Abstract
Curcumin is widely known for its anti-oxidant, anti-inflammatory and anti-proliferative activities in cell culture studies. However, poor oral bioavailability limited its efficacy in animal and clinical studies. Recently, we developed polymeric curcumin implants that circumvents oral bioavailability issues, and tested their potential against 17β-estradiol (E2)-mediated mammary tumorigenesis. Female ACI rats were administered curcumin either via diet (1,000 ppm) or via polymeric curcumin implants (two 2-cm; 200 mg each; 20% drug load) 4 days prior to grafting a subcutaneous E2 silastic implant (1.2 cm, 9 mg E2). Implants were changed after 4½ months to provide higher curcumin dose at the appearance of palpable tumors. The animals were euthanized after 3 weeks, 3 months and after the tumor incidence reached >80% (~6 months) in control animals. The curcumin administered via implants resulted in significant reduction in both the tumor multiplicity (2±1 vs 5±3; p=0.001) and tumor volume (184±198 mm3 vs 280±141 mm3; p=0.0283); the dietary curcumin, however, was ineffective. Dietary curcumin increased hepatic CYP1A and CYP1B1 activities without any effect on CYP3A4 activity whereas curcumin implants increased both CYP1A and CYP3A4 activities but decreased CYP1B1 activity in presence of E2. Since CYP1A and 3A4 metabolize most of the E2 to its non-carcinogenic 2-OH metabolite and CYP1B1 produces potentially carcinogenic 4-OH metabolite, favorable modulation of these CYPs via systemically delivered curcumin could be one of the potential mechanisms. The analysis of plasma and liver by HPLC showed substantially higher curcumin levels via implants versus the dietary route despite substantially higher dose administered.
Keywords: Curcumin, Polymeric implants, Breast cancer inhibition, Estradiol, Hepatic cytochromes
Introduction
Curcumin is a natural polyphenolic di-arylheptanoid obtained from rhizomes of Curcuma longa (turmeric) and has shown potent antioxidant, anti-inflammatory and anti-proliferative activities in various cell culture studies (1, 2). Commercially, it is available as a mixture of 3 curcuminoids; curcumin I (diferuloylmethane, 75%), curcumin II (demethoxycurucmin, 20%) and curcumin III (bisdemethoxycurcumin, 5%) (supplementary Fig. S-1) (3). All the 3-curcuminoids have been shown to possess similar anti-proliferative activities against leukemia, lung, prostate, pancreas and breast cancer cell lines (4) and cervical cancer cells (R. Munagala and R. Gupta, unpublished data). Curcumin’s ability to inhibit various enzymes (like COX-2, CYP1B1, LOX), transcription factors (like NF-kB, STAT3) and secondary messengers made it a highly desirable candidate to be developed as a drug. However, due to its poor solubility and high intestinal liver metabolism, it showed limited oral efficacy in various preclinical and clinical studies (3, 5). As a result, various advanced drug delivery systems like nanoparticles (6), liposomes (7), microparticles (8), micelles (9), hydrogels (10), micro-emulsions (11) and phospholipid mixtures (12) were developed to harness its full potential (3). However, due to rapid intestinal and hepatic metabolism, a drug delivery system of curcumin with continuous systemic administration is desired that can overcome the oral bioavailability issues.
Recently, we developed polymeric implants using poly (ε-caprolactone) (PCL) as the polymeric matrix to enable continuous administration of curcumin (for months to years) directly into the systemic circulation (13). Once grafted subcutaneously, these implants provide controlled release of curcumin continuously (“24/7”) at the implantation site from where it is systemically absorbed and distributed to various tissues (14). Biocompatibility and toxicity studies carried out using ACI rats revealed that these implants are safe and biocompatible with no apparent changes in physiological biochemistry (13). Hematological parameters (such as WBCs, RBCs, platelets, basophils, eosinophils), and biochemical parameters of liver (AST, ALP, AP, amylase) and kidney (Na+, K+, Ca2+, creatinine, BUN) functions were also found to be unaltered by continuous systemic administration of massive doses of curcumin by these implants (13). Furthermore, these implants were found to maintain much higher plasma, liver and brain concentrations for up to 3 months and required ~20-fold lower doses as compared to dietary administration (15).
Therefore, this study was designed to test chemopreventive efficacy of curcumin implants compared to curcumin delivered via diet against 17β-estradiol (E2)-induced mammary tumorigenesis in female ACI rats. Furthermore, mechanistic aspects for efficacy of curcumin implants were explored to deduce its mode of action against E2-induced mammary tumorigenesis.
Materials and Methods
Materials
Medical-grade PCL of 121,000 molecular weight (PCL-121) was purchased from SurModics Pharmaceuticals (Birmingham, AL) and curcumin (C-3 complex extracted under GMP conditions for human use), was a generous gift from Sabinsa Corporation (East Windsor, NJ). Dichloromethane (DCM), ethoxy resorufin and pyrene were from Sigma-Aldrich (St. Louis, MO), Polyethylene glycol of 8,000 molecular weight (PEG-8K) was from Fisher Scientific (Fair Lawn, New Jersey), and ethanol was from Pharmco-AAPER (Louisville, KY). Medical-grade silastic tubing (3.4 mm internal dia) was purchased from Allied Biomedical (Ventura, CA).
Methods
Preparation of Implants
Implants were prepared using solvent evaporation coupled with melt extrusion technique as described previously (13, 14). Briefly, PCL-121 and PEG-8K (65:35) were dissolved in DCM while curcumin at a drug load of 20% (w/w) was dissolved in ethanol. The two solutions were mixed and the solvents were evaporated initially at 70 °C, followed by drying at 65 °C overnight under vacuum using savant SpeedVac (Thermo-Savant, Holbrook, NY) to form a molecular dispersion of the drug in polymer. The molten polymer material was then extruded into silastic tubes (internal dia 3.4 mm) at 65 °C and cut into desired lengths to obtain cylindrical implants.
Animal Studies
The animal studies were conducted after approval from the Institutional Animal Care and Use Committee of University of Louisville. Female ACI rats (5- to 6-week old) purchased from Harlan Laboratories (Indianapolis, IN), were acclimated for 1 week under vivarium conditions followed by the administration of AIN93M control diet. After 2 weeks, all the animals were subdivided into 8 groups: 1) untreated controls (n=6), 2) sham implants (n=6), 3) curcumin implants (n=6), 4) curcumin diet (n=6), 5) E2 alone (n=25), 6) sham implants+E2 (n=25), 7) curcumin implants+E2 (n=20), and 8) curcumin diet+E2 (n=20), for the 6 month time point. While for the 3 weeks and 3 month time point, all the groups contained 6 animals each.. A 1.2-cm silastic implant containing 9 mg E2 was grafted at the back of the rats as described previously (16). All the interventions - curcumin diet (1,000 ppm), curcumin implants (two 2-cm implants, 200 mg/implant, 20% w/w drug load) and sham implants were grafted 4 days prior to E2 treatment and were continued until termination of the study. Animals were palpated weekly after 12 weeks of E2 treatment for tumor appearance and tumor incidence. The experiment was terminated when tumor incidence was >80% in E2 alone-treated animals (about 6 months of E2 treatment). Animals were euthanized by asphyxiation under CO2 and all the animals were carefully examined for the presence of mammary tumors to calculate tumor volume and multiplicity as described before (16). Blood was collected by cardiac puncture. When the rats were bled on euthanasia, blood was divided into two tubes, one for serum and in another tube containing heparin for plasma. Serum from individual animals was used for E2 analysis. Plasma from individual animals was used for prolactin measurement. The remaining plasma samples were pooled to generate enough volume for curcumin levels for the 3 week and 3 month time points and from individual animals (every third rat) for the 6-month time point. The liver was cut into small pieces, snap frozen in liquid nitrogen in cryo-vials and stored at −80 °C for tissue curcumin analysis. All the implants were also recovered, cleaned of tissue debris, dried overnight under vacuum and stored for future use to measure residual curcumin.
Analysis of Residual Curcumin in the Implants
The rate of curcumin release was determined by measuring residual curcumin in the implants recovered from animals at different time intervals. Implants were dissolved in DCM:ethanol (1:1) and an aliquot of the solution was diluted (1:10) with ethanol containing 20% DCM followed by further dilution in ethanol (1:40). The curcumin concentration was measured using a UV spectrophotometer (Spectramax M2, Molecular Devices, USA) at 430 nm and the rate of curcumin release was calculated by subtracting the residual amount from initial amount per unit time. Initial measurement of these samples by HPLC gave comparable results and hence all samples were analyzed using UV spectrophotometry (15).
Plasma Prolactin
Plasma prolactin was measured by using an EIA kit (Alpco Immunoassays, Salem, NH) following manufacturer’s protocol.
Serum E2 Analysis
Serum E2 was analyzed using Roche E170 immunoassay analyzer at the University Hospital's Clinical Chemistry facility. Estradiol II reagent kit purchased from Roche Diagnostics, Inc. (Indianapolis, IN) was used following the manufacturer’s protocol.
Microsome Extraction
Liver (~100 mg) was homogenized in 0.25 M sucrose buffer (pH 7.4) at 3,000 rpm with a polytron homogenizer. The homogenate was centrifuged at 3,000xg for 20 min at 4 °C to separate the nuclear content. The supernatant was further centrifuged at 11,000xg for 20 min at 4 °C to remove mitochondrial fraction. The post-mitochondrial supernatant was then transferred to ultracentrifuge tubes and centrifuged at 100,000xg for 1 h at 4 °C to obtain the microsomal pellet which was suspended in 1 ml sucrose buffer, aliquoted and stored at −80 °C until further use.
Cytochrome P450 Activities
Microsomal proteins were quantified by bicinchoninic acid (BCA) method (17) using BCA™ Protein Assay kit (Thermo Scientific, Rockford, IL). CYP1A and 1B1 activities were determined by EROD assay with and without a selective CYP1B1 inhibitor (pyrene). CYP3A4 activity was measured using P450-Glo™ CYP3A4 assay with Luciferin-IPA following the manufacturer’s protocol by replacing NADPH regenerating system with NADPH (5 mM).
Analysis of Plasma and Tissue Curcumin Levels
Plasma (1.5 ml) was pooled from all the animals (n=6) in each group during 3 week and 3 month time points, and from every 3rd animal from 6 month time point, and 200 µl of 0.5 M sodium acetate was added to reduce the pH to 5. Plasma was then extracted thrice with 3 ml ethyl acetate. Pooled ethyl acetate extract was dried under vacuum. The dried residue was reconstituted in 100 µl of acetonitrile (ACN), one half of which was analyzed by HPLC using Shimadzu liquid chromatography system equipped with LC-10ADVP pump, RF-10AXL fluorescence detector and a Shimadzu C18 column of 5 µm particles (250×4.6 mm). Similarly, liver tissue (~500 mg) from each animal was homogenized in 3 ml PBS (pH 7.4) and 200 µl of 0.5 M sodium acetate was added. The homogenate was then extracted twice with 2 volumes of ethyl acetate. The extract was separated, evaporated under vacuum and the residue was reconstituted in 1 ml ACN. The ACN solution was filtered through 0.45 µ glass-microfiber filter again evaporated, the residue was finally reconstituted in 100 µl ACN, and one-half of it was analyzed by HPLC. Three curcuminoids were separated using ACN and 1% citric acid (adjusted to pH 2.5) mobile phases at a flow rate of 1 ml/min with a gradient elution. The ACN concentration was increased from 0 to 30% in first 5 min, followed by an increase to 45% in next 5 to 20 min. ACN was then maintained at this ratio till 36 min. Curcumin was detected using 410 and 500 nm as excitation and emission maxima, respectively, in the fluorescence detector.
Statistical Analysis
Generalized linear model approach was used to analyze the group effect of tumor volume, cube root tumor volume and tumor number using SAS version 9.2 software. The p value of Shapiro-Wilk normality test for tumor volume and for cube root tumor volume was found to be <0.0001 and 0.7505, respectively. Hence, cube root tumor volume was considered to be distributed normally and was used to calculate statistically significant differences in tumor volumes with different interventions. The tumor-free survival (TFS) was estimated by the Kaplan-Meier method (18). Differences in the survival curves are evaluated through the estimated hazard rates using the un-weighted log-rank tests (19). The TFS time was determined as the time from the beginning of the study until the first occurrence of the tumor. All calculations were performed with SAS statistical software (SAS Institute Inc., Cary, NC).
Results and Discussion
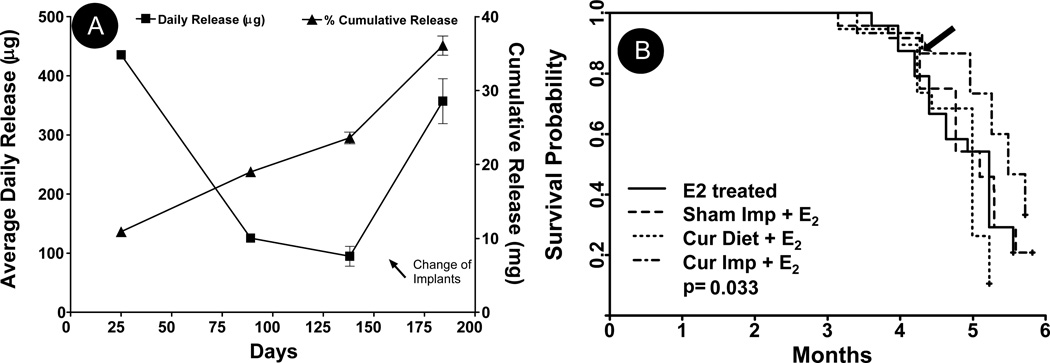
We previously showed that curcumin release from polymeric implants follow a biphasic release pattern characterized by an initial burst ranging from 7–10 days, followed by a more controlled release phase (13, 15). Implants formulated for this study also reproducibly exhibited biphasic release kinetics with 10.9 mg of curcumin released over a period of 25 days with an average release of 436±5 µg/day from each implant (Fig. 1A). However, the release was much higher (~1.8 mg; 4.5%) on day 1 that decreased slowly with time, as reported earlier (13). The release dropped significantly by 90 days with only 19 mg (~48%) cumulative curcumin released into the systemic circulation in 3 months at a rate of ~127±5 µg/day/implant between 25–90 days. Since the release was expected to drop further with time (14), implants were replaced with new implants at ~138 days when average daily drug release dropped to ~94±16 µg/day/implant. Grafting of new implants provided a burst release of curcumin at the time of tumor development and provided a higher daily drug release for the rest of the period to achieve maximum chemopreventive and chemotherapeutic activities of curcumin during the tumor initiation and promotion phases. Furthermore, no significant changes in diet intake or weight gain were observed after the implantation of second implants. The average daily drug release from new implants was 357±38 µg/day/implant from 138 to 180 days, with a total release of 36.1 mg (45%) from all implants combined (Fig. 1A).
Fig. 1.
(A) Curcumin implants placed subcutaneously were removed when the rats were anaesthetized at various time points (25, 89, 138 and 184 days). In vivo release of curcumin from a 2-cm implant (200 mg, 20% load) were plotted as cumulative curcumin released at various time points and the cumulative release were divided by the number of days they remained in the rats to derive the daily average release; data represent an average of three animals (±SD). (B) The tumor-free survival (TFS) is estimated by the Kaplan-Meier method. Differences in the survival curves are evaluated through the estimated hazard rates using the un-weighted log-rank tests. The TFS time is be determined as the time from on study until the first occurrence of tumor. The four groups represent female ACI rats treated with E2 implant (n=25) along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) (n = 20 each group). All 4 groups are significantly different (p=0.0328); these are mainly due to significant difference between the curcumin implant and curcumin diet groups (p=0.0012).The arrows in panels A and B indicate the time at which new curcumin implants were grafted.
The chemopreventive efficacy of curcumin delivered by implants or diet was measured in ACI rats implanted with silastic estrogen implants to induce mammary tumors. It has been previously shown by us and others that the mammary tumors developed are intraductal carcinoma of the comedo type. In addition, few papillary carcinoma and areas of invasion were present (16, 20, 21). Tumor incidence was measured by weekly palpation for appearance of any tumors starting 12 weeks of E2 treatment. The first tumor in all the E2-treated groups appeared between 95–110 days. Although curcumin delivered via diet did not affect the appearance of first tumor (95 days), curcumin implants delayed the tumor latency by 7 days. However, subsequent appearance of tumors was similar to control treatments suggesting insufficient doses of curcumin reaching into the systemic circulation. The implants, therefore, were changed at around 135–140 days at the peak tumor development stage to provide a burst release of curcumin when ~20% of animals in each group developed tumors. Tumor-free survival as estimated by the Kaplan-Meier method (Fig. 1B) demonstrated a significant difference (p=0.03) for all four groups, but the comparison of the curcumin diet or the curcumin implant group with respective controls did not achieve significance (p=0.299 and 0.181, respectively). However, the difference between the diet and implant group was highly significant (p=0.0012), implying curcumin delivered via the implant route was more efficient than when delivered via diet.
As is evident from Fig. 1B, the burst release of curcumin provided by new implants almost completely stopped the appearance of new tumors for at least 3 weeks and the delay was maintained during the rest of the period. Measurement of palpable tumor volume with time further showed that after a delay of 3 weeks with new set of implants, the slope of the tumor growth curve of the curcumin implants group was almost similar to the sham implants group (supplementary Fig S-2). This observation suggests that the curcumin delivered via implants did not have any effect on the growth of tumors and probably had only chemopreventive activity (as evident from delayed tumor initiation) at least in this model.
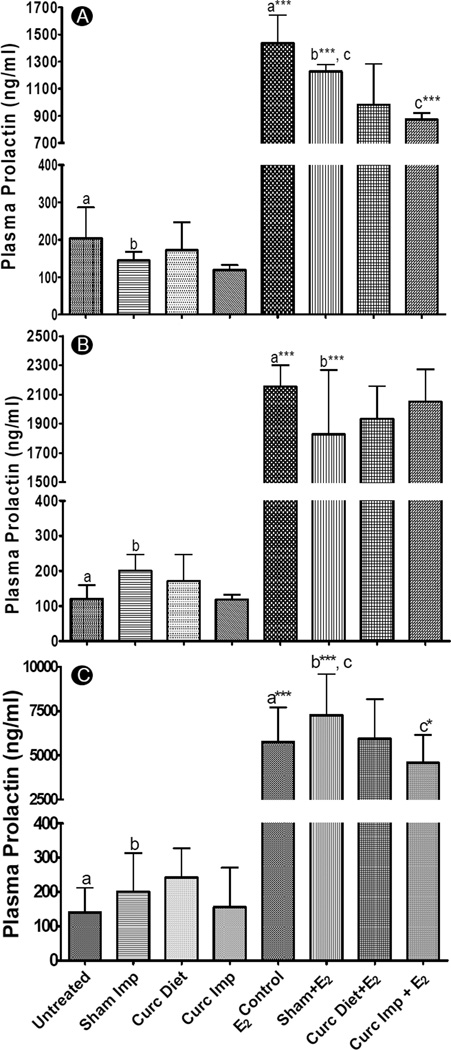
To further substantiate these findings, we also measured serum prolactin levels at all the 3 time points. Prolactin is a pituitary hormone released during late pregnancy and lactation by the presence of high E2 concentrations in systemic circulation. It has been proposed that prolactin produced by mammary tissues during carcinogenesis acts as an autocrine and paracrine growth factor for mammary tissue proliferation (22). Furthermore, prolactin release from pituitary isografts have been shown to increase mammary tumor incidence in ovariectomized mice, suggestive of its mitogenic role in promoting the growth of already transformed normal and pre-neoplastic cells (23). As evident from Fig. 2A, curcumin implants were significantly effective at reducing the plasma prolactin levels at 3 weeks (p<0.001) as compared to sham implants but were ineffective at the 3 month time point (Fig 2B). Once the old implants were replaced with new implants, the burst release of high curcumin doses again inhibited E2 -mediated prolactin release at 6 months (p<0.05) (Fig 2C). The dietary curcumin, on the other hand, was ineffective at all the time points and may need much higher doses to exert chemopreventive activity. These results suggest that release of bolus doses of curcumin at 3 weeks (from initially grafted implants) and at 6 months (from new set of implants grafted after 135 days) were required to significantly reduce plasma prolactin levels.
Fig. 2.
Plasma prolactin levels of female ACI rats treated with or without a silastic E2 implant along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) for 3 weeks (n=6) (A), 3 months (n=6) (B) and 6 months (n=6–8) (C). Plasma prolactin was measured by using an EIA kit following manufacturer’s protocol and a p value of <0.05 was considered significant. Groups compared are denominated with the same alphabets (* p<0.05, ** p<0.01 and *** p<0.001).
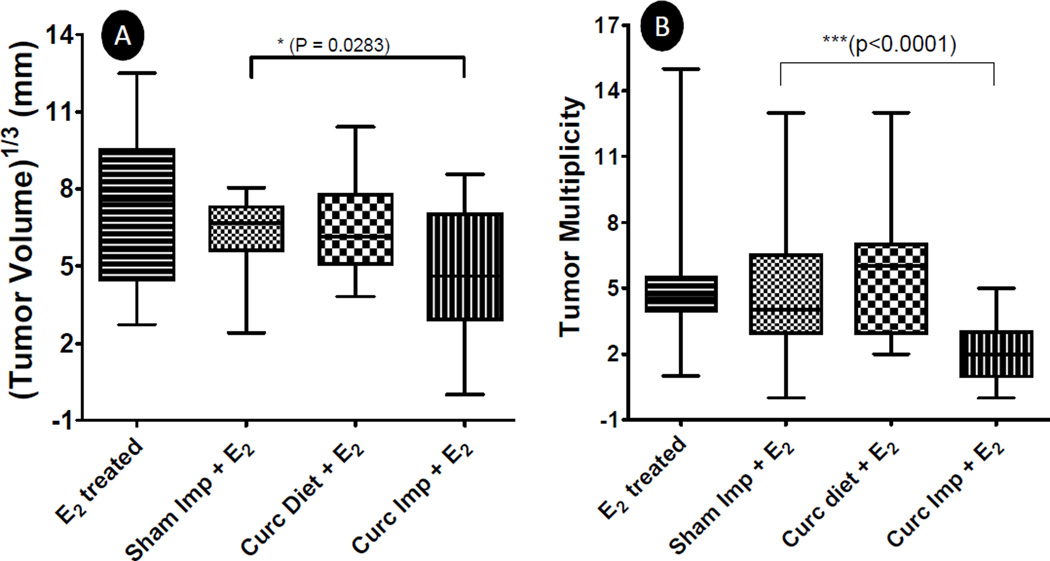
At the termination of the study, all tumors were measured to determine the average tumor volume (Fig. 3A). Curcumin diet was found to be ineffective as compared to the E2 alone-treated group. However, curcumin implants were found to modestly but significantly (p<0.05) reduce the tumor volume (by ~35%) as compared to E2 alone treatment. Since curcumin implants as such were not found to affect tumor growth kinetics in this model (supplementary Fig. S-2), it appears that this reduction in tumor volume was due to 3 weeks delay in tumor appearance after the grafting of a new set of implants. This notion is further supported from the tumor multiplicity data (Fig. 3B) as curcumin implants were highly effective (p<0.0001) in reducing the tumor multiplicity compared to sham implants (2±1 versus 5±2); dietary curcumin, however, was ineffective (Fig. 3B). These results are consistent with other studies where it has been shown in 7,12-dimethylbenz(a)anthracene-induced mammary cancer model that chemopreventive agents like indole-3-carbinol exert maximum effect when given during the pre-initiation stage (24, 25) and are more effective in reducing tumor multiplicity than tumor volume (26). Significant reduction in tumor multiplicity observed with curcumin implants, therefore, again suggests that higher doses of curcumin delivered by the next set of implants exerted maximum chemopreventive activity by blunting the initiation of new tumors. It has also been shown that potent inhibition of tumor multiplicity often results from changes in hepatic carcinogen-metabolizing enzymes that are preferentially induced or inhibited by chemopreventive agents to decrease the formation of reactive carcinogen metabolites and enhance carcinogen detoxification (26). This favorable alteration in carcinogen metabolism, then ultimately results in reduced genetic mutations blunting the neoplastic trigger.
Fig. 3.
Mammary tumor volume (A) and tumor multiplicity (B) in female ACI rats treated with a silastic E2 implant along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) for over a period of 6 months (n=20). The rats were palpated for tumor occurrence every week and when the E2-treated group achieved > 90% tumor incidence, the study was terminated. Mammary tumors were harvested, measured for tumor volume and counted for tumor multiplicity. A significant difference was observed between sham implant and curcumin implant group as indicated.
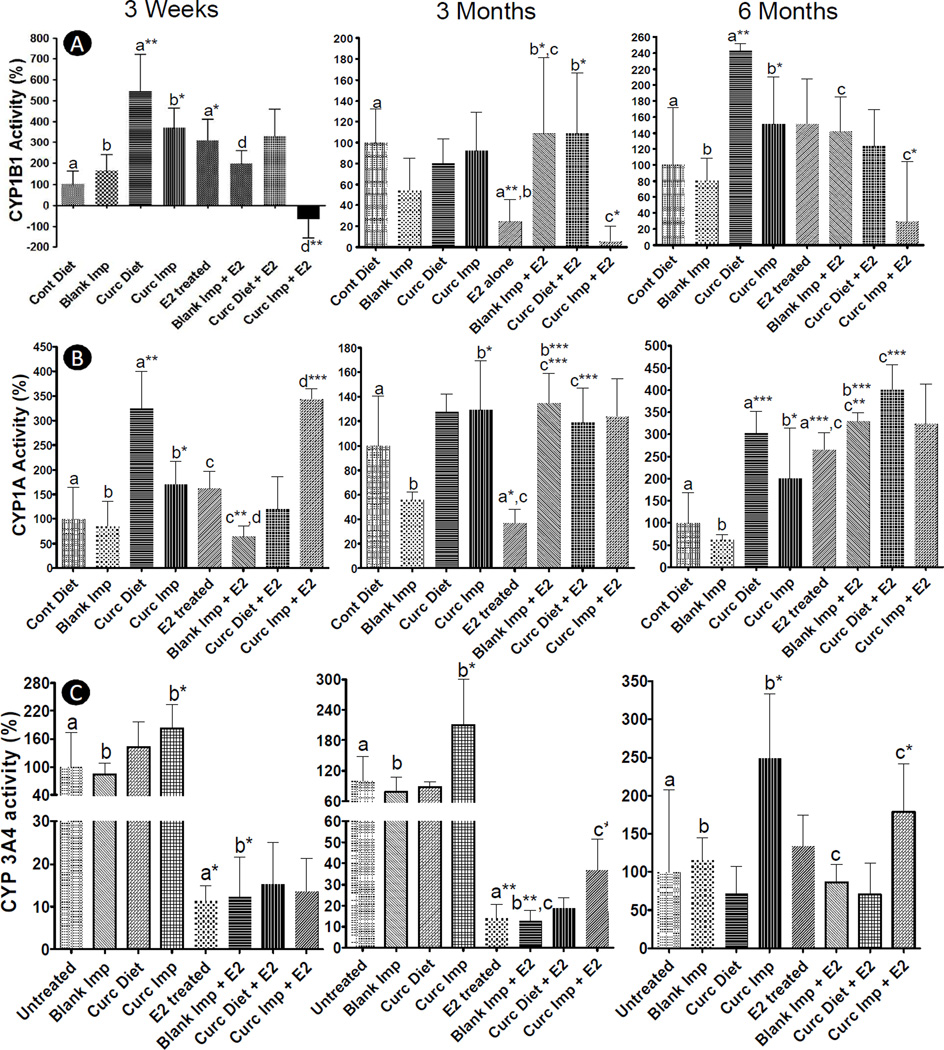
During initiation phase highly reactive carcinogenic E2 metabolites like estradiol-3,4-quinone binds with DNA leading to genetic mutations that result in neoplastic transformation of mammary cells (27). Initiation phase is followed by tumor promotion phase which is mediated by hormonal activity of E2 and 4-hydroxy E2 (4-E2) via their interaction with estrogen receptor α (ERα) and subsequent activation of proliferation signaling (28). Previous studies of estrogen-induced mammary tumorigenesis have shown that E2-mediated mammary tumorigenesis is a two-step process (27). Since curcumin implants were found to exert their effect only during the initiation stages, it appears that favorable alteration of hepatic E2-metabolizing cytochrome P450s played a significant role in their efficacy. E2 is metabolized by three distinct P450s to generate different metabolites (shown in Supplementary Fig. S-3). E2 metabolism by CYP1B1 results in formation of 4-E2 metabolite, a potent carcinogen and initiator of carcinogenesis cascade (29). On the other hand, E2 metabolism by CYP1A (1A1 and 1A2) and CYP3A4 are true detoxification pathways forming 2-hydroxy E2 (2-E2) metabolite (anti-carcinogenic) and 16α-hydroxy E2 metabolite, respectively (29). Since previous studies showed that modulation of hepatic xenobiotic-metabolizing enzymes affects tumor initiation and tumor incidence (26, 30), we extracted hepatic microsomes and analyzed their CYP1B1, CYP1A (1A1 and 1A2) and CYP3A4 activities (Fig 4A, B and C).
Fig. 4.
CYP 1B1 (A), 1A (1A1 and 1A2) (B) and 3A4(C) activities of hepatic microsomes isolated from female ACI rats treated with or without a silastic E2 implant along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) for over a period of 3 weeks, 3 months and 6 months. CYP1A and 1B1 activities were determined by EROD assay with and without a selective CYP1B1 inhibitor (pyrene). CYP3A4 activity was measured using P450-Glo™ CYP3A4 assay with Luciferin-IPA following manufacturer’s protocol by replacing NADPH regenerating system with NADPH (5 mM). Groups compared are denominated with the same alphabets (* p<0.05, ** p<0.01 and *** p<0.001).
CYP1B1 activity exhibited time-dependent kinetics with slow sustained E2 delivery by silastic implants (Fig 4A). Enzyme activity increased significantly after 3 weeks of E2 treatment when compared to untreated 3-week control animals, which decreased to less than the value in untreated controls by 3 months of treatment. At 6 months, an increased CYP1B1 activity was again observed, but the increase was insignificant due to wide variation in untreated and E2-treated animals. Both curcumin diet and curcumin implant group (in the absence of E2 implants) increased 1B1 activity both at 3 weeks and 6 months as compared to untreated control animals (p<0.05). However, in presence of E2 implants, dietary curcumin showed no effect on CYP1B1 activity at 3 weeks and 6 months, but increased it significantly at 3 months (p<0.05). Curcumin implants, on the other hand, significantly reduced CYP1B1 activity at all the time points in the presence of E2 Implants when compared with sham implants with the E2 group (p<0.05). Since CYP1B1 is known to be the major xenobiotic-metabolizing enzyme responsible for formation of 4-E2 (31), inhibition of CYP1B1 activity by systemic delivery of curcumin can result in reduced formation of 4-E2 delaying tumor initiation and blunting tumor multiplicity.
CYP1A1 activity also showed time-dependent and exposure-dependent kinetics with E2 treatment. Enzyme activity was found to increase both at 3 weeks (though insignificant) and 6 months (significant) but significantly decreased at 3 months of E2 treatment as compared to untreated animals. Both curcumin diet as well as curcumin implants increased CYP1A1 activity as compared to untreated animals in absence of E2 (p<0.05). However, in presence of E2, dietary curcumin did not have any effect on CYP1A1 activity at 3 weeks but increased significantly at both 3 and 6 months of E2 treatment (p<0.001). Curcumin implants, on the other hand, significantly increased CYP1A1 activity at 3 weeks as compared to animals treated with sham implants and E2 (p<0.001).
CYP3A4 activity significantly decreased both at 3 weeks and at 3 months of E2 treatment as compared to vehicle treatment. Although an increasing trend in CYP3A4 activity was observed at 6 months of E2 treatment, due to wide variation in untreated animals significance was not achieved. Curcumin diet was not found to exert any effect on CYP3A4 activity with or without E2, but curcumin implants significantly (p<0.05) increased CYP3A4 activity at all the time points in absence of E2. However, in presence of E2, curcumin implants increased CYP3A4 activity both at 3 weeks and 6 months (p<0.05) when high curcumin concentrations were delivered from new implants (300–400 µg/day/implant) as compared to 3 months of implantation when release was low (~120 µg/day/implant) (Fig 1A.).
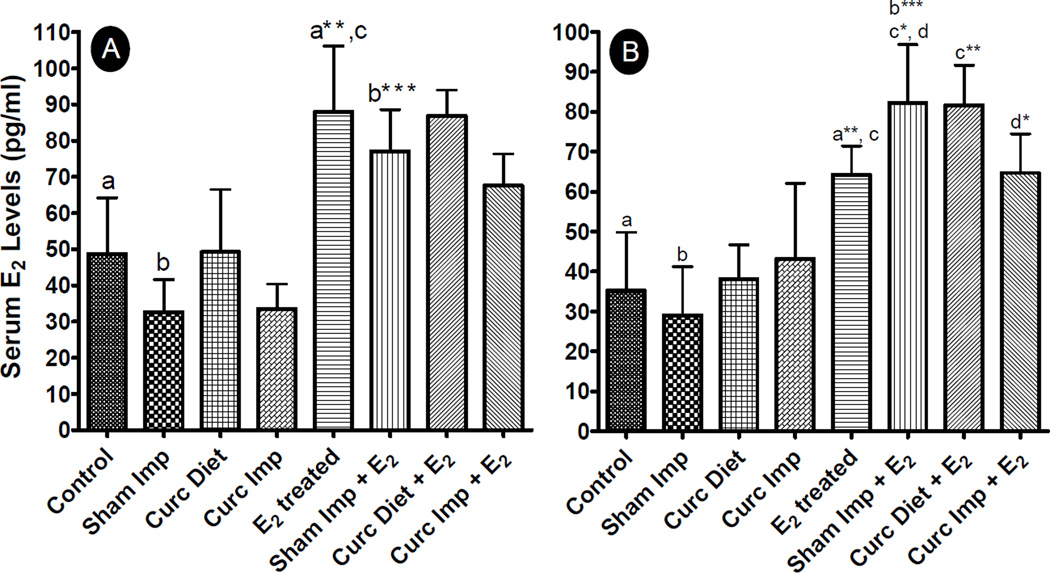
Since CYP3A4 is a major E2-detoxifying enzyme (31), an increase in its activity leads to increased metabolism and excretion of non-carcinogenic E2 metabolites from the systemic circulation and hence better therapeutic efficacy. Induction of E2 metabolism by curcumin, particularly when delivered via implants, was further supported by circulating E2 levels as reflected by measurement of E2 in serum both after 3 weeks and 3 months, as maximum alteration in enzyme activities was observed at these time points (Fig. 5). As is evident from Fig. 5, administration of E2 by silastic implants significantly increased serum E2 concentration at all the time points as compared to untreated animals. Curcumin diet and implants both did not have any effect on endogenous levels of E2, and serum estradiol concentrations were similar to untreated animals in absence of silastic E2 implants. However, in presence of exogenous E2, serum E2 concentration was found to be low with curcumin implants both at 3 weeks and 3 months when compared with sham implants+E2 group, although the decrease was significant only at 3 months (p < 0.05). Dietary curcumin, however, did not decrease the serum levels when compared with E2 alone group but in fact significantly increased serum E2 concentration (p<0.005). It is to be noted that an increased serum E2 concentration was observed in sham implants+ E2 group also when compared to E2 alone group.
Fig. 5.
Serum estradiol (E2) levels in female ACI rats treated with sham or E2 implant along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) for over a period of 3 weeks (A) and 3 months (B) (n=6 for each group). Serum E2 was measured using Estradiol II reagent kit as per manufacturer’s instructions. Groups compared are denominated with the same alphabets (* p<0.05, ** p<0.01 and *** p<0.001).
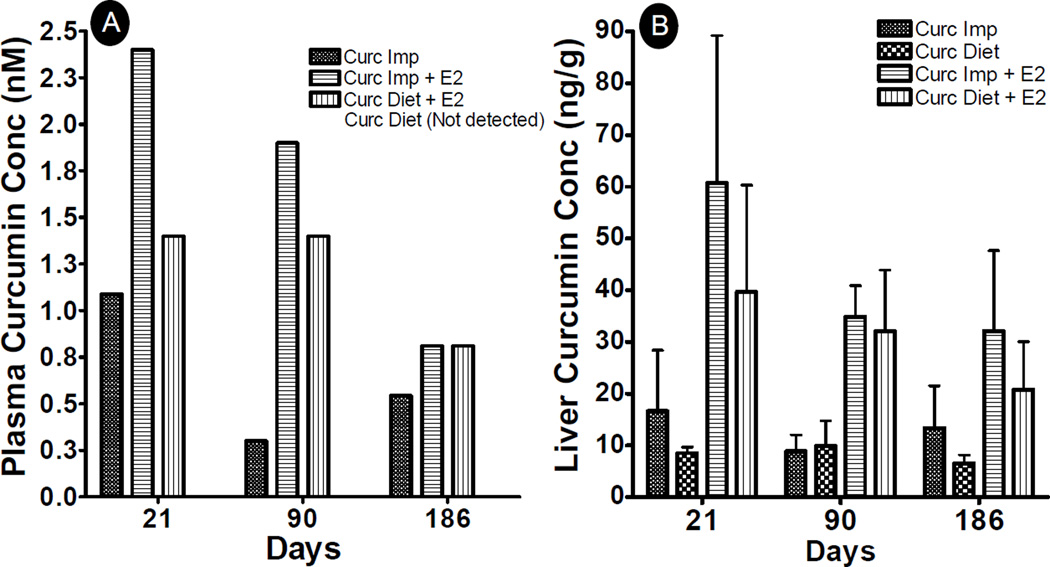
Since only curcumin delivered by implants was able to modulate E2 metabolism as compared to dietary route, we further measured curcumin levels both in plasma (Fig 6A) as well as in liver (Fig 6B) to determine if these differences in efficacy by both routes were due to differences in curcumin concentrations. Curcumin was undetectable in plasma at all the time points when given by dietary route, consistent with other studies (32), but was found to be 1.1, 0.3 and 0.6 nM in the case of curcumin implants in the absence of E2 (Fig 6A). On the other hand, significantly higher concentrations of curcumin were observed in the presence of exogenous E2 as compared to E2 only-treated animals. In the presence of E2 implants, curcumin was detected at levels of 1.4 nM at both 3 week and 3 month time points and 0.9 nM after 6 months of dietary administration and was 2.4, 1.9 and 0.9 nM after 3 weeks, 3 months and 6 months of E2 treatment with curcumin implants.
Fig. 6.
Plasma (A) and liver (B) curcumin concentrations in female ACI rats treated with sham or E2 implant along with either two sham polymeric implants (2 cm), or two curcumin implants (2 cm, 20% load) or curcumin diet (1,000 ppm) for over a period of 3 weeks, 3 months and 6 months. Plasma was pooled from all the animals (n=6) in each group during 3 week and 3 month time points, and from every 3rd animal from 6 month time point, acidified and extracted with ethyl acetate. The dried residue was reconstituted in acetonitrile and analyzed by HPLC.
Analysis of liver curcumin concentration also revealed a similar trend. Although curcumin was not undetected in plasma after dietary administration, it was found in liver tissue at all the time points. In the absence of E2, almost similar levels of curcumin were observed at 3 weeks (9±1 ng/g), 3 months (9±3 ng/g) and 6 months (9±1 ng/g) by dietary curcumin. Curcumin implants on the other hand resulted in nearly 2-fold higher liver concentration of 16.6±11.7 ng/g after 3 weeks, which decreased slightly to 8.9±3 ng/g after 3 months and increased again to 13.4±8 ng/g at 6 months treatment after replacing the implants at 135 days. As was observed in plasma, significantly increased curcumin concentrations were detected in liver tissue in the presence of E2 implants. Curcumin concentrations of 40±21 ng/g, 32±12 ng/g and 21±9 ng/g were observed by dietary route after 3 weeks, 3 months and 6 months of E2 treatment. Somewhat higher curcumin concentrations of 61±28, 35±6, and 32±16 ng/g tissue were observed by implant route after 3 weeks, 3 months and 6 months of E2 treatment, respectively. Curcumin levels in liver tissue resulting from implant versus dietary routes were not significantly different. However, we have shown in our earlier studies that curcumin levels achieved via burst release from implants (1–12 days) are higher than dietary curcumin (15). Since our studies showed that burst release was a pre-requisite to achieve chemopreventive efficacy for inhibition of tumor initiation, as demonstrated by grafting of a new set of implants, these results were in concordance with previously published studies where high doses were established to exploit curcumin’s chemopreventive potential (1, 5). It is to be noted that implants showed efficacy even when the curcumin doses delivered were around 11-, 23- and 24-fold lower than the dietary route at 3 week, 3 month and 6 month time points, respectively.
These observations also suggest that not only curcumin (at least via implant route) altered the hepatic metabolism of E2 but the presence of E2 also altered curcumin metabolism to a significant extent. Curcumin is known to get metabolized by both CYP3A4 (33) and UGT (32). It is evident from Fig. 4C that administration of E2 significantly decreased CYP3A4 activity at initial time points, consequently increasing liver curcumin concentration (Fig 6B). Measurement of UGT activity also showed a similar decrease (data not shown) suggestive of decreased hepatic curcumin metabolism.
Curcumin exerts its chemopreventive efficacy through a wide range of cellular processes including anti-inflammation, anti-proliferative and anti-tumorigenesis (34–36). Most of the activities are attributed to its antioxidant activity and inhibition of cell signalling pathways at various levels, especially through inhibition of NFκB (37) in preclinical studies. Curcumin also induces apoptosis especially in the G2 phase of cell cycle (38, 39) and blocked tumor initiation in chemically induced mammary tumors (40–42). However, it was inefficient in clinical studies up to a dose of 3.6 g per person (43) and more than 8 g was not well-tolerated (44). We demonstrate that by providing the curcumin directly into the systemic circulation using subcutaneous implants, we were able to increase the tissue bioavailability and chemopreventive efficacy. Even though there are several mechanisms by which curcumin can exert the chemopreventive potential, we had focused this study specifically on the estrogen metabolism since these rats do not develop mammary tumors without the estrogen implants.
Conclusions
Curcumin implants showed diffusion-mediated biphasic release kinetics both under in vitro as well as in vivo conditions, with a 2-fold higher drug release in vivo as compared to in vitro. Systemic delivery of Curcumin via implants significantly reduced the plasma prolactin levels, estrogen-induced mammary tumor burden and tumor multiplicity as compared to the curcumin administered in the diet. The enhanced chemopreventive efficacy of curcumin delivered via implants was found to be due to favorable modulation of hepatic CYP P450s. CYP1B1 activity was found to be significantly reduced by curcumin implants while it was increased by the curcumin diet. CYP1A and CYP3A4 activities were found to be increased significantly by implant route. Together, these data are suggestive of increased metabolism of estradiol to carcinogenic metabolites by curcumin diet and to non-carcinogenic metabolites by curcumin implants. This differential activity was found to be due to different liver and plasma concentrations from both the routes. Curcumin concentration was found to be much higher both in plasma as well as in the liver when delivered via implants as compared to the curcumin diet.
Supplementary Material
Acknowledgement
Authors are thankful to Mr. Jeyaprakash Jeyabalan, Drs., Farrukh Aqil, Radha Munagala, Gilandra K. Russell and Pengxiao Cao for assistance with tumor measurements and tissue collection during animal euthanasia at the end of the study. The authors thank Ms. Meghan Hancock, Assistant Director at the Writing Center for editiorial services.
Financial Support: This research work was supported from USPHS grants CA-118114, CA-125152, and Agnes Brown Duggan Endowment. Dr. Ramesh Gupta holds the Agnes Brown Duggan Chair in Oncological Research. Dr. Shesh Rai is supported by JG Brown Cancer Center and is the Wendell Cherry Chair in Clinical Trial Research.
Footnotes
Conflicts of Interest: Authors declare no conflicts of interest
References
- 1.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends Pharmacol Sci. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Curcumin and cancer: an "old-age" disease with an "age-old" solution. Cancer Lett. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC. Advanced Drug-Delivery Systems of Curcumin for Cancer Chemoprevention. Cancer Prev Res (Phila) 2011;4:1158–1171. doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–1773. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 5.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 6.Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur J Pharm Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 7.Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, et al. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT-independent pathway. Clin Cancer Res. 2008;14:6228–6236. doi: 10.1158/1078-0432.CCR-07-5177. [DOI] [PubMed] [Google Scholar]
- 8.Shahani K, Swaminathan SK, Freeman D, Blum A, Ma L, Panyam J. Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 2010;70:4443–4452. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Haddadi A, Molavi O, Lavasanifar A, Lai R, Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J Biomed Mater Res A. 2008;86:300–310. doi: 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]
- 10.Boriwanwattanarak P, Ingkaninan K, Khorana N, Viyoch J. Development of curcuminoids hydrogel patch using chitosan from various sources as controlled-release matrix. Int J Cosmet Sci. 2008;30:205–218. doi: 10.1111/j.1468-2494.2008.00437.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee MH, Lin HY, Chen HC, Thomas JL. Ultrasound mediates the release of curcumin from microemulsions. Langmuir. 2008;24:1707–1713. doi: 10.1021/la7022874. [DOI] [PubMed] [Google Scholar]
- 12.Marczylo TH, Verschoyle RD, Cooke DN, Morazzoni P, Steward WP, Gescher AJ. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother Pharmacol. 2007;60:171–177. doi: 10.1007/s00280-006-0355-x. [DOI] [PubMed] [Google Scholar]
- 13.Bansal SS, Kausar H, Aqil F, Jeyabalan J, Vadhanam MV, Gupta RC, et al. Curcumin Implants for Continuous Systemic Delivery: Safety and Biocompatibility. Drug Deliv Trans Res. 2011 doi: 10.1007/s13346-011-0028-0. In press. [DOI] [PubMed] [Google Scholar]
- 14.Gupta RC, Bansal SS, Aqil F, Jeyabalan J, Cao P, Kausar H, et al. Controlled-release systemic delivery - a new concept in cancer chemoprevention. Carcinogenesis. 2012;33:1608–1615. doi: 10.1093/carcin/bgs209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bansal SS, Kausar H, Vadhanam MV, Ravoori S, Gupta RC. Controlled systemic delivery by polymeric implants enhances tissue and plasma curcumin levels compared with oral administration. Eur J Pharm Biopharm. 2012;80:571–577. doi: 10.1016/j.ejpb.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravoori S, Vadhanam MV, Sahoo S, Srinivasan C, Gupta RC. Mammary tumor induction in ACI rats exposed to low levels of 17beta-estradiol. Int J Oncol. 2007;31:113–120. [PubMed] [Google Scholar]
- 17.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. 2nd ed. New York: Springer Verlag; 2003. [Google Scholar]
- 20.Shull JD, Spady TJ, Snyder MC, Johansson SL, Pennington KL. Ovary-intact, but not ovariectomized female ACI rats treated with 17beta-estradiol rapidly develop mammary carcinoma. Carcinogenesis. 1997;18:1595–1601. doi: 10.1093/carcin/18.8.1595. [DOI] [PubMed] [Google Scholar]
- 21.Jeyabalan J, Aqil F, Munagala R, Annamalai L, Vadhanam MV, Gupta RC. Chemopreventive and therapeutic activity of dietary blueberry against estrogen-mediated breast cancer. Journal of Agricultural and Food Chemistry. 2013 doi: 10.1021/jf403734j. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clevenger CV, Furth PA, Hankinson SE, Schuler LA. The role of prolactin in mammary carcinoma. Endocr Rev. 2003;24:1–27. doi: 10.1210/er.2001-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welsch CW, Jenkins TW, Meites J. Increased incidence of mammary tumors in the female rat grafted with multiple pituitaries. Cancer Res. 1970;30:1024–1029. [PubMed] [Google Scholar]
- 24.Malejka-Giganti D, Niehans GA, Reichert MA, Bliss RL. Post-initiation treatment of rats with indole-3-carbinol or beta-naphthoflavone does not suppress 7,12-dimethylbenz[a]anthracene-induced mammary gland carcinogenesis. Cancer Lett. 2000;160:209–218. doi: 10.1016/s0304-3835(00)00594-2. [DOI] [PubMed] [Google Scholar]
- 25.Malejka-Giganti D, Parkin DR, Bennett KK, Lu Y, Decker RW, Niehans GA, et al. Suppression of mammary gland carcinogenesis by post-initiation treatment of rats with tamoxifen or indole-3-carbinol or their combination. Eur J Cancer Prev. 2007;16:130–141. doi: 10.1097/01.cej.0000228401.14988.50. [DOI] [PubMed] [Google Scholar]
- 26.Grubbs CJ, Steele VE, Casebolt T, Juliana MM, Eto I, Whitaker LM, et al. Chemoprevention of chemically-induced mammary carcinogenesis by indole-3-carbinol. Anticancer Res. 1995;15:709–716. [PubMed] [Google Scholar]
- 27.Liehr JG. Dual role of oestrogens as hormones and pro-carcinogens: tumour initiation by metabolic activation of oestrogens. Eur J Cancer Prev. 1997;6:3–10. doi: 10.1097/00008469-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Zhu BT, Conney AH. Functional role of estrogen metabolism in target cells: review and perspectives. Carcinogenesis. 1998;19:1–27. doi: 10.1093/carcin/19.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Huang Z, Guengerich FP, Kaminsky LS. 16Alpha-hydroxylation of estrone by human cytochrome P4503A4/5. Carcinogenesis. 1998;19:867–872. doi: 10.1093/carcin/19.5.867. [DOI] [PubMed] [Google Scholar]
- 30.Malejka-Giganti D, Bennett KK, Culp SJ, Beland FA, Shinozuka H, Bliss RL. Suppression of 7,12-dimethylbenz[a]anthracene-induced mammary carcinogenesis by pre-initiation treatment of rats with beta-naphthoflavone coincides with decreased levels of the carcinogen-derived DNA adducts in the mammary gland. Cancer Detect Prev. 2005;29:338–347. doi: 10.1016/j.cdp.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115–124. doi: 10.1016/j.canlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, et al. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 33.Wahlang B, Pawar YB, Bansal AK. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur J Pharm Biopharm. 2011;77:275–282. doi: 10.1016/j.ejpb.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB, Sundaram C, Malani N, Ichikawa H. Curcumin: the Indian solid gold. Advances in experimental medicine and biology. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 35.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. The AAPS journal. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta SC, Patchva S, Koh W, Aggarwal BB. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clinical and experimental pharmacology & physiology. 2012;39:283–299. doi: 10.1111/j.1440-1681.2011.05648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Gupta SC, Park B, Yadav VR, Aggarwal BB. Turmeric (Curcuma longa) inhibits inflammatory nuclear factor (NF)-kappaB and NF-kappaB-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. Molecular nutrition & food research. 2012;56:454–465. doi: 10.1002/mnfr.201100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choudhuri T, Pal S, Agwarwal ML, Das T, Sa G. Curcumin induces apoptosis in human breast cancer cells through p53-dependent Bax induction. FEBS letters. 2002;512:334–340. doi: 10.1016/s0014-5793(02)02292-5. [DOI] [PubMed] [Google Scholar]
- 39.Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. The Journal of biological chemistry. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- 40.Carroll CE, Benakanakere I, Besch-Williford C, Ellersieck MR, Hyder SM. Curcumin delays development of medroxyprogesterone acetate-accelerated 7,12-dimethylbenz[a]anthracene-induced mammary tumors. Menopause. 2010;17:178–184. doi: 10.1097/gme.0b013e3181afcce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang MT, Lou YR, Xie JG, Ma W, Lu YP, Yen P, et al. Effect of dietary curcumin and dibenzoylmethane on formation of 7,12-dimethylbenz[a]anthracene-induced mammary tumors and lymphomas/leukemias in Sencar mice. Carcinogenesis. 1998;19:1697–1700. doi: 10.1093/carcin/19.9.1697. [DOI] [PubMed] [Google Scholar]
- 42.Pereira MA, Grubbs CJ, Barnes LH, Li H, Olson GR, Eto I, et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis. 1996;17:1305–1311. doi: 10.1093/carcin/17.6.1305. [DOI] [PubMed] [Google Scholar]
- 43.Sharma RA, Euden SA, Platton SL, Cooke DN, Shafayat A, Hewitt HR, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10:6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 44.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.