Abstract
Purpose: Hepatotoxicity is one of the most important side effects of the statins therapy as lipid-lowering agents. However, the mechanism(s) of hepatotoxicity induced by these drugs is not clearly understood yet, and no hepatoprotective agent has been developed against this complication.
Methods: The protective effect of N-acetylcysteine (NAC) against statins-induced cytotoxicity was evaluated by using freshly isolated rat hepatocytes. Hepatocytes were prepared by the method of collagenase enzyme perfusion via portal vein. This technique is based on liver perfusion with collagenase after removal of calcium ion (Ca2+) with a chelator (ethylene glycol tetra acetic acid (EGTA) 0.5 mM). The level of parameters such as cell death, ROS formation, lipid peroxidation, mitochondrial membrane potential (MMP) in the statins-treated hepatocytes were determined. Additionally, the mentioned markers were assessed in the presence of NAC.
Results: Incubation of hepatocytes with the statins resulted in cytotoxicity characterized by an elevation in cell death, increasing ROS generation and consequently lipid peroxidation and impairment of mitochondrial function. Administration of NAC caused reduction in amount of ROS formation, lipid peroxidation and finally, cell viability and mitochondrial membrane potential (MMP) were improved.
Conclusion: This study confirms that oxidative stress and consequently mitochondrial dysfunction is one of the mechanisms underlying the statins-induced liver injury and treating hepatocytes by NAC (200 μM) attenuates this cytotoxicity.
Keywords: Statins, Hepatotoxicity, Reactive oxygen species, Mitochondrial membrane potential, N-acetylcysteine
Introduction
Statins (3-hydroxy-3-methylglutaryl co-enzyme A reductase inhibitors) are the most prescribed agents for cholesterol lowering and consequently prevention of obstructive cardiovascular events in the world.1,2 These pharmaceuticals block the mevalonic acid pathway by inhibition of the rate limiting step in the hepatic de novo cholesterol biosynthesis.3 Severe adverse effects of the statins involving myopathy and hepatotoxicity sometimes limit their usage as lipid lowering agents. Hepatotoxicity characterized by elevation of plasma transaminases is most common and the frequency of elevation is 0.5-5% and dose dependent.4,5 However the precise molecular mechanisms underlying statins cause hepatotoxicity are not understood.2 statins are metabolized by cytochrome P450 (3A4) enzymes.1 The dose- and time-dependent impairment of mitochondrial function caused by statins has been observed in different in vitro models.6,7
Different antioxidants administration has been shown to be useful in reducing oxidative stress and cell death in hepatocytes. Efficacy of N-acetylcysteine (NAC) administration as a free radical scavenger in multiple models of oxidative stress induction has been studied.8
In the present study, isolated rat hepatocytes were exploited to gain insight into statins hepatotoxicity and to investigate the role of supplementation with NAC in reducing toxicity. Markers like reactive oxygen species (ROS), lipid peroxidation, mitochondrial membrane potential and cell death in the statins-treated freshly isolated rat hepatocytes were assessed and the results were compared to those in NAC-treated hepatocytes. The hepatoprotective properties of NAC(200 µM) supplementation against the statins-induced hepatotoxicity were related to the reduction of ROS generation, lipid peroxidation and maintenance of mitochondrial membrane potential.
Materials and Methods
Chemicals
Atorvastatin, Simvastatin and Lovastatin were purchased from sigma-aldrich. NAC was provided from Acros (New Jersey, USA). 2-vinyl pyridine, Triethanolamineand (4-(2-hydroxyethyl) 1-piperazine-ethanessulfonic acid (HEPES) were obtained from Acros (New Jersey, USA). Albumine bovine type was purchased from Roche diagnostic corporation (Indianapolis USA). Rhodamine 123, 5,5′-dithio-bis(2-nitro-benzoicacid)(DTNB), 2,7-Dichlorofluorescin diacetate (DCFDA) and clostridium histolyticum extracted Collagenase (Type II), were obtained from Sigma Aldrich (St. Louis, USA). Trichloroacetic acid (TCA), Ethyleneglycol-bis (ρ-aminoethylether)-N,N,N′,N′-tetra acetic acid (EGTA), and Trypan blue were obtained from Merck (Darmstadt, Germany). Thiobarbituric acid (TBA) was obtained from SERVA (Heidenberg, New York). All salts used for preparing buffer solutions were of analytical grade and obtained from Merck (Darmstadt, Germany).
Animals
Male Sprague–Dawley rats (weight range: 250-300g) were provided from Tabriz University of Medical Sciences, Tabriz, Iran. The animals were housed in an animal house unit (temperature 21-23°C and 50-60% relative humidity), fed a standard chow diet and water ad libitum were used. The animals were handled and used according to the animal handling protocol of Tabriz University of Medical Sciences, Tabriz, Iran which was approved by a local ethic committee.
Hepatocytes isolation
Hepatocytes were isolated from male Sprague–Dawley rats by a two-step collagenase perfusion as described previously.9 Approximately 85–90% of hepatocytes excluded Trypan blue (0.1%, w/v) at the time of isolation. The cells were suspended (1×106 cell/ml) in Krebs–Henseleit buffer containing 12.5 mM HEPES and incubated under a stream of 95% O2 and 5% CO2 in continuously rotating round-bottomed 50 ml flasks at 37°C water bath. Hepatocytes were kept under the relevant atmosphere for 30 minute to achieve equilibrium between gas and liquid phases before the addition of chemicals.
Cell viability
After hepatocyte isolation process, cell viability was assessed by the extent of plasma membrane intactness as determined by Trypan blue exclusion test.10 Viability was determined immediately after isolation and after one, two and three hours after statins incubation. In experiments NAC (200 µM) and statins were added contemporarily (There were no significant difference between before and co-adding of chemicals).
Reactive oxygen species (ROS) formation
To assess the rate of hepatocytes ROS formation during statins metabolism, 2, 7-dichlorofluorescein diacetate (DCF-DA) 1.6 µM was added to the hepatocyte incubate. DCFH-DA became hydrolyzed to non-fluorescent dichlorofluorescein (DCFH) in hepatocytes. Dichlorofluorescin then reacted with reactive oxygen species to form the highly fluorescent dichlorofluorescein. One ml (106 cells) of hepatocytes was taken and the fluorescence intensity was measured using a Jasco® FP-750 spectrofluorometer with excitation and emission wavelengths of 500 and 520 nm, respectively.11
Lipid peroxidation
Hepatocyte lipid peroxidation was determined by measuring the amount of thiobarbituric acid-reactive substances (TBARS) such as malondialdehyde (MDA), formed during the decomposition of lipid hydroperoxides by following the absorbance at 532 nm in a Pharmacia Biotech Ultrospec 2000 spectrophotometer after treating a 1.0 ml aliquot of hepatocyte suspension (106 cells/ml) with trichloroacetic acid (70%, w/v) and boiling the supernatant with thiobarbituric acid (0.8%, w/v) for 20 min.12
Mitochondrial membrane potential assay
Mitochondrial membrane potential (MMP) in hepatocytes was assessed by monitoring the uptake of rhodamine 123, a cationic dye, as described.13,14 Isolated cells were extracted, and then resuspended in original media containing 1.5µM rhodamine 123. After 10 min of incubation, the cells were centrifuged and the supernatant was measured with a Jasco FP-750 spectrofluorimeter. The amount of dye remaining in the supernatant was inversely proportional to the membrane potential of the cells. The results are reported as the difference in fluorescence intensity between control and treated cells and expressed as percentage of control.
Statistical analysis
The results are shown as mean ±SD, measured at least for 3 different batches of hepatocytes. The differences among the control and experimental groups were determined by one way ANOVA followed by Tukey’s post hoc analysis and a p<0.05 was considered significant.
Results
By adding different concentrations of atorvastatin, simvastatin and lovastatin to isolated rat hepatocytes and by use of trypan blue exclusion test, the LC50s of statins (the statin concentration that caused 50% death after 120 minutes) were found as 450µM for atorvastatin, simvastatin 200µM and lovastatin 200µM (data not shown). As shown in Table 1, simvastatin 200µM is the most cytotoxic statin, because the amount of dead cell in third hour is more noticeable. Additionally, an optimum effective dose for NAC that provided an appropriate protection was found as 200µM. Hepatocytes were treated with NAC at the same time that statins were added. It was found that NAC (200µM) effectively prevented cell death induced by the statins (Table 1). Markers such as ROS formation, lipid peroxidation, and mitochondrial membrane potential were assessed to investigate the mechanism by which NAC protects hepatocytes against statins-induced toxicity and elucidate the cause of cell death induced by statins. A significant amount of reactive oxygen species were formed when hepatocytes were treated with atorvastatin (Figure 1), simvastatin (Figure 2) and/or lovastatin (Figure 3) and supplementation with NAC (200µM) significantly reduced the statins-induced ROS generation (Figure 1-3).
Table 1. Prevention of statins cytotoxicity by NAC.
| Cytotoxicity (% Trypan blue uptake) | |||
| Addition Incubation time (min) | 60 | 120 | 180 |
| Control (only hepatocytes) | 18±1 | 22±2 | 24±1 |
| +NAC(N-acetylcysteine) 200 µM | 15±1 | 17±1 | 21±1 |
| +Atorvastatin 450 µM | 36±1a | 52±2a | 68±1a |
| +NAC200 µM | 23±1b | 29±1b | 38±2b |
| +Simvastatin 200 µM | 38±2a | 54±2a | 88±2a |
| + NAC200 µM | 25±1b | 33±2b | 47±2b |
| +Lovastatin 200 µM | 39±2a | 52±2a | 74±3a |
| + NAC200 µM | 28±2b | 38±2b | 45±4b |
| Statin doses are LD50 (The statins concentration caused 50% death after 120 minutes). | |||
| NAC (200 µM) was added at the time of statins addition. | |||
| Data represent Mean±SE for three independent experiments. | |||
| a: Significantly different from control group (P<0.05). | |||
| b: Significantly different from statins-treated group (P<0.05). | |||
Figure 1 .
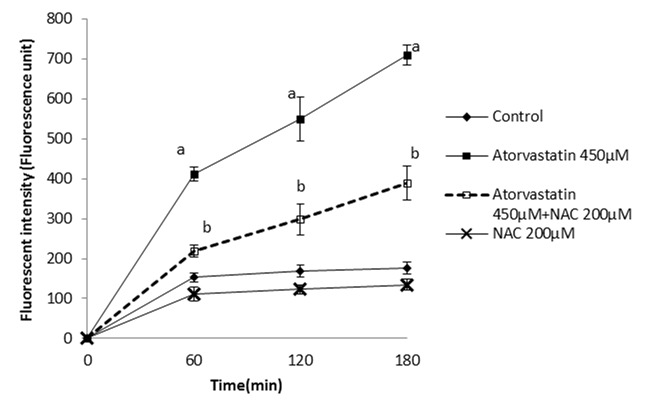
Atorvastatin- induced ROS formation in isolated rat hepatocytes and the protective effect of NAC.
NAC (200 µM) was added at the time of Atorvastatin(450 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a: significant VS control group(p<0.05).
b: significant VS Atorvastatin treated group(p<0.05).
Figure 2 .

Simvastatin- induced ROS formation in isolated rat hepatocytes and the protective effect of NAC.
NAC (200 µM) was added at the time of Simvastatin (200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a: significant VS control group(p<0.05).
b: significant VS Simvastatin - treated group(p<0.05).
Figure 3 .
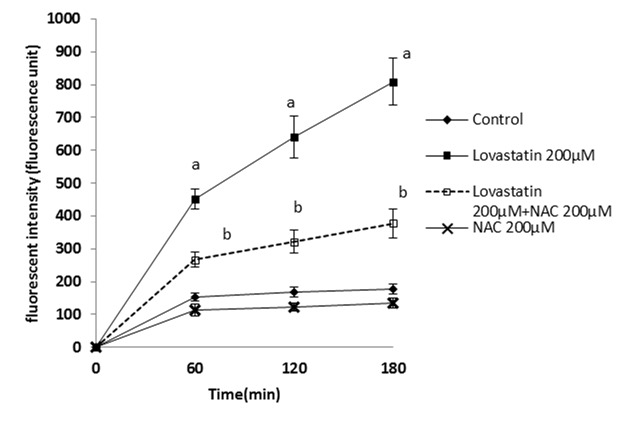
Lovastatin- induced ROS formation in isolated rat hepatocytes and the protective effect of NAC.
NAC(200 µM) was added at the time of Lovastatin(200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b: significant VS Lovastatin - treated group(p<0.05).
It has been shown that ROS formation is usually followed by lipid peroxidation.15 Lipid peroxidation was determined after 120 and 180 minutes after the statins treatment and it was found that NAC (200µM) significantly prevented lipid peroxidation in the statins-treated hepatocytes (Figure 4-6).
Figure 4 .
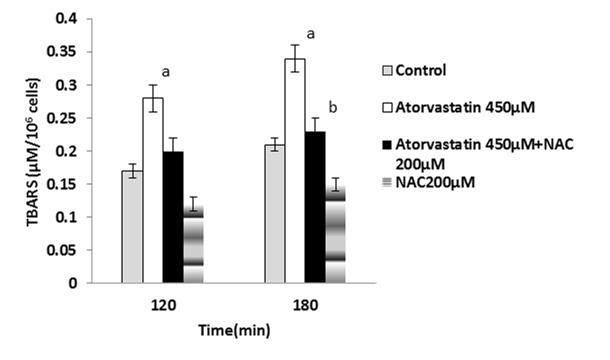
Atorvastatin-induced lipid peroxidation and the protective effect of NAC.
NAC (200 µM) was added at the time of Atorvastatin(450 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b : significant VS Atorvastatin treated group(p<0.05).
Figure 6 .
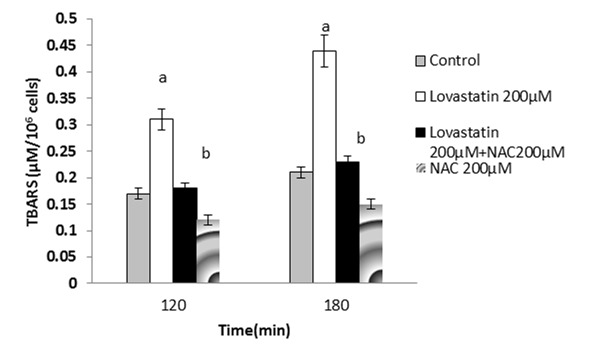
Lovastatin- induced lipid peroxidation and the protective effect of NAC.
NAC (200 µM) was added at the time of Lovastatin (200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b :significant VS Lovastatin - treated group(p<0.05).
The effect of statins on mitochondria as an essential organelle in energy production was assessed. It was found that statins caused mitochondrial membrane potential (MMP)depression (Figure 7-9). NAC supplementation showed a significant elevation of mitochondrial membrane potential.
Figure 7 .
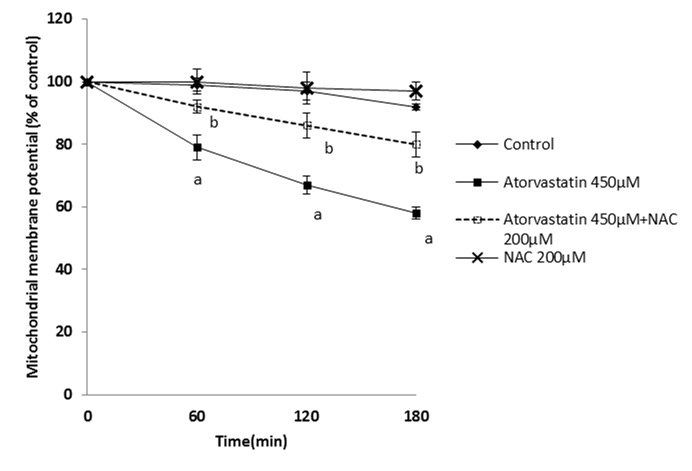
Effect of Atorvastatin on mitochondrial membrane potential and the protective role of NAC.
NAC (200 µM) was added at the time of Atorvastatin (450 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b: significant VS Atorvastatin -treated group(p<0.05).
Figure 9.
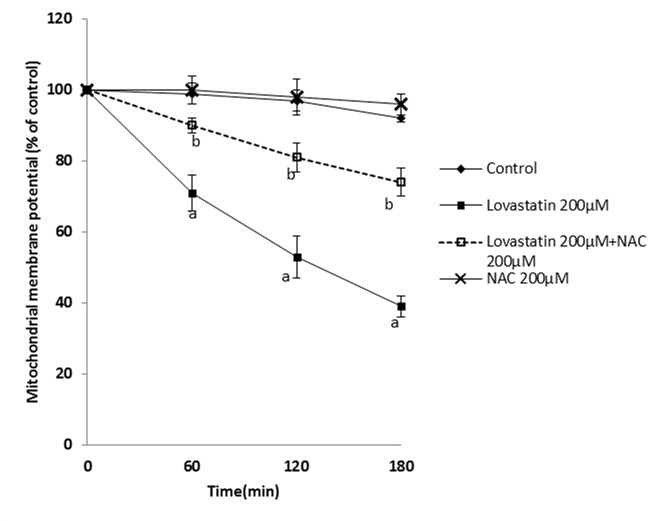
Effect of lovastatin on mitochondrial membrane potential and the protective role of NAC.
NAC (200 µM) was added at the time of lovastatin (200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b: significant VS lovastatin -treated group(p<0.05).
Discussion
The results of our study showed the beneficial protective effects of NAC against statin-induced hepatotoxicity. Inhibition of the respiratory chain (complex I and III), depolarization of mitochondrial membrane7 and releasing of Ca2+, are known as the results of statins exposure.16 The statins are metabolized by liver cytochrome P450 (CYP 3A4) enzymes.1 The cytochrome P450 include a superfamily of monooxygenases whose members function critically in xenobiotics catabolism.17 The major metabolic reactions include oxidation of aliphatic carbons, alkenyl groups and aromatic rings. After oxidation, subsequent reactions involve N-dealkylations, dehydration to form C=C double bonds, dehydrogenation of primary alcohols to an aldehyde and then a carboxylic acid, and finally glutathione conjugation of epoxides.18 Mitochondria or cytochrome P450-dependent metabolism act as ROS production systems and participate in cell death processes.19 Our data showed that treating hepatocytes with the statins produces a significant amount of ROS, induces lipid peroxidation, reduces mitochondrial membrane potential and promotes cytotoxicity as compared to the control group and these effect are dose- and time-dependent (different doses of the statins were assessed but data are not shown). Our results are in accordance with the previous study.7 The amount of ROS formation by Simvastatin was more than that with the others. The intracellular ROS generation is accompanied with cell death in cultured hepatocytes.20 The efficacy of N-acetylcysteine (NAC) administration as a free radical scavenger in multiple models of oxidative stress induction has been studied.8,12 The administration of N-acetylcysteine (NAC), as an excellent source of intracellular cysteine and free radical scavenger, has been shown to have clinical applications in conditions such as HIV infection, cancer, heart disease, as well as in smoking, kidney and liver diseases.21 Adding NAC to the cells incubated with the statins resulted in prevention of ROS formation, lipid peroxidation, mitochondrial dysfunction, and finally improving the cells survival. Therefore, reactive oxygen species formed during the statins metabolism are scavenged by NAC and this may have a role in its protective effects in statins cytotoxicity.
Figure 5 .
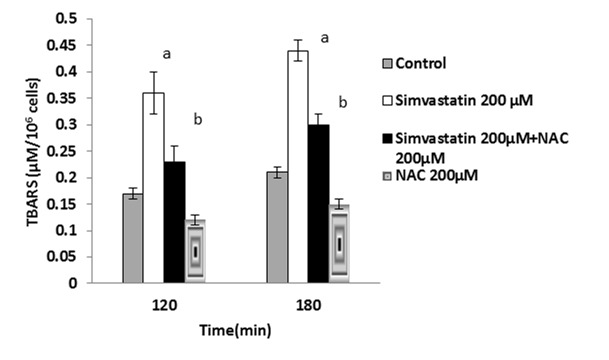
Simvastatin- induced lipid peroxidation and the protective effect of NAC.
NAC (200 µM) was added at the time of Simvastatin(200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b: significant VS Simvastatin - treated group(p<0.05).
Figure 8 .

Effect of simvastatin on mitochondrial membrane potential and the protective role of NAC.
NAC (200 µM) was added at the time of Simvastatin(200 µM) addition.
Data are shown as Mean±SE for three independent experiments.
a : significant VS control group(p<0.05).
b:significant VS Simvastatin -treated group(p<0.05).
Conclusion
In summary, in accordance with a previous study7, we have shown that, statins act as the inducers of oxidative stress that severely inhibits mitochondria respiration and decrease mitochondrial membrane potential in rat hepatocytes. They also promote peroxidation of lipids and subsequently induce cytotoxicity and cell death. The treatment of hepatocytes with NAC protected cells from the statins-induced ROS production, lipid peroxidation, mitochondrial impairment and cell death. Hence, supplementation with NAC may be an effective therapeutic strategy for the prevention and treatment of clinical conditions caused by statins.
Acknowledgements
The authors thank Biotechnology and Drug Applied Research Centers of Tabriz University of Medical Sciences, Tabriz-Iran, for providing facilities to carry out this study. This research was a part of Narges Abdoli’s PhD thesis that was supported by students’ research committee. The authors are thankful to the students’ research committee of Tabriz University of Medical Sciences, Tabriz-Iran, for providing supports to the study.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Ellesat KS, Tollefsen KE, Asberg A, Thomas KV, Hylland K. Cytotoxicity of atorvastatin and simvastatin on primary rainbow trout (Oncorhynchus mykiss) hepatocytes. Toxicol In Vitro . 2010;24(6):1610–8. doi: 10.1016/j.tiv.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Kubota T, Fujisaki K, Itoh Y, Yano T, Sendo T, Oishi R. Apoptotic injury in cultured human hepatocytes induced by HMG-CoA reductase inhibitors. Biochem Pharmacol . 2004;67(12):2175–86. doi: 10.1016/j.bcp.2004.02.037. [DOI] [PubMed] [Google Scholar]
- 3.Guzman M, Cortes JP, Castro J. Effects of lovastatin on hepatic fatty acid metabolism. Lipids . 1993;28(12):1087–93. doi: 10.1007/BF02537075. [DOI] [PubMed] [Google Scholar]
- 4.Kromer A, Moosmann B. Statin-induced liver injury involves cross-talk between cholesterol and selenoprotein biosynthetic pathways. Mol Pharmacol . 2009;75(6):1421–9. doi: 10.1124/mol.108.053678. [DOI] [PubMed] [Google Scholar]
- 5.Hsu I, Spinler SA, Johnson NE. Comparative evaluation of the safety and efficacy of HMG-CoA reductase inhibitor monotherapy in the treatment of primary hypercholesterolemia. Ann Pharmacother . 1995;29(7-8):743–59. doi: 10.1177/106002809502907-818. [DOI] [PubMed] [Google Scholar]
- 6.Callegari S, Mckinnon RA, Andrews S, De Barros Lopes MA. Atorvastatin-induced cell toxicity in yeast is linked to disruption of protein isoprenylation. FEMS Yeast Res . 2010;10(2):188–98. doi: 10.1111/j.1567-1364.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 7.Abdoli N, Heidari R, Azarmi Y, Eghbal MA. Mechanisms of the statins cytotoxicity in freshly isolated rat hepatocytes. J Biochem Mol Toxicol . 2013;27(6):287–94. doi: 10.1002/jbt.21485. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez R, Ferrin G, Hidalgo AB, Ranchal I, Lopez-Cillero P, Santos-Gonzalez M. et al. N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem Biol Interact . 2009;181(1):95–106. doi: 10.1016/j.cbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Heidari R, Babaei H, Eghbal M. Mechanisms of methimazole cytotoxicity in isolated rat hepatocytes. Drug Chem Toxicol . 2013;36(4):403–11. doi: 10.3109/01480545.2012.749272. [DOI] [PubMed] [Google Scholar]
- 10.Heidari R, Babaei H, Eghbal MA. Ameliorative effects of taurine against methimazole-induced cytotoxicity in isolated rat hepatocytes. Sci Pharm . 2012;80(4):987–99. doi: 10.3797/scipharm.1205-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidari R, Babaei H, Eghbal MA. Cytoprotective effects of taurine against toxicity induced by isoniazid and hydrazine in isolated rat hepatocytes. Arh Hig Rada Toksikol . 2013;64(2):15–24. doi: 10.2478/10004-1254-64-2013-2297. [DOI] [PubMed] [Google Scholar]
- 12.Heidari R, Babaei H, Roshangar L, Eghbal MA. Effects of enzyme induction and/or glutathione depletion on methimazole-induced hepatotoxicity in mice and the protective role of N-acetylcysteine. Adv Pharm Bull . 2014;4(1):21–8. doi: 10.5681/apb.2014.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidari R, Babaei H, Eghbal MA. Cytoprotective Effects of Organosulfur Compounds against Methimazole Induced Toxicity in Isolated Rat Hepatocytes. Adv Pharm Bull . 2013;3(1):135–42. doi: 10.5681/apb.2013.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidari R, Babaei H, Eghbal MA. Amodiaquine-induced toxicity in isolated rat hepatocytes and the cytoprotective effects of taurine and/or N-acetyl cysteine. Res Pharm Sci . 2014;9(2):97–105. [PMC free article] [PubMed] [Google Scholar]
- 15.Benzie IF. Lipid peroxidation: a review of causes, consequences, measurement and dietary influences. Int J Food Sci Nutr . 1996;47(3):233–61. doi: 10.3109/09637489609012586. [DOI] [PubMed] [Google Scholar]
- 16.Liantonio A, Giannuzzi V, Cippone V, Camerino GM, Pierno S, Camerino DC. Fluvastatin and atorvastatin affect calcium homeostasis of rat skeletal muscle fibers in vivo and in vitro by impairing the sarcoplasmic reticulum/mitochondria Ca2+-release system. J Pharmacol Exp Ther . 2007;321(2):626–34. doi: 10.1124/jpet.106.118331. [DOI] [PubMed] [Google Scholar]
- 17.Tafazoli S, O'brien PJ. Peroxidases: a role in the metabolism and side effects of drugs. Drug Discov Today . 2005;10(9):617–25. doi: 10.1016/S1359-6446(05)03394-5. [DOI] [PubMed] [Google Scholar]
- 18.Caron G, Ermondi G, Testa B. Predicting the oxidative metabolism of statins: an application of the MetaSite algorithm. Pharm Res . 2007;24(3):480–501. doi: 10.1007/s11095-006-9199-7. [DOI] [PubMed] [Google Scholar]
- 19.Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol . 2007;47:143–83. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Aragon D, Ariza J, Villalba JM. Dicoumarol impairs mitochondrial electron transport and pyrimidine biosynthesis in human myeloid leukemia HL-60 cells. Biochem Pharmacol . 2007;73(3):427–39. doi: 10.1016/j.bcp.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev . 1998;3(2):114–27. [PubMed] [Google Scholar]


