Abstract
Background
Hippocampal long term potentiation (LTP) is impaired following repeated morphine administration paired with a novel context. This procedure produces locomotor sensitization that can be abolished by blocking Ca2+-permeable 2-amino-3-(3-hydroxy-5-methyl-isoxazol-4-yl) propanoic acid receptors (AMPARs) in the hippocampus. However, the mechanisms underlying LTP impairment remain unclear. Here, we investigate the role of N-methyl-D-aspartate receptors (NMDARs), AMPARs and small conductance Ca2+-activated potassium type 2 (SK2) channels in LTP induction after context-dependent sensitization to morphine.
Methods
Mice were treated with saline or escalating doses of morphine (5, 8, 10 and 15 mg/kg) every 12 hours in a locomotor activity (LMA) chamber and a challenge dose of 5mg/kg morphine was given one week later. After the challenge the hippocampi were removed to assay phosphatase 2A (PP2A) activity, NMDAR and SK2 channel synaptic expression or to perform electrophysiological recordings.
Results
Impaired hippocampal LTP, which accompanied morphine-induced context-dependent sensitization, could not be restored by blocking Ca2+-permeable AMPARs. Context-dependent sensitization to morphine altered hippocampal NMDAR subunit composition and enhanced the SK2 channel-mediated negative feedback on NMDAR. Increased PP2A activity observed following context-dependent sensitization suggests that the potentiated SK2 channel effect on NMDAR was mediated by increased SK2 sensitivity to Ca2+. Finally, inhibition of SK2 channel or PP2A activity restored LTP.
Conclusions
Our studies demonstrate that the SK2 channel-NMDAR feedback loop plays a role in opiate-induced impairment of hippocampal plasticity and that the positive modulation of SK2 channels occurs via increases in PP2A activity. This provides further evidence that SK channels play a role in drug-induced plasticity.
Keywords: long term potentiation, hippocampus, PP2A, apamin, locomotor activity, SK2-NMDA loop
Introduction
Long lasting associations between drugs of abuse and the context in which they are taken often result in cravings leading to addiction relapse (1). Using rodent models of locomotor sensitization, a measure of how consistent use of a drug such as morphine produces enhanced craving (2), brain areas and molecular mechanisms involved in producing drug craving can be determined. Previously, we have demonstrated that context-dependent morphine treatment produces locomotor sensitization. This behavior is dependent on increased insertion of GluA1-containing (Ca2+-permeable) AMPARs in the hippocampus and is accompanied by impaired long-term potentiation (LTP) (3). Several studies demonstrate that chronic morphine alters hippocampal LTP and long-term depression (LTD) (4–10), however, none of the studies have established the mechanisms underlying this impairment.
Drugs of abuse target neural circuitry involved in learning and memory forming long lasting drug associations and alter the signaling machinery involved in synaptic plasticity (11, 12). Context-dependent morphine may particularly impact hippocampal plasticity as the hippocampus is responsible for processing contextual information (13–16). Moreover, morphine-induced impairments in LTP may contribute to relapse by making it difficult to unlearn associations that drive cravings. Therefore, understanding the mechanism underlying LTP impairment may reveal therapeutic targets that can be used to prevent relapse. LTP is induced by NMDAR mediated Ca2+ influx and insertion of new AMPARs resulting in enhanced synaptic signaling (17–19). Previous studies have reported increased Ca2+-permeable AMPARs in the nucleus accumbens and hippocampus following withdrawal from cocaine self-administration and context-dependent morphine, respectively (3, 20, 21). However, none of these studies examined the role of individual NMDAR subunits in terms of context-dependent morphine.
There are pathways involved in the modulation and maintenance of LTP. In particular, SK channels modulate NMDAR activity via a negative feedback loop where Ca2+ entry via NMDARs activates SK channels and K+ flux then hyperpolarizes the membrane resulting in NMDAR inactivation (22). Of the three types of SK, SK1 and SK2 subunits are expressed in CA1 pyramidal neurons (23). SK2 channel internalization from the postsynaptic density (PSD) is involved in LTP at CA1 synapses (24) and SK2 channel overexpression impacts synaptic plasticity and memory in the hippocampus (25). In terms of drug abuse, increases in neuronal excitability following alcohol withdrawal, mediated by apamin-sensitive SK channels, occur in ventral tegmental area dopaminergic neurons. Similarly, SK2 and SK3 channels in nucleus accumbens core neurons and SK2 channels in hippocampal CA1 pyramidal neurons, were shown to be involved in alcohol-associated plasticity (26–28). Additionally, SK channels regulate excitability after cocaine withdrawal and cannabinoid tolerance (29, 30). To date, no study has investigated SK2-mediated inhibition of NMDARs in terms of drugs of abuse or the role of SK2 channels in opiate dependence. Our studies are the first to demonstrate increased SK2 channel-mediated inhibition of NMDAR after context-dependent morphine, leading to LTP impairment in the hippocampus. These findings highlight a role for SK2 channels in the modulation of abnormal synaptic plasticity induced by opiates and introduce new targets for treatment strategies to promote opiate abstinence. They further emphasize SK channels as key players in drug-induced plasticity in the brain.
Materials and Methods
Animals and morphine treatment
Protocols were approved by the IACUC at Columbia University, according to NIH's Guide for the Care and Use of Laboratory Animals. Adult (6–8 weeks) male C57BL/6 mice (Harlan) were maintained on a 12 hour light/dark cycle. Mice randomly assigned into groups received morphine or saline paired with a novel context (a 41.5 × 41.5 × 30 cm LMA chamber equipped with photobeams (AccuScan Instruments)). Sixty minutes before morphine administration mice received an intraperitoneal (IP) injection of saline in the LMA chamber to habituate. Next, IP injections of saline or escalating doses of morphine (5, 8, 10 and 15 mg/kg) were given and LMA was recorded for 90 minutes. Doses were given every 12 hours. One week later mice received the 5mg/kg challenge dose in the LMA chamber. After measuring sensitization, hippocampi were harvested for electrophysiological or subcellular fractionation experiments.
Biochemistry
Standard western blotting procedures were used to determine receptor expression in homogenate and post synaptic density (PSD) fractions. PP2A activity was measured using the PP2A immunoprecipitation phosphatase assay kit (Millipore, Temecula, CA). Detailed methods can be found in Methods and Materials section of Supplement 1.
Electrophysiology
Mice were euthanized by decapitation and brains were submerged in an ice-cold cutting solution containing (mM) sucrose 175, MgCl2 7, KCl 2.5, NaH2PO4 1.2, CaCl2 0.5, NaHCO3 26, and glucose 25 (3). 350–400 μm brain slices were held in ACSF for 60 min then transferred to the recording chamber. Solutions were bubbled with 95% O2/5% CO2 continuously during dissection, incubation, and recording.
A concentric bipolar stimulating electrode placed in the Schaffer collateral (SC) pathway was used to evoke synaptic responses and a glass Ag/AgCl electrode filled with ACSF recorded extracellular field excitatory postsynaptic potentials (fEPSP) in the stratum radiatum of CA1. Initially, a stimulus–response curve was constructed by recording fEPSPs after stimulating the slice with increasing intensities. Next, baseline synaptic responses were recorded for 20 min. LTP was induced by two high-frequency stimulation trains (HFS; 100 Hz for 1 s) separated by a 20 s inter-train interval. During LTP recording, pulses at an intensity eliciting 50% of a maximum slope were used. In pharmacological experiments, either 100 μM NASPM, 50 nm okadaic acic or 100 nm apamin was applied after obtaining a stable recording and incubated for 10 min prior to applying HFS.
Visualized whole-cell patch-clamp recordings were made from CA1 or CA3 area with recording pipettes containing (mM): Cs-methylsulphonate 130, Na-methylsulphonate 10, EGTA 10, CaCl2 1, HEPES 10, QX-314·Cl 5, spermine tetrahydrochloride 0.1, Mg2+-ATP 2. Osmolarity was adjusted to 280–290 osmol l−1 and pH to 7.35–7.4 with CsOH. Open pipette resistance was 2–4 MΩ, and access resistance during recordings was <20 MΩ. Evoked excitatory postsynaptic currents (EPSCs) were recorded using Axopatch 200B, (Axon Instruments, Union City, CA). To isolate NMDA-mediated responses the experiments were performed in the presence of AMPA/kainate, GABAA and GABAB receptor antagonists: 20 μM 6,7-Dinitroquinoxaline-2,3-dione (DNQX), 100 μM picrotoxin (PTX) and 2μM (2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl](phenylmethyl)phosphinic acid hydrochloride (CGP55845) and the cells were held at + 40 mV. Medium after hyper polarization (mAHP) currents were recorded in CA1 neurons voltage clamped at holding potentials −55 mV following 80 ms voltage steps to −10 mV applied every 20 s, with recording pipettes containing (mM): potassium methylsulphate 150, KCl 10, HEPES 10, NaCl 4, Mg2ATP 4, and Na4GTP 0.4 (31). In these experiments, the mAHP current was studied in isolation following block of slow AHP currents by inclusion of 100 μM 8Br-cAMP in the electrode. The records were filtered at 1 kHz. Data acquisition and analysis were performed using Clampex and Clampfit 10 (Axon Instruments).
Data Analysis
Data was analyzed by unpaired t-test except in the LTP studies with NASPM, okadaic acid and apamin which were analyzed by one-way ANOVA followed by Tukey post hoc tests. All data are represented as mean ± S.E.M.
Results
Blockade of Ca2+-permeable AMPARs does not restore LTP impaired following morphine-induced context-dependent sensitization
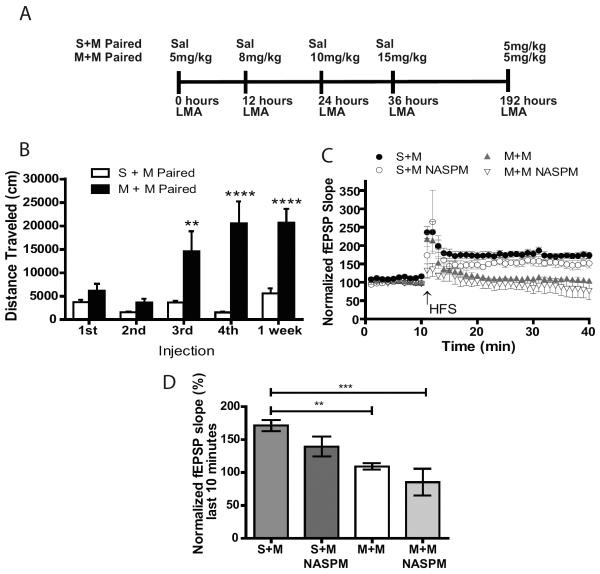
Previously, we demonstrated that increased Ca2+-permeable AMPARs in the hippocampus mediate context-dependent locomotor sensitization to morphine (3). Furthermore, we showed that LTP impairment in sensitized mice. Here, we examined whether LTP impairment at the SC-CA1 synapse is mediated by an increase in Ca2+-permeable AMPARs. In the morphine paired condition, where mice received morphine (5, 8, 10 and 15mg/kg) in the LMA chamber context every 12 hours (Figure 1A), mice had LMA increases after the 10 and 15 mg/kg doses (Figure 1B). In one week a 5mg/kg challenge of morphine produced LMA equal to the 15mg/kg dose one week earlier (Figure 1B). As described previously (3), in the morphine unpaired condition, where administration was not paired with the LMA chambers, mice showed no changes in LMA (Supplement: Figure S1) indicating that locomotor sensitization to morphine was context-dependent.
Figure 1.
Locomotor sensitization to morphine is associated with impaired LTP that cannot be reversed by blocking Ca2+ permeable AMPARs. A) LMA is measured after mice receive escalating doses of 5, 8, 10 and 15mg/kg of morphine or saline in a LMA chamber every 12 hours. One week later the morphine paired and saline paired mice are given a challenge dose of 5 mg/kg morphine (M+M and S+M, respectively). B) Paired M+M mice have an increase in LMA at the 10 and 15mg/kg dose of morphine compared to paired S+M (n=15–20/group, unpaired t-test, p<0.01 and p<0.0001, respectively). Significant increases in LMA occur when paired M+M mice received a low challenge dose of 5mg/kg morphine one week later (n=15–20/group, unpaired t-test p<0.0001). C) Summary plot of EPSP slope relative to baseline before and after HFS (2×- 100Hz, 1s duration separated by 20s). D) Bar graph representing normalized EPSP slope during the final 10 minutes of recording indicating that in paired M+M mice there is impaired LTP at the SC-CA1 synapses compared to saline paired mice (S+M). Impairment in LTP is not restored by bath application of Ca2+ permeable AMPAR antagonist 100μM NASPM (n=3–6/group, One-way ANOVA p<0.001, Tukey post-hoc test, S+M vs M+M p<0.001, S+M vs M+M NASPM p<0.0001). Error bars are mean ± SEM.
LTP is impaired in mice that received morphine paired with the LMA chamber (Figure 1C). As Ca2+-permeable AMPARs saturation at the synapse may occlude LTP induction, to determine if increases in Ca2+-permeable AMPARs underlie LTP impairment hippocampal slices were treated with the Ca2+-permeable AMPAR antagonist NASPM (100 μM) for 10 minutes prior to LTP induction. Blocking Ca2+-permeable AMPARs did not alter LTP (Figure 1 C– D) demonstrating that these receptors do not play a role in LTP impairment following context-dependent morphine.
Context-dependent administration of morphine affects NMDAR subunit composition
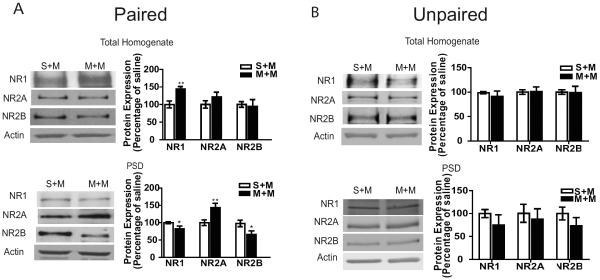
Next, we investigated NMDARs following context-dependent sensitization to morphine given their role in LTP induction (17–19). We performed subcellular fractionation on hippocampal tissue to determine NMDAR composition in total homogenate and PSD fractions. NMDAR subunit, NR1, NR2A and NR2B, expression was determined in mice that received morphine or saline paired with the LMA chambers followed by 5mg/kg morphine challenge one week later (M+M and S+M, respectively). In M+M mice there were increased NR1 levels in the homogenates compared to S+M mice and no changes in NR2A or NR2B (Figure 2A). In PSD fractions, there were decreased NR1 and NR2B levels and increased NR2A levels in M+M compared to S+M mice (Figure 2A). A decrease in the NR1 subunit suggests an overall decrease in NMDARs at the PSD since this subunit is necessary for NMDAR formation, while changes in NR2A and NR2B subunits imply a change in the configuration of NMDAR, such as an increase in NR2A-NR1 tetramers at the synapse.
Figure 2.
Expression of NMDAR subunits is altered in the total homogenate and PSD fractions from paired M+M mice, but not in the unpaired M+M group. A) (Left panel) Representative western blots of NMDAR subunits in total homogenate and PSD fractions from hippocampus of paired S+M and paired M+M mice. (Right panel) Summary graphs representing the % of saline treatment as the mean ±SEM. There is an increase in NR1 expression in the total homogenate fraction from paired M+M compared to paired S+M mice (n=10–15/group, p<0.001). In the PSD fraction there is a decrease in NR1, an increase in NR2A and a decrease in NR2B expression in paired M+M mice compared to S+M (n=10/group, p<0.05, n=10/group, p<0.01 and n=8/group, p<0.05 respectively). B) (Left panel) Representative western blots of NMDAR subunits in total homogenate and PSD fractions from hippocampus of unpaired S+M and unpaired M+M mice. (Right panels) Summary graphs representing the % of saline treatment as the mean ±SEM. There is no significant change in NMDAR subunits in the hippocampi from the unpaired M+M mice (n=4/group). Data were analyzed by comparing the morphine to saline groups using an unpaired t-test.
To determine if the alterations in NMDAR subunit expression are due to an association with context-dependent morphine or morphine treatment alone, subcellular fractionation was performed in a morphine unpaired group. Here, we found that in addition to not developing locomotor sensitization (Supplement: Figure S1) there was no change in NMDAR subunit expression in either total homogenate or PSD fractions (Figure 2B) indicating that changes in NMDAR subunits are driven by the combination of pairing escalating doses of morphine with a specific context. Additionally, a saline or an acute morphine challenge does not alter NMDAR subunit expression in the PSD (Supplement: Figure S2) indicating that NMDAR subunit composition is affected by both prior morphine associations and the sensitized response that occurs after the morphine challenge.
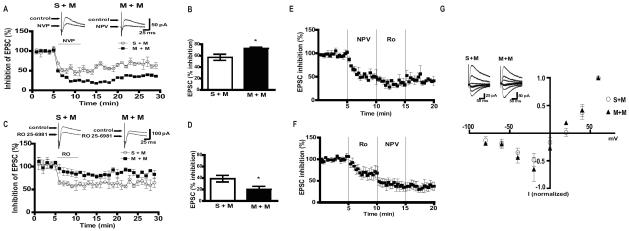
Next, we investigated functional changes in NMDARs, using whole-cell patch-clamp recordings from CA1 pyramidal neurons. Pharmacologically isolated NMDAR-mediated EPSCs were recorded from hippocampal slices in the presence of either 0.5μM RO 25-6981 or 0.4μM NVP (NR2B and NR2A antagonists, respectively). In support of our expression data, inhibition of NMDAR currents by NR2A antagonist NVP was increased in M+M compared to S+M mice (Figure 3A and B) indicating that there were more NR2A containing NMDARs. Furthermore, NMDAR currents from M+M mice were less sensitive to RO 25-6981 (Figure 3C–D) indicating that there were less NR2B containing NMDARs in M+M mice compared to S+M. Slow washout of both NVP and RO 25-6981 in slices was described before (32). To examine the specificity of each antagonist effect on NMDAR-EPSCs in CA1 neurons we performed occlusion experiments in naive mice. When NVP application was followed by RO 25-6981, initial 50% decrease in NMDAR-EPSCs produced by NVP was reduced by subsequent application of RO 25-6981 by additional 20% (Figure 3E). In a separate set of experiments, 30% inhibition by RO 25-6981 was further reduced after subsequent application of NVP by 30 % (Figure 3F). Maximal inhibition by both antagonists was obtained within five minutes of application (Figure 3 A–B). These experiments demonstrated that the occlusion is minimal, and therefore, the effect of each blocker reflects inhibition of respective subunit). To determine if this effect was regionally specific we examined the effects of NVP and RO 25-6981 on NMDAR-EPSCs in CA3 pyramidal neurons. We found no significant difference between S+M and M+M (Supplement: Figure S4A–B). Although in CA1 there were significant changes in sensitivity to NR2A and NR2B antagonists, current-voltage relationships (I-V curves) of NMDAR-EPSCs were not different in M+M verses S+M neurons (Figure 3G). This result was consistent with the observation that single cell deletion of either NR2A or NR2B subunits did not affect I-V curves of NMDAR EPSCs (33).
Figure 3.
NMDAR currents are altered in mice that display context-dependent sensitization to morphine. A) (Upper panel) Representative traces and (lower panel) time course of % inhibition of NMDAR EPSCs by NVP in CA1 pyramidal cells from paired S+M and paired M+M mice. B) Summary of % inhibition of EPSCs indicating that neurons recorded in the slices from M+M mice are more sensitive to NR2A subunit antagonist NVP (n=5/group, p<0.05). C) (Upper panel) Representative traces and (lower panel) time course of % inhibition of NMDAR EPSCs by RO 25-6921. D) Summary of % inhibition of EPSCs indicating that neurons from paired M+M mice are less sensitive to RO 25-6921 compared to S+M (n=5/group, p<0.05). E) Average time course of NMDA-EPSCs during the application of NVP followed by RO 25-6981(n=4/group). F) Average time course of NMDA-EPSCs during the application of RO 25-6981 followed by NVP (n=4/group). G) I–V curves and representative traces of NMDAR EPSCs from S+M and M+M mice (n=4–5/group). Data were analyzed using unpaired t-test.
NMDAR subunit composition regulates SK2 channel-mediated mAHP currents
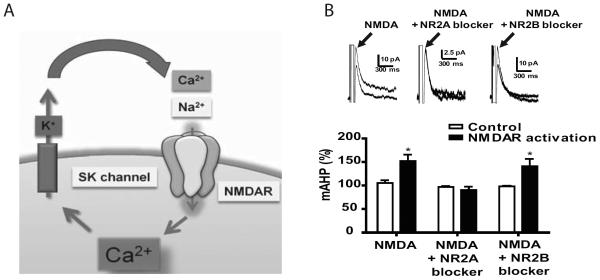
Our data indicate that context-dependent sensitization to morphine triggers an increase in NR2A-containing NMDARs. However, NR2A has been implicated in LTP induction and not LTP impairment (34, 35). One possibility is that other proteins that modulate NMDARs may impair LTP following context-dependent morphine. Previous studies have demonstrated that dendritic NMDARs are regulated by SK2 channels in the hippocampus via a negative feedback loop (Figure 4A; (22)) and that SK2 channels are involved in LTP induction (24). Our current studies demonstrate that NR2A subunits are increased after context-dependent sensitization. However, no studies have examined whether changes in NMDAR composition impacts SK2 channel function. To determine if NR2A and NR2B subunits differentially activate SK2 channels, we measured mAHP currents in the presence of NMDA plus NR2A antagonist NVP or NR2B antagonist RO 25-6981. In the presence of NMDA alone mAHP currents were enhanced (Figure 4B, p<0.05) confirming that NMDARs activate SK2 channels. This enhancement was blocked by NPV and remained intact in the presence of Ro 25-6981 (Figure 4B, p<0.05). Therefore, our data demonstrate that SK2 channels were preferentially activated by NR2A containing NMDARs.
Figure 4.
Small conductance Ca2+dependent K+ channel is activated by NR2A-containing NMDAR. A) Schematic of NMDAR-SK2 receptor interactions. Ca2+ entry through NMDARs opens SK2 channels allowing K+ to hyperpolarize the membrane thus inactivating NMDARs. B) (Top Panel) Representative traces of mAHP currents in the presence of NMDA, NMDA plus NR2A antagonist and NMDA plus NR2B antagonist. (Bottom panel) Summary graph indicating that mAHP currents are enhanced by NMDA (unpaired t-test p<0.05), the effect that can be blocked by NR2A, but not NR2B subunit inhibition (unpaired t-test p<0.05, n= 4–5/group).
SK2-mediated negative feedback on NMDAR is enhanced following context-dependent sensitization
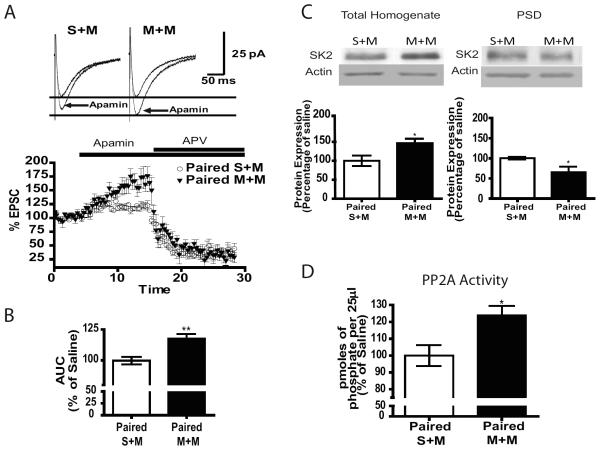
We investigated whether context-dependent sensitization to morphine alters the SK2-NMDAR feedback loop. NMDAR-EPSCs were evoked in CA1 pyramidal cells by stimulation of SCs and were pharmacologically isolated by applying AMPA/kainate, GABAA and GABAB receptor antagonists. Bath application of SK2 channel antagonist apamin (100 nm) potentiated NMDAR-mediated synaptic transmission which is consistent with previous studies (22). In M+M mice this effect was significantly increased compared S+M (Figure 5A–B). The summary plot shows the effect of apamin, and the specific NMDAR antagonist APV (50 μM) confirms SK2-NMDAR feedback loop activation (Figure 5A), indicating potentiated negative feedback of SK2 channel on NMDAR function following context-dependent sensitization to morphine.
Figure 5.
SK2 channel-mediated down-regulation of NMDAR function is increased in paired M+M mice. A) (Top Panel) Representative traces showing that apamin-induced potentiation of NMDAR-mediated EPSCs is increased in paired M+M versus S+M. (Bottom panel) Summary plot of EPSC amplitude compared to baseline after application of apamin followed by NMDAR antagonist APV. B) Bar graph representing the area under the curve in paired S+M versus M+M cells showing a significant increase in the M+M group (n=4/group, unpaired t-test, p<0.01). C) (Top panel) Representative western blots of the SK2 channel and PP2A in total homogenate and PSD fractions from hippocampus of S+M and M+M mice. (Bottom panel) Summary graphs representing the mean ±SEM. Mice that receive morphine paired with context followed by a morphine challenge (M+M) have increased SK2 channels in the total homogenate fraction (n=6–7/group, p<0.05) and decreased in the PSD fraction (n=5–6/group, p<0.01). D) Graph demonstrating increased PP2A activity in the paired M+M group. (n=6–8/group, p<0.05). Data analyzed using unpaired t-tests.
To determine whether the increased effect of SK2 channel on NMDAR function is related to increased SK2 expression at the synapse, we performed subcellular fractionation. In the homogenates of M+M mice SK2 channel expression was increased compared to S+M (Figure 5C). However, acute morphine decreased SK2 expression (S+S vs S+M) and there was no significant difference in SK2 expression between the S+S and M+M treated groups (Supplement: Figure S4A). Therefore, the increased expression between M+M and S+M groups may reflect a decrease caused by acute morphine. In fact, in the PSD fraction SK2 levels are decreased in M+M compared S+M mice (Figure 5C). Morphine treatment alone does not change the expression of SK2 channels since there were no changes in the unpaired group (Supplement: Figure S4B). These data indicate that enhancement of the SK2 channel effect on NMDAR-mediated synaptic transmission was not driven by increases in the expression of SK2 channels.
It has been shown that SK2 channel Ca2+ sensitivity is modulated by phosphorylation and dephosphorylation by casein kinase 2 and PP2A, respectively. In the latter case, dephosphorylation by PP2A increases SK2 channels Ca2+ sensitivity and therefore increases SK2 channel activation (36, 37). To determine whether SK2 channels may be overactivated via increased dephosphorylation by PP2A, we investigated PP2A activity in M+M and S+M mice. Following context-dependent sensitization to morphine PP2A activity in hippocampal extracts was increased (Figure 5D, n=6–8/group, p<0.05). Therefore, dephosphorylation by PP2A may enhance SK2 channel activity in the hippocampus of M+M mice.
SK2 channels mediate LTP impairment following context-dependent sensitization via increased PP2A activity
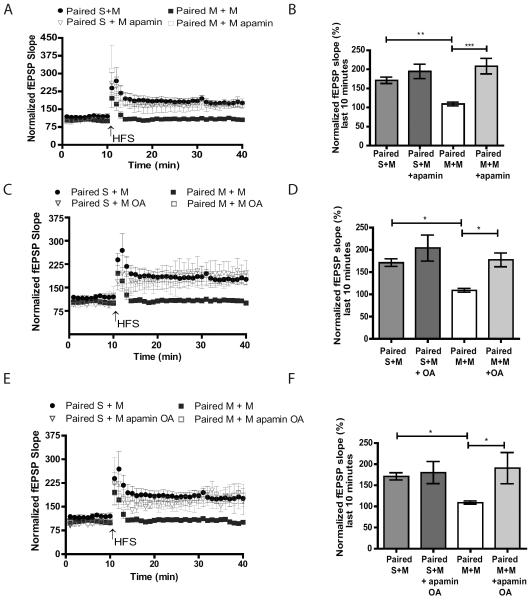
SK2 channel overexpression impairs synaptic plasticity and hippocampal memory formation (25). We have demonstrated that the SK2 channel modulation of NMDAR currents and PP2A activity are increased after context-dependent sensitization to morphine. These data suggest that SK2 channel activity may underlie LTP impairment in CA1 observed in our experiments. LTP was investigated following ten minute incubation with SK2 channel antagonist apamin (100 nM), PP2A inhibitor okadaic acid (OA, 50nM) or in the presence of both drugs in hippocampal slices from M+M and S+M mice. LTP in M+M slices was restored by apamin (Figure 6A–B), OA (Figure 6B– C) or apamin plus OA (Figure 6E– F) treatment. The % increase from baseline after HFS was similar in the three groups (208.8± 20.3 % in the M+M apamin group, 177.6±15.52 % in the M+M OA group, and 190.8±36.88% in the M+M apamin plus OA group). This suggests that OA and apamin are likely to be acting on the same target. Therefore, we conclude that enhanced SK2 channel control over NMDAR contributes to LTP impairment via increased PP2A activity following context-dependent sensitization to morphine.
Figure 6.
Both SK2 and PP2A antagonists restore LTP impaired after context-dependent sensitization to morphine. A, C and E) Hippocampal slices were incubated with either apamin (A), OA (B) or apamin plus OA (E). Plot of fEPSP slope relative to baseline before and after HFS (2×- 100Hz, 1s duration separated by 20s). B, D and F) Bar graph representing the average of the normalized fEPSPs 20 to 30 minutes after HFS indicating that mice that display increases in LMA (M+M) have impaired LTP that is restored after bath application of apamin (B) n=3–6/group, One-way ANOVA p<0.0001, Tukey post-hoc test, S+M vs M+M p<0.001 , M+M vs M+M apamin p<0.0001), OA (D) n=4–7/group, One-way ANOVA p<0.01, Tukey post-hoc test, S+M vs M+M p<0.05, M+M vs M+M OA p<0.05) or apamin plus okadiac acid (F) n=3–7/group, One-way ANOVA p<0.01, Tukey post-hoc test, S+M vs M+M p<0.05, M+M vs M+M apamin plus OA p<0.05).
Discussion
The development of drug craving due to environmental cues is driven by neuroplastic changes in brain areas that process drug cues and reward (38). The current study describes a novel role for NMDAR subunits and SK2 channels in hippocampal LTP impairment in mice that express context-dependent sensitization to morphine. Morphine induced LTP impairment may have consequences in drug dependence and craving since contextual driven memories have been associated with drug craving in abstinent opiate abusers (1). Here, we demonstrate that there is an increase in NR2A-containing NMDARs in the hippocampus observed only after context dependent-sensitization to morphine. Additionally, we show that NR2A-containing NMDARs activate SK2-mediated mAHP currents, and that negative feedback regulation by SK2 channels on NMDAR is enhanced after context-dependent sensitization to morphine. Moreover, PP2A activity is increased which may contribute to enhanced SK2 channel activity. Finally, SK2 channel and PP2A antagonists restore hippocampal LTP in the slices from morphine sensitized mice.
Previously (3), we demonstrated that locomotor sensitization is not a general effect of morphine alone, but only occurs when morphine administration is paired with a novel context. In fact, others have reported that contextual cues such as olfactory and auditory cues can determine the degree of locomotor sensitization resulting from repeated morphine treatment (39, 40). The present studies further this concept by demonstrating that context-dependent sensitization to morphine alters SK2 channels and NMDAR subunits in the hippocampus, which does not occur when the same morphine treatments are not paired with a novel context. Additionally, our previous study showed a similar effect for the AMPAR GluA1 subunit which was increased when morphine was paired with LMA chambers and unaltered in the unpaired group (3).
Previously, we have shown that overexpression of a GluA1 S845 mutant in the hippocampus attenuates locomotor sensitization, indicating that GluA1 trafficking plays a role in the development of locomotor sensitization. However, in the present study inhibition of Ca2+-permeable AMPARs does not restore the LTP impairment observed following sensitization. In an attempt to elucidate the mechanisms underlying LTP impairment, we implemented studies to characterize NMDAR subunit expression and function in the hippocampus following context-dependent sensitization to morphine and found that NR1 and NR2B subunits are decreased and NR2A subunits increased. Since NR2A subunits are more highly expressed in the adult hippocampus (41–43) this increase may reflect decreases in the number of NR2B-NR2B homomers and NR2A-NR2B heteromers and an increase in NR2A-NR2A homomers (44). Additionally, increases in NR2A subunits may contribute to the development of locomotor sensitization since NR2A inhibition has been reported to blunt locomotor sensitization to cocaine (45). Our whole-cell patch-clamp experiments confirmed an increase in NR2A contribution to EPSCs in M+M mice and indicated that this increase occurred in the CA1 area of hippocampus, but not in the CA3.
Previous studies have shown that NR2A and NR2B subunits play different roles in the mechanisms underlying neuroplasticity with the NR2A subunit being critical for LTP induction (34, 35). Based on this evidence an increase in NR2A containing receptors found in the present study would not impair LTP induction, therefore, it appears that additional mechanisms are playing a role in the observed LTP impairment.
SK2 channels modulate learning and memory such that consequent to LTP induction, SK2 channels are removed from the PSD in a PKA dependent manner (24) and SK2 overexpression impairs spatial learning and LTP (25). It has been shown that an increase in PKA activity occurs in the hippocampus after chronic morphine treatment (7) and in our previous studies we have demonstrated an increase in GluA1 phosphorylation at the PKA site (3) following context-dependent locomotor sensitization. PKA dependent GluA1 and SK2 phosphorylation has opposing effects, such that PKA-dependent phosphorylation increases GluA1exocytosis and promotes SK2 endocytosis (46). Our previous studies demonstrated that context-dependent sensitization increases GluA1 trafficking to the PSD (3) and our current studies indicate that SK2 channels are removed from the PSD. Therefore, it is likely that escalating doses of morphine result in increased PKA activity leading to the insertion of GluA1 as well as removal of SK2 channels from the PSD.
SK2 channels form a negative feedback loop with NMDARs and increased SK2 channel activation regulates LTP induction (22). In the present study, we find increased SK2 regulation of NMDARs following context-dependent morphine resulting in LTP impairment. This increase occurs despite a decrease in SK2 expression in the PSD. One possible explanation for this discrepancy is that the patch-clamp experiments were performed in the CA1 while the whole hippocampus was used to measure SK2 expression, so it is possible that SK2 channels may be increased at the PSD in the CA1 pyramidal cell layer of the hippocampus since small changes in one particular cell type would not be detected by western blot analysis. Additionally, these differences may be explained by the fact that SK2 channel activation can be modulated by kinases and phosphatases that modulate the SK2 channel's Ca2+ sensitivity. While phosphorylation by CK2 results in SK2 channel inactivation by decreasing its Ca2+ sensitivity, dephosphorylation of SK2 channel by PP2A increases its Ca2+ sensitivity thus increasing its activity (37). Previous studies have shown an increase in phosphatase activity after chronic morphine treatment that was blocked by a PP2A inhibitor (47). Therefore, increased PP2A activity would result in increased activation of SK2 channels. We show that PP2A activity is increased following context-dependent sensitization to morphine and that blocking PP2A with OA restores LTP to normal levels. Moreover, LTP impairment is restored by treatment with the SK2 antagonist apamin further demonstrating that context-dependent sensitization to morphine overactivates the SK2-NMDAR feedback via increased PP2A activity and is responsible for LTP impairment. This idea is further corroborated by our data showing that the blockade of SK2 channels with apamin fully restores LTP to the extent observed in saline paired controls.
To test whether overall induction of synaptic plasticity was impaired after context-dependent morphine we performed additional experiments where we applied metabotropic glutamate receptor (mGluR) agonist DHPG (50 μM) known to induce LTD in hippocampal CA1. We found that mGluR1/5 dependent LTD was not impaired, but was significantly reduced after context-dependent morphine (Supplement: Figure S5.) This could be explained by internalization of Ca2+-impermeable AMPARs that were reported previously in morphine-sensitized mice (3). Additionally, it could be triggered by Ca2+-dependent PKC activation induced by mGluRs. Overactivation of SK2 channels could also contribute to regulation of intracellular Ca2+ levels via negative feedback on L-type voltage-gated Ca2+ channels (48, 49). Further studies, including Ca2+ imaging, are needed to fully characterize underlying changes in intracellular Ca2+.
Conclusion
Long lasting associations between drugs of abuse and the context in which they are taken often result in cues that lead to drug craving and ultimately relapse. Our research attempts to define the key signaling components that are critical for synaptic plasticity and that are altered after associations between morphine and context are formed. We have demonstrated that context-dependent sensitization to morphine enhances the feedback effect of SK2- channels on NMDARs in the hippocampus. This mechanism has been shown to modulate synaptic plasticity and therefore is a potential target to prevent abnormal opiate-induced plasticity. These alterations in synaptic plasticity may be the first step in a cascade leading to long-lasting modifications in brain function that could consequently control drug-related behaviors. In fact, our data demonstrates that inhibition of the SK2 channel function can restore LTP impairment that occurs following context-dependent sensitization to morphine. By understanding these mechanisms therapies can be developed to prevent drug relapse triggered by contextual cues associated with previous drug use.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DA025036 and DA027460 (J.A.M.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interests The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, et al. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001;158:1680–6. doi: 10.1176/appi.ajp.158.10.1680. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Portugal GS, Fakira AK, Melyan Z, Neve R, Lee HT, et al. Hippocampal GluA1-containing AMPA receptors mediate context-dependent sensitization to morphine. J Neurosci. 2011;31:16279–91. doi: 10.1523/JNEUROSCI.3835-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bao G, Kang L, Li H, Li Y, Pu L, Xia P, et al. Morphine and heroin differentially modulate in vivo hippocampal LTP in opiate-dependent rat. Neuropsychopharmacology. 2007;32:1738–49. doi: 10.1038/sj.npp.1301308. [DOI] [PubMed] [Google Scholar]
- 5.Hosseinmardi N, Fathollahi Y, Naghdi N, Javan M. Theta pulse stimulation: a natural stimulus pattern can trigger long-term depression but fails to reverse long-term potentiation in morphine withdrawn hippocampus area CA1. Brain Res. 2009;1296:1–14. doi: 10.1016/j.brainres.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Pourmotabbed A, Motamedi F, Fathollahi Y, Mansouri FA, Semnanian S. Involvement of NMDA receptors and voltage-dependent calcium channels on augmentation of long-term potentiation in hippocampal CA1 area of morphine dependent rats. Brain Res. 1998;804:125–34. doi: 10.1016/s0006-8993(98)00676-3. [DOI] [PubMed] [Google Scholar]
- 7.Pu L, Bao GB, Xu NJ, Ma L, Pei G. Hippocampal long-term potentiation is reduced by chronic opiate treatment and can be restored by re-exposure to opiates. J Neurosci. 2002;22:1914–21. doi: 10.1523/JNEUROSCI.22-05-01914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salmanzadeh F, Fathollahi Y, Semnanian S, Shafizadeh M, Kazemnejad A. Dependence on morphine leads to a prominent sharing among the different mechanisms of long-term potentiation in the CA1 region of rat hippocampus. Brain Res. 2003;963:93–100. doi: 10.1016/s0006-8993(02)03947-1. [DOI] [PubMed] [Google Scholar]
- 9.Velisek L, Stanton PK, Moshe SL, Vathy I. Prenatal morphine exposure enhances seizure susceptibility but suppresses long-term potentiation in the limbic system of adult male rats. Brain Res. 2000;869:186–93. doi: 10.1016/s0006-8993(00)02384-2. [DOI] [PubMed] [Google Scholar]
- 10.Yang SN, Huang LT, Wang CL, Chen WF, Yang CH, Lin SZ, et al. Prenatal administration of morphine decreases CREBSerine-133 phosphorylation and synaptic plasticity range mediated by glutamatergic transmission in the hippocampal CA1 area of cognitive-deficient rat offspring. Hippocampus. 2003;13:915–21. doi: 10.1002/hipo.10137. [DOI] [PubMed] [Google Scholar]
- 11.Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;33:267–76. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 13.Becker JT, Walker JA, Olton DS. Neuroanatomical bases of spatial memory. Brain Res. 1980;200:307–20. doi: 10.1016/0006-8993(80)90922-1. [DOI] [PubMed] [Google Scholar]
- 14.Miller VM, Best PJ. Spatial correlates of hippocampal unit activity are altered by lesions of the fornix and endorhinal cortex. Brain Res. 1980;194:311–23. doi: 10.1016/0006-8993(80)91214-7. [DOI] [PubMed] [Google Scholar]
- 15.Olton DS, Branch M, Best PJ. Spatial correlates of hippocampal unit activity. Exp Neurol. 1978;58:387–409. doi: 10.1016/0014-4886(78)90096-1. [DOI] [PubMed] [Google Scholar]
- 16.Olton DS, Walker JA, Gage FH. Hippocampal connections and spatial discrimination. Brain Res. 1978;139:295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- 17.Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–8. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- 18.Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–7. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- 19.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, et al. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–6. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 20.Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, et al. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–21. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011;31:5737–43. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–9. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 23.Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–69. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–7. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, et al. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–53. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopf FW, Bowers MS, Chang SJ, Chen BT, Martin M, Seif T, et al. Reduced nucleus accumbens SK channel activity enhances alcohol seeking during abstinence. Neuron. 2010;65:682–94. doi: 10.1016/j.neuron.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 28.Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol Psychiatry. 2011;69:625–32. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, et al. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–31. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nazzaro C, Greco B, Cerovic M, Baxter P, Rubino T, Trusel M, et al. SK channel modulation rescues striatal plasticity and control over habit in cannabinoid tolerance. Nat Neurosci. 2012;15:284–93. doi: 10.1038/nn.3022. [DOI] [PubMed] [Google Scholar]
- 31.Melyan Z, Wheal HV, Lancaster B. Metabotropic-mediated kainate receptor regulation of IsAHP and excitability in pyramidal cells. Neuron. 2002;34:107–14. doi: 10.1016/s0896-6273(02)00624-4. [DOI] [PubMed] [Google Scholar]
- 32.Wu LJ, Xu H, Ren M, Cao X, Zhuo M. Pharmacological isolation of postsynaptic currents mediated by NR2A- and NR2B-containing NMDA receptors in the anterior cingulate cortex. Mol Pain. 2007;3:11. doi: 10.1186/1744-8069-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll RA. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu SB, Ma L, Guo HJ, Feng B, Guo YY, Li XQ, et al. Gentiopicroside Attenuates Morphine Rewarding Effect through Downregulation of GluN2B Receptors in Nucleus Accumbens. CNS Neurosci Ther. 2012;18:652–8. doi: 10.1111/j.1755-5949.2012.00338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E, et al. Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci. 2004;24:7821–8. doi: 10.1523/JNEUROSCI.1697-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–76. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bildl W, Strassmaier T, Thurm H, Andersen J, Eble S, Oliver D, et al. Protein kinase CK2 is coassembled with small conductance Ca(2+)-activated K+ channels and regulates channel gating. Neuron. 2004;43:847–58. doi: 10.1016/j.neuron.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 38.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atalla A, Kuschinsky K. Effects of blockade of glutamate NMDA receptors or of NO synthase on the development or the expression of associative or non-associative sensitization to locomotor activation by morphine. J Neural Transm. 2006;113:1–10. doi: 10.1007/s00702-005-0298-0. [DOI] [PubMed] [Google Scholar]
- 40.Niu H, Zheng Y, Rizak JD, Fan Y, Huang W, Ma Y, et al. The effects of lesion of the olfactory epithelium on morphine-induced sensitization and conditioned place preference in mice. Behav Brain Res. 2012;233:71–8. doi: 10.1016/j.bbr.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 41.Foster KA, McLaughlin N, Edbauer D, Phillips M, Bolton A, Constantine-Paton M, et al. Distinct roles of NR2A and NR2B cytoplasmic tails in long-term potentiation. J Neurosci. 2010;30:2676–85. doi: 10.1523/JNEUROSCI.4022-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel A, Fritschy JM, Mohler H, Benke D. NMDA receptor heterogeneity during postnatal development of the rat brain: differential expression of the NR2A, NR2B, and NR2C subunit proteins. J Neurochem. 1997;68:469–78. doi: 10.1046/j.1471-4159.1997.68020469.x. [DOI] [PubMed] [Google Scholar]
- 43.Sanz-Clemente A, Matta JA, Isaac JT, Roche KW. Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron. 2010;67:984–96. doi: 10.1016/j.neuron.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Hallaq RA, Conrads TP, Veenstra TD, Wenthold RJ. NMDA di-heteromeric receptor populations and associated proteins in rat hippocampus. J Neurosci. 2007;27:8334–43. doi: 10.1523/JNEUROSCI.2155-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schumann J, Matzner H, Michaeli A, Yaka R. NR2A/B-containing NMDA receptors mediate cocaine-induced synaptic plasticity in the VTA and cocaine psychomotor sensitization. Neurosci Lett. 2009;461:159–62. doi: 10.1016/j.neulet.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Lin MT, Lujan R, Watanabe M, Frerking M, Maylie J, Adelman JP. Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors. J Neurosci. 2010;30:11726–34. doi: 10.1523/JNEUROSCI.1411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gabra BH, Bailey CP, Kelly E, Sanders AV, Henderson G, Smith FL, et al. Evidence for an important role of protein phosphatases in the mechanism of morphine tolerance. Brain Res. 2007;1159:86–93. doi: 10.1016/j.brainres.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu L, Zhang Q, Timofeyev V, Zhang Z, Young JN, Shin HS, et al. Molecular coupling of a Ca2+-activated K+ channel to L-type Ca2+ channels via alpha-actinin2. Circ Res. 2007;100:112–20. doi: 10.1161/01.RES.0000253095.44186.72. [DOI] [PubMed] [Google Scholar]
- 49.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–5. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.