Abstract
In systemic sclerosis (SSc), a common and etiologically mysterious form of scleroderma (defined as pathologic fibrosis of the skin), previously healthy adults acquire fibrosis of the skin and viscera in association with autoantibodies [1]. Familial recurrence is extremely rare and causal genes have not been identified. While the onset of fibrosis in SSc typically correlates with the production of autoantibodies, whether they contribute to disease pathogenesis or simply serve as a marker of disease remains controversial and the mechanism for their induction is largely unknown [2]. The study of SSc is hindered by a lack of animal models that recapitulate the etiology of this complex disease. To gain a foothold in the pathogenesis of pathologic skin fibrosis, we chose to study stiff skin syndrome (SSS), a rare but tractable Mendelian disorder that shows childhood onset of diffuse skin fibrosis with autosomal dominant inheritance and complete penetrance. We showed that SSS is caused by heterozygous missense mutations in the gene (FBN1) encoding fibrillin-1, the major constituent of extracellular microfibrils [3]. Notably, SSS mutations all localize to the only domain in fibrillin-1 that harbors an Arg-Gly-Asp (RGD) motif needed to mediate cell-matrix interactions by binding to cell-surface integrins [3]. Here we show that mouse lines that harbor analogous amino acid substitutions in fibrillin-1 recapitulate aggressive skin fibrosis that is prevented by integrin-modulating therapies and reversed by antagonism of the pro-fibrotic cytokine transforming growth factor β (TGFβ). Mutant mice show skin infiltration of pro-inflammatory immune cells including plasmacytoid dendritic, T helper, and plasma cells, and autoantibody production; these findings are normalized by integrin-modulating therapies or TGFβ antagonism. These data show that alterations in cell-matrix interactions are sufficient to initiate and sustain inflammatory and pro-fibrotic programs and highlight novel therapeutic strategies.
Fibrillin-1 contributes to the regulation of TGFβ, a cytokine that has been descriptively linked to many fibrotic diseases including both SSS and SSc [3, 4]. TGFβ is secreted from the cell in the context of a large latent complex (LLC) that includes the active cytokine bound to a dimer of its processed N-terminal propeptide, latency-associated peptide (LAP), which in turn binds to latent TGFβ-binding proteins (LTBPs) [5]. Studies in mouse models and in vitro have shown that fibrillin-1 directly interacts with LTBPs, allowing sequestration of the LLC by microfibrils [5].
Mutations throughout the FBN1 gene also cause Marfan Syndrome (MFS), a disorder characterized by bone overgrowth, ocular lens dislocation, and aortic dilatation [6]. Failed matrix sequestration of the LLC in fibrillin-1-deficient patients and mice promotes increased activation of and signaling by TGFβ. SSS mutations are specifically localized to the 4th transforming growth factor-β binding protein-like domain (TB4) of fibrillin-1, which encodes the RGD motif through which fibrillin-1 binds integrins αvβ3, α5β1, and αvβ6 [3, 5].
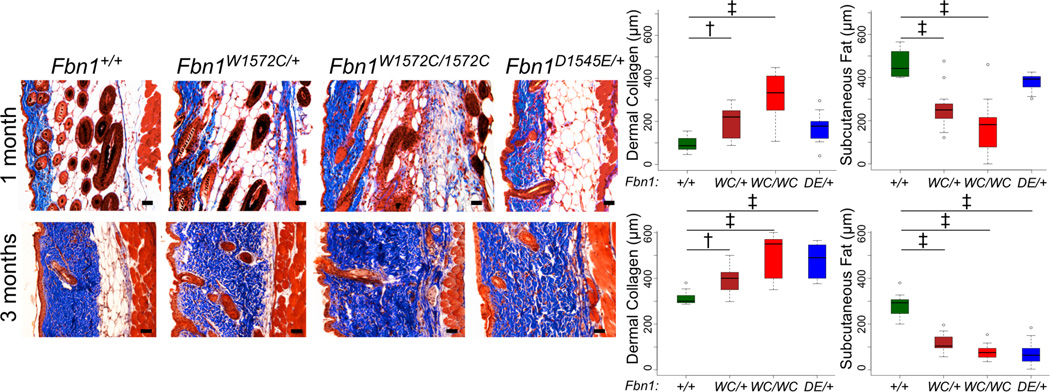
To determine if failed interaction between integrins and fibrillin-1 is sufficient to initiate skin fibrosis, two Fbn1-targeted knock-in mouse models were generated: one with SSS-associated change W1572C (the mouse equivalent of human W1570C) and the other with an RGD to RGE substitution (D1545E) predicted to cause an obligate loss of integrin binding to fibrillin-1 (Figure S1). Mice heterozygous for either mutation phenocopy SSS with increased deposition of collagen by 1 month of age and reduction of subcutaneous fat by three months of age (Figures 1 and S2A,B). While homozygosity for D1545E causes embryonic lethality before embryonic day 10.5, mice homozygous for W1572C are viable and show accelerated skin fibrosis when compared to heterozygous littermates (Figures 1 and S2A,B). As seen in patients with SSS or SSc [3], mutant mice show disorganized and excessive microfibrillar aggregates in the dermis with sparsely distributed elastin (Figure S2C). Freshly isolated cells from mutant dermis show increased surface levels of integrin α5β1 and integrin αvβ3 in its active conformation (as assessed using WOW-1 antibody) by flow cytometry (Figure 2A). There was no corresponding increase in either total β3 integrin or integrin β5, a subtype that can cross-react with WOW-1 (Figure S3). Based on these data, we hypothesized that disrupted cell-matrix interaction in SSS results in compensatory upregulation of specific integrins at the surface of dermal cells, and that integrins represent a possible therapeutic target for this disease.
Figure 1. SSS mouse models show skin fibrosis.
Masson’s trichrome staining of back skin sections from male mice (genotypes indicated) at 1 month (top panels) and 3 months (bottom panels) of age demonstrates progressive loss of subcutaneous fat and an expanded zone of dense dermal collagen in mutant animals. Quantification of the thickness of the zones of dermal collagen and subcutaneous fat in wild-type and mutant mice at 1 (top panels) and 3 (bottom panels) months of age is shown. Similar findings were observed in mutant female mice (Figure S2A,B). 1 month males: n = 9 (+/+), 10 (WC/+), 10 (WC/WC), 9 (DE/+); 3 month males: n = 13 (+/+), 9 (WC/+), 9 (WC/WC), 9 (DE/+). Scale bars, 50 µm. * p<0.05, ** p<0.01, † p<0.001, ‡ p<0.0001. DE = D1545E. WC = W1572C.
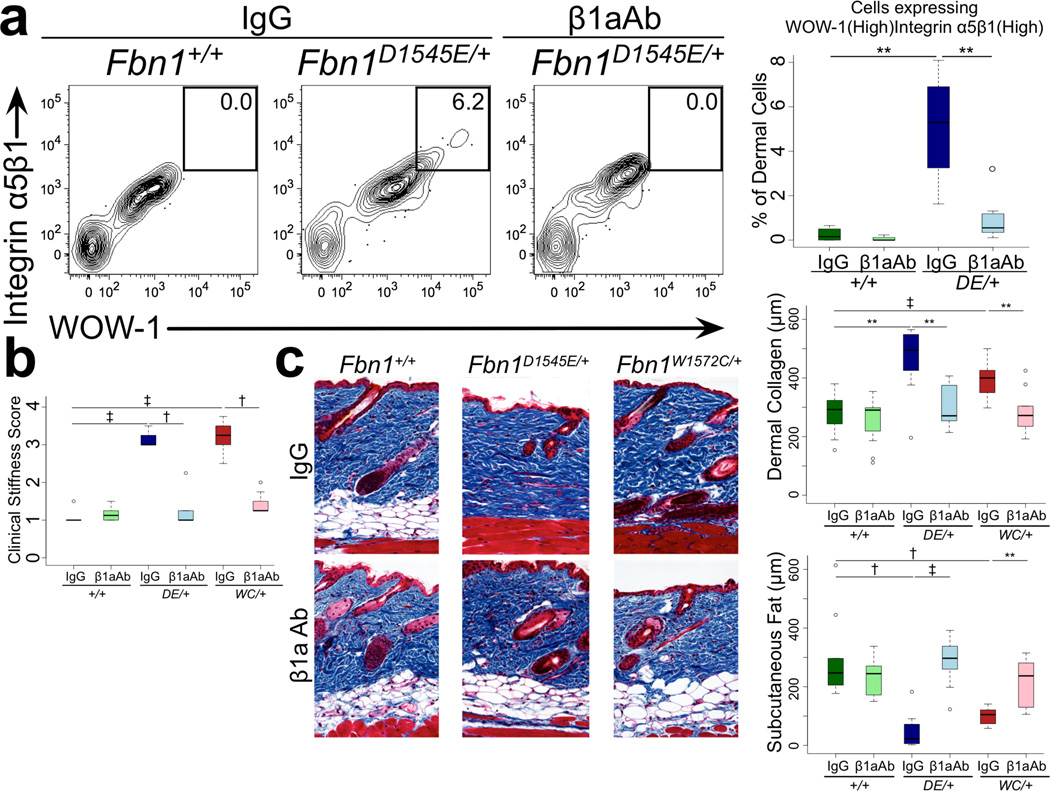
Figure 2. Integrin-modulating interventions prevent skin fibrosis.
(A) Flow cytometry of cells derived from the dermis reveals a unique population expressing both α5β1 and active β3 integrins (monitored using WOW-1 antibody) in mutant mice that is eliminated upon treatment with β1aAb but not an isotype-matched control (IgG). Representative contour plots are shown. An agonist and antagonist of β3 integrin activation were used to attest to the specificity of the WOW-1 antibody (Figure S16B). Isotype control-treated: n = 5 (Fbn1+/+), 7 (Fbn1D1545E/+); β1aAb-treated: n = 4 (Fbn1+/+), 7 (Fbn1D1545E/+). (B) Clinical assessment demonstrated that β1aAb prevented skin stiffness in mutant animals when compared to those treated with an isotype-matched control (IgG). (C) Masson’s trichrome staining reveals reduced skin collagen and preservation of subcutaneous fat in β1aAb-treated mutants. Isotype control-treated: n = 12 (Fbn1+/+), 9 (Fbn1D1545E/+), 8 (Fbn1W1572C/+); β1aAb-treated: n = 12 (Fbn1+/+), 10 (Fbn1D1545E/+), 10 (Fbn1W1572C/+). DE = D1545E. WC = W1572C.
We next investigated whether mimicking integrin-matrix ligand (i.e. fibrillin-1) interactions in mutant mice using a β1 integrin-activating antibody (β1aAb, 9EG7) offered therapeutic potential for the treatment of SSS. Twelve weeks of β1aAb treatment normalized integrin expression, skin stiffness and distensibility and skin architecture in SSS mouse models (Figure 2A–C, S4). In keeping with a pathogenic role for β3 integrin, we found that targeted introduction of haploinsufficiency or complete deficiency for β3 integrin in SSS mice normalized skin stiffness, collagen deposition and subcutaneous fat by three months of age (Figure S5A,B). By five months of age, 8 of 67 (12%) of Itgb3-targeted animals developed focal dermal and epidermal thickening (irrespective of Fbn1 genotype) reminiscent of the aberrant wound healing previously described in β3 integrin-deficient mice (Figure S5C) [7].
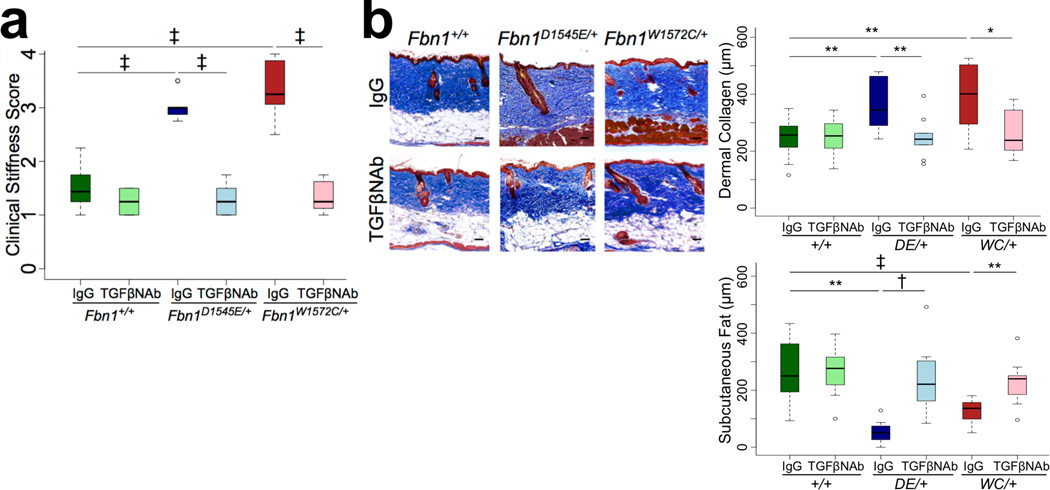
To assess for a pathogenic contribution for TGFβ, SSS mice were treated for twelve weeks with a panspecific TGFβ neutralizing antibody (NAb, 1D11) or isotype-matched control IgG after establishment of dense fibrosis at twelve weeks of age. Clinical (Figure 3A) and histological (Figure 3B) findings confirmed full reversal of skin stiffness and restoration of skin architecture in NAb-treated animals. Potential mechanisms for enhanced TGFβ activity include excessive concentration of latent TGFβ by the abnormally abundant microfibrillar aggregates in the dermis or excessive integrin-mediated activation (release) of TGFβ from its latent complex [8]. To address this, we used flow cytometry to monitor mutant mice for increased cell surface expression of the 3 integrin subtypes (αvβ5, αvβ6, αvβ8) known to support potent TGFβ activation [6]; this was not observed (Figure S6A). In addition, immunofluorescence analysis of skin in mutant mice did not reveal increased expression of free TGFβ1 (Figure S6B), which is known to be activated by integrins through interaction with the RGD sequence in its LAP (LAP1). There was an increase in total (free and active) TGFβ2 (Figure S6B), which has not been demonstrated to be activated by integrins (presumably due to the absence of an RGD sequence in LAP2) [6]. Furthermore, there was excessive concentration of both LAP1 and LAP2 in the dermis of mouse models of SSS, suggesting accumulation of the LLC for TGFβ1 and TGFβ2, respectively. While we cannot exclude a contribution of integrin-mediated TGFβ activation, these data suggest that enhanced TGFβ bioavailability prominently contributes to increased TGFβ activity in mutant mice.
Figure 3. A panspecific transforming growth factor β-neutralizing antibody reverses established skin fibrosis.
(A) Clinical assessment showing that stiffness was fully normalized by TGFβ-neutralizing antibody (TGFβNAb) treatment, commencing at three months of age and lasting twelve weeks. (B) Histologic and morphometric analyses using Masson’s trichrome stain. Isotype control-treated: n = 14 (Fbn1+/+), 9 (Fbn1D1545E/+), 8 (Fbn1W1572C/+). TGFβNAb-treated: n = 14 (Fbn1+/+); 10 (Fbn1D1545E/+); 8 (Fbn1W1572C/+). DE = D1545E. WC = W1572C. Scale bars, 50 µm. * p<0.05, ** p<0.01, † p<0.001, ‡ p<0.0001.
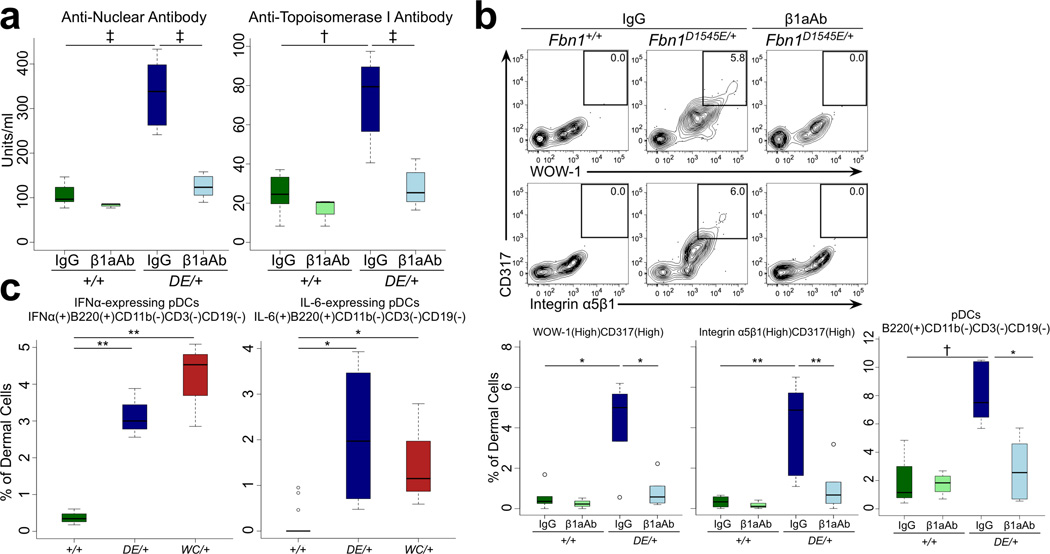
As seen in SSc, SSS mouse models show circulating anti-nuclear and anti-topoisomerase I antibodies (Figures 4A, S7). The finding that the deep dermal fibrosis seen in early SSS (Figure 1) co-localizes with high expression of active β3 integrin and accumulation of CD45(+) marrow-derived cells (Figure S8A) prompted speculation that an infiltrating class of immune cells might contribute to disease progression. In keeping with this hypothesis, nearly all dermal cells expressing high levels of α5β1 and active β3 integrins in SSS mice are CD317(+) plasmacytoid dendritic cells (pDCs) (Figure 4B). SSS mice show enrichment for cells that are CD11b(−)CD3(−)CD19(−)B220(+)SiglecH(+)Ly6C(high) in the dermis, further validating this identity (Figures 4B, S8B–D) [9]. As is characteristic for mature and active pDCs, these dermal cells express the pro-inflammatory cytokines interleukin (IL)-6 and interferon (IFN)-α (Figures 4C, S8E) [9]. There is also dermal polarization toward pro-inflammatory T helper (Th) cell populations, including CD4(+)IL-4(+) Th2, CD4(+)IL-17(+) Th17, and CD4(+)IL-9(+) Th9 cells (Figure S9A). In keeping with Th2, Th9, and/or Th17-skewing, there was also increased expression of IL-9, IL-13, and IL-22 by CD3(+) dermal cells (Figure S9A–C). There was no corresponding increase in either IFN γ (+)CD4(+) Th1 or FoxP3(+)CD4(+) T-regulatory (Treg) cells in mutant animals (Figure S9D). Finally, the dermis of SSS mice also shows infiltration with B220(high)CD19(+) activated B cells and CD138(+)B220(low)CD19(+) plasma cells (Figure S10). These abnormalities, including circulating autoantibodies and immune cell infiltration/activation, were normalized upon treatment of mutant mice with β1aAb (Figure 4, S9, S10). A similar response was seen in association with reversal of skin fibrosis upon treatment with TGFβNAb (Figure S11).
Figure 4. Immunologic abnormalities in SSS mice are prevented by integrin-modulating therapies.
(A) Increased circulating levels of anti-nuclear and anti-topoisomerase I antibodies by enzyme-linked immunosorbent assay (ELISA) in Fbn1D1545E/+ mice at 3 months of age are normalized upon treatment with β1aAb but not an isotype-matched control (IgG). Isotype control-treated: n = 6 (Fbn1+/+), 4 (Fbn1D1545E/+); β1aAb-treated: n = 4 (Fbn1+/+), 10 (Fbn1D1545E/+). (B) The cells expressing high α5β1 integrin in the dermis of mutant mice are CD317(high) cells that fail to accumulate upon treatment with β1aAb but not an isotype-matched control (IgG). The CD317(high) cells that accumulate in the dermis of mutant mice are B220(+)CD3(−)CD19(−) plasmacytoid dendritic cells and (C) express both IFNα and IL-6. For panels B–C: Isotype control-treated: n = 5 (Fbn1+/+), 7 (Fbn1D1545E/+); β1aAb-treated: n = 4 (Fbn1+/+), 7 (Fbn1D1545E/+). For panel E: n = 5 (Fbn1+/+), 4 (Fbn1D1545E/+), 4 (Fbn1DW1572C/+). DE = D1545E. WC = W1572C. * p<0.05, ** p<0.01, † p<0.001, ‡ p<0.0001.
We hypothesized that altered presentation of the fibrillin-1 RGD sequence might directly influence integrin expression by and the performance of pDCs. In keeping with this hypothesis, we found that wild-type spleen derived pre-pDCs showed increased adherence and activation (IFN-α and IL-6 expression) when plated on the matrix expressed by SSS murine embryonic fibroblasts (MEFs) as compared to control MEFs (Figure S12).
SSc fibroblasts demonstrated increased cell-surface presentation of total β1 integrin (Figure S13) and active β3 integrin (as monitored by WOW-1 staining) in comparison to controls, whereas levels of total β3 and β5 integrins were normal (Figure S14A). Treatment with β1aAb TS2/16, which promotes and stabilizes integrin β1-ligand interactions, normalized active β3 integrin cell-surface levels (Figure S14A). Treatment with β3 integrin-blocking antibody (β3bAb) did not significantly reduce cell-surface presentation of total β1 integrin (Figure S13). Human SSc cells in culture showed decreased levels of microRNA-29 (miR-29) (Figure S14B), a small regulatory RNA that is repressed by TGFβ and is known to inhibit expression of multiple matrix elements and to suppress fibrosis in selected disease states [10,11]. Treatment with β1aAb normalized miR-29 expression and attenuated expression of types I and III collagen in SSc fibroblasts in a dose-dependent manner (Figure S14B). SD208, an antagonist of the kinase activity of the type I TGFβ receptor subunit (TβRI), also normalized collagen and miR-29a expression (Figure S14C).
In addition to canonical (Smad-dependent) signaling, TGFβ can also initiate so-called noncanonical cascades, prominently including extracellular signal regulated kinase (ERK1/2) [5]. SSc fibroblasts showed normal Smad3 phosphorylation (pSmad3) in response to stimulation with TGFβ1 that was not influenced by integrin-modulating therapies, but uniquely showed TGFβ1-dependent phosphorylation of ERK1/2 (pERK1/2), when compared to control fibroblasts, that was normalized upon treatment with either β1aAb or β3bAb (Figure S14D,E). The activation of ERK1/2 in SSc fibroblasts was seen within 5 minutes of TGFβ1 stimulation and was inhibited by pretreatment with SD208, suggesting a relatively direct response (Figure S14D,E). In keeping with a pathogenic contribution of pERK1/2, treatment of SSc fibroblasts with U0126, an inhibitor of the mitogen-activated-protein kinase/ERK kinase (MEK), increased miR-29a levels and reduced collagen expression in SSc fibroblasts (Figure S14F). Both SSS mouse models show excessive activation of ERK1/2 in CD317(+) pDCs and other dermal cells (Figure S14G). Treatment of Fbn1D1545E/+ mice with the MEK-inhibitor RDEA119 prevented skin stiffness, dermal collagen accumulation, and loss of subcutaneous fat (Figure S14H,I).
This study shows that point mutations specifically in the sole integrin-binding domain of fibrillin-1 are sufficient to recapitulate the SSS phenotype in mice and to initiate many findings reminiscent of SSc including dermal fibrosis, autoantibody production, high IFN-α expression, Th2 and Th17 polarization, and accumulation of activated B cells and plasma cells in the skin [1,2, 4,12,13]. While prior studies have reported autoantibodies and subdermal fibrosis in tight skin (Tsk) mice harboring a large central duplication in Fbn1, there are no direct human correlates and both the mechanism and pathogenic relevance remain unclear [14, 15]. In SSS, all of these processes can be functionally linked to altered integrin expression and/or function since they are prevented by integrin-modulating therapies. While skin fibrosis was observed in mice upon conditional silencing of β1 integrin expression in keratinocytes [16], targeting of Itgb1 in fibroblasts afforded relative protection against bleomycin-induced skin fibrosis [17]. This apparent discrepancy has not been mechanistically explained.
A comparison of MFS and SSS highlights the complicated role of the extracellular matrix in cytokine regulation. Unlike MFS, where a deficiency of fibrillin-1 is seen, SSS mutations promote increased deposition of abnormal microfibrillar aggregates that fail to make contact with neighboring cells but retain the ability to bind to the TGFβ LLC, findings also seen in SSc [3]. This results in decreased or increased concentration of latent TGFβ in tissues in MFS or SSS, respectively [3, 5]. In MFS, it is posited that decreased LLC concentration is offset by increased TGFβ activation, but that this may occur in a tissue-specific manner [5,6]. The relative deficiency of microfibrils and hence latent TGFβ in MFS would mandate ongoing TGFβ production to support high signaling, whereas the high dermal concentration of TGFβ in SSS might allow a more sustained enhanced signaling state. Curiously, this does not appear to occur in all tissues where fibrillin-1 is expressed, perhaps due to different repertoires of expressed integrin subtypes that vary in their sensitivity to conformational changes induced by SSS mutations and/or tissue-specific differences in the regulation of microfibrillar assembly. The stiffened ECM in SSS could support mechanical traction-based activation of the excessive amounts of latent TGFβ in the dermis, a plausible feed-forward mechanism for the observed fibrosis [8]. Thus the level of TGFβ signaling in a given tissue may, at least in part, be determined by integration of both positive and negative regulation by microfibrils [5,6].
While the cell type that first detects and responds to aberrant presentation of the RGD sequence in fibrillin-1 remains unknown, it is interesting to speculate involvement of pre-pDCs that normally perform a surveillance function for viral pathogens at low concentrations in the skin. Prior work has shown that α5β1 integrin influences DC adhesion, migration, and maturation, and that migration is inhibited by β1aAb, at least in part through podosome disassembly [18]. Furthermore, a specific role for α5β1 integrin in pre-pDC chemotaxis and trafficking has been demonstrated [19]. It is therefore evident that pre-pDCs are informed by and respond to their matrix environment, with fibrillin-1 potentially serving as a prominent informant. In keeping with this, our in vitro observations (Figure S12) suggest that an altered matrix environment, devoid of any systemic influence, is sufficient to promote pDC recruitment and activation. Whether this relates to loss of a physiologic inhibitory signal by normal microfibrils or a pathogenic gain-of-function by abnormal microfibrillar aggregates seen in SSS and SSc remains to be determined.
pDCs are a major source of IFN-α and are capable of inducing Th2-and Th17-skewing, autoreactive B cell and plasma cell differentiation, and autoantibody production (Figure S15) [9,12,20–22] and have also previously been implicated in multiple autoimmune processes (including SSc) [9,12,20–22]. Although pDCs can contribute to both tolerogenic Treg or auto-inflammatory Th17 cell commitment, in vitro experiments suggest that TGFβ-treated pDCs favor the latter via a Smad dependent mechanism [23]. While the altered matrix environment in SSS likely contributes to excessive TGFβ activity early in the course of disease, TGFβ induces its own production and activation by pDCs, as well as IL-6 secretion (known prerequisites for Th17 polarization) [23]. pDCs can also induce either Th1 or Th2 skewing via IL-6/IFN-α- or OX40L/IL-4-dependent mechanisms, respectively (Figure S15) [9]. pDCs in a Th2 environment become activated and show enhanced IL-4 secretion, constituting a potential feed-forward mechanism for maintenance of a Th2 response [24]. In the context of high TGFβ-signaling, this might also allow for Th9-skewing [25]. Th2-, Th17- and pDC- related cytokines, including IL-4, IL-6, IL-13, IL-17 and IFN-α, have been prominently implicated in the fibrotic response in diverse disease states, including SSc [1,2, 4, 9,12,13,20–22]. To our knowledge, this is the first study that implicates TGFβ in pDC recruitment.
While many studies have highlighted the contribution of integrins to fibrotic disease [8], the focus has been on the ability of certain integrins to release (activate) TGFβ1 or TGFβ3 from the LLC through a direct interaction with RGD sequences in LAP1 and LAP3 [8]. Multiple observations in this study suggest that enhanced TGFβ bioavailability, rather than activation, may be the primary determinant of increased TGFβ activity in SSS and perhaps SSc. Our in vitro data in SSc fibroblasts suggest that cell surface integrins can influence the inherent signaling properties of the TGFβ receptor complex in response to free and active TGFβ. While the initiating pathogenic event in SSc remains unknown, this study provides evidence for a cell autonomous signaling defect that is maintained in culture. In theory, this could relate to primary but poorly penetrant genetic alterations or fixed epigenetic modifications, both of which may require a major environmental trigger.
Activation of ERK1/2 has previously been implicated in the TGFβ-mediated fibrotic response in general and specifically in SSc fibroblasts [26–28]. Asano and colleagues previously observed that constitutive ERK1/2 signaling in SSc fibroblasts drives expression of integrin αvβ3. Both αvβ3 and TGFβ were required for excessive collagen production [28]. Despite overlapping observations and the common conclusion that αvβ3 represents an attractive therapeutic target, this study places ERK1/2 activation downstream of both TGFβ and enhanced active αvβ3 expression in SSc fibroblasts and uniquely shows phenotypic rescue upon ERK antagonism in an in vivo model of scleroderma. Furthermore, we show prominent ERK1/2 signaling in pDCs in SSS mice, a described prerequisite for the stabilization, nuclear export and translation of IFN-α mRNA [29], and for toll-like receptor-mediated expression of inflammatory cytokines [30]. While prior work associated low levels of miR-29, a negative regulator of collagen expression, with fibrotic diseases including post-injury cardiac fibrosis [10] and SSc [11], this study is the first to offer a pathogenic sequence for scleroderma that integrates structural matrix elements, integrins, TGFβ signaling, ERK activation, and miR-29.
SSS mouse models demonstrate the potential to reverse established dermal fibrosis and suggest several therapeutic strategies including β1 integrin activation and blockade of β3 integrin, TGFβ or ERK signaling. When paired with the ability to perform pre-clinical trials in the first described mouse models of a genetically-defined human presentation of scleroderma, the potential for therapeutic advancement seems promising.
Methods Summary
Patients were recruited from the Johns Hopkins Hospital. All protocols were performed in compliance with the Johns Hopkins School of Medicine Institutional Review Board after informed consent. Full methods and any associated references are available in Supplementary Materials.
Supplementary Material
Acknowledgements
This work was supported by grants to H.C.D. from the Scleroderma Research Foundation, the National Institutes of Health (RO1- AR41135 and PO1- AR049698), the National Marfan Foundation, the Smilow Center for Marfan Syndrome Research, and the Howard Hughes Medical Institute. The authors are very grateful to those who contributed skin biopsies to this study and those who provided reagents for this study including Sanford Shattil, Kathleen Flanders, Craig J. Thomas, Samarjit Patnaik, and Juan J. Marugan.
Footnotes
Author Contributions
E.M.G, F.M.W., and H.C.D. aided in experimental design and interpretation of the data. E.M.G. performed enzyme-linked immunosorbent assays. F.M.W. obtained patient skin samples (The Scleroderma Center of Johns Hopkins University School of Medicine) and provided valuable guidance and clinical expertise. S.C.F. assisted in drug trials in vivo and mouse sera collection. E.C.D. performed electron microscopy. E.E.G. generated mouse models and performed all other experiments. D.L.H. aided in complete blood count analysis, mouse surgery, and histopathology. E.E.G. and H.C.D. wrote the paper.
The authors declare no competing financial interests.
References
- 1.Mayes MD, et al. Prevalence, incidence, survival, and disease characteristics of systemic sclerosis in a large US population. Arthritis Rheum. 2003;48:2246–2255. doi: 10.1002/art.11073. [DOI] [PubMed] [Google Scholar]
- 2.Harris ML, Rosen A. Autoimmunity in scleroderma: the origin, pathogenetic role, and clinical significance of autoantibodies. Curr Opin Rheumatol. 2003;15:778–784. doi: 10.1097/00002281-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Loeys BL, Gerber EE, Riegert-Johnson D, et al. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci Transl Med. 2010;2:23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varga J, Pasche B. Transforming growth factor β as a therapeutic target in systemic sclerosis. Nat Rev Rheumatol. 2009;5:200–206. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle JJ, Gerber EE, Dietz HC. Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett. 2012;586:2003–2015. doi: 10.1016/j.febslet.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–316. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reynolds LE, et al. Accelerated re-epithelialization in beta3-integrin-deficient-mice is associated with enhanced TGF-beta1 signaling. Nat. Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 8.Munger JS, Sheppard D. Cross talk among TGF-β signaling pathways, integrins, and the extracellular matrix. Cold Spring Harb Perspect Biol. 2011;3:a005017. doi: 10.1101/cshperspect.a005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Rooij E, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurer B, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 12.Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol. 2010;6:40–49. doi: 10.1038/nrrheum.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of Disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:679–685. doi: 10.1038/ncprheum0346. [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto M, et al. CD19-dependent B lymphocyte signaling thresholds influence skin fibrosis and autoimmunity in the tight-skin mouse. J. Clin. Invest. 2002;109:1453–1462. doi: 10.1172/JCI15078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bona C, Rothfield N. Autoantibodies in scleroderma and tight skin mice. Curr Opin Immunol. 1994;6:931–937. doi: 10.1016/0952-7915(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 16.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19(15):3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu S, et al. Loss of beta1 integrin in mouse fibroblasts results in resistance to skin scleroderma in a mouse model. Arthritis Rheum. 2009;60(9):2817–2821. doi: 10.1002/art.24801. [DOI] [PubMed] [Google Scholar]
- 18.van Helden SF, et al. A critical role for prostaglandin E2 in podosome dissolution and induction of high-speed migration during dendritic cell maturation. J. Immunol. 2006;177:1567–1574. doi: 10.4049/jimmunol.177.3.1567. [DOI] [PubMed] [Google Scholar]
- 19.Zou W, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 20.Ding C, Cai Y, Marroquin J, Ildstad ST, Yan J. Plasmacytoid dendritic cells regulate autoreactive B cell activation via soluble factors and in a cell-to-cell contact manner. J. Immunol. 2009;183:7140–7149. doi: 10.4049/jimmunol.0901175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jego G, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 22.Fleming JN, et al. Capillary Regeneration in Scleroderma: Stem Cell Therapy Reverses Phenotype. PLoS ONE. 2008;3:e1452. doi: 10.1371/journal.pone.0001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saas P, Perruche S. Functions of TGF-β-exposed plasmacytoid dendritic cells. Crit Rev Immunol. 2012;32:529–553. doi: 10.1615/critrevimmunol.2013005868. [DOI] [PubMed] [Google Scholar]
- 24.Bratke K, Klein C, Kuepper M, Lommatzsch M, Virchow JC. Differential development of plasmacytoid dendritic cells in Th1- and Th2-like cytokine milieus. Allergy. 2011;66:386–395. doi: 10.1111/j.1398-9995.2010.02497.x. [DOI] [PubMed] [Google Scholar]
- 25.Dardalhon V, et al. IL-4 inhibits TGF-β -induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nature Immunology. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakerakanti SS, Bujor AM, Trojanowska M. CCN2 is required for the TGF-β induced activation of Smad1-Erk1/2 signaling network. PLoS ONE. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, et al. Heparan sulfate-dependent ERK activation contributes to the overexpression of fibrotic proteins and enhanced contraction by scleroderma fibroblasts. Arthritis Rheum. 2008;58:577–585. doi: 10.1002/art.23146. [DOI] [PubMed] [Google Scholar]
- 28.Asano Y, et al. Increased expression of integrin αvβ3 contributes to the establishment of autocrine TGF-β signaling in scleroderma fibroblasts. J. Immunol. 2005;175:7708–7718. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- 29.Watarai H, et al. PDC-TREM, a plasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc. Natl. Acad. Sci. U.S.A. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.