Abstract
Purpose: Severe oxidative stress is an important event that occurs in patients with sepsis. The body has extensive and multiple defense mechanisms against the reactive oxygen species (ROS) produced during inflammation and sepsis. One of these mechanisms includes a group of enzymes that utilize selenium as their cofactor. The purpose of this study is investigating of Selenium effect on oxidative stress factors in animal model of sepsis.
Methods: Sepsis was induced by caecal ligation and puncture (CLP) method. 30 Male Wistar rats were divided into following groups: sham group; CLP group; 100 μg/kg Selenium- treated CLP group. 12 hours after inducing sepsis animals were killed and lungs were removed. One of the lungs was frozen in liquid nitrogen and kept at -70°C for enzymatic activity analysis and the other was kept in formalin 10% until tissue section preparation performed for histopathological studies.
Results: The Myeloperoxidase (MPO) activity was decreased in Selenium- treated CLP group. Inflammation score of lung tissue was lowered in Selenium- treated CLP group, but it wasn’t statically significant. Level of glutathione peroxidase (GPx) was higher in CLP and Selenium- treated CLP groups.
Conclusion: It seems that Selenium has protective effect on lung inflammation during acute lung injury. Also it may improve some stress oxidative profile during CLP model of sepsis.
Keywords: Sepsis, Selenium, CLP, Oxidative stress
Introduction
Sepsis is a systemic inflammatory syndrome occurring due to extraordinary immune system response to the microorganism or its toxin in bloodstream.1 Sepsis is one of the most important public health concerns. Sepsis affects persons of all ages, and is the leading cause of morbidity and mortality in intensive care unit (ICU). When sepsis is associated with acute organ dysfunction, results from inflammation and activation of procoagulant response, it is called severe sepsis.2
In patients with severe sepsis, an early decrease in plasma selenium concentrations occurs that could be as a result of a defect in antioxidant defenses. Oxidative stress and free radicals might cause the development of multiple organ failure in patients with septic shock, so during severe oxidative stress like sepsis or septic shock, the requirement of selenium might be increased.3 Selenium is a part of three major Se-containing proteins: selenoprotein-P, glutathione peroxidase (GPx), and albumin. As a part of GPx, which is considered to be the most important antioxidant enzyme system preventing injury to cells, Selenium serves as a free radical scavenger and plays an important role in reducing oxidative damage.4
There is a good evidence that Selenium content is low in critically ill patients and patients with conditions of inflammation and high oxidative stress are at risk of its deficiency.5 However, selenium can also be considered as a pro- oxidative agent, which may become toxic to cells and damage them. It is suggested that this toxicity is due to the pro-oxidant ability of selenium compounds to catalyze the oxidation of thiols and produce superoxide (O2–), which causes depletion of intracellular glutathione, excessive oxidative stress, and organ damage.
Therefore, to clarify the mechanism of selenium effect on sepsis and oxidative-stress pathway, we aimed to investigate the antioxidant effects of Selenium in animal model of sepsis on oxidative-stress biomarkers and evaluating histopathology properties of lung tissue.
Materials and Methods
Animals
The experiments were carried out on male Wistar rats weighing 270-300g and each group included ten rats. Animals were housed in standard polypropylene cages, four per cage, under a 12:12 h light/dark schedule at an ambient temperature of 23±2 °C. Animals had free access to food and water. All experiments were carried out under ethical guidelines for the care and use of laboratory animals (National Institutes of Health Publication No 85-23, revised 1985).
The rats were divided into three groups: (I) sham group, rats were subjected to laparotomy without any other manipulation; (II) CLP group, rats were subjected to CLP without any treatment; (III) 100 µg/kg Selenium- treated CLP group, rats received Selenium 0.5 hours after CLP. Selenium was administered i.p. The rats had free access to food and water after surgery, until they were killed.
Surgical procedure
Animal model of sepsis was induced through caecal ligation and one-hole puncture (CLP). Anaesthesia was induced through the intraperitoneal administration of ketamine (80–100 mg/kg) and xylazine (5–15 mg/kg). The abdomen was shaved and the peritoneum was opened. The caecum was ligated with a 4/0 silk ligature just distal to the ileocaecal valve. One puncture was made with an 18-gauge needle through the caecum, and the caecum was returned to the peritoneal cavity. The abdominal incision was then closed with a 4/0 sterile silk suture. The wound was treated by phenylbutazone to ensure analgesia.
The sham-operated group received laparotomy, but the caecum was not ligated or perforated. All the animals were given prewarmed normal saline (2ml/100g body weight) subcutaneously after surgery for fluid resuscitation.7
Specimen Collection
All three groups were anaesthetized after 12 h and lungs were removed quickly from all the rats and washed in ice-cold saline. Half the tissues were kept at -70°C for biochemical analyses, and the other half of the tissues were fixed in 10% formalin solution for histopathological analyses.
Measurement of Myeloperoxidase (MPO) activity
Myeloperoxidase (MPO) activity was measured according to the method of Bradley et al.8 The lung tissues were chopped in l of 50 mM potassium phosphate buffer (pH=6), including 0.5% hexa-decyl-trimethyl-ammonium-bromide (HTAB) and homogenized for 3 min at 8500 rpm. The homogenates were sonicated for 10 seconds, frozen and thawed 3 times then centrifuged at 45000 rpm, in 4°C for 45 min. The supernatant (100 μl) was added to 2.9 ml of phosphate buffer (50 mM; pH=6) including 0.167 mg/ml of O-dianisidine dihydrochloride and 0.0005% hydrogen peroxide. Five minutes later the reaction was stopped after addition 0.1 ml of 1.2 M hydrochloric acid and absorbance was measured spectrophotometrically at 400 nm. The concentrations were calculated by using calibration curve and expressed as units of MPO in mg weight of wet tissue (U/mg).
Measurement of Malondialdehyde (MDA)
Lipid per oxidation in the rat lung tissues was assayed by determination of the MDA levels according to the method of Olgen et al.9 The tissues were homogenized in 1.15% KCl to achieve a 10% (W/V) homogenate, then were centrifuged and 1 ml of each supernatant was added to a mixture containing 3 ml of O- phosphorous acid (1%) and 1 ml of thiobarbituric acid (TBA; 0.67%) in an aqueous solution. The reaction mixture was heated for 60 min up to 90°C, and then was cooled in a room temperature. Then, 3 ml of n-butanol was added to each tube, and the tubes were shaken and then centrifuged. The absorbance of n-butanol phase was measured spectrophotometrically at 532 nm and the amount of thiobarbituric acid reactant substances (TBARS) was calculated from a calibration curve and reported as nmol MDA/ mg tissue.
Measurement of Superoxide Dismutase (SOD)
The rat lung tissues were homogenized in 1.15% KCl solution, 10% (w/v). The homogenates were centrifuged and the supernatants were used for SOD assay according to the method of Paoletti et al.10 This method employs xanthine and xanthine oxidase to generate the superoxide radicals reacting with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. The SOD activity was assayed by the degree of inhibition of the formazin production. The absorbance was measured at 505 nm; one unit of SOD was defined as the amount of the enzyme that caused a 50% inhibition of the INT reduction. Tissue SOD was measured by Randox commercial kit (Randox Laboratories Ltd., Crumlin, UK) and was reported as Unit/mg protein.
Measurement of Glutathione Peroxidase (GPx)
GPx activity was measured according to the method of Paglia and Valentine.11 The rat lung tissues were homogenized in 1.15% KCl solution, 10% (w/v). The homogenates were centrifuged and the supernatants were used for GPx assay. GPx catalyzes the oxidation of glutathione by cumene hydroperoxide. In the presence of glutathione reductase and nicotinamide adenine dinucleotide phosphate-oxidase (NADPH), the oxidized glutathione is converted to the reduced form with a concomitant oxidation of NADPH to NADP+. Tissue GPx was measured by Randox commercial kit at 340 nm and were reported as Unit/mg protein.
Data analysis
Data are expressed as the mean ± SD. One-way ANOVA test was used to find out differences in tissue extract oxidative stress levels in each measured time. P<0.05 was accepted as statistically significant differences.
Results
SOD activity, GPx levels, MDA levels and MPO enzymatic activity were evaluated in all lung tissues. There was no significant difference between groups in tissue SOD enzyme activity (P>0.05) (Figure 1).
Figure 1 .
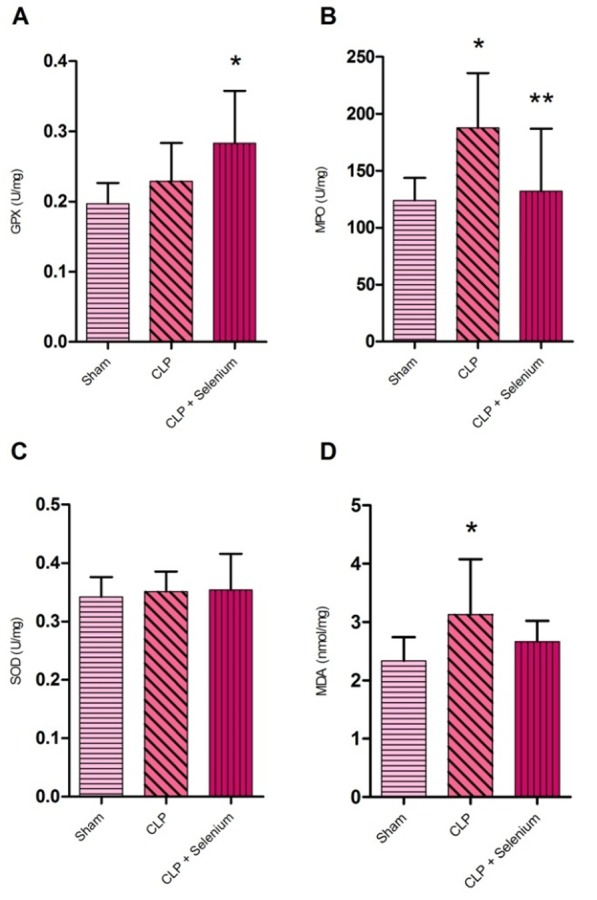
Lung tissue oxidative stress parameters measured 12 hours after caecal ligation and puncture (n= 10). (A) GPx level. (B) MPO activity. (C) SOD level. (D) MDA level. *P<0.05 compared to sham group. **P<0.05 compared to control group. Values are expressed as mean ± SD.
The level of lung tissue MDA was significantly higher in the CLP group compared to sham group. MDA level has a relative decrease in Selenium- treated CLP group compared to CLP group but it was not significant (P>0.05) (Figure 1).
GPx activity was increased significantly in Selenium- treated CLP group compared to sham group (P<0.05) (Figure 1).
The activity of MPO in lung tissue was significantly higher in the CLP group. Treatment with Selenium significantly depressed the elevation in MPO activities (P<0.05) (Figure 1).
According to our analysis, there was a significant difference between sham group and CLP group, in terms of inflammation scores (P<0.05). Also histopathology studies showed decrease in lung tissue damage in Selenium- treated CLP group compared to CLP group, but it wasn’t statistically significant (P>0.05) (Figure 2).
Figure 2 .
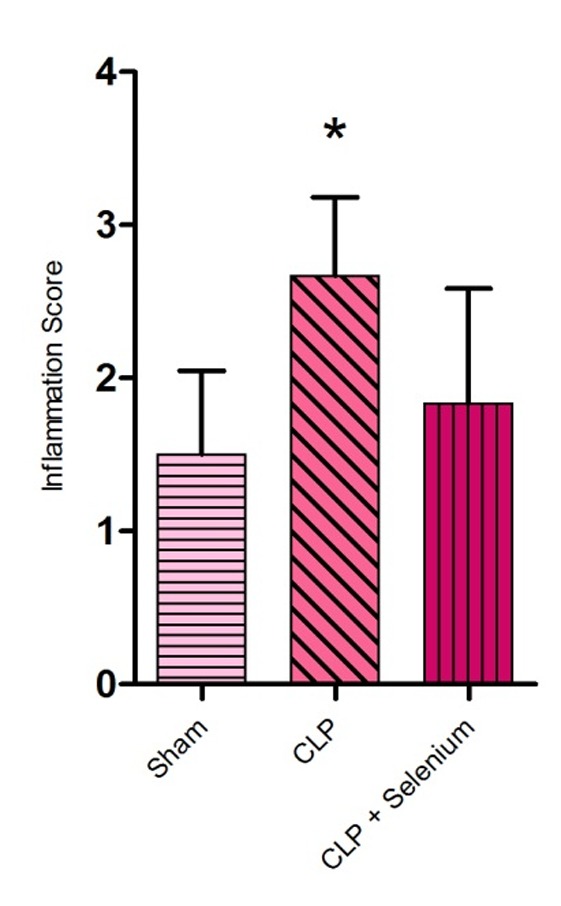
Lung tissue Inflammation scores measured 12 hours after caecal ligation and puncture (n = 6). *P<0.05 compared to sham group. Values are expressed as mean ± SD.
Discussion
Sepsis is an important health problem because it is complicated and difficult to treat and causes high mortality and morbidity; thus, it is important to diagnose and treat it as soon as possible. Microorganisms and their toxins cause the release of cytokines and the activation of inflammatory systems.12
Oxidative stress is the imbalance between oxidants and antioxidants at the cellular level. This imbalance can cause oxidative damage which includes oxidative modification of cellular macromolecules, cell death, as well as structural tissue and organ damage.13 Many antioxidants, such as selenium have been used to prevent defect of antioxidant defences in inflammatory and immune-related disease.
In septic patients, Selenium deficiency and defect of GPx detoxifying status has been proved, which correlates directly with high mortality rate.5 Also it has been shown that Selenium replacement has improved the ability of immune system in oxidative stress conditions.14 A study around the ability of Selenium to suppress respiratory problems showed that administration of selenium can decrease sepsis- induced change in lung tissue and improve GPx status to oppose oxidative stress.12
Considering recent studies of Selenium effects on systemic inflammatory response factors, we aimed to evaluate Selenium antioxidative effects on lung tissue parameters in septic rats to realize that whether it is able to improve outcome in septic patients or not. The MPO activity is a marker of neutrophil accumulation in lung tissue that is affected strongly in sepsis.15 In present study, decreasing level of MPO activity by treatment with Selenium may indicate that Selenium suppresses the severity of sepsis. Regarding to this result, lung inflammation following induction of sepsis was decreased in Selenium- treated CLP group. Obviously, lower neutrophil accumulation will result in lower tissue inflammation.
Superoxide anion (O2._) is the substrate of SOD that is converted to hydrogen peroxide (H2O2). SOD over activation in conditions like inflammation and sepsis, causes the accumulation of the H2O2 which could react with metal ions and generate hydroxyl radicals (OH.) that is suggested to be the most dangerous radical.16Studies show an increase in SOD levels after sepsis as a feedback of ROS rise.17 In this study SOD had no rise after 12 h after CLP in CLP group compared to sham group.
Our results showed that treatment with Selenium has improved GPx levels. GPx detoxifies H2O2 by reducing it to water. It also protects cytosolic organelles from oxidative damage by preventing lipid peroxidation.15 Increasing in GPx level by prescription of Selenium may enable body to use antioxidative capacity in order to confront oxidative stress induced by invasive microorganism during sepsis.
Oxidized lipids and proteins play an important role in destruction and damaging cell membranes.15 Secondary lipid peroxidation products have toxic effects and can prolong and potentiate the primary free-radical initiated oxidative damage.18 Lipid peroxidation has been found in rats with sepsis, and MDA levels have been increased in patients with septic shock.19 Lung tissue level of MDA was increased in septic rats compared to sham group. Also treatment with Selenium lowered MDA tissue levels but depression was not statistically significant. In conclusion, Selenium is seemed to partially overcome to lung manifestation of sepsis. However, more evidence is needed to prove its definite effect on process of sepsis.
Acknowledgments
The authors would like to acknowledge with gratitude Dr Nasrin Maleki and Dr Hamed Hamishehkar for their valuable comments. We also thank Amir M. Vatankhah for their helpful technical assistance (Drug Applied Research Center). This study was financially supported by grant no. 91/83 from the Drug Applied Research Center of Tabriz University of Medical Sciences.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Kumar V, Sharma A. Is neuroimmunomodulation a future therapeutic approach for sepsis? Int Immunopharmacol. 2010;10(1):9–17. doi: 10.1016/j.intimp.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Bernard GR, Vincent JL, Laterre PF, Larosa SP, Dhainaut JF, Lopez-Rodriguez A. et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald J, Galley HF, Webster NR. Oxidative stress and gene expression in sepsis. Br J Anaesth. 2003;90(2):221–32. doi: 10.1093/bja/aeg034. [DOI] [PubMed] [Google Scholar]
- 4.Carlos WG, Curtis Ramsey M, Fraiz J. Selenium in Early Sepsis: A Marker for Change? Advances in Sepsis. 2008;6(3):99–102. [Google Scholar]
- 5.Strachan S, Wyncoll D. Selenium in critically ill patients. J Intensive Care Soc. 2009;10:38–43. [Google Scholar]
- 6.Heyland DK. Selenium supplementation in critically ill patients: can too much of a good thing be a bad thing. Crit Care. 2007;11(4):153. doi: 10.1186/cc5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc. 2008;4(1):31–6. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78(3):206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 9.Olgen S, Coban T. Antioxidant evaluations of novel N-H and N-substituted indole esters. Biol Pharm Bull. 2003;26(5):736–8. doi: 10.1248/bpb.26.736. [DOI] [PubMed] [Google Scholar]
- 10.Paoletti F, Aldinucci D, Mocali A, Caparrini A. A sensitive spectrophotometric method for the determination of superoxide dismutase activity in tissue extracts. Anal Biochem. 1986;154(2):536–41. doi: 10.1016/0003-2697(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 11.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70(1):158–69. [PubMed] [Google Scholar]
- 12.Atli M, Erikoglu M, Kaynak A, Esen HH, Kurban S. The effects of selenium and vitamin E on lung tissue in rats with sepsis. Clin Invest Med. 2012;35(2):E48–54. doi: 10.25011/cim.v35i2.16288. [DOI] [PubMed] [Google Scholar]
- 13.Lykkesfeldt J, Svendsen O. Oxidants and antioxidants in disease: oxidative stress in farm animals. Vet J. 2007;173(3):502–11. doi: 10.1016/j.tvjl.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Xu HB, Mei WD, Dong ZM, Liao BL. Study of the oxidative metabolic function and chemotaxis of neutrophils from patients with cancer influenced by selenium yeast. Biol Trace Elem Res. 1990;25(3):201–9. doi: 10.1007/BF02990415. [DOI] [PubMed] [Google Scholar]
- 15.Ozturk E, Demirbilek S, Begec Z, Surucu M, Fadillioglu E, Kırımlıoglu H. et al. Does leflunomide attenuate the sepsis-induced acute lung injury? . Pediatr Surg Int . 2008;24(8):899–905. doi: 10.1007/s00383-008-2184-y. [DOI] [PubMed] [Google Scholar]
- 16.Andrades M, Ritter C, Moreira JC, Dal-Pizzol F. Oxidative parameters differences during non-lethal and lethal sepsis development. J Surg Res. 2005;125(1):68–72. doi: 10.1016/j.jss.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Warner BW, Hasselgren PO, James JH, Bialkowska H, Rigel DF, Ogle C. et al. Superoxide dismutase in rats with sepsis. Effect on survival rate and amino acid transport. Arch Surg. 1987;122(10):1142–6. doi: 10.1001/archsurg.1987.01400220052010. [DOI] [PubMed] [Google Scholar]
- 18.Toklu HZ, Tunali Akbay T, Velioglu-Ogunc A, Ercan F, Gedik N, Keyer-Uysal M. et al. Silymarin, the antioxidant component of Silybum marianum, prevents sepsis-induced acute lung and brain injury. J Surg Res. 2008;145(2):214–22. doi: 10.1016/j.jss.2007.03.072. [DOI] [PubMed] [Google Scholar]
- 19.Koksal G, Sayilgan C, Aydin S, Oz H, Uzun H. Correlation of plasma and tissue oxidative stresses in intra-abdominal sepsis. J Surg Res. 2004;122(2):180–3. doi: 10.1016/j.jss.2004.07.246. [DOI] [PubMed] [Google Scholar]


