Abstract
Purpose: Curcumin is a hydrophobic polyphenol isolated from dried rhizome of turmeric. Clinical usefulness of curcumin in the treatment of cancer is limited due to poor aqueous solubility, hydrolytic degradation, metabolism, and poor oral bioavailability. To overcome these limitations, we proposed to fabricate curcumin-piperine, curcumin-quercetin and curcumin-silibinin loaded polymeric nanoformulation. However, unfavourable combinations of drug-drug and drug-excipient may result in interaction and rises the safety concern. Hence, the present study was aimed to assess the interaction of curcumin with excipients used in nanoformulations.
Methods: Isothermal stress testing method was used to assess the compatibility of drug-drug/drug-excipient.
Results: The combination of curcumin-piperine, curcumin-quercetin, curcumin-silibinin and the combination of other excipients with curcumin, piperine, quercetin and silibinin have not shown any significant physical and chemical instability.
Conclusion: The study concludes that the curcumin, piperine, quercetin and silibinin is compatible with each other and with other excipients.
Keywords: Compatibility Study, Curcumin, Piperine, Polymeric Nanoparticles, Quercetin, Silibinin
Introduction
Curcumin is a hydrophobic polyphenol isolated from dried rhizome of turmeric (Curcuma Longa Linn & zingiberaceae family), which is responsible for various pharmacological activities including anti-cancer, anti-oxidant, anti-bacterial, anti-fungal, anti-viral and anti-inflammatory and expected to have medicinal benefits in arthritis, psoriasis, diabetes, acquired immunodeficiency syndrome, cardiovascular diseases, multiple sclerosis, cancer and lung fibrosis.1,2 However, clinical usefulness of curcumin in the treatment of cancer is limited due to poor aqueous solubility, hydrolytic degradation in alkaline pH, metabolism via glucuronidation and sulfation in the liver and in intestine, and poor oral bioavailability. These limitations results in decreased therapeutic efficacy or absence of therapeutic efficacy in in-vivo studies.2 Though there are many novel approaches to overcome these limitations, nanotechnology (Particle size <1000 nm) is the most recent and offer significant improvement.3,4 Hence, to overcome these limitations we proposed to fabricate curcumin-piperine, curcumin-quercetin and curcumin-silibinin loaded polymeric nanoformulation. However, unfavourable combinations of drug-drug and drug-excipient may result in interaction, which leads to physical instability or chemical instability. Physical instability refers to changes in the characteristics of a drug that do not involve chemical bond formation or breakage in the drug structure, which can be identified by changes in the organoleptic parameters such as appearance, form etc. Chemical instability refers to changes in the chemical structure of the drug molecule resulting in drug degradation, reduced drug content and formation of other molecule such as degradation products. Both physical and chemical instability may cause safety concerns. Hence, a thorough drug-drug/drug-excipient compatibility study is mandatory.5 The present study was aimed to assess the physical and chemical instability of curcumin with various excipients to be used in the proposed nanoformulations.
Materials and Methods
Materials
Poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) polymer was obtained from Degussa, India. Curcumin, Piperine, Quercetin, Silibinin and Poloxamer 188 were obtained from Sigma-Aldrich, India. β-cyclodextrin was obtained from Himedia Laboratories, India.
Drug-drug/drug-excipient compatibility study
Isothermal stress testing method is used to assess the compatibility of drug-drug/drug-excipient.6 Briefly, about 100 mg of pure drugs and excipients were weighed separately and in combination as shown in Table 1. Individual drugs (Sample 1-4), individual excipients (Sample 5-7) and drug-drug/drug-excipient combinations (Sample 8-22) were transferred in to an appropriately labelled glass vial. Subsequently, 10 µL of ultra pure water (Milli-Q Academic, Milli-Pore) was added to each vial and mixed using a glass capillary, which was left inside the vial after mixing. Each vial was sealed properly and placed in hot air oven (T26/HAO-L, Technico) at 50°C for 4 weeks. To identify the physical instability, organoleptic parameters of samples (1-22) such as colour and texture were observed initially and at the end of 1st, 2nd, 3rd and 4th week. To identify the chemical instability, samples (1-22) were divided into two parts at the end of 4th week. First part of samples were used to record the Fourier-Transform Infrared (FT-IR) spectrum using FT-IR Spectrometer (Nicolet iS5, Thermo Scientific). Disappearance of absorption bands or reduction of the band intensity combined with the appearance of new bands give a clear evidence for interactions.5,6 The second part of samples (sample 1-4 and 8-22) were separately mixed with 10 mL of methanol and sonicated (Ultrasonic cleaner, Lark) for 5 minutes followed by filtration through 0.22 µm membrane and analysed using the developed High Performance Liquid Chromatography (HPLC) methods in triplicate.7-9
Table 1. Summary of drug-drug/drug-excipient compatibility study.
| S.No. | Samples | Colour and Texture | Week | Assay (%) | % RSD | |||
| 1 | 2 | 3 | 4 | |||||
| 1 | Curcumin* | Bright yellow-orange powder | NSVC | 98.43 | 0.91 | |||
| 2 | Piperine* | Faint yellow-off-white crystals | NSVC | 98.98 | 1.23 | |||
| 3 | Quercetin* | Yellow-green powder | NSVC | 99.11 | 1.98 | |||
| 4 | Silibinin* | White powder | NSVC | 99.56 | 0.63 | |||
| 5 | Polymer* | White powder | NSVC | - | - | |||
| 6 | Poloxamer 188* | White powder | NSVC | - | - | |||
| 7 | ß-cyclodextrin* | White powder | NSVC | - | - | |||
| 8 | Curcumin + Piperine@ | Bright yellow-orange powder | NSVC | 97.91 & 98.17 | 1.39 & 0.86 | |||
| 9 | Curcumin + Quercetin@ | Yellowish green powder | NSVC | 98.14 & 100.52 | 0.76 & 0.63 | |||
| 10 | Curcumin + Silibinin@ | Bright yellow powder | NSVC | 99.02 & 98.71 | 0.49 & 1.59 | |||
| 11 | Curcumin + Polymer@ | Bright yellow powder | NSVC | 100.17 | 1.79 | |||
| 12 | Curcumin + Poloxamer@ | Bright yellow powder | NSVC | 99.49 | 0.61 | |||
| 13 | Curcumin + ß-cyclodextrin@ | Bright yellow powder | NSVC | 100.41 | 0.52 | |||
| 14 | Piperine + Polymer@ | White to off-white powder | NSVC | 98.42 | 0.65 | |||
| 15 | Piperine + Poloxamer@ | White to off-white powder | NSVC | 99.83 | 1.66 | |||
| 16 | Piperine + ß-cyclodextrin@ | White to off-white powder | NSVC | 100.10 | 0.73 | |||
| 17 | Quercetin + Polymer@ | Faint greenish powder | NSVC | 98.29 | 1.19 | |||
| 18 | Quercetin + Poloxamer 188@ | Faint greenish powder | NSVC | 97.84 | 1.21 | |||
| 19 | Quercetin + ß-cyclodextrin@ | Faint greenish powder | NSVC | 100.76 | 0.76 | |||
| 20 | Silibinin + Polymer@ | White powder | NSVC | 99.56 | 0.63 | |||
| 21 | Silibinin + Poloxamer 188@ | White powder | NSVC | 98.09 | 1.55 | |||
| 22 | Silibinin + ß-cyclodextrin@ | White powder | NSVC | 100.50 | 1.75 | |||
| *Refers to 100 mg of samples; @Refers to 100 mg + 100 mg of samples in combination; NSVC: No significant visual changes; Polymer: Poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) | ||||||||
Results and Discussion
The organoleptic parameters (colour and texture) of samples have not shown any significant visual changes throughout the storage period (Table 1). Hence, there were no physical instabilities in drug-drug/drug-excipient combinations.
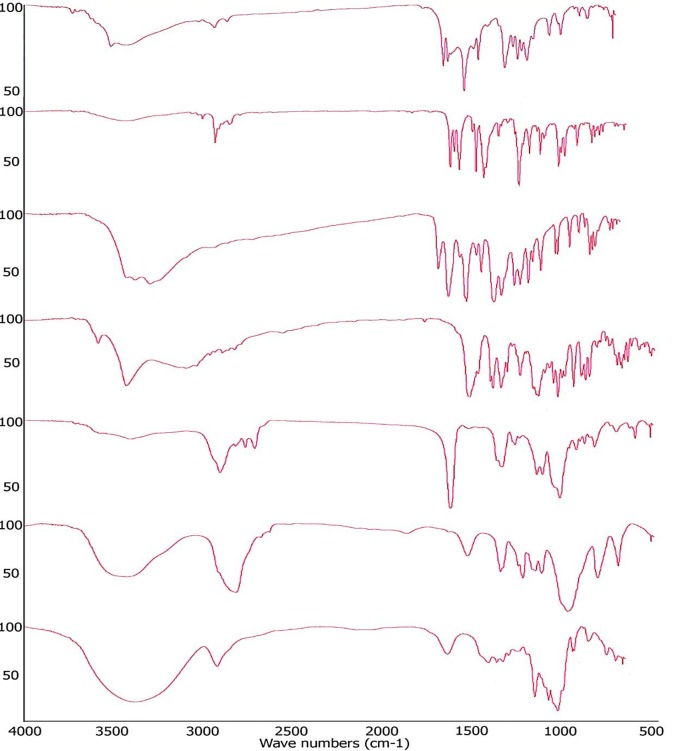
The FT-IR spectrum of samples (1-22) have shown characteristic absorption bands. Curcumin showed characteristic absorption bands at (a) 3726 and 3509 cm-1 (O-H stretch); (b) 2924 cm-1 (C-H stretch); (c) 1627 cm-1 (C=C stretch); (d) 1602 cm-1 (symmetric aromatic ring stretch); (e) 1509 cm-1 (C=O stretch); (f) 1429 cm-1 (C-H asymmetric stretch); (g) 1281, 1233, 1206, 1184 and 1153 cm-1 (C–O stretch); (h) 1026 cm-1 (C–O-C stretch ); (i) 963 cm-1 (trans-CH vibration); and (j) 856 cm-1 (C-C skeleton vibration). Piperine showed characteristic absorption bands at (a) 3009 cm-1 (aromatic C-H stretch); (b) 2940 cm-1 (asymmetric & symmetric CH2 stretch); (c) 2861 cm-1 (aliphatic C-H stretch); (d) 1633 cm-1 (-CO-N stretch); (e) 1611 cm-1 (symmetric & asymmetric C=C stretch); (f) 1584, 1510 and 1491 cm-1 (aromatic C=C stretch benzene ring); (g) 1448 cm-1(CH2 bend); (h) 1252 and 1194 cm-1 (asymmetrical =C-O-C stretch); (i) 1132 cm-1 (in-plane bend of phenyl C-H); (j) 1032 cm-1 (symmetrical =C-O-C stretch); (k) 930 cm-1 (C-O stretch); (l) 997 cm-1 (C-H bend of trans -CH=CH-); and (m) 847, 831 and 804 cm-1 (out-of-plane C-H bend). Quercetin showed characteristic absorption bands at (a) 3299 cm-1 (Phenolic OH stretch); (b) 1672 cm-1 (C=0 Aryl ketonic stretch); (c), 1615, 1550, 1512 and 1429 cm-1(aromatic ring stretch); (d) 1360, 1316, 1244, 1212 cm-1 (-C-OH deformation vibration); and (e) 1165, 1141 and 1094 cm-1 (-C-OH stretch). Silibinin showed characteristic absorption bands at (a) 3606, 3455, 3137 and 2946 cm-1(OH stretch); (b) 1873 and 1637 cm-1 (C=O stretch); (c) 1522, 1510, 1468 and 1435 cm-1 (skeleton vibration of aromatic C=C ring stretch); (d) 1366 cm-1 (OH in plane bend); (e) 1269, 1234, 1211 and 1189 cm-1 (C-O-C stretch); (f) 1166, 1143, 1128, 1082, 1041 and 1018 cm-1 (in plane = C-H bend); (g) 997, 956, 911 and 893 cm-1 (O-H out plane bend); and (h) 850, 834, 824, 812, 793, 774, 733, 703 and 668 cm-1 (in plane = C-H bend). Poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methyl methacrylate) showed characteristic absorption bands at (a) 2957 cm-1 (CHX stretch); (b) 2822 and 2772 cm-1 (CH3 stretch, dimethylamino groups); (c) 1731 cm-1 (C=O stretch); (d) 1457 and 1388 cm-1 (CHX stretch); (e) 1272, 1241 and 1149 cm-1 (C=O stretch); (f) 1061 cm-1 (C-N stretch); and (g) 1017 and 966 cm-1 (CH3 rocking). Poloaxmer 188 showed characteristic absorption bands at (a) 3445 cm-1 (O-H stretch); (b) 2874 cm-1 (C-H stretch); (c) 1467 cm-1 (C-H deformation); (d) 1348 cm-1 (in-plane OH bend); (e) 1282, 1250, 1108 and 951 cm-1 (C-O stretch); and (f) 842 cm-1 (C-C skeletal vibration. β-cyclodextrin showed characteristic absorption bands at (a) 3385 cm-1 (O-H stretch); (b) 2924 cm-1 (C-H stretch); (c) 1414 and 1368 cm-1 (C-H deformation vibration); (d) 1157, 1080 and 1028 cm-1 (C-O stretch); and (e) 947, 859, 755, 706 and 668 cm-1 (C-H deformation vibration). FT-IR spectrum of pure drug and excipients were displayed in Figure 1. Similarly, FT-IR spectrum of samples (8 to 22) showed characteristic absorption bands which were comparable with absorption bands of individual sample. Hence, there were no chemical instabilities in drug-drug/drug-excipient combinations.
Figure 1 .
FT-IR spectrum of pure drugs and excipients
The assay of the samples (8-22) were well within ±2% of control samples (1-4) and the %RSD of assay was less than 2% (Table 1). Hence, there were no chemical instabilities in drug-drug/drug-excipient combinations.
Conclusion
The combination of curcumin-piperine, curcumin-quercetin, curcumin-silibinin and the combination of other excipients with curcumin, piperine, quercetin and silibinin have not shown any significant physical and chemical instability. Hence, the study concludes that the curcumin, piperine, quercetin and silibinin is compatible with each other and with other excipients.
Conflict of Interest
The authors report no conflicts of interest.
References
- 1.Moorthi C, Kiran K, Manavalan R, Kathiresan K. Preparation and characterization of curcumin-piperine dual drug loaded nanoparticles. Asian Pac J Trop Biomed . 2012;2(11):841–8. doi: 10.1016/S2221-1691(12)60241-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moorthi C, Kathiresan K. Curcumin–Piperine/Curcumin–Quercetin/Curcumin–Silibinin dual drug-loaded nanoparticulate combination therapy: A novel approach to target and treat multidrug-resistant cancers. J Med Hypotheses Ideas . 2013;7(1):15–20. [Google Scholar]
- 3.Moorthi C, Kathiresan K. Fabrication of dual drug loaded polymeric nanosuspension: Incorporating analytical hierarchy process and data envelopment analysis in the selection of a suitable method. Int J Pharm Pharm Sci . 2013;5(2):499–504. [Google Scholar]
- 4.Moorthi C, Kathiresan K. A Step-by-step optimization process to fabricate narrow sized dual drug loaded polymeric nanoparticles using modified nanoprecipitation technique. Nano Biomed Eng . 2013;5(3):107–15. [Google Scholar]
- 5.Narang AS, Desai D, Badawy S. Impact of excipient interactions on solid dosage form stability. Pharm Res . 2012;29(10):2660–83. doi: 10.1007/s11095-012-0782-9. [DOI] [PubMed] [Google Scholar]
- 6.Liltorp K, Larsen TG, Willumsen B, Holm R. Solid state compatibility studies with tablet excipients using non thermal methods. J Pharm Biomed Anal . 2011;55(3):424–8. doi: 10.1016/j.jpba.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Moorthi C, Senthil kumar C, Mohan S, Kiran K, Kathiresan K. Application of validated RP-HPLC-PDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. J Pharm Res . 2013;7(3):224–9. [Google Scholar]
- 8.Moorthi C, Kathiresan K. Reversed phase high performance liquid chromatographic method for simultaneous estimation of curcumin and quercetin in pharmaceutical nanoformulation. Int J Pharm Pharm Sci . 2013;5(3):622–5. [Google Scholar]
- 9.Moorthi C, Kathiresan K. Simultaneous estimation of curcumin and silibinin using validated RP-HPLC-PDA method and its application in pharmaceutical nanoformulation. Int J Pharm Pharm Sci . 2013;5(3):475–8. [Google Scholar]