Abstract
Purpose: Gardenia jasminoides is a traditional medicinal plant rich in anti-inflammatory flavonoids and phenolic compounds and used for the treatment of inflammatory diseases and pain. In this present study, antioxidant potential of Gardenia jasminoides leaves extract was evaluated by using various antioxidant assays.
Methods: Various antioxidant assays such as 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay, reducing power and total antioxidant capacity expressed as equivalent to ascorbic acid were employed. Moreover, phenolic compounds were detected by high-performance liquid chromatography (HPLC) coupled with diode-array detection.
Results: The methanol extract showed significant free radical scavenging activities in DPPH radical scavenging antioxidant assays compared to the reference antioxidant ascorbic acid. Total antioxidant activity was increased in a dose dependent manner. The extract also showed strong reducing power. The total phenolic content was determined as 190.97 mg/g of gallic acid equivalent. HPLC coupled with diode-array detection was used to identify and quantify the phenolic compounds in the extracts. Gallic acid, (+)-catechin, rutin hydrate and quercetin have been identified in the plant extracts. Among the phenolic compounds, catechin and rutin hydrate are present predominantly in the extract. The accuracy and precision of the presented method were corroborated by low intra- and inter-day variations in quantitative results in leaves extract.
Conclusion: These results suggest that phenolic compounds and flavonoids might contribute to high antioxidant activities of Gardenia jasminoides leaves.
Keywords: Gardenia jasminoides, DPPH, Free radical, Reducing power
Introduction
The role of oxygen derived free radicals in the pathogenesis of a number of degenerative diseases is well known.1,2 The oxidation induced by reactive oxygen species can result in cell membrane disintegration, membrane protein damage and DNA mutation, which can further initiate and propagate the development of many diseases, such as cancer, liver injury and cardiovascular disease.3 Mammalian body possesses antioxidant defense mechanisms such as catalase, superoxide dismutase, glutathione peroxidase enzymes and antioxidant nutrients i.e. vitamin e, ascorbic acid which arrest the damaging properties of reactive oxygen species (ROS).4,5 Certain chemicals and contaminants exposure may lead to an increase in free radicals generation in the body beyond its capacity to control them, and ultimately cause irreversible damage to tissues and cellular components.6 Recent investigations have shown that the antioxidant properties of plants could be correlated with oxidative stress defense and different human diseases like aging process etc.7,8 Currently, the possible toxicity of synthetic antioxidants has been criticized. It is generally assumed that frequent consumption of plant-derived phytochemicals from vegetables, fruit, tea, and herbs may contribute to shift the balance toward an adequate antioxidant status.9 In this respect, flavonoids and other polyphenolic compounds have received greatest attention. Gardenia jasminoides Ellis (family Rubiaceae) is an evergreen shrub grows all over Bangladesh. They are used as contraceptive, febrifuge, analgesic, diuretic, larvicide, antihypertensive, antibacterial, anxiolytic, antiplasmodial and for the treatment of headaches.10 It has also been used for the cure of febrile diseases, jaundice, acute conjunctivitis, epistaxis, haematemesis, pyogenic infections and ulcers of the skin.11 The fruits of Gardenia jasminoides are included in oriental herbal medicines and in traditional formulations. It is used for the treatment of inflammation, jaundice, headache, edema, fever and hepatic disorders.12 In addition, its pigments are used as food colorants in oriental countries.13 The pharmacological actions of Gardenia jasminoides, such as protection against oxidative damage, cytotoxic effects, anti-inflammatory activity, and fibrolytic activity have been described previously.14,15 The fruits extracts of Gardenia jasminoides showed potent antioxidant activities by using in vitro antioxidant assay systems.16,17 The bioactive compound isolated from Gardenia jasminoides is crocin and the relationship between total crocin contents and antioxidant activity of ethanol extracts were investigated which showed a positive correlation with the crocin content.17 However, very few reports have been found in literature which showed the analysis of phenolic compounds present in Gardenia jasminoides.18
As a part of our ongoing investigations about natural antioxidants from local medicinal plants of Bangladesh,19-21 in this paper, we analyzed the phenolic compounds present in Gardenia jasminoides leaves extracts by using HPLC-diode-array detection system and evaluated antioxidant activities in vitro by using DPPH radical scavenging assay, reducing power and total antioxidant capacity assays.
Materials and Methods
Chemicals
DPPH (1, 1-diphenyl, 2-picrylhydrazyl), TCA (trichloroacetic acid) and ferric chloride were obtained from Sigma Chemical Co. USA. Ascorbic acid was obtained from SD Fine Chem. Ltd., Biosar, India. Ammonium molybdate was purchased from Merck, Germany. Gallic acid (GA), (+)-catechin hydrate (CH), vanillic acid (VA), caffeic acid (CA), (-)-epicatechin (EC), p-coumaric acid (PCA), rutin hydrate (RH), ellagic acid (EA), and quercetin (QU) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Acetonitrile (HPLC), methanol (HPLC), acetic acid (HPLC), and ethanol was obtained from Merck (Darmstadt, Germany).
Plant material
Leaves of Gardenia jasminoides were collected from Dhaka, Bangladesh in May, 2013, and identified by the expert of the National Herbarium of Bangladesh. The accession number of the plant is 32545; a voucher specimen (GR-SK-05.12.12) for this collection has been retained in the National Herbarium, Dhaka, Bangladesh.
Extraction
The shade-dried leaves were coarsely powdered and extracted with 95% methanol by a Soxhlet apparatus at 45°C. The solvent was completely removed by rotary evaporator and obtained greenish gummy exudates (percentage recovery approximately 12%). This crude extract was used for further investigation for potential antioxidant properties.
Phytochemical screening
The freshly prepared extract of Gardenia jasminoides was qualitatively tested for the presence of chemical constituents. Phytochemical screening of the extract was performed using the following reagents and chemicals: alkaloids with Dragendorffs reagent, Mayer’s reagent and Fehling’s Solutions, flavonoids with the use of Mg and HCl; tannins with ferric chloride and potassium dichromate solutions, steroids with sulfuric acid and saponins with ability to produce suds. Gum was tested using Molish reagents and concentrated sulfuric acid. These were identified by characteristic color changes using standard procedures.19
Determination of total phenolic content
The total phenolic content of the extract was determined by the modified Folin-Ciocaltu method.22 Briefly, 1.0 ml of each extract (1 mg/ml) was mixed with 5 ml Folin-Ciocaltu reagent (1:10 v/v distilled water) and 4 ml (75g/l) of sodium carbonate. The mixture was vortexed for 15 second and allowed to stand for 30 min at 40°C for color development. The absorbance was read at 765 nm with a spectrophotometer. Total phenolic content was determined as mg of gallic acid equivalent per gram using the equation obtained from a standard gallic acid calibration curve y = 6.2548x - 0.0925, R2=0.9962.
High Performance Liquid Chromatography (HPLC) Analysis of the Extract
High performance liquid chromatography (HPLC) system
Chromatographic analyses were carried out on a Thermo Scientific Dionex UltiMate 3000 Rapid Separation LC (RSLC) systems (Thermo Fisher Scientific Inc., MA, USA), coupled to a quaternary rapid separation pump (LPG-3400RS), Ultimate 3000RS auto sampler (WPS-3000) and rapid separation diode array detector (DAD-3000RS). Phenolic compounds were separated on an Acclaim® C18 (4.6 x 250 mm; 5µm) column (Dionix, USA) which was controlled at 30°C using a temperature controlled column compartment (TCC-3000). Data acquisition, peak integration, and calibrations were performed with Dionix Chromeleon software (Version 6.80 RS 10).
Chromatographic conditions
The phenolic composition of the methanol extract of Gardenia was determined by HPLC, as described previously with some modifications.23 The mobile phase consisted of acetonitrile (solvent A), acetic acid solution at pH 3.0 (solvent B), and methanol (solvent C). The system was run with the following gradient elution program: 0 min, 5%A/95%B; 10 min, 10%A /80%B /10%C; 20 min, 20%A /60%B /20%C and 30min, 100%A. There was a 5 min post run at initial conditions for equilibration of the column. The flow rate was kept constant throughout the analysis at 1 ml/min and the injection volume was 20 µl. For UV detection, the wavelength program was optimized to monitor phenolic compounds at their respective maximum absorbance wavelengths as follows: λ 280 nm held for 18.0 min, changed to λ 320 nm and held for 6 min, and finally changed to λ 380 nm and held for the rest of the analysis and the diode array detector was set at an acquisition range from 200 nm to 700 nm. The detection and quantification of GA, CH, VA, CA, and EC was done at 280 nm, of PCA, RH, and EA at 320 nm, and of QU at 380 nm, respectively.
Standard and sample preparation
A stock standard solution (100µg/ml) of each phenolic compound was prepared in methanol by weighing out approximately 0.0050 g of the analyte into 50 ml volumetric flask. The mixed standard solution was prepared by dilution the mixed stock standard solutions in methanol to give a concentration of 20 µg/ml for each polyphenols except caffeic acid (8 µg/ml) and quercetin (6 µg/ml). All standard solutions were stored in the dark at 5°C and were stable for at least three months.
The calibration curves of the standards were made by serial dilution of the stock standards (five set of standard dilutions) with methanol to yield 1.25 - 20 µg/ml for GA, CH, VA, EC, PCA, RH, EA; 0.5 - 8.0 µg/ml for CA, and 0.375 - 6.0 µg/ml for QU. The calibration curves were constructed from chromatograms as peak area vs. concentration of standard.
A solution of methanolic extract of Gardenia jasminoides at a concentration of 5 mg/ml was prepared in ethanol by vortex mixing (Branson, USA) for 30 min. The samples were stored in the dark at low temperature (5°C). Spiking the sample solution with phenolic standards was done for additional identification of individual polyphenols.
Prior to HPLC analysis, all solutions (mixed standards, sample, and spiked solutions were filtered through 0.20 µm nylon syringe filter (Sartorius, Germany) and then degassed in an ultrasonic bath (Hwashin, Korea) for 15 min.
Peak characterization and quantification
The compounds were identified by comparing with standards of each identified compound using the retention time, the absorbance spectrum profile and also by running the samples after the addition of pure standards. Quantification was performed by establishing calibration curves for each compound determined, using the standards. Linear calibration curves for standards (peak area vs concentration) were constructed with R2 exceeding 0.995. Data are reported as means ± standard deviations of triplicate independent analyses.
Antioxidant Activity Test
DPPH radical scavenging activity
Qualitative analysis DPPH radical scavenging activity: The methanol extract was applied on a TLC plate as a spot (100 µg/ml) for chromatographic separation of the extract using the mobile phase methanol:chloroform (95:5, v/v). It was allowed to develop the chromatogram for 30 minutes. After completion of the chromatogram the whole plate was sprayed with DPPH (0.15 % w/v) solution using an atomizer. The color changes (yellowish color development on pinkish background on the TLC plate) were noted as an indicator of the presence of antioxidant substances.
Quantitative analysis for DPPH radical scavenging activity: The free radical scavenging capacity of the extracts was determined using DPPH.19 DPPH solution (0.004% w/v) was prepared in 95% methanol. Metahnol extract of Gardenia jasminoides was mixed with 95 % methanol to prepare the stock solution (5 mg/ml). Freshly prepared DPPH solution (0.004% w/v) was taken in test tubes and Gardenia jasminoides extracts was added followed by serial dilutions (1 μg to 500 μg) to every test tube so that the final volume was 3 mL and after 10 min, the absorbance was read at 515 nm using a spectrophotometer (HACH 4000 DU UV – visible spectrophotometer). Ascorbic acid was used as a reference standard and dissolved in distilled water to make the stock solution with the same concentration (5 mg/ml). Control sample was prepared containing the same volume without any extract and reference ascorbic acid. 95 % methanol was served as blank. % scavenging of the DPPH free radical was measured by using the following equation:
% Scavenging Activity= [(Absorbance of the control – Absorbance of the test sample)/ Absorbance of the control] X 100.
The inhibition curve was plotted for duplicate experiments and represented as % of mean inhibition ± standard deviation.
Determination of total antioxidant capacity: The antioxidant activity of the extract was evaluated by the phosphomolybdenum method according to the procedure describe by Prieto and his colleagues.24 The assay is based on the reduction of Mo (VI)–Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at acid pH. 0.3 ml extract was combined with 3 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes containing the reaction solution were incubated at 95°C for 90 min. Then the absorbance of the solution was measured at 695 nm using a spectrophotometer (HACH 4000 DU UV – visible spectrophotometer) against blank after cooling to room temperature. Methanol (0.3 ml) in the place of extract was used as the blank. The antioxidant activity is expressed as the number of equivalents of ascorbic acid.
Reducing power: The reducing power of Gardenia jasminoides was determined according to the method described by Oyaizu.25 Different concentrations of crude extract (100 μg – 1000 μg) in 1 ml of distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50°C for 20 min. A portion (2.5 ml) of trichloroacetic acid (10%) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml. 0.1%) and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as the standard. Phosphate buffer (pH 6.6) was used as blank solution. The absorbance of the final reaction mixture of three parallel experiments was taken and is expressed as mean ± standard deviation.
Results and Discussion
In the past few years, there has been growing interest in the involvement of reactive oxygen species (ROS) in several pathological situations. ROS produced in vivo include superoxide radical (O2•-), hydrogen peroxide (H2O2) and hypochlorous acid (HOCl). H2O2 and O2•- can interact in the presence of certain transition metal ions to yield a highly-reactive oxidizing species, the hydroxyl radical (•OH).26 In this respect, polyphenolic compounds like flavonoids and phenolic acids are getting much interest because of their potent antioxidant activity.27 Phenolic compounds and flavonoids have been reported to show antioxidant activity in biological systems, acting as scavengers of singlet oxygen and free radicals.28,29 Many plants contain substantial amounts of antioxidants including Vitamin C and E, carotenoids, flavonoids, tannins and thus can be utilized to scavenge the excess free radicals from the human body.30-32 Preliminary phytochemical screening of the Gardenia jasminoides showed the presence of flavonoids and tannins (Table 1).
Table 1. Phytochemical Screening of Gardenia jasminoides.
| Groups | Status* |
| Glycosides | + |
| Alkaloid | + |
| Flavonoids | + |
| Tannins | ++ |
| Gums | + |
| Saponin | - |
| Steroid | ++ |
| (+) means presence in a single method test, (++) means presence experimented in two methods and (-) = absence. | |
Gardenia jasminoides leaves extract was also evaluated for their antioxidant activity by using various in vitro assays. DPPH is relatively stable nitrogen centered free radical that can easily accept an electron or hydrogen radical to form a more stable diamagnetic molecule.32 The DPPH radical scavenging activity of Gardenia jasminoides extracts are given in Figure 1. This activity was increased by increasing the concentration of the sample extract. DPPH antioxidant assay is developed based on the ability of 1, 1-diphenyl-2-picryl-hydrazyl (DPPH), a stable free radical, to decolorize in the presence of antioxidants. The DPPH radical contains an odd electron, which is responsible for a visible deep purple color of DPPH in alcoholic solution and the color intensity can be measured at absorbance 515 nm.32,33 When DPPH accepts an electron donated by an antioxidant compound, the DPPH is decolorized, which can be quantitatively measured from the changes in absorbance. Moreover, the DPPH radical scavenging activity has been shown to be directly related with the total phenolic content present in the extracts as suggested by many previous reports.34,35
Figure 1 .
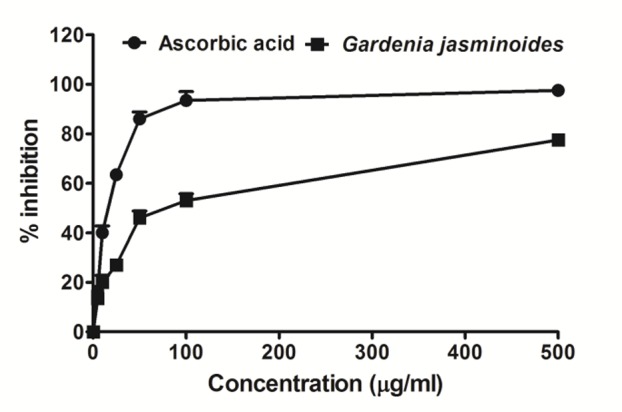
DPPH radical scavenging activity of the methanol extracts Gardenia jasminoides. DPPH radical scavenging activity was increased by increasing the concentration of the sample extract. Data was represented as Mean ± SD of duplicate experiments.
Total antioxidant capacity of the Gardenia jasminoides extract, expressed as the number of equivalents of ascorbic acid was shown in Table 2. The phosphomolybdenum method was developed based on the reduction of Mo (VI) to Mo (V) by the antioxidant compound which may produce a green phosphate/Mo (V) complex. The color intensity of the phosphate/Mo (V) complex can then be measured with a maximal absorption at 695 nm.
Table 2. Contents of polyphenolic compounds in the methanol extract of Gardenia jasminoides.
| Polyphenolic compound | Gardenia jasminoides | |
| Content | % RSD | |
| GA | 9.06(mg/100 g of dry extract) | 0.96 |
| CH | 141.02(mg/100 g of dry extract) | 3.05 |
| RH | 72.06(mg/100 g of dry extract) | 1.25 |
| QU | 19.06(mg/100 g of dry extract) | 0.97 |
| Total phenolic content (mg of gallic acid equivalent per g of dry extract) | 190.97 ± 10.37 | - |
| Average absorbance at 765 nm | 141.02(mg/100 g of dry extract) | 3.05 |
| GA, gallic acid; CH, (+)-catechin; RH, rutin hydrate; QU, quercetin. | ||
Reducing power of the extracts has been investigated based on the conversion of Fe3+ to Fe2+ transformation in the presence of extract samples using the method previously described by Oyaizu.25 The reducing properties are generally associated with the presence of reductones,36 which have been shown to exert antioxidant action by breaking the free radical chain by donating a hydrogen atom. Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. Figure 2 shows the reducing capabilities of the plant extract where reducing power of Gardenia jasminoides extract was increased in a concentration dependent manner compared to ascorbic acid. Increased absorbance in samples in the reducing power assay implies that extracts are capable of donating hydrogen atoms in a dose dependent manner.33,37 The reducing power of extract of Gardenia jasminoides was found remarkable and the reducing power of the extract was observed to rise as the concentration of the extract gradually increased. Moreover, the amount of total phenolic compound was found quite high in the methanol extract of Gardenia jasminoides (190.97 ± 10.37 mg of gallic acid equivalent). Thus higher inhibition values and reducing ability of the methanol extract might be due to the high concentration of phenolic compounds present.37
Figure 2 .
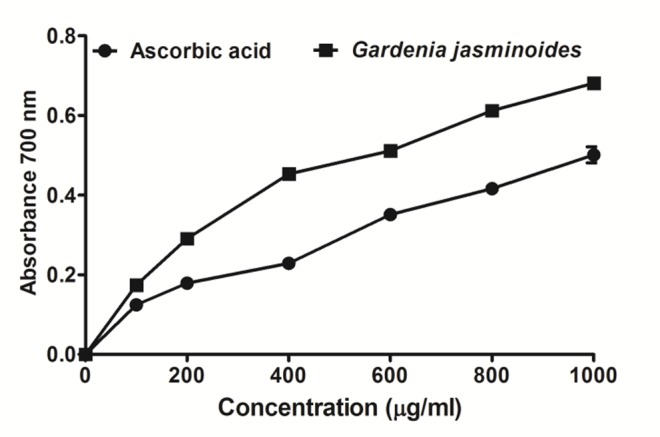
Reducing power of the crude plant extract of Gardenia jasminoides. An increase in absorbance in the reducing power method implies that extracts are capable of donating hydrogen atoms in a dose dependent manner. Data was represented as Mean ± SD of duplicate experiments.
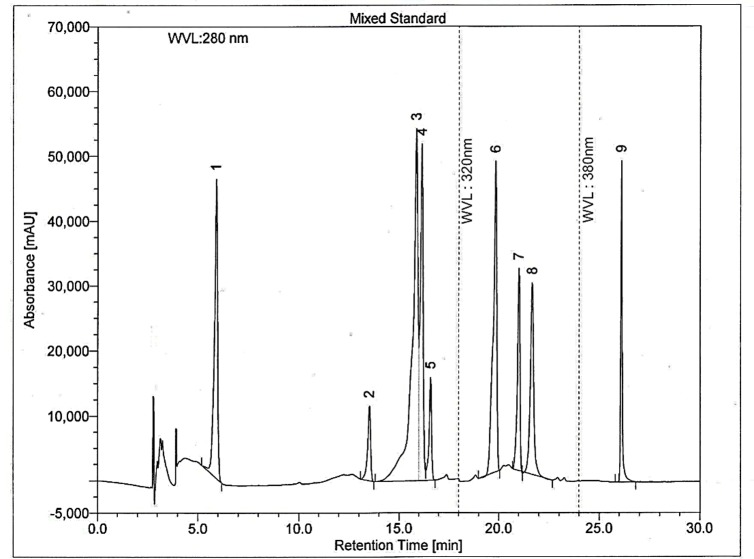
Among the many separation systems, the HPLC analysis includes the use of a binary solvent system containing acidified water and a polar organic solvent to specifically measure polyphenolic concentrations.38,39 For method development, we had followed the previously reported method23 with some modifications. In this study we used C18 column with 250 mm length, and rapid separation LC (RSLC) systems while other authors used C18 column with 150mm length, and HP 1090, series II, liquid chromatography systems to determine the polyphenolic contents. In this study we also used nine different phenolic standards to compare with the chromatograms produced by the unknown Gardenia jasminoides extracts while earlier investigators23 used six standards for identifying phenolic compounds. From Figure 3, it can be observed that a good separation can be achieved within 30 min using the above condition described. Symmetrical, sharp and well-resolved peaks were observed for the nine polyphenolic standards. The elution order and the retention times for GA, CH, VA, CA, EC, PCA, RH, EA, and QU were 6.16, 13.54, 15.90, 16.16, 16.60, 19.85, 21.01, 21.65, and 26.10 min respectively.
Figure 3 .
HPLC chromatogram of a standard mixture of polyphenolic compounds. Peaks: 1, gallic acid; 2, (+)-catechin; 3, vanillic acid; 4, caffeic acid; 5, (–)-epicatechin; 6, p-coumaric acid; 7, rutin hydrate; 8, ellagic acid; 9, quercetin.
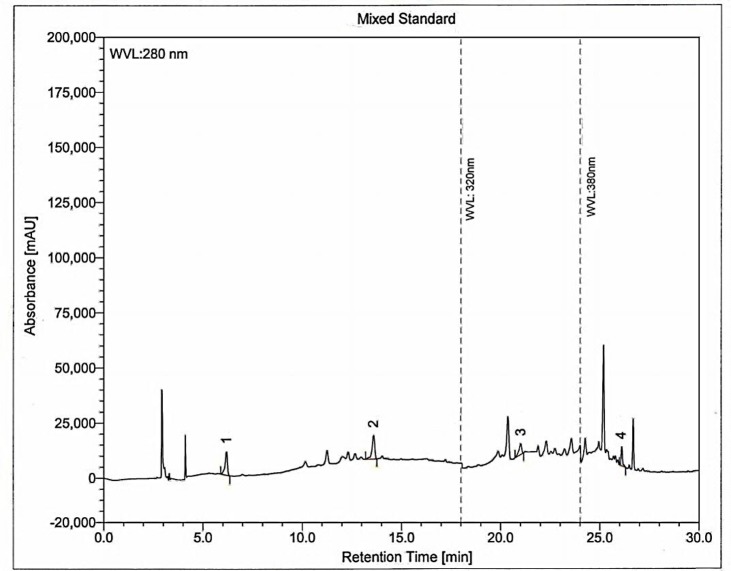
Phenolic compounds are very important plant constituents because of their scavenging ability due to their hydroxyl groups. In HPLC analysis, the presence of gallic acid, (+)-catechin, rutin hydrate and quercetin were confirmed in Gardenia jasminoides extracts. Figure 3 and 4 show the HPLC chromatograms obtained from standards and Gardenia jasminoides extracts respectively. Out of the four phenolic compounds catechin was found in the highest concentration compared to others amounting 141.02 mg/g in Gardenia jasminoides extracts whereas rutin hydrate was found 72.06 mg/g (Table 3). Catechin and rutin both showed beneficial effects such as antioxidant, anti-ageing and may prevent cardiovascular complecations.40-42 Their beneficial effects are attributed to their ability to reduce oxidative stress, lipid peroxidation, free radical generation and low density lipoprotein (LDL) cholesterol-oxidation.40,42 Moreover, other phenolic compounds found in the extracts such as gallic acid and quercetin also possess beneficial effects on human health and eases oxidative stresses.27 Literature reviews suggests that the chemical constituents present in Gardenia plants are triterpenes,43 carotenoids, e.g. crocins,17 iridoid glycosides, quinic acid derivatives, amides and fatty acids.10 However, very few reports suggest the presence of polyphonic compounds in Gardenia jasminoides. Our results confirmed the presence of catechin and rutin hydrate (Figure 5) present in abundant in the extract of Gardenia jasminoides. Radical scavenging activity can thus be explained by the presence of flavonoids, catechin and rutin hydrate in this plant.
Figure 4 .
HPLC chromatogram of Gardenia jasminoides leaves extract. Peaks: 1, gallic acid; 2, (+)-catechin; 3, rutin hydrate; 4, quercetin.
Table 3. Total antioxidant capacity of methanol leaf extract of Gardenia jasminoides Ells.xxsupaxysup .
| Materials | Concentration (µg/ml) | Equivalent to ascorbic acid (µg/mg) |
| Methanol extract of Gardenia jasminoides | 100 | 1.00 ± 0.15 |
| 200 | 1.99 ± 0.07 | |
| 400 | 2.80 ± 0.15 | |
| 600 | 3.41± 0.14 | |
| 800 | 5.37 ± 0.14 | |
| aTotal antioxidant capacity of the Gardenia jasminoides extract, expressed as the number of equivalents of ascorbic acid. Values are the average of triplicates experiments and represented as mean± standard deviation. | ||
Figure 5 .
HPLC separated phenolic compounds from Gardenia jasminoides leaves extract. 1, gallic acid; 2, (+)-catechin; 3, quercetin and 4, rutin hydrate.
Conclusion
This is the first report on the phytochemical and antioxidant potentials of Gardenia jasminoides leaves extract. Our results clearly indicate that Gardenia jasminoides leaves extract possess antioxidant activities which are comparable to the standard antioxidant vitamin C. This study showed that the phenolic compounds and flavonoid contents are responsible for its free radical scavenging activity. These antioxidant substances may be responsible for anti-inflammatory and chemoprotective properties of Gardenia jasminoides leaves extract as well as justify the use of this plant’s extract in folkloric remedies.
Acknowledgements
This project was supported by the Department of Pharmacy, Stamford University Bangladesh.
Conflict of Interest
The authors report no conflict of interest.
References
- 1.Cross CE, Halliwell B, Borish ET, Pryor WA, Ames BN, Saul RL. et al. Oxygen radicals and human disease. Ann Intern Med . 1987;107(4):526–45. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 2.Khatoon M, Islam E, Islam R, Rahman AA, Alam AK, Khondkar P. et al. Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Res Notes . 2013;6(1):121. doi: 10.1186/1756-0500-6-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao K, Yin M. Individual and combined antioxidant effects of seven phenolic agents in human erythrocyte membrane ghosts and phosphatidylcholine liposome systems: importance of the partition coefficient. J Agric Food Chem . 2000;48(6):2266–70. doi: 10.1021/jf990946w. [DOI] [PubMed] [Google Scholar]
- 4.Halliwell B, Aeschbach R, Loliger J, Aruoma OI. The characterization of antioxidants. Food Chem Toxicol . 1995;33(7):601–17. doi: 10.1016/0278-6915(95)00024-v. [DOI] [PubMed] [Google Scholar]
- 5.Sies H. Strategies of antioxidant defense. Eur J Biochem . 1993;215(2):213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 6.Flora SJS. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2(4):191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev . 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging . 2007;2(2):219–36. [PMC free article] [PubMed] [Google Scholar]
- 9.Halliwell B. Oxidative stress, nutrition and health. Experimental strategies for optimization of nutritional antioxidant intake in humans. Free Radic Res. 1996;25(1):57–74. doi: 10.3109/10715769609145656. [DOI] [PubMed] [Google Scholar]
- 10.Fu X-M, Chou G-X, Wang Z-T. Iridoid glycosides from Gardenia jasminoides Ellis. Helv Chim Acta . 2008;91(4):646–53. [Google Scholar]
- 11.Lee SJ, Oh PS, Lim KT. Hepatoprotective and hypolipidaemic effects of glycoprotein isolated from Gardenia jasminoides ellis in mice. Clin Exp Pharmacol Physiol . 2006;33(10):925–33. doi: 10.1111/j.1440-1681.2006.04466.x. [DOI] [PubMed] [Google Scholar]
- 12.Koo HJ, Lim KH, Jung HJ, Park EH. Anti-inflammatory evaluation of gardenia extract, geniposide and genipin. J Ethnopharmacol . 2006;103(3):496–500. doi: 10.1016/j.jep.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 13.Spears K. Developments in food colourings: the natural alternatives. Trends Biotechnol . 1988;6(11):283–8. [Google Scholar]
- 14.Jagadeeswaran R, Thirunavukkarasu C, Gunasekaran P, Ramamurty N, Sakthisekaran D. In vitro studies on the selective cytotoxic effect of crocetin and quercetin. Fitoterapia . 2000;71(4):395–9. doi: 10.1016/s0367-326x(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 15.Tseng TH, Chu CY, Huang JM, Shiow SJ, Wang CJ. Crocetin protects against oxidative damage in rat primary hepatocytes. Cancer Lett . 1995;97(1):61–7. doi: 10.1016/0304-3835(95)03964-x. [DOI] [PubMed] [Google Scholar]
- 16.Debnath T, Park PJ, Deb Nath NC, Samad NB, Park HW, Lim BO. Antioxidant activity of Gardenia jasminoides Ellis fruit extracts. Food Chem . 2011;128(3):697–703. [Google Scholar]
- 17.Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y. et al. Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem. 2008;109(3):484–92. [Google Scholar]
- 18.He W, Liu X, Xu H, Gong Y, Yuan F, Gao Y. On-line HPLC-ABTS screening and HPLC-DAD-MS/MS identification of free radical scavengers in Gardenia (Gardenia jasminoides Ellis) fruit extracts. Food Chem . 2010;123(2):521–8. [Google Scholar]
- 19.Alam M, Ghani A, Subhan N, Rahman M, Haque M, Majumder M. et al. Antioxidant and membrane stabilizing properties of the flowering tops of Anthocephalus cadamba. Nat Prod Commun . 2008;3:65–7. [Google Scholar]
- 20.Alam M, Nyeem M, Awal M, Mostofa M, Alam M, Subhan N. et al. Antioxidant and hepatoprotective action of the crude methanolic extract of the flowering top of Rosa damascena. Oriental Pharm Exp Med . 2008;8:164–70. [Google Scholar]
- 21.Talukder FZ, Khan KA, Uddin R, Jahan N, Alam MA. In vitro free radical scavenging and anti-hyperglycemic activities of Achyranthes aspera extract in alloxan-induced diabetic mice. Drug Discov Ther . 2012;6(6):298–305. [PubMed] [Google Scholar]
- 22.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem . 2003;51(3):609–14. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 23.Chuanphongpanich S, Phanichphant S. Method development and determination of phenolic compounds in broccoli seeds samples. Chiang Mai J Sci . 2006;33:103–7. [Google Scholar]
- 24.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem . 1999;269(2):337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 25.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jap J Nutr . 1986;44:307–15. [Google Scholar]
- 26.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radical Biol Med . 1989;6(6):593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 27.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev . 2009;2(5):270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot . 2003;91 Spec No:179–94. doi: 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmsen PK, Spada DS, Salvador M. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem . 2005;53(12):4757–61. doi: 10.1021/jf0502000. [DOI] [PubMed] [Google Scholar]
- 30.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol . 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Andre C, Larondelle Y, Evers D. Dietary antioxidants and oxidative stress from a human and plant perspective: A review. Curr Nutr Food Sci . 2010;6:2–12. [Google Scholar]
- 32.Mai W, Chen D, Li X. Antioxidant activity of Rhizoma cibotii in vitro. Adv Pharm Bull . 2012;2(1):107–14. doi: 10.5681/apb.2012.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asirvatham R, Christina AJ, Murali A. In Vitro Antioxidant and Anticancer Activity Studies on Drosera Indica L. (Droseraceae) Adv Pharm Bull. 2013;3(1):115–20. doi: 10.5681/apb.2013.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med . 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middha SK, Usha T, Pande V. HPLC evaluation of phenolic profile, nutritive content, and antioxidant capacity of extracts obtained from punica granatum fruit peel. Adv Pharmacol Sci . 2013;2013:296236. doi: 10.1155/2013/296236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of Harng Jyur (Chrysanthemum morifolium Ramat) LWT - Food Sci Technol . 1999;32(5):269–77. [Google Scholar]
- 37.Ganjewala D, Gupta AK. Study on Phytochemical composition, antibacterial and antioxidant properties of different parts of Alstonia scholaris Linn. Adv Pharm Bull . 2013;3(2):379–84. doi: 10.5681/apb.2013.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem . 2003;51(3):571–81. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- 39.Tsao R, Yang R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: towards a total phenolic index using high-performance liquid chromatography. J Chromatogr A . 2003;1018(1):29–40. doi: 10.1016/j.chroma.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome - a review. Phytochemistry . 2009;70(1):11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 41.Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med . 2004;37(3):304–17. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 42.Augustyniak A, Bartosz G, Cipak A, Duburs G, Horakova L, Luczaj W. et al. Natural and synthetic antioxidants: an updated overview. Free Radic Res . 2010;44(10):1216–62. doi: 10.3109/10715762.2010.508495. [DOI] [PubMed] [Google Scholar]
- 43.Suksamrarn A, Tanachatchairatana T, Kanokmedhakul S. Antiplasmodial triterpenes from twigs of Gardenia saxatilis. J Ethnopharmacol . 2003;88(2-3):275–7. doi: 10.1016/s0378-8741(03)00261-7. [DOI] [PubMed] [Google Scholar]