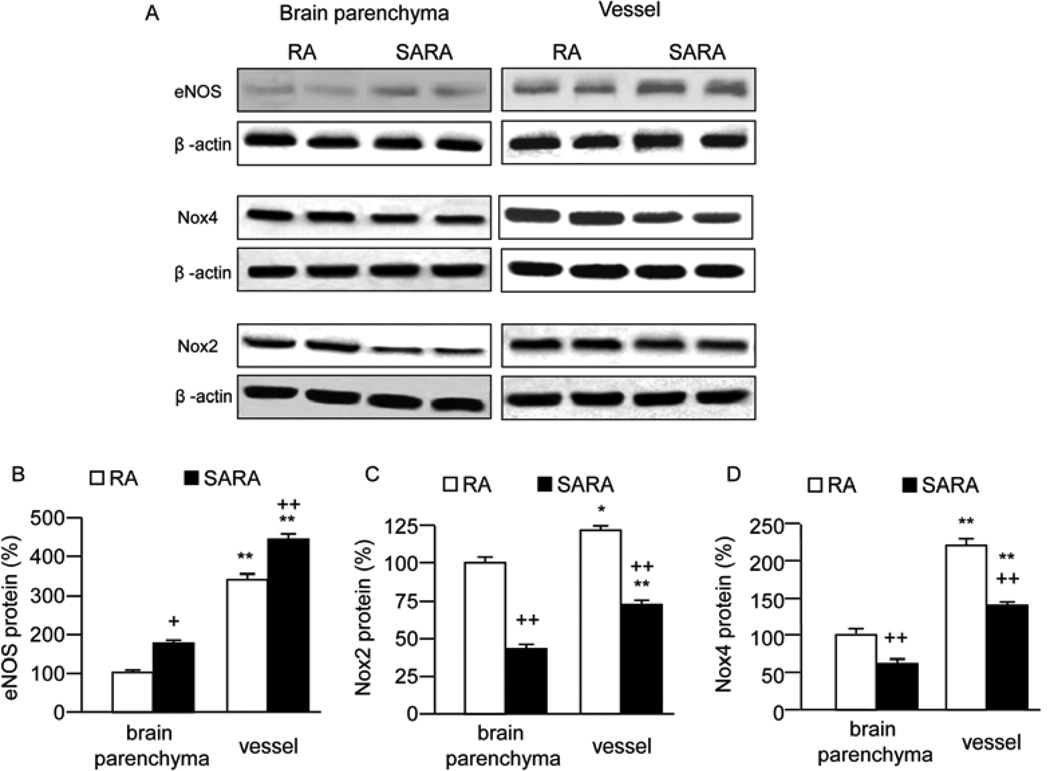
The ROS immune-staining was lower, while the NO levels were higher in SARA mice than those in RA mice (P<0.05; Fig. 3). The eNOS expression of SARA mice was higher than RA mice both in cerebral vessel and vessel-depleted brain parenchyma fractions (P<0.05 or 0.01; Fig. 4A). The Nox 2, 4 expressions of SARA mice was lower than RA mice both in cerebral vessel and vessel-depleted brain parenchyma fractions (P<0.05 or 0.01; Fig. 4B and 4C).
Fig. 3.
Levels of ROS and NO in RA and SARA mice. A, The representative pictures of ROS and NO production in the brain (cortex area) of RA and SARA mice. ROS and NO production were measured by DHE staining (red) and DAF-FM DA staining (green), respectively. Scale bars: 200 µm. B, Summarized data on ROS and NO production. *P<0.05, **P<0.01 vs. RA, n=10/group. ROS: reactive oxygen species; DHE: dihydroethidium; NO: nitric oxide; DAF-FM DA: 4-amino-5-methylamino-2’,7’-difluorofluorescein diacetate.
Fig. 4.
Expressions of eNOS, Nox2, 4 in RA and SARA mice. Brains from RA and SARA mice were removed to isolate the cerebral blood vessel and vessel-depleted brain parenchyma. The expressions of eNOS, Nox2,4 were analyzed by Western blot. A, Representative bands for eNOS and Nox 2,4 expressions in the brain of RA and SARA mice. B, Summarized data on eNOS and Nox 2,4 expressions. *P<0.05, **P<0.01 vs. RA, n=10/group.
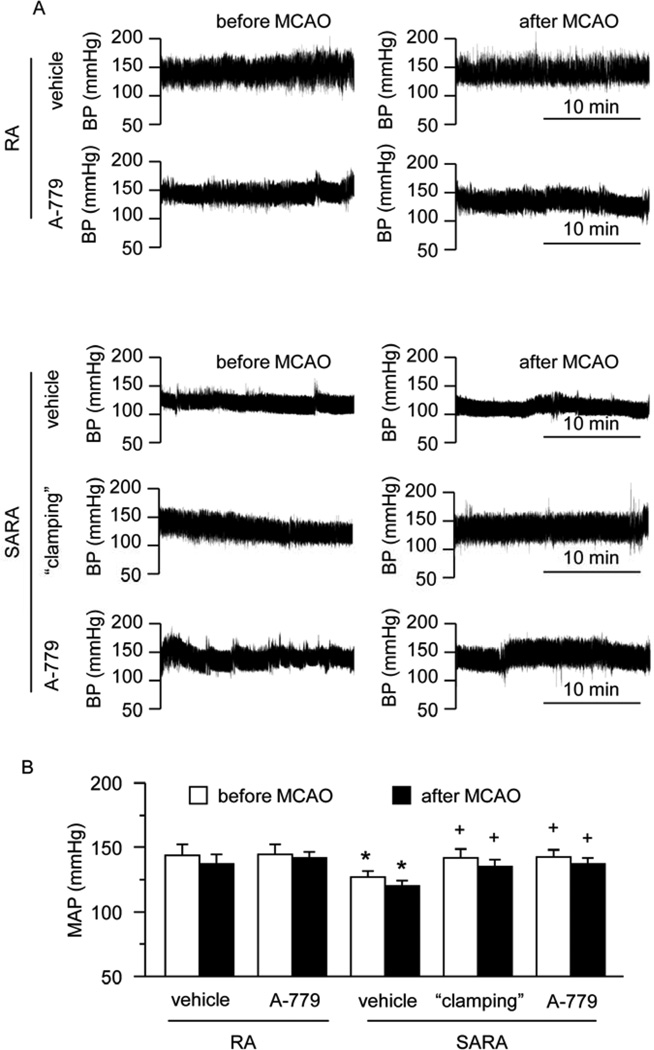
3.4. Both peripheral infusion of NE and icv infusion of A-779 can increase the MAP of SARA mice to a similar level as RA mice
As shown in Fig. 5, there is no different between the MAP before and after MCAO. SARA mice had lower MAP than RA mice (119 ± 6 mmHg vs. 134 ± 11 mmHg, P<0.05). To “clamp” the BP in SARA mice similarly as in RA mice, we chose to use a continuous infusion of NE. As we expected, after “clamping” for 7 days, the MAP in SARA group was shown to increase to the same level as that in RA group (P>0.05). After icv infusion of A-779 for 7 days, the MAP was significantly increased (P<0.05), and was similar to that in the “clamping” group (P>0.05), suggesting that Ang (1–7) stimulation of the MasR is important for the central regulation of BP.
Fig. 5.
MAP levels of RA and SARA mice with or without “clamping” before and after MCAO surgery. A, Representative raw BP traces at 9AM before and after stroke. B, Summarized data on MAP in different treatment groups during day and night time. *P<0.05 vs. RA vehicle; +P<0.05 vs. SARA vehicle, n=10/group. MAP: mean arterial pressure; icv: intracerebroventricular.
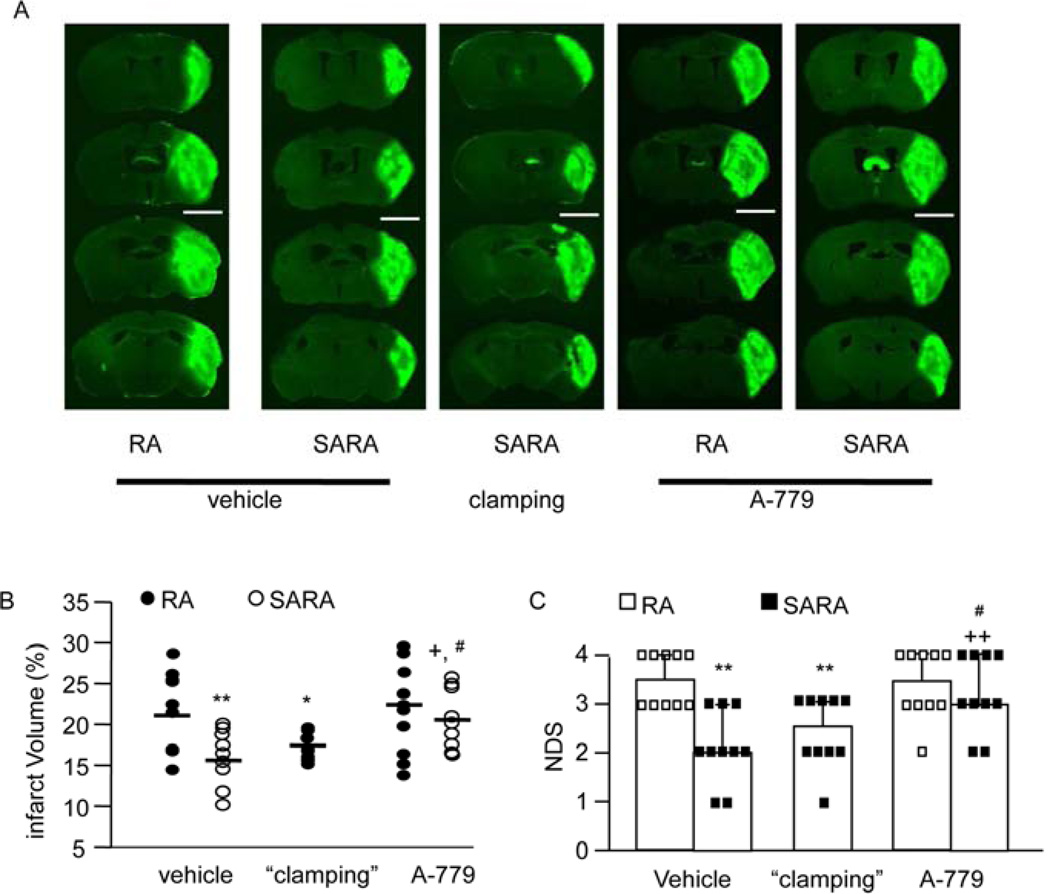
3.5. Neuronal over-expression of ACE2 decreases ischemia induced cerebral injury and improves neurological function in a BP-independent manner
On day 2 after MCAO, there was no mortality across different groups. The stroke volume in SARA mice was significantly less than in RA mice (15.3 ± 0.6 vs. 21.5 ± 1.0%, SARA mice vs. RA mice, P<0.05; Fig. 6A and 6B). After BP “clamping”, the MCAO-induced infarct volume in SARA mice was similar as those before BP “clamping” (17.2 ± 0.5 vs. 15.3 ± 0.6%, “clamping” vs. vehicle, P>0.05). A-779 significantly increased the infarct volume (P<0.05). Similarly, SARA mice had better neurological function after MCAO (P<0.05; Fig. 6C). BP “clamping” did not change the NDS of SARA mice (P>0.05; Fig. 6C). In contrast, A-779 significantly increased the NDS (P<0.05). These data suggest that reduction of the infarct volume and improvement of neurological function is independent of BP level and requires the activation of MasR signaling pathways.
Fig. 6.
Effects of neuronal over-expression of ACE2 on ischemic cerebral injury and neurological function. A, Representative Fluoro-J staining images in the peri-infarct area of RA mice and SARA mice following MCAO. Scale bars: 3.5 mm. B, MCAO-induced cerebral injury in RA mice and SARA mice before or after BP “clamping”. BP “clamping” was applied to SARA mice. C, The NDS of SARA mice and RA mice following MCAO. *P<0.05, **P<0.01 vs. RA vehicle; +P<0.05, ++P<0.01 vs. vehicle, #P<0.05 vs. SARA “clamping”, n=10/group. NDS: neurological deficit scores.
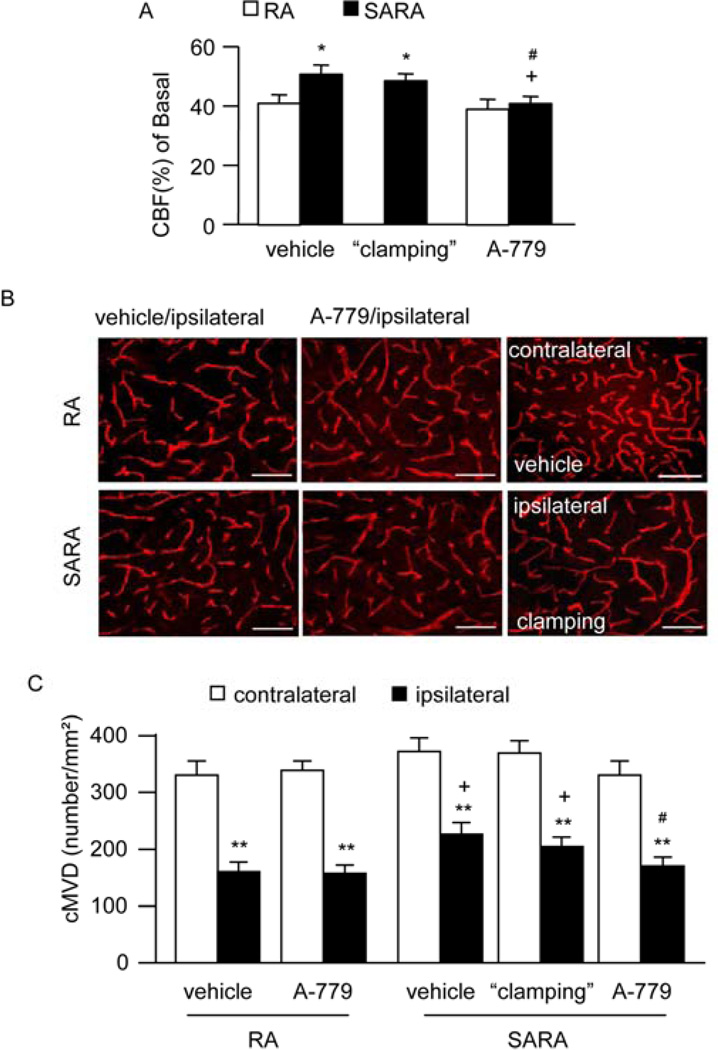
3.6. Neuronal over-expression of ACE2 preserves CBF in the peri-infarct area in a BP-independent manner
SARA mice had better preservation of CBF in the peri-infarct area (18.9 ± 1.0% vs. 50.2 ± 4.2%, for SARA mice and RA mice, respectively; P<0.05; Fig. 7A). BP “clamping” did not change the CBF in the peri-infarct area of SARA mice (P>0.05; Fig. 7A). In contrast, A-779 significantly decreased the CBF in the peri-infarct area (P<0.05). These data suggest that preservation of CBF is independent of BP level and dependent on the activation of MasR signaling pathways.
Fig. 7.
Effects of neuronal over-expression of ACE2 on CBF and cMVD after MCAO-induced ischemia. A, The relative CBF in the peri-infarct area of SARA mice and RA mice following MCAO. *P<0.05 vs. RA vehicle; +P<0.05 vs. vehicle, #P<0.05 vs. SARA “clamping”. B, Representative pictures of CD31 staining (red) for cMVD in each group. Scale bars: 50 µm. C, Summarized data on cMVD. BP “clamping” was applied to SARA mice. **P<0.01 vs. contralateral; +P<0.05 vs. RA mice, #P<0.05 vs. vehicle, n=10/group. CBF: cerebral blood flow; cMVD: cerebral microvascular density; icv: intracerebroventricular.
3.7. Neuronal over-expression of ACE2 increases cMVD in the peri-infarct area in a BP-independent manner
There is no different between RA mice and SARA mice on the basal level of cMVD (in contralateral). The cMVD in the peri-infarct area of SARA mice was significantly higher than those of RA mice (P<0.05; Fig. 7B). BP “clamping” had no effect on cMVD in the peri-infarct area of SARA mice (P>0.05; Fig. 7B). Interestingly, A-779 was able to decrease the cMVD in the peri-infarct area (P<0.05). These data suggest that elevation of cMVD is independent of BP level and dependent on the activation of MasR signaling pathways. Altogether with the data on the increased angiogenic factors in SARA mice, these data imply that the increased level of angiogenic factors might contribute to the increase of cMVD.
4. Discussion
There are three major findings in the present study. Firstly and importantly, MCAO-induced brain injury is decreased in the SARA mice when compared with the chronically hypertensive RA mice. Blockade of the ACE2/Ang (1–7)/MasR pathway in the brain partially abolishes the beneficial effects of ACE2 overexpression. Secondly, the protective effect of neuronal ACE2 over-expression is BP-independent. This is evidenced by the similar brain injury after BP “clamping”. Thirdly, the mechanisms of the cerebral protective effect of ACE2 is probably due to the up-regulation of eNOS/NO, angiogenic factors, and down-regulation of Nox/ROS expression. The data from this study suggest that activation of brain ACE2/Ang (1–7)/MasR pathway could protect from cerebral ischemic injury.
Transgenic models have the advantage of providing stable and chronic expression of target genes and offer a useful tool for studying the role of the RAS in ischemic stroke. Our previous study demonstrates that brain injury is exaggerated in the RA mice model following MCAO-induced ischemic stroke (Chen et al. 2009). To investigate the critical role of central ACE2, Xia, et al. (Xia et al. 2009) generated the triple transgenic SARA mice. They found that neuronal ACE2 overexpression exhibits protective effects on spontaneous baroreflex sensitivity, parasympathetic tone and BP. In the present study, we found the levels of MAP in SARA mice to be lower compared to the RA, which is consistent with previous data (Xia et al. 2009). The main mechanism involved is thought to be the ACE2 cleaving of Ang II into Ang (1–7). Since Ang II causes cerebral vasoconstriction, activation of brain ACE2 activity in SARA mice could accelerate Ang II degradation and Ang (1–7) production, which results in inhibition of AT1R-mediated vasoconstriction and lower BP. Based on this, we measured the Ang II and Ang (1–7) levels in the plasma, brain parenchyma and cerebral vessel. Our data show that the level of Ang (1–7) in the brain parenchyma is increased, while the Ang II level is decreased. This is consistent with previous study showing that after chronic ACE2 over-expression in the mouse brain, the formation of Ang (1–7) is the major pathway involved in BP regulation (Feng et al. 2010).
Previous study shows the presence of ACE2 in the mouse brain, including in regions involved in the central regulation of vascular function (Doobay et al. 2007). Diz et al (Diz et al. 2008) reported that pharmacological inhibition of ACE2 in the dorsal brain stem results in the reduction of the reflex bradycardia in anesthetized rats, suggesting the importance of ACE2 in the central regulation of BP. However, very few studies have investigated the role of central ACE2 expression/activity in ischemic stroke. The main goal of this study is to assess the effects of ACE2 over-expression on Ang II-mediated responses in ischemic stroke. Our data provide the first evidence that neuronal overexpression of ACE2 counteracts Ang II-mediated responses on MCAO-induced ischemic stroke by showing the deceased infarct volume, lower neurological deficits, increased CBF and elevated cMVD in the peri-infarct area of SARA mice. Elevated cMVD in the peri-infarct area could be a mechanism for increased CBF. Interestingly, neuronal over-expressing ACE2 had no effect on cMVD in contralateral side (basal level of cMVD). That could be explained by the protective effects of ACE2 on injury. Moreover, blockade of the MasR with A779 reversed those effects, which provides further evidence for the protective effects of ACE2/Ang (1–7)/MasR activation on ischemic stroke. Taken together, these findings suggest a critical role for ACE2 in the central protection of ischemic stroke.
Recent clinical evidence suggests that the beneficial effect of AT1R blockade on ischemic stroke can be observed independent of the effects on BP and cerebral flow (Dahlof et al. 2002). This conclusion is also supported by a study of cultured embryonic neurons showing that cells from AT1R−/− mice had less damage after oxygen-glucose deprivation(Walther et al. 2002). However, data supporting a BP-independent effect of ACE2 on ischemic stroke are lacking. To exclude the possible influence of low BP-mediated improvements in SARA mice, BP “clamping” was performed in the triple transgenic mice, to maintain a high BP level in these mice. Accordingly, we found that BP “clamping” did not change the beneficial effects of ACE2 over-expression on ischemic stroke. This finding supports the novel concept that ACE2 could exhibit protective effects on ischemic stroke by a BP-independent manner. In addition, the reduction in infarct volume is more striatal than cortex, which is the area was reflected by the improvement of CBF with laser Doppler. Therefore, the reduction in infarct volume in striatal area might suggest that the protective effects of ACE2 overexpressing could be independent on the improvement of CBF. Although this deduce need further investigation, it is well supported by our a previous report showing Ang II overproduction exaggerates ischemic injury by using oxygen and glucose deprivation (OGD) experiment on brain slices, which indicate the effect of Ang II on ischemic stroke is blood flow-independent (Chen et al. 2009). Taken together, our current findings suggest that ACE2 could induce both blood flow dependent (cortex) and independent (stratum) protective effect on ischemic stroke.
As mentioned above, ACE2 plays a central role in Ang (1–7) formation and our data show a decreased Ang II/Ang (1–7) ratio in the brain of SARA mice. Based on these, we consider the beneficial effects of ACE2 over-expression on ischemic stroke that might come from both decreased Ang II and increased level of Ang (1–7). Since the detrimental effect of Ang II on ischemic stroke has been confirmed in our previous study (Chen et al. 2009), decreased Ang II could account for the protective effects of ACE2 on ischemic stroke. Besides the reduction of Ang II’s detrimental effects, the increased production of Ang (1–7) should be an important direct factor contributing to the protective effects. Ang (1–7) is the main angiotensin peptide in the CNS and is widely expressed in different regions of the brain, including the hypothalamus, hippocampus, amygdala and many others (Becker et al. 2007). Recent studies show that central administration of Ang (1–7) or an ACE2 activator diminishes the cerebral infarct size and behavioral deficits (Mecca et al. 2011, Jiang et al. 2012), which provide evidence to support our results.
There are several possible underlying mechanisms for the beneficial effect of ACE2 over-expression on ischemic stroke. One of the possible molecular pathways involved in these mechanisms is the synthesis of NO, a crucial factor for vessel dilation that is modulated by Ang (1–7). Indeed, Zhang et al. have shown that central administration of Ang (1–7) up-regulates eNOS expression and consequently increases NO release in ischemic tissues in rats (Zhang et al. 2008). ACE2 overexpression also increased eNOS and NO levels in cerebrospinal fluid of mice (Feng et al. 2010). During the early stages of cerebral ischemia, eNOS-derived NO is beneficial as it promotes collateral circulation and microvascular flow (Veltkamp et al. 2002). Interestingly, our data add new information to this concept, showing that neuronal ACE2 over-expression up-regulates the expression of eNOS and elevates NO production in the brain tissue. The other possible mechanism involved is the oxidative stress pathway. Nox-derived ROS play a key role in Ang II-mediated detrimental effects on ischemic stroke (Doughan et al. 2008). A recent study also demonstrates that the cerebroprotective action of Ang (1–7) is mediated by its anti-inflammatory effects through a reduction of oxidative stress (Jiang et al. 2012). Our results showed neuronal ACE2 over-expression down-regulates the expression of Nox2/Nox4 and alleviates the ROS production, which is consistent with a previous study showing ACE2-mediated reduction of oxidative stress in the central nervous system (Xia et al. 2011). Furthermore, we deepen the research by studying the levels of angiogenic factors in the brain of SARA mice. Indeed, our results show that over-expression of ACE2 in the brain could up-regulate the expressions of VEGF, FGF, IFN-γ and IL-6 in the brain. VEGF was demonstrated to exert neuroprotective action and associated with reduced neurological deficits after focal cerebral ischemia (Gora-Kupilas and Josko 2005). EGF is a potent growth factor which enhances proliferation and survival of neural progenitor cells (Ramasamy et al. 2013). Previous clinical studies in patients with ischemic stroke suggest that IL-6 induces an excessive inflammatory response, which may aggravate ischemic cerebral damage (Beamer et al. 1995). In contrast, accumulating paradoxical experimental data have revealed that IL-6 can promote a pro-survival signaling pathway and then involved in cytoprotection and angiogenesis (Lecour and James 2011). Therefore, our study adds a new mechanism for cerebroprotective action of ACE2 after ischemic stroke.
In conclusion, our results establish the importance of central ACE2 in the regulation of eNOS/NO and Nox/ROS, and its potential as a novel target for the prevention and treatment of ischemic stroke.
Limitations and Translational Potential of the Study
In this study, we examined the effects of neural over-expressing ACE2 on ischemic stroke. The possible impact of Ang II and ACE activity feedback was not explored (Schunkert et al. 1993). We revealed that eNOS/NO and Nox/ROS pathways are the mechanisms underlying the protective effects of ACE2. Nevertheless, other mechanisms such as inflammation cytokines and MMP need further investigation. In addition, the effects of A779 on ischemic stroke injury were tested by administrating A779 seven days before MCAO, which let the animal recover from the surgery of icv infusion kit implantation and to allow the drug fully enters into brain tissue. Of note, this approach could induce other changes in the brain.
The present work is intended to inform future therapeutic approaches to the management of stroke. The mode of experimentation we have used also lends itself as an experimental paradigm not only of the immediate protective effects on stroke events but also potentially for the exploration of longer term cognitive impairment effects following stroke such as is very relevant for vascular dementia. In this study we focused mainly on motor effects, yet a longer study including cognitive measures might provide equally thought provoking results in relation to protection against vascular event induced cognitive impairment.
Highlights.
ACE2 shows protective effects on ischemic brain injury.
The protective effect of ACE2 is blood pressure-independent.
ACE2 up-regulates eNOS/NO expression, and increases angiogenic factor level
ACE2 down-regulates Nox/ROS expression.
Acknowledgements
This work was supported by the National Heart, Lung, and Blood Institute (HL-098637 to Y.C., and HL093178 to E.L.), and American Heart Association (13POST14780018 to J.C.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beamer NB, Coull BM, Clark WM, Hazel JS, Silberger JR. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann. Neurol. 1995;37:800–805. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- Becker LK, Etelvino GM, Walther T, Santos RA, Campagnole-Santos MJ. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol Heart Circ. Physiol. 2007;293:H1416–H1424. doi: 10.1152/ajpheart.00141.2007. [DOI] [PubMed] [Google Scholar]
- Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am. J. Physiol Heart Circ. Physiol. 2009;296:H1660–H1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen S, Chen Y, Zhang C, Wang J, Zhang W, Liu G, Zhao B, Chen Y. Circulating endothelial progenitor cells and cellular membrane microparticles in db/db diabetic mouse: possible implications in cerebral ischemic damage. Am. J. Physiol Endocrinol. Metab. 2011;301:E62–E71. doi: 10.1152/ajpendo.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao X, Chen S, Zhang C, Chen J, Yi D, Shenoy V, Raizada MK, Zhao B, Chen Y. Angiotensin-converting enzyme 2 priming enhances the function of endothelial progenitor cells and their therapeutic efficacy. Hypertension. 2013;61:681–689. doi: 10.1161/HYPERTENSIONAHA.111.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Li G, Zhang W, Wang J, Sigmund CD, Olson JE, Chen Y. Ischemia-induced brain damage is enhanced in human renin and angiotensinogen double-transgenic mice. Am. J. Physiol Regul. Integr. Comp Physiol. 2009;297:R1526–R1531. doi: 10.1152/ajpregu.91040.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen H, Hoffmann A, Cool DR, Diz DI, Chappell MC, Chen AF, Morris M. Adenovirus-mediated small-interference RNA for in vivo silencing of angiotensin AT1a receptors in mouse brain. Hypertension. 2006;47:230–237. doi: 10.1161/01.HYP.0000200259.01947.bb. [DOI] [PubMed] [Google Scholar]
- Chrysant SG. Possible pathophysiologic mechanisms supporting the superior stroke protection of angiotensin receptor blockers compared to angiotensin-converting enzyme inhibitors: clinical and experimental evidence. J. Hum. Hypertens. 2005;19:923–931. doi: 10.1038/sj.jhh.1001916. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de FU, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Ann TE, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp. Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ. Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD, Lazartigues E. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ. Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Lessard A, Capone C, Milner TA, Iadecola C. The neurovascular dysfunction induced by angiotensin II in the mouse neocortex is sexually dimorphic. Am. J. Physiol Heart Circ. Physiol. 2008;294:H156–H163. doi: 10.1152/ajpheart.01137.2007. [DOI] [PubMed] [Google Scholar]
- Gora-Kupilas K, Josko J. The neuroprotective function of vascular endothelial growth factor (VEGF) Folia Neuropathol. 2005;43:31–39. [PubMed] [Google Scholar]
- Guo YJ, Li WH, Wu R, Xie Q, Cui LQ. ACE2 overexpression inhibits angiotensin II-induced monocyte chemoattractant protein-1 expression in macrophages. Arch. Med. Res. 2008;39:149–154. doi: 10.1016/j.arcmed.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36:1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- Iwanami J, Mogi M, Tsukuda K, Min LJ, Sakata A, Jing F, Iwai M, Horiuchi M. Low dose of telmisartan prevents ischemic brain damage with peroxisome proliferator-activated receptor-gamma activation in diabetic mice. J. Hypertens. 2010;28:1730–1737. doi: 10.1097/HJH.0b013e32833a551a. [DOI] [PubMed] [Google Scholar]
- Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of angiotensin-(1–7) unmasked during combined treatment with lisinopril and losartan. Hypertension. 1998;31:699–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- Jiang T, Gao L, Guo J, Lu J, Wang Y, Zhang Y. Suppressing inflammation by inhibiting the NF-kappaB pathway contributes to the neuroprotective effect of angiotensin-(1–7) in rats with permanent cerebral ischaemia. Br. J. Pharmacol. 2012;167:1520–1532. doi: 10.1111/j.1476-5381.2012.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjeldsen SE, Lyle PA, Kizer JR, Dahlof B, Devereux RB, Julius S, Beevers G, de FU, Fyhrquist F, Ibsen H, Kristianson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn SM, Harris KE, Wedel H. The effects of losartan compared to atenolol on stroke in patients with isolated systolic hypertension and left ventricular hypertrophy. The LIFE study. J. Clin. Hypertens. 2005;7:152–158. doi: 10.1111/j.1524-6175.2005.04254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H, Hirata M, Kudo Y, Kikuchi K, Nagano T. Visualization of oxygen-concentration-dependent production of nitric oxide in rat hippocampal slices during aglycemia. J. Neurochem. 2001;76:1404–1410. doi: 10.1046/j.1471-4159.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- Lecour S, James RW. When are pro-inflammatory cytokines SAFE in heart failure? Eur. Heart J. 2011;32:680–685. doi: 10.1093/eurheartj/ehq484. [DOI] [PubMed] [Google Scholar]
- Mecca AP, Regenhardt RW, O'Connor TE, Joseph JP, Raizada MK, Katovich MJ, Sumners C. Cerebroprotection by angiotensin-(1–7) in endothelin-1-induced ischaemic stroke. Exp. Physiol. 2011;96:1084–1096. doi: 10.1113/expphysiol.2011.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Saavedra JM. Angiotensin II AT(1) blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- Ramasamy S, Narayanan G, Sankaran S, Yu YH, Ahmed S. Neural stem cell survival factors. Arch. Biochem. Biophys. 2013;534:71–87. doi: 10.1016/j.abb.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Saavedra JM, Benicky J, Zhou J. Mechanisms of the Anti-Ischemic Effect of Angiotensin II AT( 1 ) Receptor Antagonists in the Brain. Cell Mol. Neurobiol. 2006;26:1099–1111. doi: 10.1007/s10571-006-9009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schunkert H, Ingelfinger JR, Hirsch AT, Pinto Y, Remme WJ, Jacob H, Dzau VJ. Feedback regulation of angiotensin converting enzyme activity and mRNA levels by angiotensin II. Circ. Res. 1993;72:312–318. doi: 10.1161/01.res.72.2.312. [DOI] [PubMed] [Google Scholar]
- Sierra C, Coca A, Schiffrin EL. Vascular mechanisms in the pathogenesis of stroke. Curr. Hypertens. Rep. 2011;13:200–207. doi: 10.1007/s11906-011-0195-x. [DOI] [PubMed] [Google Scholar]
- Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002;33:2704–2710. doi: 10.1161/01.str.0000033132.85123.6a. [DOI] [PubMed] [Google Scholar]
- Walther T, Olah L, Harms C, Maul B, Bader M, Hortnagl H, Schultheiss HP, Mies G. Ischemic injury in experimental stroke depends on angiotensin II. FASEB J. 2002;16:169–176. doi: 10.1096/fj.01-0601com. [DOI] [PubMed] [Google Scholar]
- Williams WM, Chung YW. Evidence for an age-related attenuation of cerebral microvascular antioxidant response to oxidative stress. Life Sci. 2006;79:1638–1644. doi: 10.1016/j.lfs.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Williams WM, Reichman M, McNeill TH. Cerebral microvascular and parenchymal phospholipid composition in the mouse. Neurochem. Res. 1988;13:743–747. doi: 10.1007/BF00971597. [DOI] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Suda S, Bindom S, Feng Y, Gurley SB, Seth D, Navar LG, Lazartigues E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS. One. 2011;6:e22682. doi: 10.1371/journal.pone.0022682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Gao L, Lazartigues E, Zucker IH. Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension. 2011;58:1057–1065. doi: 10.1161/HYPERTENSIONAHA.111.176636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto E, Tamamaki N, Nakamura T, Kataoka K, Tokutomi Y, Dong YF, Fukuda M, Matsuba S, Ogawa H, Kim-Mitsuyama S. Excess salt causes cerebral neuronal apoptosis and inflammation in stroke-prone hypertensive rats through angiotensin II-induced NADPH oxidase activation. Stroke. 2008;39:3049–3056. doi: 10.1161/STROKEAHA.108.517284. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu J, Shi J, Lin X, Dong J, Zhang S, Liu Y, Tong Q. Central administration of angiotensin-(1–7) stimulates nitric oxide release and upregulates the endothelial nitric oxide synthase expression following focal cerebral ischemia/reperfusion in rats. Neuropeptides. 2008;42:593–600. doi: 10.1016/j.npep.2008.09.005. [DOI] [PubMed] [Google Scholar]