Abstract
Bryant and colleagues follow the development of Glybera (alipogene tiparvovec), the first gene therapy product approved in the European Union, from early preclinical studies through the approval process. They review key data from human and animal studies with an emphasis on issues that will be critical to other gene therapy products. The article concludes with an analysis of the complex review process that eventually led to Glybera's approval.
Introduction
Development of the first commercially-approved gene therapy product in the West represents a remarkable medical achievement. The product Glybera, or alipogene tiparvovec, is based on an adeno-associated viral (AAV) vector to replace the gene responsible for the expression of lipoprotein lipase (LPL), which is deficient in some patients who have extremely elevated levels of serum triglycerides causing recurrent and life-threatening pancreatitis. Glybera received marketing authorization for a subset of patients with LPL deficiency from the European Commission (EC) in November 2012. A summary of the early preclinical research forming the basis for the clinical development of this product was reviewed by the principal scientists in Human Gene Therapy (Kastelein et al., 2013).
The goal of this review is to chronicle the clinical development of Glybera from proof-of-concept in animal models through review and eventual authorization by the European Medicines Agency (EMA). As is the case with most pioneering efforts, the path to success was not easy and was associated with multiple twists and turns, rejections, and iterations. Our review and critique of this process was made possible because of the incredible transparency that the scientists, physicians, and regulators provided about the preclinical and clinical data and the deliberations of the EMA and its associated committees. The research teams published virtually every relevant preclinical and clinical experiment in peer-reviewed journals, and the EMA made available detailed summaries of its reviews. This review provides background about the disease and the vector, followed by summaries and critiques about the preclinical and clinical studies and the multiple iterations of regulatory reviews.
The Disease
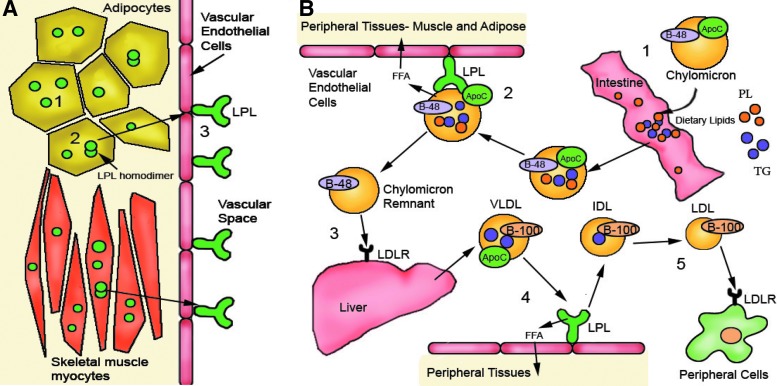
Familial lipoprotein lipase deficiency (LPLD) is a rare, autosomal recessive disorder affecting about 1 person per million (Brunzell, 1999). The underlying pathophysiology of LPLD is a lack of functionally active lipoprotein lipase (LPL), which plays an important role in triglyceride (TG), chylomicron (CM), and very low–density lipoprotein (VLDL) metabolism (Fig. 1). LPL is produced primarily in skeletal muscle and adipose tissue, where it is secreted as a homodimer. This active homodimer is then transported across the vascular endothelium and immobilized on the luminal surface of blood vessels. In the vascular lumen, LPL interacts with circulating chylomicrons, the triglyceride-rich particles that carry lipids absorbed by the intestine. LPL converts TGs found in the core of chylomicrons into free fatty acids (FFAs) that can be taken up and used by peripheral tissues. The chylomicron remnant is then endocytosed by hepatocytes via interaction with apolipoprotein E on the chylomicron surface. The chylomicron remnant is subsequently salvaged and repackaged into VLDL by the liver and secreted into the plasma to supply endogenously-synthesized lipids to the peripheral tissues. LPL then participates in the conversion of VLDL to LDL in the plasma (Mead et al., 2002). Without LPL, chylomicrons cannot be depleted of their dietary fats, leading to severe hypertriglyceridemia and hyperchylomicronemia. These lipids then accumulate and lead to pathology in various tissues, although the exact relationship between lipid accumulation and disease presentation has not been established.
FIG. 1.
Production and function of lipoprotein lipase (LPL). (A) LPL is synthesized primarily in adipocytes, but also in skeletal muscle, heart, lung, liver, and other organs to a lesser degree (1). LPL forms homodimers in the cell before being secreted (2) and translocated to the vascular endothelial surface. LPL is then anchored to heparan sulfate proteoglycans that line the vascular endothelial cell surface (3). (B) Dietary fats entering the intestine are packaged into chylomicrons in enterocytes (1). The chylomicrons are then secreted into the lymphatic space. After finding their way back into the blood, chylomicrons encounter LPL anchored to the vascular endothelial surface. ApoCII on the surface of the chylomicron serves as a cofactor for LPL (2), allowing LPL to hydrolyze the triglyceride (TG) core of the chylomicron to produce free fatty acids (FFAs). These FFAs can then be taken up by peripheral tissues. The chylomicron remnant, depleted of TG, is taken up by the liver via low-density lipoprotein receptor (LDLR) binding to ApoE (3) and then transformed into very low–density lipoprotein (VLDL). At this point, ApoB-48 is exchanged for ApoB-100. VLDL is then secreted into the plasma, where it can contact LPL again. On this interaction, LPL hydrolyzes the VLDL TG core and produces FFA (4), an essential step in the conversion to intermediate-density lipoprotein (IDL). IDL can then be further converted into low-density lipoprotein (LDL), which can be taken up by peripheral cells via LDLR for use by the tissues (5). ApoC, apolipoprotein CII; B-48, B-100, apolipoproteins B-48 and B-100; LDLR, low-density lipoprotein receptor; PL, phospholipid.
The first symptoms of LPLD present during childhood as severe abdominal pain or failure to thrive, and patients may exhibit eruptive xanthomas, lipemia retinalis, and hepatosplenomegaly. The most severe manifestation of LPLD is acute pancreatitis, which is the primary cause of morbidity and mortality in these patients (Frossard et al., 2008). Recurrent pancreatitis can lead to exocrine pancreatic insufficiency and diabetes. Despite the presence of these outward symptoms, the diagnosis of LPLD is dependent on molecular sequencing of the gene, as the phenotype cannot be clinically distinguished from other causes of hyperchylomicronemia. The currently accepted treatment for LPLD includes severe dietary restriction of fat intake to 10–15% of total daily calories and supplementation with medium-chain triglycerides, which are metabolized through a chylomicron-independent pathway. Notably, even with strict compliance to the diet and close monitoring by clinicians, TG levels rarely drop out of the range (greater than 1000–2000 mg/dl) that is considered to confer an elevated risk of pancreatitis (Yadav and Pitchumoni, 2003; Gaudet et al., 2010). Therefore, there is a clear need for an effective treatment for LPLD.
The Vector
A naturally-occurring truncated human LPL variant, LPLS447X, was selected as a transgene because of its higher activity in vivo (Ross et al., 2005). To deliver the 1.9-kb LPLS447X transgene (Gotoda et al., 1989), an adeno-associated viral (AAV) vector was selected. Adeno-associated viruses, first described in 1965 (Atchison et al., 1965), are nonpathogenic, single-stranded DNA viruses with genomes of approximately 4.7 kb. To date, dozens of AAVs have been isolated from a variety of mammalian species (Asokan et al., 2012). Of these, AAV2, a common human isolate, has been the most frequently used vector for research purposes. As a result, it is also the most characterized AAV serotype regarding its safety profile as a vehicle for transgene delivery in human gene therapy studies.
The AAV genome is composed of two inverted terminal repeats (ITRs) that flank two viral genes, rep and cap. Whereas the ITRs are required, cis-acting elements, the nonstructural rep-encoded proteins, and the structural cap-encoded proteins, responsible for viral replication and tropism, respectively, can be supplied in trans during virus production (Asokan et al., 2012). This not only allows for a large portion of the AAV genome to be replaced with a therapeutic transgene but also the packaging of an AAV2-derived genome by capsid (Cap) proteins from other serotypes, resulting in AAV2 with altered tropism and immunogenicity. The recombinant vector used for this study, AAV1-LPLS447X, was produced in this way, using Cap proteins from AAV serotype 1 and ITRs from AAV2 (Rip et al., 2005).
The first infectious clone of AAV1 was characterized in 1999 in Jim Wilson's laboratory by then-postdoctoral fellow Weidong Xiao in an effort to develop AAV vectors with improved performance over that achieved with AAV2 (Xiao et al., 1999). The frequency of neutralizing antibodies (NAbs) to AAV1 in normal human subjects was less than that observed with AAV2. In addition to the lower seroprevalence, AAV1 vectors transduce skeletal muscle with substantially higher efficiency than is observed with AAV2. Given that skeletal muscle is a site for physiological expression of LPL, the efficiency of transduction in this tissue made AAV1 an attractive vector for delivery of the LPL transgene (Rip et al., 2005).
Preclinical
Preclinical models
Two LPL-deficient animal models were used for preclinical testing of AAV1-LPLS447X: an LPL knockout mouse and a naturally-occurring feline model carrying a mutant allele encoding a catalytically inactive LPL protein. A summary of the salient features of these animal models is provided in Table 1. Development of the LPL knockout mouse model was complicated by the fact that complete LPL knockout is fatal in the neonatal period in this species. Rescue of LPL–/– mice at birth by intramuscular delivery of an adenoviral vector carrying a human LPLS447X transgene prevented immediate lethality and allowed mice to survive to adulthood. Rescued adult mice developed an LPL-deficient phenotype, which included hypertriglyceridemia, high LDL cholesterol, and low HDL cholesterol. These Ad-LPLS447X-rescued mice were used for initial studies of the efficacy of AAV-mediated delivery of the LPLS447X transgene. Although the development of these mice provided a convenient model to study changes in disease-specific parameters in response to AAV1-LPLS447X, interpretation of the results is compromised by residual expression of low levels of human LPL from the initial Ad-mediated rescue. This model is further complicated by the presence of significant local muscle pathology including fiber degeneration, lipid accumulation, and collagen deposition, which may have resulted from the initial vector delivery or from localized LPL overexpression.
Table 1.
Summary of Disease Parameters in Preclinical Modelsa
| Mouse | Cat | Human | |
|---|---|---|---|
| Genotype | LPL−/− | LPLG412R (catalytically inactive mutant) | Multiple |
| Ad rescue at birth | Yes | No | No |
| Immune response to hLPL | No | Yes | No |
| Hypertriglyceridemia | Yes | Yes | Yes |
| Pancreatitis | No | Yes | Yes |
| Muscle damage | Yes | No | No |
The murine lipoprotein lipase deficiency (LPLD) model exhibits several characteristics distinct from the disease in humans, including a requirement for adenovirus-mediated rescue of LPL expression at birth, preexisting muscle damage and lipid accumulation, and absence of pancreatitis. The feline model more faithfully recapitulates the features of the human disease.
Ad, adenovirus; hLPL, human lipoprotein lipase.
The second preclinical model of LPLD studied was an LPL-deficient cat (Ross et al., 2006) (Table 1). These animals are naturally homozygous for an LPLG412R mutation that results in complete lack of LPL catalytic function and vastly elevated serum triglycerides. Unlike LPL–/– mice, LPL-deficient cats do not require additional Ad-LPLS447X rescue to survive to adulthood, which is more similar to the human disease state (Table 1). LPLD cats also recapitulate the most serious clinical sequelae of LPLD—severe, recurrent pancreatitis—and lack the unusual muscle phenotype present in the murine model.
Efficacy and stability in preclinical models
In both the murine and feline preclinical studies of AAV1-LPLS447X gene therapy, transgene expression and activity were primarily evaluated indirectly via reduction in total serum triglycerides and resolution of the visible turbidity of the lipemic plasma that is characteristic of LPLD. Although LPL protein and activity were also directly measured in serum immediately after administration of heparin, which liberates the enzyme from its heparan sulfate scaffold on the vascular endothelium, reduction in serum triglycerides proved to be a more sensitive marker for the presence of LPL. Notably, chylomicron metabolism, the primary aberrant pathway associated with LPLD (Fig. 1), was not characterized in animal models. A potential reduction in pancreatitis in the cat model of LPLD was also not evaluated, given that the duration of the feline studies was insufficient to effect notable changes in the progression of the pancreatic pathology observed in LPLD cats. However, given the known association between serum triglycerides and pancreatitis in humans, it was assumed that triglycerides would provide a useful surrogate marker for a reduction in pancreatitis risk.
In the first study of gene therapy in the LPLD mouse, neonates rescued with Ad-LPLS447X were treated at 10–16 months of age with AAV1-LPLS447X (Ross et al., 2004; and see Table 2). Two cohorts of animals were studied, each receiving a different dose of vector (8×1011 and 8×1012 genome copies [GC]/kg) injected intramuscularly. The low-dose group exhibited a modest decrease in triglycerides without detectable serum LPL protein, whereas the high-dose animals had detectable serum LPL protein, as well as a reduction in serum triglycerides to levels observed in LPL+/− mice associated with resolution of visible plasma lipemia. Although postheparin serum LPL protein gradually declined, correction of serum triglycerides persisted for the duration of the 52-week study. Treated mice demonstrated rapid clearance of an intravenous lipid bolus 8 months after vector administration, indicating significant functional LPL expression (Table 3). These results determined the minimally effective dose (MED) for AAV1-LPLS447 at which a significant decrease in serum TG levels was observed in mice to be 8×1011 GC/kg.
Table 2.
Comparison of Experimental Design Between Preclinical and Clinical Studiesa
| Mouse (Ad) | Mouse (AAV) | Cat | Human | |
|---|---|---|---|---|
| Vector | Adenovirus | AAV1 | AAV1 | AAV1 |
| First exposure to hLPL | P0 | P0 | 2–8 yr old | Embryonic |
| Low vector dose | None | 8×1011 GC/kg | 1×1011 GC/kg | 3×1011 GC/kg |
| Medium vector dose | None | None | 5×1011 GC/kg | None |
| High vector dose | 1×109 PFU | 8×1012 GC/kg | 1×1012 GC/kg | 1×1012 GC/kg |
| Volume per injection | 75 μl | 25 μl | 1 ml | 500 μl |
| No. of injections | 4 | 36 | 10 | ∼60 |
| Immunosuppressed | No | No | Yes | Yes |
LPL transgene delivery was tested in murine and feline models of lipoprotein lipase deficiency (LPLD). The total vector dose and number of injections varied considerably among the preclinical studies using AAV1. Immunosuppression was initially introduced in the feline studies in order to attenuate the humoral immune response to the human LPL protein. Immunosuppression was carried forward into human trials in order to block suspected cytotoxic T cell responses to AAV1 capsid, which were blamed for preventing long-term efficacy in AMT-010-01.
AAV, adeno-associated virus; Ad, adenovirus; GC, genome copies; hLPL, human lipoprotein lipase; P0, postnatal day 0; PFU, plaque-forming units.
Table 3.
Summary of Results from Preclinical and Clinical Studiesa
| Mouse | Catb | Humanb | |
|---|---|---|---|
| MED | 8×1011 GC/kg | 1×1011 GC/kg | 1×1012 GC/kg |
| Immune response | No | Yes | No |
| Duration of measurable LPL activity in plasma | <52 wk | 4 wk | N/R |
| Maximal duration of LPL+/− range triglyceride levels | >52 wkc | 10 wkc | Variedd |
The minimally effective vector dose was reasonably predicted by the feline lipoprotein lipase deficiency (LPLD) model, although the magnitude and duration of serum triglyceride (TG) reduction were not predicted by preclinical studies. Importantly, the antibody response against human LPL observed in the feline studies was not replicated in human trials. Establishing the MED in human is complicated because no subjects who received the lower dose were analyzed for postprandial chylomicrons, which is the only reliable metabolic marker of efficacy.
Immunosuppression regimen.
Duration of study.
Did not compare with LPL heterozygotes.
GC, genome copies; LPL, lipoprotein lipase; MED, minimally effective dose; N/R, not reported.
A subsequent study was performed in the feline model of LPLD. Animals treated with doses as low as 1×1011 GC/kg exhibited a rapid correction of serum triglycerides to levels equivalent to those of LPL+/– cats (Table 2). These results were complicated by the presence of a robust humoral immune response to the transgene product, which limited the duration of the effect to several weeks. The addition of a cyclophosphamide regimen only modestly attenuated this response; therefore, long-term evaluation of expression and efficacy was not carried out in this model. The use of a human LPL variant transgene in the feline model complicated the interpretation of the humoral immune response, which is a potential complication in human studies. However, the emergence of these responses in the cat model made it impossible to evaluate the longevity of the treatment and its long-term safety and efficacy. Another noteworthy observation in the feline model is a minimally effective dose 8-fold lower than that identified in mice (Table 3). Possible reasons include species differences in LPL substrate-binding affinity, variation in efficiency of transgene expression, changes in vector tropism, and variability in the half-life of LPL protein. The discrepancy could also be explained by other differences in the two models, including preexisting muscle damage in the mouse, the presence of baseline LPLS447X in control and experimental mice, and differences in the number and volume of vector injections (Tables 1 and 2).
Safety in preclinical models
Formal assessments of safety were conducted in preparation for the first clinical trial under conditions of Good Laboratory Practices (GLP) (Rip et al., 2005). Wild-type mice in a C57BL/6 background were selected for an evaluation of maximally tolerated dose (MTD) and dose-limiting toxicities. Three doses of vector were administered to male and female mice, with the highest dose of 1×1013 GC/kg being 100-fold greater than the MED used in the feline efficacy study. Up to 90 days posttreatment, the authors reported no significant changes in the health of the mice or their eating patterns. Although the overall health of the mice was not affected, minor inflammation was observed in muscle histology sections at injection sites and female mice were found to have a reduced body weight gain of about 30%, hypothesized to be due to increased energy expenditure after treatment. The investigators concluded that the treatment is safe and the MTD was not achieved even at the highest dose that could be administered. To determine the biodistribution of the vector, two doses, 1×1011 and 1×1013 GC/kg, were given to male and female mice. Tissue samples of the injected mice were analyzed for vector DNA 7, 28, and 90 days after injection. At the 90-day time point, vector DNA was observed in the highest concentration at the injection site but was also observed in the liver and inguinal lymph nodes. In addition, mice were also observed to have small, but detectible, dose-dependent levels of vector DNA in the gonads at the day 90 time point.
Summary of Glybera Clinical Trial Data
AMT-010-01
This first safety and efficacy trial for AAV1-LPLS447X was initiated by Amsterdam Molecular Therapeutics (AMT) in July 2005 (Fig. 2). Eight subjects met the eligibility criteria, which included (1) LPL activity ≤20% of normal, (2) mutations in the gene encoding LPL, (3) an LPL mass >5% of normal, and (4) TG levels >10 mmol/liter. Before vector delivery, subjects participated in an observational study (PREP-01) during which they maintained a low-fat diet controlled by a dietitian in order to assess baseline levels of TGs. These data showed that dietary control did not lead to significant reduction or stabilization of serum triglycerides (EMA, 2012, p. 41). After completion of the observational study, the subjects were split into two dose cohorts, with the low-dose cohort receiving AAV1-LPLS447X at 1×1011 GC/kg and the high-dose cohort receiving 3×1011 GC/kg. The vector was administered via multiple injections (either 40 or 60), depending on dose cohort, into the quadriceps muscles. The primary objective of the trial was to establish efficacy and safety of AAV1-mediated delivery of LPL. The primary efficacy end point was defined as a reduction in individual median fasting plasma TG to a level ≤10 mmol/liter or a reduction in median fasting plasma TG greater than or equal to 40%. The subjects were evaluated at 12 weeks and then again at 18 to 31 months after vector administration for long-term follow-up. No serious adverse events were reported over the evaluation period, and the most common adverse event reported was general muscle pain at the injection site. At 12 weeks after vector administration, 50% of subjects had reached the primary efficacy end point of a median decrease in TG levels compared with baseline TGs. LPL protein and activity were detected in muscle biopsies taken between 26 and 36 weeks after administration, but there was no discussion of LPL detection in the blood. However, the long-term follow-up showed a loss in efficacy regarding TG levels that was attributed to an immune response to the AAV1 capsid, as CD8+ T cell responses against the AAV capsid have previously been reported to affect transgene expression in a trial of AAV2 in the liver of patients with hemophilia B (Mingozzi et al., 2003). However, antibodies against the transgene were not detected. Conclusions reached from AMT-010-01 included the need for a higher dose to boost transgene expression and the use of an immunosuppressive regimen to curtail the immune response to the vector. Overall, the effect of the therapy appeared to be transient, based on serum triglycerides, which highlights one of the chief concerns about this product being limited to a single administration (Stroes et al., 2008).
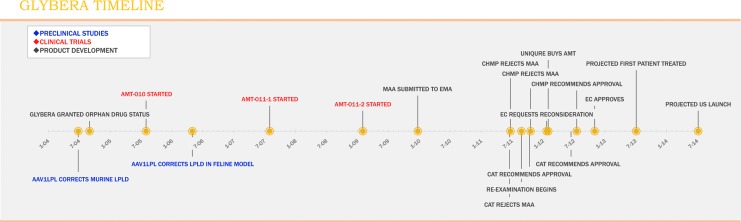
FIG. 2.
Timeline of events related to approval of Glybera. Note: Preclinical studies are listed by date of publication. CHMP, Committee for Human Medicinal Products; EC, European Commission; CAT, Committee for Advanced Therapies; EMA, European Medicines Agency; MAA, Marketing Authorization Application.
AMT-011-01
In August 2007, after completion of AMT-010-01, Amsterdam Molecular Therapeutics initiated a second trial, AMT-011-1 (enumerated differently to reflect a changed manufacturing process), to assess efficacy and long-term safety in a larger number of subjects (National Institutes of Health [NIH], 2010; and see Fig. 2). Importantly, the HEK293 system used to manufacture AAV1-LPLS447X for the first study was replaced by a baculovirus production system to increase vector yield, despite the fact that this slightly decreased the potency of the vector in vivo in mice and by up to 4-fold in vitro (EMA, 2012, p. 25). This final AAV1-LPLS447X therapeutic vector is referred to as alipogene tiparvovec. Similar to the first trial, participants were first enrolled in the PREP-02 observational study to be monitored for a minimum of 4 months before the clinical study. In addition to the prior eligibility criteria, participants were also required to have a history of pancreatitis in order to examine the effect of Glybera on clinical outcomes. Fourteen of the 22 subjects enrolled in the observational study received a single series of injections of Glybera. Six subjects received a dose of 3×1011 GC/kg, four of whom were placed on an immunosuppressive regimen consisting of cyclosporine A at 3 mg/kg per day and mycophenolate mofetil at 2 g/day in an attempt to attenuate potential cellular immune responses against the vector or the LPL transgene. Eight subjects received a dose of 1×1012 GC/kg and were also placed on immunosuppression, which was initiated at the time of injection and discontinued at 12 weeks. Seven subjects consented to a muscle biopsy at 26 weeks.
Seven subjects (half of the total) met the primary end point for this study, a reduction in median plasma TG of at least 40% between 3 and 12 weeks. Four of those subjects also met the secondary efficacy end point of a median fasting plasma TG level ≤10 mmol/liter between 3 and 12 weeks. TG reductions were statistically significant in the study group as a whole as well as for the high-dose group; however, by 26 weeks, plasma TG in all subjects returned to baseline; this was interpreted to mean that the immune suppression did not increase the duration of efficacy of Glybera. All subjects experienced a mild to moderate injection site reaction, and muscle biopsies at 26 weeks demonstrated lipid accumulation and immune infiltrates at the injection site. The authors noted that the severity of the injection site reaction appeared to be correlated with the level of LPL expression in muscle tissue, but no quantitative relationship was identified. There were several adverse events reported during 2 years of follow-up, but none were attributed to vector administration (see Glybera Approval, below). In addition, shedding of the virus in saliva, urine, and semen was minimal and dropped below the level of detection by week 10; the conclusion was that alipogene tiparvovec is considered safe.
Because of the transient effect on plasma TG, the authors also examined posttreatment chylomicron and VLDL lipoprotein fractions in order to determine whether Glybera may have a prolonged effect on another aspect of lipoprotein metabolism. In the high-dose cohort, VLDL was significantly increased at 12 weeks postinjection. In addition, total cholesterol and TG content in the VLDL fractions were increased at 12 and 52 weeks along with corresponding decreases in total cholesterol and TG content of the chylomicron fraction. Because LPL is an essential enzyme in the conversion of chylomicrons to VLDL, these data suggest that there was a sustained increase in plasma LPL activity despite the lack of long-term effect on total plasma TG.
This study was not designed to evaluate overall clinical benefit regarding specific disease symptoms; however, the authors did record incidence of pancreatitis and self-reported symptoms by the subjects enrolled. One episode of pancreatitis occurred during the PREP study and two episodes occurred during 2 years of follow-up. The authors stated that this constitutes a 5-fold decrease in pancreatitis incidence after administration of Glybera, as compared with patient history before treatment, although the numbers are too low to reach statistical significance. Moreover, subjects reported improvement in clinical signs of the disease such as “capacity to eat more food” and “increased energy levels.” However, these claims must be interpreted with caution as they are based on a small sample size and subjective reports. Altogether, this clinical trial demonstrated that Glybera is well-tolerated and associated with a transient decrease in total plasma TG and sustained alterations in chylomicron metabolism (Gaudet et al., 2013).
AMT-011-02
In February 2009, five subjects with LPLD were enrolled in a third trial to measure the effect of Glybera on chylomicron metabolism and to validate the use of postprandial chylomicrons (pp-CM) as a replacement for total plasma TG as a primary efficacy end point (NIH, 2009; and see Fig. 2). All subjects received a one-time series of injections at a dose of 1×1012 GC/kg. In addition to the immunosuppression regimen used in AMT-011-01, a single dose of intravenous methylprednisolone, was administered 30 min before the vector. The enrollment criteria for the study remained the same as in AMT-011-01.
To assess the effect of Glybera on chylomicron metabolism, the authors performed a challenge study to measure postprandial plasma lipids and metabolites before treatment and 14 weeks after receiving Glybera. Each subject ingested a study meal containing [3H]palmitic acid, and hourly blood samples were drawn to analyze tracer in plasma lipoprotein fractions. Data from individuals with LPLD were compared with data from healthy control subjects for the first six time points. Before dosing, LPL-deficient subjects displayed markedly elevated postprandial plasma TG levels and increased TG content of the chylomicron fraction. At 14 weeks posttreatment, postprandial chylomicron clearance and triglyceride content of the chylomicron lipoprotein fraction were similar to those of normal subjects. This study strongly suggests that Glybera results in functional LPL expression 14 weeks after treatment and that this LPL is able to exert an effect on lipid metabolism, despite plasma TG levels typically returning to baseline by this time point. Three subjects were challenged again at 52 weeks, and again demonstrated accelerated chylomicron clearance, although the effect was less pronounced than at the 14-week time point (EMA, 2012, p. 56). The results of this study established a new efficacy end point and suggested that LPL expression persists despite the transient decrease in plasma TG (Carpentier et al., 2012; EMA, 2012, p. 87).
AMT-011-03
Evidence of metabolic efficacy in conjunction with the preliminary evidence that Glybera may reduce the incidence of pancreatitis in LPLD prompted further investigation of the effect of Glybera on pancreatitis. In a retrospective study including 17 of the 22 subjects from all prior clinical trials, the authors reported a decrease in the number of episodes of pancreatitis after treatment when evaluating events during a 3-year period before and after Glybera delivery. When comparing 3 years before and after treatment, the hazard ratio was estimated to be between 0.41 and 0.49. However, the significance of this decrease is dependent on the length of time used for the analysis. This retrospective analysis is further complicated by the variability in the incidence of pancreatitis events, both before and after treatment. Although some of the subjects had only single events in the past, 3 subjects accounted for up to 11 of the 41 pretreatment events. Several (8 of the 17) subjects were pancreatitis-free after treatment but also had no incidents of pancreatitis in the equivalent time period before treatment (EMA, 2012, p. 60). Meanwhile, a majority of the episodes in the follow-up occurred in three subjects. Although these data remain unpublished, the EMA review documents include this analysis, stating that it is “insufficient to provide evidence of efficacy for a reduction in the rate of pancreatitis” but that it suggests a potential benefit (EMA, 2012). A longer period of follow-up will be necessary to evaluate the effect on acute pancreatitis.
Glybera Approval
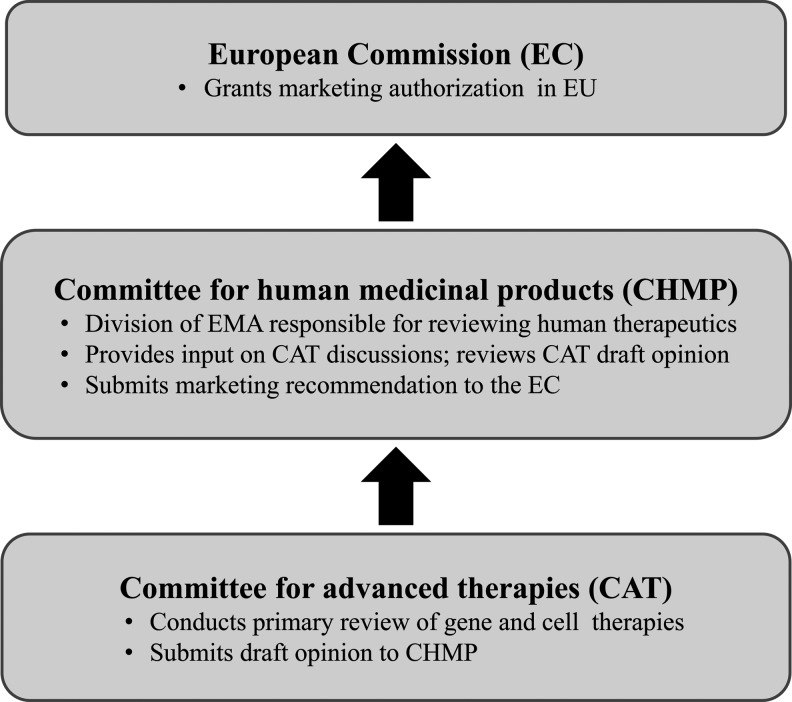
Although multiple pathways for drug approval exist in the European Union, novel biological agents are required to follow a centralized review process overseen by the EMA (Fig. 3). Approval through this pathway leads to a recommendation to the European Commission (EC), which has the authority to grant marketing authorization in all EU member states. Within the EMA, drugs for human use are evaluated by the Committee for Human Medicinal Products (CHMP), which carries out the scientific review and makes final recommendations to the EC for marketing approval. For Glybera, the primary responsibility for review fell on a relatively new subdivision of the EMA, the Committee for Advanced Therapies (CAT), which was formed in 2009 specifically to provide expert evaluation of gene and cell therapies. The CAT is charged with carrying out the initial review of products that fall within its scope and submitting a draft recommendation to the CHMP for (or against) approval. The CHMP provides oversight and input during CAT deliberations and has the authority to adopt or reject a CAT draft opinion. The deliberations of both the CAT and the CHMP are publicly available and have been published (EMA, 2012).
FIG. 3.
Centralized approval process in the European Union (EU). The scientific review of human therapeutics is carried out by the Committee for Human Medicinal Products (CHMP), a division of the European Medicines Agency (EMA). For gene and cell therapies, a primary review is performed by the Committee for Advanced Therapies (CAT), an independent specialist committee composed of five CHMP members and representatives selected by EU member states, as well as European Commission (EC)-appointed patient and clinician representatives. Although the CAT adopts a draft opinion on marketing applications, the CHMP is ultimately responsible for making a recommendation to the EC, which has the final authority to grant marketing approval.
In December 2009, AMT submitted the marketing authorization application for Glybera to the EMA (Fig. 2). Given that a gene therapy had never been approved in the West, it is noteworthy that two of the most likely sources of concern for this type of product—safety issues related to viral vector-mediated gene transfer and the process of manufacturing and characterizing a viral vector—did not prove to be major obstacles to approval. The initial review brought up several of these expected theoretical concerns, including insertional mutagenesis, immune toxicity, tumorigenicity, germ-line transmission of vector sequences, and manufacturing issues such as contaminating DNA sequences and in vitro assessment of potency (EMA, 2012, p. 30). After some discussion with the applicant and submission of additional data, all of these issues were addressed to the satisfaction of both the CAT and CHMP.
In the examination of the cumulative clinical trial data, safety proved to be of limited concern; most subjects experienced only local discomfort after treatment, and muscle biopsy findings of localized inflammation and changes in fiber architecture were not believed to be clinically significant. However, it was noted that functional tests on injected muscle should have been performed to rule out any lasting effect. One subject was diagnosed with suspected deep venous thrombosis and pulmonary embolism after treatment, which was thought to be potentially related to receiving a large number of intramuscular injections. Another subject experienced transient polyarthritis and myositis at the time of discontinuation of immunosuppression, but this was deemed to have most likely been caused by a preexisting condition. The 12-week course of immunosuppression was identified as a potential source of serious risk, particularly because it had not prevented the observed local inflammatory infiltrate, indicating that its inclusion was unjustified. Still, it became clear through the review process that both the CAT and CHMP would deem the safety risks acceptable so long as efficacy could be demonstrated.
Demonstrating efficacy, however, proved to be the primary hurdle for the approval of Glybera. On the basis of data from animal models and expert opinion suggesting that serum triglycerides are a useful surrogate marker of pancreatitis risk, reduction in triglycerides was selected as the primary efficacy end point in the clinical trials. Problems with this approach became apparent even in the observational studies. In PREP-01 in particular, the CAT noted high variability of baseline fasting triglyceride levels in these subjects despite their following a strict diet (EMA, 2012, p. 41). In the interventional studies, 50% of subjects transiently met the 40% reduction in serum fasting TG level end point; however, triglycerides were highly variable throughout the study and improvements were not sustained in any subject. On the basis of these data, the CAT stated that “the plasma levels of triglycerides (TGs) are a poor predictor of efficacy,” with which the CHMP “agreed that TG plasma levels are not the appropriate biomarker for efficacy” (EMA, 2012, p. 88). The CAT accepted a change in primary end point to functional measures of postprandial chylomicron (pp-CM) metabolism, recognizing the “evolving scientific knowledge” of this rare disease. However, when this end point was examined in AMT-011-02, observations were extended to 52 weeks in only three subjects, which was not sufficient to establish long-term efficacy (EMA, 2012, p. 56). Although analysis of the retrospective data by blinded, independent experts on the basis of revised Atlanta criteria suggested a reduction in incidence of pancreatitis (AMT-011-03), the CAT and CHMP brought attention to the fact that the majority of pancreatic events occurred in 3 of the 17 subjects they evaluated; therefore, the number of events in the other subjects was too limited to make any robust conclusions (EMA, 2012, p. 60). Before the initiation of the clinical studies, the EMA had suggested that protocols be put in place to objectively evaluate all potentially relevant clinical parameters (such as abdominal pain and organomegaly). Instead, subjects self-reported improvement in these categories, and the fact that AMT relied on these subjective reports was considered to be a “major deficiency” in their application (EMA, 2012, p. 52). Because decreases in triglycerides in this trial were transient, and no long-term efficacy was seen or reported in an objective fashion, the CAT suggested that it would likely be unacceptable to promote this drug as a life-long cure. In June 2011, based on the results of the preclinical and clinical trials, the CAT declared that the risk–benefit balance was negative because of the potential risks of multiple muscle injections along with immunosuppressants as well as the lack of accompanying efficacy data. The CHMP agreed, saying that AMT did not sufficiently demonstrate safety and sustained efficacy. They recommended refusal of the Glybera application, citing three particular causes for concern: the lack of evidence for a long-lasting effect on TG or pp-CM levels, insufficient evidence of reduction in incidence of pancreatitis, and significant risks associated with the inclusion of immune suppression, especially with insufficient justification of its inclusion (EMA, 2012, p. 85).
In October 2011, AMT requested a reexamination of their application and included rebuttals to the three primary concerns. They acknowledged that TG levels may not be the ideal end point for efficacy but contended that the switch to pp-CM metabolism should resolve this issue. In addition, they committed to a postapproval study of all previously-dosed subjects in order to confirm appropriateness of this end point and argued that this should be sufficient for approval. To address the validity of the pancreatitis end point, AMT put together a panel of experts in order to more objectively evaluate the pancreatitis data to increase its relevance despite the small number of patients. Clinicians were also consulted to evaluate the relative risk of immunosuppression, which was felt to pose minimal risk given the limited duration of treatment. After consideration of this response, the CAT accepted the relevance of the new pp-CM end point and efficacy regarding incidence of pancreatitis in the smaller subset of patients with recurrent pancreatitis. They therefore gave a positive opinion for Glybera for the treatment of this more restricted group of patients with LPLD (EMA, 2012, p. 103). The CHMP, however, determined that there were not enough data points to sufficiently show efficacy for either pp-CM or pancreatitis and subsequently recommended refusal of the application. Interestingly, they did agree that concerns associated with immunosuppression would have been considered resolved if sufficient efficacy had been demonstrated.
In January 2012, the European Commission requested that the CHMP reassess their position on the risk–benefit balance of Glybera in patients with recurrent pancreatitis. The CHMP requested additional clinical data, such as rates of diabetes and hospitalization, but did not change its opinion that Glybera was not suitable for authorization. After this third refusal in April 2012, the EMA reported that the CHMP had breached the review protocol by issuing its response to the EC request without first allowing the CAT to adopt a draft opinion. The prior decision was therefore considered void, and the review process was initiated for a fourth time. The CAT and CHMP had open dialogue sessions with representatives from AMT (now uniQure) to discuss their residual concerns. The CAT maintained their opinion that although there was a need to add to efficacy data postmarketing, approval under exceptional circumstances was justified for the restricted patient population (EMA, 2012, p. 122). The CHMP then simply stated agreement with the CAT opinion that “Glybera is indicated for adult patients diagnosed with familial lipoprotein lipase deficiency and suffering from severe or multiple pancreatitis attacks despite dietary fat restrictions.” However, a large proportion of the members of the CHMP had dissenting opinions, arguing that the agreed-on postmarketing evaluations of the effect of Glybera on pp-CM should have been performed before EMA approval. After the CHMP recommendation, the European Commission approved Glybera for marketing in the EU in November 2012. Provisions of the approval included the enactment of a pharmacovigilance program, a risk management plan, biannual periodic safety update reports, and the formation of a registry of every patient dosed with Glybera.
Summary
The pursuit of successful gene therapy in 2013 as defined by products that treat, cure, or prevent diseases in human beings is indeed a “venture into the unknown.” The story of Glybera as depicted in this review highlights the challenges of pioneering efforts in the field of novel human biopharmaceuticals. To be frank, the Glybera story is not elegant or tidy; the product does not cure the disease, and some may say its clinical effectiveness is still in question. However, this research clearly broke new ground and set the stage for the next wave of gene therapy products. Our detailed accounting of events associated with the development and regulatory review of this product afforded the opportunity to reflect on lessons learned that may inform future efforts. We have done so as third parties not directly involved in the clinical development of the product, although one of us (J.M.W.) invented the AAV vector used in the final product.
The selection of LPLD as a target for an early gene therapy product makes sense. Identification of the transgene was obvious because this is gene replacement for a recessive monogenic disease. The investigators, however, used a variant of LPL associated with hyperactivity (LPLS447X) rather than a gene that encodes the normal version of the protein, which is a reasonable strategy to improve efficacy. Small- and large-animal models for LPLD are available and were used in developing the preclinical data to support the first human study. The metabolic consequences of LPL expression, that is, a drop in serum triglycerides, could be used as a biomarker for efficacy and for stability of transgene expression and potentially as a surrogate for predicting clinical efficacy (i.e., reduced risk of pancreatitis). There are no effective treatments for LPLD, and it leads to severe and often life-threatening pancreatitis, indicating a substantial unmet need.
The investigators performed extensive studies in two animal models of LPLD. As is often the case, the mouse model did not completely reproduce the phenotype in humans in that a complete deficiency led to neonatal lethality. Injection of adenovirus expressing human LPLS447X into newborn pups was a clever way to temporarily rescue the mice before administration of the AAV vector. However, the immunogenic nature of adenoviral vectors likely confounded the interpretation of AAV gene transfer by eliciting transgene-specific immune responses and possibly inducing inflammation in muscle before intramuscular AAV delivery. The feline model of LPLD is more authentic than the mouse model in terms of phenotype and proved to be a useful way to establish activity of the vector in terms of the MED. The cats did develop antibodies against the human transgene that interfered with the assessment of durability of expression, which turned out to be a key question in the human trials. It is unclear whether the outcome in the feline experiments would have been different if the vector expressed a feline version of the LPL gene. It is interesting that the MED in the larger animal model is lower than that observed in the mouse model, which is different from what has been observed in preclinical studies for other AAV gene therapies such as hemophilia B, for which the MED was much higher in the canine model in comparison with the mouse model (Manno et al., 2006). It is difficult to correlate the MED in LPL-deficient cats with that observed in humans because the end points for efficacy used in the human dose escalation study (i.e., serum triglycerides) were flawed; studies at the low dose were not informative. However, the dose of vector tested in the human efficacy trials (i.e., 1012 GC/kg) was informative because it significantly improved clearance of postprandial chylomicrons. This dose was 10-fold higher than the MED in cats.
The design and interpretation of the human clinical trials turned out to be the most substantial challenge. One significant limitation in designing efficacy trials in familial LPLD is the limited number of patients available for participation. Familial LPLD is characterized as an ultra-orphan disease with a prevalence of only 1 per million (Brunzell, 1999). The investigators further limited participation in the trial in several ways including the requirement that subjects have substantial levels of circulating endogenous (but defective) LPL enzyme as a way to avoid transgene-specific B and T cell responses. The scarcity of potential subjects makes it difficult to enroll sufficient numbers required to show statistical significance. In addition, it is difficult to conduct placebo-controlled randomized studies because of the low subject numbers as well as ethical concerns regarding the risks of sham injections or injections with empty vector. One solution to this problem is solid natural history data to allow for comparisons with historical controls and/or comparative measures of efficacy before and after treatment. Unfortunately, natural history data were limited for this disease; the team had to generate these data before initiating their clinical programs. The primary efficacy end point of a stable reduction of serum triglycerides was not consistently achieved and, when it was observed, it was not durable. Ongoing analysis of the natural history data concluded that serum triglycerides were simply too variable in these patients to be a useful end point for assessing transgene engraftment, requiring that the investigators reevaluate the design of their ongoing clinical development program. This led to the proposal to measure postprandial kinetics of serum chylomicrons before and after gene therapy, which made biological sense. The data were quite compelling in the few subjects in which it was measured. This, coupled with the demonstration of gene transfer by polymerase chain reaction and LPL protein expression by immunohistochemistry in biopsied muscle after gene transfer, confirmed without question that immediately after vector injection, transgene-derived LPLS447X was expressed and impacted positively on chylomicron metabolism. The problem is that postprandial chylomicron accumulation is not a validated end point; that is, there are no existing data that correlate these measurements with improved clinical outcomes. A retrospective analysis of patients, using the revised Atlanta classification of acute pancreatitis to evaluate the impact of gene therapy on the incidence of pancreatitis, was conducted in an attempt to generate clinical efficacy data. The original trials were not powered to achieve statistical significance for this variable manifestation of the disease and the data collection regarding pancreatitis had flaws, both of which limited the usefulness of the exercise.
A number of important lessons emerged from the clinical development of Glybera including the value of extensive natural history data as a prelude to the design of clinical trials. Many orphan disease advocacy groups have proactively established patient registries to address this need. The experience with Glybera also illustrates the importance of selecting the best primary efficacy end point and the difficulty in progressing clinical development when this end point is not achieved.
Much has been written about the potential toxicity of AAV gene therapy, with a focus on adverse immune responses. It is encouraging that the EMA did not view immune toxicity as a barrier to registration. What was ironic is that the most substantial safety concern emanated from the investigators' attempt to mitigate potential immune toxicity with concurrent immune suppressive therapy. They initiated the phase 1 trial without the intent to immune suppress. A constellation of findings emerged from this study that suggested immune responses may be extinguishing expression (and therefore efficacy), based on the eventual return of triglycerides to pretreatment levels concurrent with the presence of inflammation in muscle biopsies. The appearance of T cells to the vector capsid, based on enzyme-linked immunospot assays of peripheral blood, implicated their role in these findings. A course of immune suppression with cyclosporine and mycophenolate mofetil was included in the subsequent trial, with a high-dose injection of steroids added in the third trial. The EMA concluded that the value of including immune suppression was not supported by data and that it added significant additional risk. In retrospect, this conclusion seems justified. One flaw in the rationale for introducing immune suppression in the second trial was the conclusion from the phase 1 trial that transgene expression was lost, based on a return of triglycerides to background levels, which ultimately was determined to be an unreliable biomarker of LPL expression. In fact, in the phase 1 trial, late biopsy specimens confirmed ongoing transgene expression. The introduction of immune suppression in subsequent trials made little difference in terms of the appearance of capsid-specific T cells, the kinetics of triglyceride changes, or inflammation in muscle biopsies. Incorporating immune suppression into AAV clinical trials should be based on solid preclinical and clinical data. The prophylactic treatment of AAV gene therapy research subjects with immune-suppressing drugs without strong justification should be discouraged.
One aspect of the Glybera review that was closely watched by the gene therapy community was the response of the EMA to issues related to manufacturing of the vector, which fall under the umbrella of what is called chemistry, manufacturing, and controls (CMC). A necessary consequence of being the first to market is that there is limited information as to how the product should be manufactured and tested for identity, potency, and purity. Validated assays with associated reference standards do not exist for many of these parameters. Furthermore, the regulatory agencies have not established a complete set of criteria for “releasing” the product for use in humans. Analysis of the clinical data was furthermore confounded by the change in manufacturing method from a standard transfection approach for the phase 1 trial to the more scalable baculovirus approach for the additional trials and the commercial product. A change in manufacturing process during clinical development is not uncommon and will be the norm in gene therapy because large-scale commercial manufacturing processes are still in development. The EMA worked collaboratively with the sponsor to evaluate comparability of products made by the various processes without the need for substantial additional studies. It is encouraging that CMC concerns did not get in the way of market authorization, although this will be a constantly-evolving landscape with the rigor of product characterization being raised as we learn more and product indications move into less lethal diseases.
In summary, the first market authorization of a gene therapy product in the West signifies a true milestone in the field of gene therapy, representing a victory for the community of scientists and physicians who have helped shape this field over the last 3 decades. The team of scientists, physicians, and business leaders who spearheaded this effort should be applauded for their persistence and resilience, and the EMA should be recognized for their patience, flexibility, and transparency. The fact is that this is only the beginning, and a modest beginning at that. The “approval” of Glybera was associated with substantial postmarketing requirements that allow commercial sale of the product concurrent with an ongoing evaluation of its safety and efficacy.
Acknowledgments
The authors thank Martha Turnista for being instrumental throughout the process of compiling this manuscript. Funding for this work was provided by: NIDDK DK047757, NHLBI HL059407, NHLBI HHSN268201200041C, NICHD HD057247, Bill & Melinda Gates Foundation 51061, ReGenX Biosciences SRA, and NEI EY020792.
Author Disclosure Statement
J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. J.M.W. is an inventor on the AAV1 patent and will receive royalties from the sales of Glybera.
References
- Asokan A. Schaffer D.V. Samulski R.J. The AAV vector toolkit: Poised at the clinical crossroads. Mol. Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison R.W. Casto B.C. Hammon W.M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Brunzell J.D. Familial lipoprotein lipase deficiency. In: Pagon R.A., editor; Bird T.D., editor; Dolan C.R., editor; Stephens K., editor; Adam M.P., editor. GeneReviews. University of Washington Press; Seattle, WA: 1999. [PubMed] [Google Scholar]
- Carpentier A.C. Frisch F. Labbe S.M., et al. Effect of alipogene tiparvovec (AAV1-LPLS447X) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. J. Clin. Endocrinol. Metab. 2012;97:1635–1644. doi: 10.1210/jc.2011-3002. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency (EMA) Assessment Report: Glybera. European Medicines Agency; London: 2012. [Google Scholar]
- Frossard J.L. Steer M.L. Pastor C.M. Acute pancreatitis. Lancet. 2008;371:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- Gaudet D. De Wal J. Tremblay K., et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler. Suppl. 2010;11:55–60. doi: 10.1016/j.atherosclerosissup.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Gaudet D. Methot J. Dery S., et al. Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: An open-label trial. Gene Ther. 2013;20:361–369. doi: 10.1038/gt.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoda T. Senda M. Gamou T., et al. Nucleotide sequence of human cDNA coding for a lipoprotein lipase (LPL) cloned from placental cDNA library. Nucleic Acids Res. 1989;17:2351. doi: 10.1093/nar/17.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelein J.J. Ross C.J. Hayden M.R. From mutation identification to therapy: Discovery and origins of the first approved gene therapy in the Western world. Hum. Gene Ther. 2013;24:472–478. doi: 10.1089/hum.2013.063. [DOI] [PubMed] [Google Scholar]
- Manno C.S. Pierce G.F. Arruda V.R., et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Mead J.R. Irvine S.A. Ramji D.P. Lipoprotein lipase: Structure, function, regulation, and role in disease. J. Mol. Med. (Berl.) 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- Mingozzi F. Liu Y.L. Dobrzynski E., et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J. Clin. Invest. 2003;111:1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health (NIH) Efficacy and safety of human lipoprotein lipase (LPL)[S447X] expressed by an adeno-associated viral vector in LPL-deficient subjects. 2009. http://www.clinicaltrials.gov/ct2/show/NCT00891306 http://www.clinicaltrials.gov/ct2/show/NCT00891306
- National Institutes of Health (NIH) Safety and efficacy in LPL-deficient subjects of AMT-011, an adeno-associated viral vector expressing human lipoprotein lipase [S447X] 2010. http://www.clinicaltrials.gov/ct2/show/NCT01109498 http://www.clinicaltrials.gov/ct2/show/NCT01109498
- Rip J. Nierman M.C. Sierts J.A., et al. Gene therapy for lipoprotein lipase deficiency: Working toward clinical application. Hum. Gene Ther. 2005;16:1276–1286. doi: 10.1089/hum.2005.16.1276. [DOI] [PubMed] [Google Scholar]
- Ross C.J. Twisk J. Meulenberg J.M., et al. Long-term correction of murine lipoprotein lipase deficiency with AAV1-mediated gene transfer of the naturally occurring LPLS447X beneficial mutation. Hum. Gene Ther. 2004;15:906–919. doi: 10.1089/hum.2004.15.906. [DOI] [PubMed] [Google Scholar]
- Ross C.J. Liu G. Kuivenhoven J.A., et al. Complete rescue of lipoprotein lipase-deficient mice by somatic gene transfer of the naturally occurring LPLS447X beneficial mutation. Arterioscler. Thromb. Vasc. Biol. 2005;25:2143–2150. doi: 10.1161/01.ATV.0000176971.27302.b0. [DOI] [PubMed] [Google Scholar]
- Ross C.J. Twisk J. Bakker A.C., et al. Correction of feline lipoprotein lipase deficiency with adeno-associated virus serotype 1-mediated gene transfer of the lipoprotein lipase S447X beneficial mutation. Hum. Gene Ther. 2006;17:487–499. doi: 10.1089/hum.2006.17.487. [DOI] [PubMed] [Google Scholar]
- Stroes E.S. Nierman M.C. Meulenberg J.J., et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler. Thromb. Vasc. Biol. 2008;28:2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- Xiao W. Chirmule N. Berta S.C., et al. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav D. Pitchumoni C.S. Issues in hyperlipidemic pancreatitis. J. Clin. Gastroenterol. 2003;36:54–62. doi: 10.1097/00004836-200301000-00016. [DOI] [PubMed] [Google Scholar]