Abstract
We employed event-related potentials to examine the feedback-related negativity (FRN), during a non-learning reward versus non-reward task. We compared 10–12-year-old, 13–14-year-old, and 15–17-year-old youth (n = 91). Age effects included a larger FRN for younger age groups, regardless of feedback type, and a decrease in peak latency for feedback, across age groups as a linear trend. Males showed larger responses irrespective of feedback type and longer latency for rewarded feedback. Source modeling revealed reward/non-reward differences in the anterior cingulate cortex (ACC) and orbitofrontal cortex, most strongly in the subgenual ACC. Males showed more subgenual ACC activity for feedback overall.
The interplay between expectancy, action, and outcome feedback are central to models of reinforcement learning (Holroyd & Coles, 2002; Schultz & Dickinson, 2000). Such learning relies heavily on the adaptive use of both positive and negative performance feedback in order to guide behavior. This performance monitoring system is critical for meeting the main social and educational demands that children and adolescents regularly encounter. Dysfunction in this system is associated with the development of clinical problems where the regulation of hedonically driven behavior is paramount, including attention deficit hyperactivity disorder (ADHD), substance abuse, and depression (Holroyd, Baker, Kerns, & Muller, 2008; Nelson, Patrick, Collins, Lang, & Bernat, 2011; Santesso et al., 2008). In particular, adolescence is a development period characterized by increased risk taking behavior compared to childhood and adulthood. This increase possibly reflects the relatively earlier functional development of limbic affective/hedonic circuitry compared to prefrontal brain regions (Casey, Getz, & Galvan, 2008). Nonetheless, relatively few studies examine the electrophysiological responses to performance feedback across the child and adolescent developmental period.
The last decade has seen rapid gains in our understanding of reward processing at the neuroanatomical, neurochemical, and neurophysiological levels of analysis (Schultz, 2006, 2007). At the neuroanatomical level, the broad class of reward processing, learning, and decision making is subserved by a network of subcortical and cortical structures including at least the basal ganglia, amygdala, orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC). Neurochemically, dopamine neurons located in the midbrain (substantia nigra and ventral tegmental area) project to the striatum, the dorsal and ventral prefrontal cortex, as well as to other structures. These neurons show phasic excitatory activity in the presence of primary rewards and are depressed by reward omission as well as by cues predicting reward omission. In turn, the midbrain dopamine system is thought to provide learning signals to other brain regions to represent outcome expectations and to adaptively guide behavior (Holroyd, Coles, & Nieuwenhuis, 2002; Schultz, Dayan, & Montague, 1997).
A growing body of work supports the use of event-related potentials (ERPs) as a means of studying the neurophysiology of reward processing, framed within reinforcement learning theory. ERPs, with their temporal precision, can inform our understanding of the neural processes that support reward processing with greater temporal precision than most other imaging techniques. Beginning with Miltner, Braun, and Coles (1997) studies have employed simple gambling, risk taking, and decision-making games to study neural responses to positive and negative outcomes (e.g., Fein & Chang, 2008; Oberg, Christie, & Tata, 2011; Polezzi, Sartori, Rumiati, Vidotto, & Daum, 2010). The feedback-related negativity (FRN) is an ERP response peaking between 200 and 300 msec post-feedback that shows greater amplitude for perceived worse outcomes (loss, non-reward) than for rewarded outcomes. Extrapolating from prediction error research with primates, the FRN is thought to be generated when transient dips in midbrain dopamine levels signal disinhibitory neurons in the ACC (Holroyd & Coles, 2002). ERP source modeling studies localize the FRN to the ACC in adults (Gehring & Willoughby, 2002; Luu, Tucker, Derryberry, Reed, & Poulsen, 2003). Recently, feedback source modeling studies have begun to examine subregions of the ACC as well as other brain regions relevant for reward processing, learning and decision making such as the OFC (Santesso, Dzyundzyak, & Segalowitz, 2011; Segalowitz et al., 2012). The OFC represents reward value of primary reinforcers (taste, touch, texture, and facial expression) and aids in associating these reinforcers with other stimuli in the environment, including not only visual and auditory stimuli, but also abstract stimuli such as money (Rolls & Grabenhorst, 2008). The OFC also distinguishes between reward and punishment, playing a critical role in the reversal of stimulus–reward associations (reversal learning).
DEVELOPMENTAL STUDIES OF THE FRN
In developmental research, ERPs hold the potential to chart changes in responding within the neural circuitry that supports reward processing. Several studies have examined developmental changes in the FRN for reward versus loss feedback. Eppinger, Mock, and Kray (2009) employed a probabilistic learning task to study the FRN among 10- to 12-year-old children and 19- to 24- year-old adults. They observed that compared to adults, children showed larger FRNs, but only in response to negative feedback—ERP amplitudes for positive feedback were similar across the children and adults they studied. A second relevant study by Hämmerer, Li, Müller, and Lindenberger (2011) employed a reinforcement learning task to examine the FRN among 9- to 11-year-old children, 13–14-year-old adolescents, young adults 30–40 years old and older adults 65–75 years. They found that for children 9–11, the FRN was less differentiated for reward versus loss, showing larger FRNs to both negative and positive feedback compared to 13–14-year-old adolescents and to young and older adults. Similar to adults, their adolescent group did differentiate between rewards and losses. Santesso, Dzyundzyak, and Segalowitz (2011) examined the FRN in 16–17-year-old adolescents and adults 18–29 years of age in a non-learning feedback reward task. They found comparable FRN magnitudes for the late adolescent group and the young adult group. However, in a study comparing 14–17-year-old adolescent males and young adult males (22–26 yrs.), Zottoli and Grose-Fifer (2011) did observe larger FRNs in their adolescent group and greater differentiation between win and loss among their young adult group. Across the three of the four studies, we generally see larger FRNs in childhood and smaller adult-like FRN responses appearing in adolescence. However, the rapid developmental changes taking place from early to late adolescence underscore the importance of examining late childhood through later adolescence in the same study. Here we report on a developmental study of the FRN directly comparing 10–12-year-old, 13–14-year-old, and 15–17-year-old youth on a chance-based reward versus non-reward FRN task.
FRN latency has received little attention in the developmental literature, with only one study to date addressing ERP latency effects (Zottoli & Grose-Fifer, 2011). This is surprising given that one of the relative strengths of the ERP approach is its temporal resolution. In their study, Zottoli and Grose-Fifer (2011) observed a statistical trend (p = .08), with adults tending to have shorter latencies than adolescents. However their study may not have been sufficiently powered to test for this effect (n = 18 per group). From a developmental perspective, latency differences in feedback processing could provide evidence for changes in processing efficiency across development. In fact, a significant body of data already documents developmental changes in ERP latency (decreases) related to cognitive development (Courchesne, 1978; Friedman, Putnam, & Sutton, 1990; Johnstone, Barry, Anderson, & Coyle, 1996). Thus, in line with this previous work and the trend observed by Zottoli and Grose-Fifer (2011), we might expect to see a decrease in FRN latency across our three age groups, consistent with more rapid feedback processing across development (Handy, 2005).
Learning and Non-Learning FRN Tasks
An important issue that can affect the magnitude of the FRN across a task is whether or not the task involves learning. Recent work by Muller and colleagues (Muller, Moller, Rodriguez-Fornells, & Munte, 2005) has shown that as participants learn the mapping of choices and outcomes, they come to rely less on the external feedback and more on internal error awareness. Both theEppinger et al. (2009) andHämmerer et al. (2011) studies involved tasks that required learning a stimulus–response mapping. In these types of tasks, participants initially rely on the external feedback, but over learning they are thought to rely more on internal error awareness, which tends to reduce the size of the FRN (see Muller et al., 2005; Santesso et al., 2011). Indeed,Eppinger et al. (2009) andHämmerer et al. (2011) speculated that the children in their studies showed larger FRNs than older groups due to a greater reliance on external feedback cues as opposed to internal representations of feedback emerging from learning.Santesso et al. (2011) suggest that because there may be a shift across development from reliance on external feedback to internal feedback, developmental FRN effects could be contaminated by differential learning effects. Thus, using a non-learning FRN task may be optimal for examining developmental differences in the FRN in the absence of learning effects. In the present study, we address this issue directly by using a non-learning reward based feedback task.
Sex Differences in Reward Processing
Recently, findings have emerged in the neuroimaging and electroencephalogram (EEG) literatures on reward processing suggesting that sex should be considered as an important source of variability (Kamarajan et al., 2008). Such differences may arise, for example, as a function of reward type/context (social vs. non-social, Spreckelmeyer et al., 2009), with reward circuitry in males significantly more engaged by video game–like tasks than females (Hoeft, Watson, Kesler, Bettinger, & Reiss, 2008). Moreover, mental disorders for which reward processing is a core aspect, such as ADHD and depression, have marked sex differences in prevalence, emerging in childhood and early adolescence respectively (Kessler, McGonagle, Swartz, Blazer, & Nelson, 1993; Waddell & McCarthy, 2010). Recently, in a sample of at-risk 15-year-olds, we reported that ERP responses in males differentiated more between the reward and loss conditions than in the females (Crowley et al., 2009). The effect of differential response to feedback (loss vs. reward) was over twice as large for males, although this finding emerged in a high-risk sample and may not generalize to low-risk youth. On the other hand,Santesso et al. (2011) reported that females displayed a larger FRN than males, which was accounted for by greater response to “win” feedback. The remaining three developmental studies of the FRN did not include sex in their reported statistical analyses (Eppinger et al., 2009; Hämmerer et al., 2011). Overall, emerging work clearly warrants inclusion of sex as a factor in reward processing studies.
The Present Study
The present study examined developmental and sex differences in the FRN from middle childhood through adolescence across three groups of children, 10–12 yrs., 13–14 yrs., and 15–17 yrs. We hypothesized that FRN amplitude would decrease across our three age groups, consistent with previous work. In addition, we predicted that females would show a smaller amplitude response to feedback as compared to males. In line with previous developmental studies on ERP latency, and the FRN developmental latency trend observed by Zottoli and Grose-Fifer (2011), we expected to see a decrease in FRN latency across our three age groups. Finally, we conducted an ERP source analysis to examine neural activity underlying reward and non-reward feedback processing using low-resolution electromagnetic tomography (LORETA).We expected ACC regions and OFC regions, previously reported on, to be differentially responsive to reward and non-reward feedback conditions, with greater activation (nanoamperes) for reward (Santesso et al., 2011). We used a four-choice selection feedback task similar to Holroyd’s (Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003) and our previous work (Crowley, Wu, Bailey, & Mayes, 2009; Crowley, Wu, Crutcher, et al., 2009).
MATERIAL AND METHODS
Participants
The initial sample consisted of 113 children (57 females and 56 males), recruited via a mass mailing list provided by a credit mailing company, Experian, targeting the towns surrounding New Haven, CT. Children were fluent in English and had no evidence of serious mental illness (psychosis, autism, bipolar disorder) assessed via a parental telephone screen. Children carrying a diagnosis of ADHD, per parent report, were also excluded (n = 3). Children were intellectually in the normal range (verbal IQ mean = 112.30, SD = 12.47, range 82–139). The mean age of the children was 13.54 yrs. (SD = 1.88 yrs., range = 10.10–17.77 yrs.). Among the total sample (n = 110), 91 children provided sufficient artifact-free ERP data for this report. Handedness was evaluated using the Edinburgh Handedness Inventory (Oldfield, 1971) yielding a mean handedness quotient of .54 (SD = .50, range, −1.00 −1.00) on a scale where more positive scores indicate greater degrees of right-handedness. Ten of the children in the sample were left-handed (scores below −.40) and seven of these provided sufficient ERP data. Among the final sample of 91 children was 10.6% African American, 6.4% Hispanic, 5.3% Asian, 2.1% Native American, and 75.8% Caucasian. Children were grouped by age into three groups: 10–12 years; 13–14 years; and 15–17 years (see Table 1 for age and sex breakdowns). This research was approved by the Yale University School of Medicine Human Investigation Committee.
TABLE 1.
Sample Characteristics: Age and Sex by Group
| Group | 10–12 Years | 13–14 Years | 15–17 Years |
|---|---|---|---|
| Age M (SD) | 11.63 (.74) | 13.92 (.59) | 16.00 (.75) |
| Males/Females | 15/21 | 18/14 | 14/9 |
| Total N | 36 | 32 | 23 |
Intellectual Functioning
All children completed the Vocabulary and Similarities subscales of the Wechsler Abbreviated Scale of Intelligence (WASI, Wechsler, 1999), which were used to estimate a verbal IQ based on standard norms. Prior validation studies have indicated that the WASI two-subtest IQ shows strong correlations with longer measures of intelligence (Wechsler, 1999).
ERP Reward-Feedback Task
A gambling task modeled after Holroyd et al. (2003) presented the participant with four lifelike balloon images of different colors (red, green, orange, blue) that randomly appeared in different serial positions along a row centered on the screen. The object of the game, called “Money Maker,” was to select balloons, one at a time, to win money. Participants responded with their right and left middle and index fingers on a four-button response pad. Participants were told to try to win as much money as possible and that they would receive this money at the end of the game. After each selection, all the balloons disappeared, and either a green dollar sign (indicating a reward of 10 cents), or a white square (indicating a non-reward) appeared. Subsequent to balloon selection on each trial, feedback was delayed 1 to 1.2 seconds. Feedback lasted 1,000 msec followed by a 1,000–1,200 msec crosshair, and a 100 msec blank screen before the balloons reappeared. Participants made balloon choices at self-paced intervals.
Although there were four options (balloons) on a given trial, feedback was rigged to have the probability of 50% reward and 50% non-reward outcomes across the task. Feedback was random, meaning that there was no pattern of certain balloons predicting specific outcomes, but children were led to believe that some people “can figure out a pattern some of the time.” Participants were reminded to look at the screen and not at their hands, as they would in video game play (to reduce eye-movement artifact).
Participant earnings were displayed numerically on the screen, centered just below the middle two balloons. There were four blocks of trials with approximately 30 trials in each block. After each block, a clear glass coin jar appeared to reflect the cumulative winnings to that point. Realistic dime images appeared in the jar, one by one, each followed by a coin sound. Prior to beginning the game, there were 3 practice trials, which introduced the coin jar. A total of 120 trials (60 per condition) were administered for the purpose of computing ERPs. Three additional trials were added such that the total winnings were $6.30 for each participant. Participants received this payment as part of a larger compensation ($60), for a study on stress and decision making.
Procedures
After obtaining institutional review board (IRB)–approved parental permission and child assent, each child was seated 24 inches in front of a 19-inch computer LCD monitor. Each child’s head circumference was measured to determine the appropriate net size and to mark Cz as the juncture of the halfway point between nasion to inion and left and right preauricular notches. Next, a Hydrocel high-density array of 128 Ag/AgCl electrodes arranged into a net (Geodesic Sensor Net, EGI Inc.) was placed on the child’s head using standard procedures. Before this, the net was soaked in warm potassium chloride solution (KCl) that served as the electrolyte (concentration: 1.5 tsp. per liter of water). The KCl solution enabled EEG collection even through hair and without the need for abrading the participant’s scalp.
Brain wave data were recorded through the Netstation v.4.4 software package (EGI, Inc.) and EGI high impedance amplifiers, sampling at 250 Hz (EGI, Inc. Series 300 amplifier). The online filters were set at .1–100 Hz. All electrodes were referenced to Cz for recording and then re-referenced offline for data analysis. All impedances remained at or under 40 kΩ as indicated by impedance measures made immediately before and after the test session. The E-prime v.2.0 (PST, Inc.) software package controlled the stimulus presentation. Each child’s EEG and behavior were continuously monitored across the session so that stimulus presentation occurred only when the child was sitting still and looking at the monitor.
Offline post-processing occurred in the Netstation v.4.4 software package (EGI, Inc.). The EEG data was first processed through a 0.3 Hz first order high-pass filter and a 30 Hz low-pass filter. Then it was segmented to epochs that contained a 100 msec pre-stimulus baseline and a 900 msec post-stimulus interval. Bad eye channels were manually marked and interpolated by surrounding channels. In the next step, artifact rejection was applied, in which bad segments (threshold 200 µV) were marked. Epochs with any eye blink or eye movement (threshold 150 µV) were rejected. Epochs with more than 10 bad channels (40% or more segments marked bad) were rejected as well. Then the remaining bad segments were replaced by surrounding channels. The single trial data were re-referenced from the vertex (Cz) to an average reference of all electrodes because the latter was thought to be a better representation of a true zero (Junghofer, Elbert, Tucker, & Braun, 1999). The data was baseline corrected to the 100 msec pre-stimulus interval. Finally, single trial data was averaged respectively for each condition (reward and non-reward). Participants providing at least twenty-five artifact free trials per condition were included (n = 47). Data for participants with fewer than 25 artifact free trials per condition received additional preprocessing with statistical eye-blink removal (blink threshold 14 µV/msec) (Gratton, Coles, & Donchin, 1983). Participants whose data yielded 25 good trials per condition with this additional approach (n = 44, 24 male) were then included in the overall statistical analysis (n = 91). The participants receiving artifact removal were not significantly different from those not receiving artifact removal in terms of reward/non-reward ERP amplitude, reward/non-reward ERP latency, age, sex or IQ, ts < 1.73.
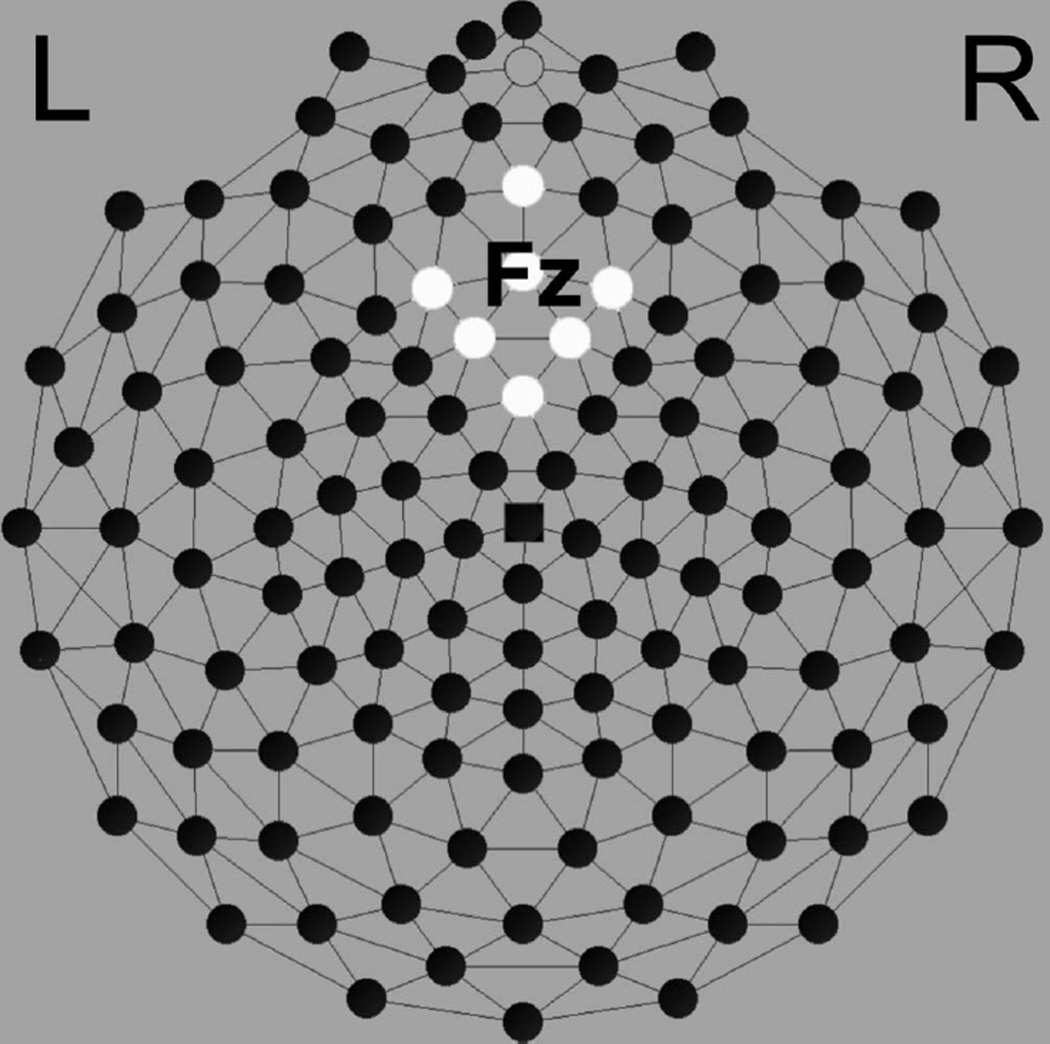
Past work on the feedback negativity has localized the FRN to the medial frontal region along the midline at site Fz (10-10 system). We relied on the average signal of seven electrodes over the midline in this region, specifically electrode numbers 11 (Fz), 16, 19, 12, 5, 4, and 6 (see Figure 1). For ERP analysis, the FRN amplitude was defined as the mean ± 25 msec around the negative peak amplitude between 200 and 350 msec within our electrode cluster. Latency for the negative peak of the FRN was assessed over the same channels and in the same 200–350 msec window.
FIGURE 1.
Electrical Geodesics 128-electrode dense array, channels surrounding Fz.
Source Analysis
Source estimation was accomplished using GeoSource, Version 1.0.1, electrical source imaging software (Electrical Geodesics, Eugene, OR). Following Luu, Tucker, and colleagues (Luu, Shane, Pratt, & Tucker, 2009; Poulsen, Luu, Crane, Quiring, & Tucker, 2009), neural source estimates of scalp-derived ERPs were computed using the distributed linear inverse minimum norm approach with sLAURA constraints (Grave de Peralta Menendez, Murray, Michel, Martuzzi, & Gonzalez Andino, 2004). Within GeoSource, the forward model applies a finite difference model (FDM) for accurate computation of the lead field in relation to head tissues. For tissue segmentation, the FDM employs a high-resolution T1-weighted magnetic resonance imaging (MRI) image and whole head computed tomography (CT) scan. Tissue compartments in the default FDM used here were constructed from the Colin27 MRI average (Holmes et al., 1998). A whole-head CT scan of this same individual, whose Talairach-transformed head closely matches the Montreal Neurological Institute average MRI (MNI305), was also used. Within the FDM, conductivity values were 0.25 S/m(Siemens/meter) for the brain, 1.8 S/mfor the cerebral spinal fluid, 0.018 S/m for the skull, and 0.44 S/m for the scalp (Ferree, Eriksen, & Tucker, 2000). Distributed dipole placement relied on the probabilistic map of the MNI305 average brain. Within this probabilistic atlas, cortical gray matter was parceled into 7 mm voxels. Each dipole served as a source location with three orthogonal orientations, resulting in a total of 2,447 source dipole triplets. Resulting estimated source activation voxel intensities and orientations were displayed superimposed on MRI slice views of the Talairach-transformed Colin27 brain. Five source regions corresponding to the scalp ERP effects were selected based on previously published source analysis of error and feedback monitoring (Pizzagalli, Peccoralo, Davidson, & Cohen, 2006; Santesso, et al., 2011). Source waveforms within each Brodmann area (BA) were generated from the above models. These source waveforms were then analyzed using mean amplitude measures (nanoamperes) within Brodmann areas, averaged over the time course of the 200–350 msec ERP window used to capture the FRN. Specifically we focused on BA 10 (anterior prefrontal cortex/orbitofrontal cortex), BA 11 (orbitofrontal cortex), BA 24 (ventral posterior cingulate cortex), BA 25 (subgenual ACC), and BA 32 (dorsal anterior cingulate cortex).
RESULTS
Analytic Overview
For both the ERP and source data, analyses employed repeated measures ANOVAs, and all F-tests are reported with the Greenhouse-Geisser correction (Greenhouse & Geisser, 1959). Repeated measures ANOVAs consisted of Condition (reward vs. non-reward) as the within subjects factor and Sex (Male vs. Female) and Age Group (10–12 yrs., 13–14 yrs., 15–17 yrs.) as between subjects factors. Analyses for source data included an additional within subjects factor, Region (BA 10, BA 11, BA 24, BA 25, BA 32).
ERP Data
Amplitude
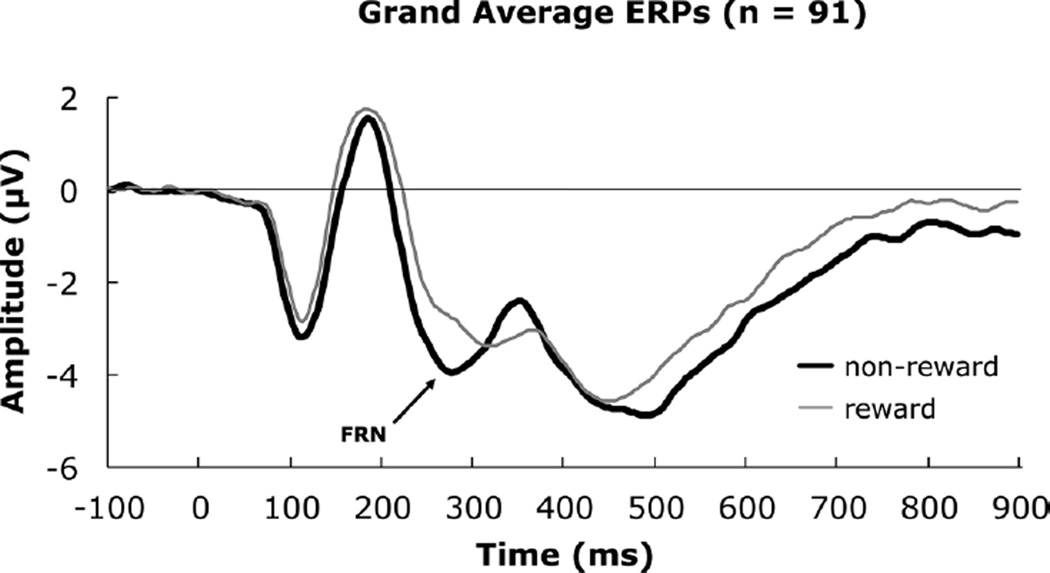
Means and standard errors for FRN amplitude and latency for reward, nonreward, and average feedback are presented in Table 2 by age group, gender, and total sample. ERP results are illustrated by average waveforms following reward and non-reward (overall, Figure 2), by sex (Figure 3), and by age (Figure 4) for the medial frontal cortical region around Fz (Geodesic Hydrocel Net, Figure 1). Based on our frontocentral electrode cluster, the FRN is clearly visible at ~280 msec post-feedback (Figure 2). We used an interval 200–350 msec to capture the FRN, computing the negative peak amplitude in this time window.
TABLE 2.
Mean and Standard Error for FRN Amplitude (µV) and Latency (msec) by Condition, Age, and Sex
| 10–12 Years |
13–14 Years |
15–17 Years |
||||
|---|---|---|---|---|---|---|
| Amplitude | Latency | Amplitude | Latency | Amplitude | Latency | |
| Reward | −4.91 (0.60) | 294.60 (4.54) | −4.81 (1.00) | 292.05 (6.51) | −2.13 (0.40) | 284.77 (6.99) |
| Non-reward | −5.42 (0.64) | 284.40 (4.15) | −5.68 (1.04) | 279.75 (4.95) | −2.94 (0.31) | 265.29 (5.22) |
| Ave. Feedback | −5.17 (0.59) | 289.50 (3.79) | −5.24 (1.01) | 285.90 (5.01) | −2.53 (0.32) | 275.03 (5.12) |
| Males |
Females |
Total Sample |
||||
| Amplitude | Latency | Amplitude | Latency | Amplitude | Latency | |
| Reward | −5.17 (0.76) | 298.89 (4.91) | −3.11 (0.41) | 283.03 (4.39) | −4.17 (0.45) | 291.22 (3.39) |
| Non-reward | −5.82 (0.82) | 279.67 (4.25) | −3.89 (0.35) | 276.08 (3.69) | −4.88 (0.47) | 277.93 (2.82) |
| Ave. Feedback | −5.49 (0.77) | 289.28 (3.88) | −3.50 (0.35) | 279.55 (3.61) | −4.53 (0.44) | 284.58 (2.69) |
Note. Feedback-related negativity (FRN) amplitudes and latencies were derived from seven channels centered on Fz (see Figure 1).
FIGURE 2.
Grand average event-related potentials depicting the feedback-related negativity for the total sample.
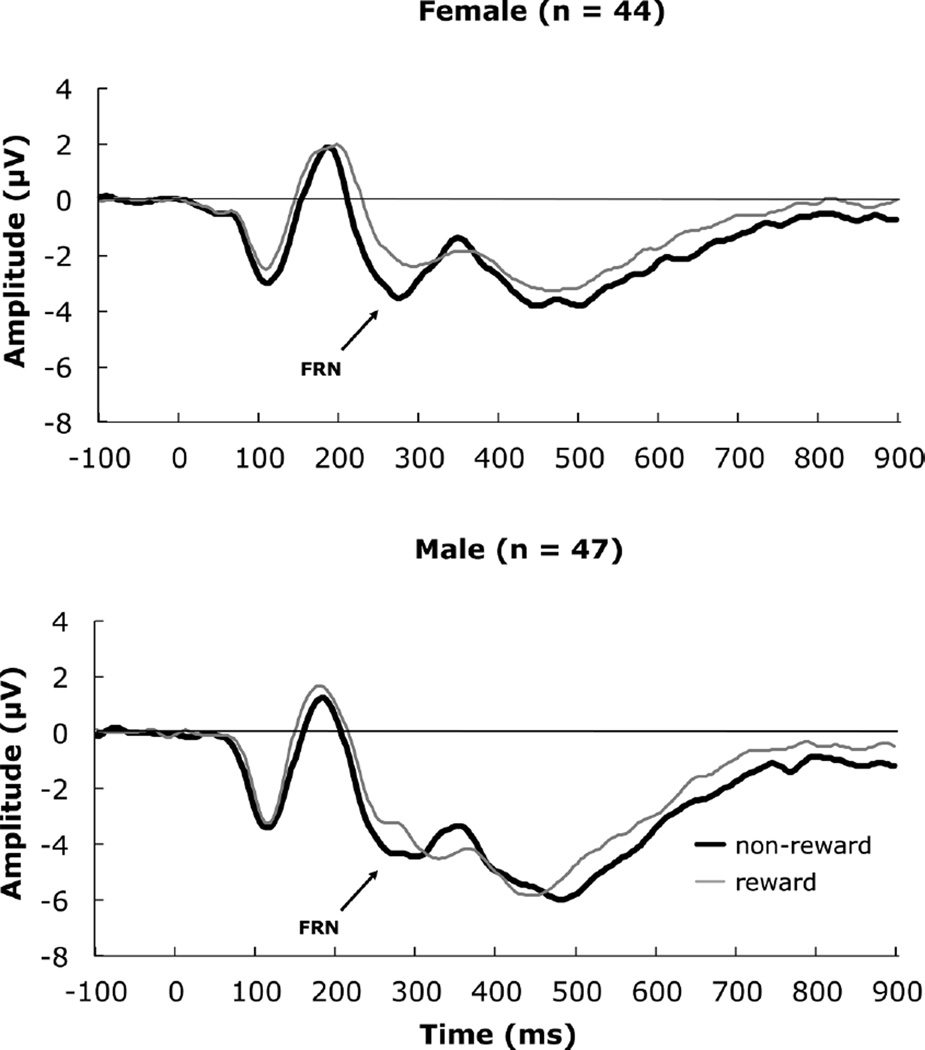
FIGURE 3.
Grand average event-related potentials depicting the feedback-related negativity for females and males.
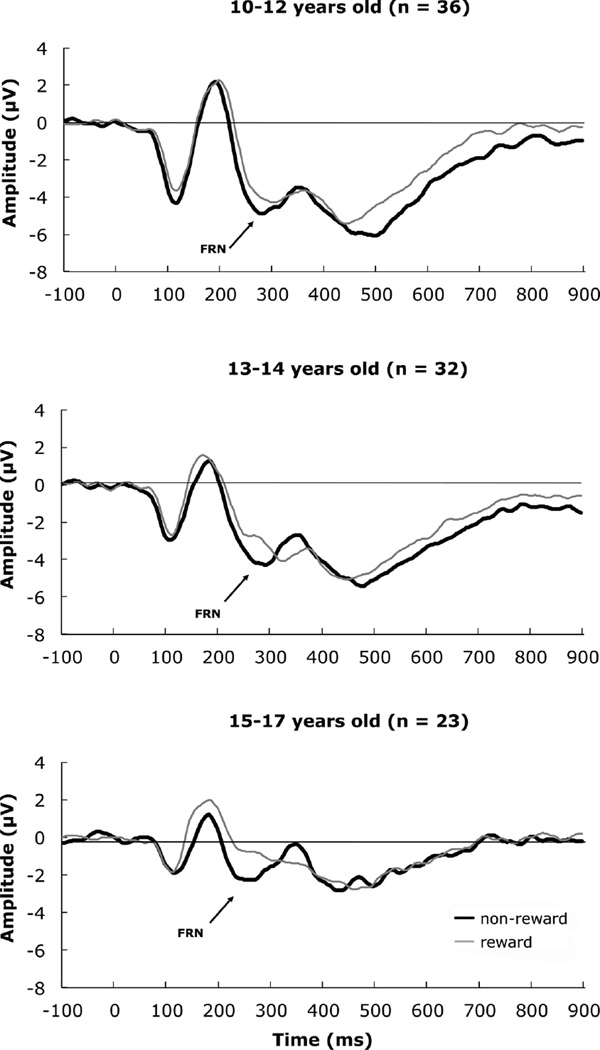
FIGURE 4.
Grand average event-related potentials depicting the feedback-related negativity by age bracket.
The repeated measures analysis of variance indicated the condition main effect was significant for amplitude, F(1, 85) = 8.90, p = .004, ηp2 = .10, 1-β = .84. A pairwise comparison indicated that the non-reward condition had a more negative amplitude than the reward condition, mean difference = −.71, SE = .22. A significant Sex effect, F(1, 85) = 5.40, p = .02, ηp2 = .06, 1-β = .63 indicated that males had a more negative amplitude than females, mean difference = −1.99, SE = .86. The Age Group effect was also significant, F(2, 85) = 3.86, p = .03, ηp2= .08, 1-β = .68.
Given that there was no Condition by Age Group interaction, we tested the effect of feedback, collapsed across condition. The Levene test (2, 88) = 2.97, p = .056, suggested the homogeneity of variance assumption was close to being violated within a linear contrast. Thus, we moved directly to post hoc pairwise tests. Comparisons with the 15–17-year-old group were tested with equal variances not assumed. The 10–12-year-old group had a more negative amplitude than the 15–17-year-old group, t(51.21) = −3.94, p = .0002. The 13–14-year-old group had a more negative amplitude than the 15–17-year-old group, t(38.88) = −2.56, p = .015. The 10–12-year-old group was comparable to the 13–14-year-old-group, t(66) = .064, p = .949. No other effects, including interactions, were significant, Fs < 1.0, ps > .05.
Latency
The Condition main effect was significant for Latency: F(1, 85) = 16.89, p < .001, ηp2 = .17, 1-β = .98. A pairwise comparison indicated that the non-reward condition had a shorter latency than the reward condition, mean difference = −13.29 msec, SE = 3.15. A main effect of Sex for latency was observed, F(1, 85) = 4.67, p = .03, ηp2= .05, 1-β = .57. This was qualified by a significant Condition × Sex interaction, F(1, 85) = 4.05, p = .047, ηp2 = .05, 1-β = .51. Post hoc tests (independent t-tests of the sex effect in each condition) indicated that in the non-reward condition there was no sex difference, t(89) = .64, p = .53. However in the reward condition, males had a significantly longer latency than females, t(89) = 2.40, p = .02, mean difference = 15.87, SE = 6.61.
There was a significant main effect for latency related to Age Group, F(2, 85) = 3.31, p = .04, ηp2 = .07, 1-β = .61.We tested our developmental hypothesis that age would be associated with a decrease in latency, within a linear contrast. Because Condition did not interact with Age Group, we tested the age effect collapsed across conditions. This test indicated a significant linear effect for reduction in ERP latency from 10–12 yrs. to 15–17 years, F(1, 88) = 4.60, p < .05, η2 = .05, the quadratic contrast was not significant, F(1, 88) = .42, p = .52. Pairwise comparison indicated that 10–12-year-olds, had a longer latency than 15–17-year-olds, mean difference = 17.35, SE = 6.80, p = .04. The 13–14-year-olds did not differ with the 15–17-year-olds or the 10–12-yearolds. Neither the interaction of Condition × Sex, F(1,85) = 4.05, ns, nor the Condition × Age Group interaction was significant, F(2,85) = .26, ns.
Source analyses
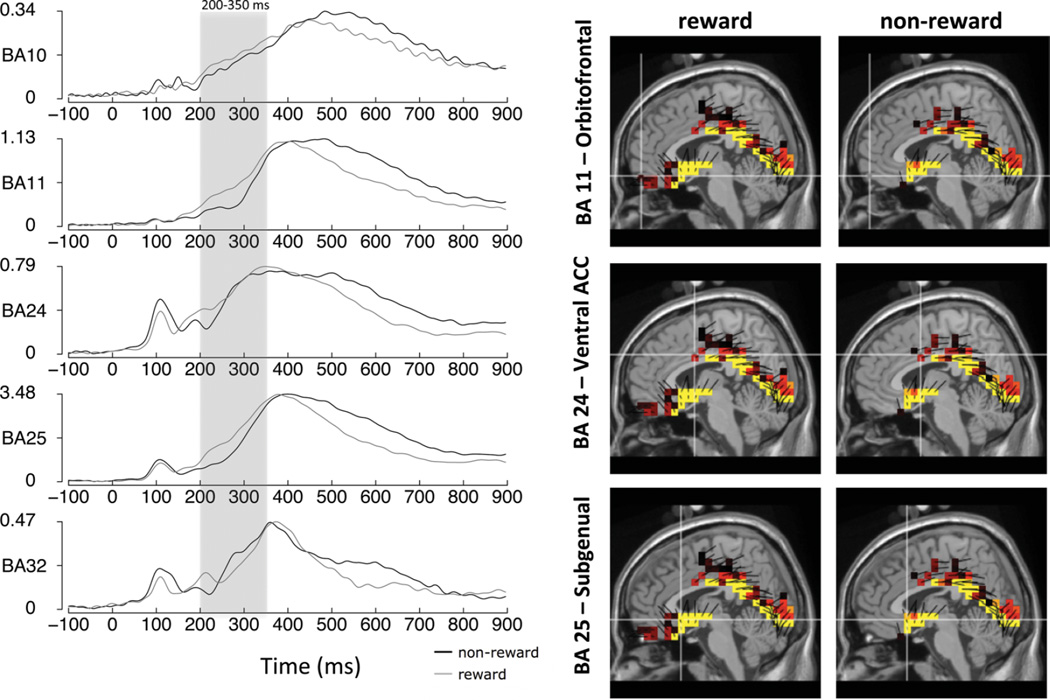
A repeated measures analysis of variance revealed a Condition main effect, F(1, 85) = 34.05, p < .001, ηp2 = .29, 1-β = .99, and a Region main effect, F(4, 82) = 155.39, p < .001, ηp2 = .88, 1-β > .99. These effects were qualified by a Condition × Region interaction, F(4, 82) = 12.13, p < .001, ηp2 = .37, 1-β > .99. Post Hoc tests consisted of paired samples t-test of the condition difference effect between regions. We tested these with a Bonferroni correction (.05/10 comparisons = .005 corrected threshold). Table 3 presents the condition means, standard errors, condition differences by Brodmann area and t-tests. Overall, the condition difference at BA 25, subgenual ACC was larger than, and significantly different from, the condition changes at all the other regions (all p’s < .001, Table 3 right panel). Thus, the Condition × Region interaction was largely driven by this effect. As a follow-up, related post hoc analyses of the condition effects revealed significant differences at BA 11 (orbitofrontal cortex), BA 24 (Ventral ACC) and BA 25 (subgenual ACC) (all p’s < .001, Table 3 left panel, Bonferroni corrected). Figure 5 displays the source waves for all 5 Brodmann areas examined (left) and source images for Brodmann yielding significant condition differences (right).
TABLE 3.
Frontal Source Means and Standard Errors for Feedback With Regional Difference Tests
| Frontal Source by Condition (over 200–350 msec) |
Regional Difference Tests |
|||||||
|---|---|---|---|---|---|---|---|---|
| Region | Reward | Non-reward | t | p | Difference | Difference Comparison | t | p |
| BA 10 | 0.392 (0.019) | 0.360 (0.017) | 2.42 | .018 | .032 (.013) | BA 25 vs. BA 10 | 6.88** | <.001 |
| BA 25 vs. BA 11 | 7.32** | <.001 | ||||||
| BA 11 | 0.863 (0.039) | 0.762 (0.037) | 3.48* | <.001 | .101 (.029) | BA 25 vs. BA 24 | 6.48** | <.001 |
| BA 25 vs. BA 32 | 7.11** | <.001 | ||||||
| BA 24 | 0.865 (0.043) | 0.765 (0.031) | 3.46* | <.001 | .100 (.029) | BA 24 vs. BA 10 | 2.38 | .020 |
| BA 24 vs. BA 11 | − 0.04 | .970 | ||||||
| BA 25 | 2.412 (0.093) | 1.911 (0.081) | 7.27* | <.001 | .501 (.069) | BA 24 vs. BA 32 | 3.16** | .002 |
| BA 32 vs. BA 10 | 0.01 | .920 | ||||||
| BA 32 | 0.558 (0.023) | 0.525 (0.019) | 1.79 | .077 | .034 (.019) | BA 32 vs. BA 11 | − 2.43 | .017 |
| BA 10 vs. BA 11 | − 2.69 | .009 | ||||||
Note. BA10 (anterior prefrontal/orbitofrontal cortex), BA11 (orbitofrontal cortex), BA24 (ventral posterior cingulate cortex), BA25 (subgenual cortex), and BA32 (dorsal anterior cingulate cortex). Difference = Reward – Non-reward. (Frontal source condition effects tested at
p < .01; Regional differences tested at
p < .005, Bonferroni corrected.)
FIGURE 5.
Source waveforms for Brodmann areas BA 10 (anterior prefrontal cortex), BA 11 (orbitofrontal cortex), BA 24 (ventral ACC), BA 25 (subgenual cortex), and BA 32 (dorsal ACC) (left), measurement units in nanoamperes. Note that, for emphasis, the source waveform y-axes vary from plot to plot. Shown on the right are S-low-resolution electromagnetic tomography (LORETA) source models for regions (BA 11, BA 24, BA 25) that were significantly different across reward and non-reward conditions. (color figure available online)
The main effect of Region was qualified by a Region×Sex interaction, F(4, 82) = 3.54, p = .012, ηp2 = .14, 1-β = .84. A post hoc test of the sex effect in each region (collapsed across condition) indicated that in BA 25, subgenual ACC was most likely responsible for the Sex × Region interaction, males (M = 2.35, SE = .11) showed greater activity (more positive value) than females (M = 1.96, SE = .11), t(89) = 2.51, p = .014. There were no significant sex differences for the other regions (BA 10, t(89) = .72, BA 11, t(89) = .16, BA 24, t(89) = .07, BA 32, t(89) = .85).
DISCUSSION
In the present study, we investigated developmental differences in responding to reward versus non-reward feedback during a non-learning feedback task. We compared three age groups of children and adolescents: 10–12-year-olds, 13–14-year-olds, and 15–17-year-olds. We examined the ERP correlates of external feedback processing (FRN), relying on a four-choice reward-based decision-making task similar to our previous work (Crowley, Wu, Crutcher, et al., 2009), but replacing a loss condition with a non-reward condition. That is, among four balloons, some contained a reward while others were empty (non-reward). As with a number of previous studies, we observed that the FRN was larger for non-rewarded feedback compared to rewarded feedback. We also observed the FRN for non-reward had a significantly shorter latency than for the reward condition, with non-reward yielding a 13.29 msec faster peak latency than reward. Source modeling indicated condition specific differences related to reward versus non-reward in BA 25 (subgenual ACC), BA 24 (ventral ACC), and BA 11 (orbitofrontal cortex), with the subgenual ACC emerging as the strongest regional effect and also the region differentiating males and females, but not age groups. Differences in Brodmann areas 32 (dorsal ACC) and BA 10 (orbitofrontal cortex) did not reach significance.
Developmental Effects
Two sets of developmental effects emerged from the data. First, irrespective of reward versus non-reward feedback type, FRNs were larger in our youngest group (10–12 yrs.) and our early adolescent group (13–14 yrs.) compared to our older adolescent group (15–17 yrs.). These results were similar to Hämmerer et al. (2011) and Zottoli and Grose-Fifer (2011). Recently,Santesso et al. (2011) suggested a possible distinction between FRN tasks that involve learning and those that involve guessing/gambling, with the former possibly confounding learning effects with developmental effects on the FRN. Here we show that the magnitude of feedback responses differentiated our child/young adolescent group from our older adolescent group on a guessing-type task, suggesting that developmental effects, as those reported for learning-type tasks (Eppinger et al., 2009; Hämmerer et al., 2011; Zottoli and Grose-Fifer, 2011), are not likely a function of differences in rates or processes of learning. Second, we observed a significant decrease in peak latency across the 10–17 year developmental window we examined here. Consistent with Eppinger et al. (2009) andHämmerer et al. (2011), perhaps an increasing reliance on internal representations of feedback confers a processing advantage, which manifests in a shorter feedback latency across development. This is the only study we know of to report statistically significant developmental latency effects on the FRN. Future developmental studies of the FRN should include peak latency as an outcome measure.
Sex Effects
The ERP literature on the FRN seldom includes examination of sex differences. We observed a significant main effect of sex on ERP amplitude, indicating that males produced a larger FRN irrespective of feedback type. This finding resembled our previous work with 15-year-old high-risk adolescents, using a paradigm that involved loss rather than non-reward. A sex difference also emerged for latency to peak, with males showing a longer latency for reward feedback only.
Previously we suggested that sex differences in the FRN might be accounted for by the type of paradigm employed (Crowley, Wu, Crutcher, et al., 2009), with video game formats possibly engaging males (see Hoeft, et al., 2008) and social tasks engaging females more (Fukushima & Hiraki, 2006). Viewed against the developmental data presented here, however—with younger children showing both larger FRN responses and longer latencies—it could just as easily be argued that the larger FRNs and the longer latency for reward feedback for males reflect a maturational difference, with males lagging behind females in terms of the neural architecture supporting the FRN. As another alternative, in a basic way, the sexes differ in terms of gonadal hormones testosterone and estrogen, which have been implicated in sex differences in reward processing (Dreher et al., 2007; Hermans et al., 2010). Future work will need to account for fluctuations in gonadal hormones, and possibly pubertal timing (Marceau, Ram, Houts, Grimm, & Susman, 2011), to isolate contributing factors for FRN differences across the sexes. Finally, we observed a sex effect in our source analyses, specific to the subgenual ACC, with males generally showing greater activation in this region compared to females, irrespective of feedback type. Subgenual ACC functioning has been linked tomood disorders (Drevets, Savitz, &Trimble, 2008) with some effects specifically related to hormone regulation among women with a history of major depression (Holsen et al., 2011). It will be interesting to know whether the sex difference we observed in subgenual ACC activation for reward processing tracks aspects of depressive symptomatology, particularly those related to hedonic capacity.
Two main study limitations should be acknowledged. First, we did not include an adult comparison group in this report. Thus, we do not know whether or not our later adolescent FRN ERPs reflect “mature” responses. However, previous developmental work with the FRN has shown late adolescent and adult FRNs have comparable magnitude (Eppinger et al., 2009; Hämmerer et al., 2011; Santesso et al., 2011). Second, because we did not assess gonadal hormones for this report, we cannot ascertain the impact of this aspect of neurobiological function on the sex differences we observed.
In summary, our work has several important implications. First, our data suggest that the feedback monitoring system continues to develop across the range of participants we studied. Because we used tightly constructed age brackets, we can say that the maturational changes on the FRN appear to emerge in the transition from early (13–14 yrs.) to middle adolescence (15–17 yrs.). This was evident for FRN amplitude, as well as for FRN latency, which linearly decreased with age group. Second, developmental effects reported here do not appear to be an artifact of learning, as we used a chance-based task. Third, sex was an important explanatory factor with males generally producing larger feedback responses, longer ERP latencies for rewarded feedback, greater ERP feedback response overall, and greater feedback activity localized to the subgenual ACC. Future work is needed to unpack these sex differences, which may have implications for how reward processing is studied across the sexes. Clearly sex needs to be included as a factor in analyses and reported in studies of ERP feedback processing.
Acknowledgments
This research was supported by NARSAD Young Investigator Award (MJC), Yale Interdisciplinary Research Consortium on Stress, Self-Control and Addiction Pilot project funding (MJC) through 1UL1RR024925-01 (R. Sinha); NIDA grants RO1-DA-06025 (LCM), DA-017863 (LCM), and KO5 (LCM), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM). This publication was also made possible by CTSA grant number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Contributor Information
Michael J. Crowley, Yale Child Study Center, Yale School of Medicine, New Haven, Connecticut
Jia Wu, Yale Child Study Center, Yale School of Medicine, New Haven, Connecticut.
Rebecca E. Hommer, Yale Child Study Center, Yale School of Medicine, New Haven, Connecticut
Mikle South, Departments of Psychology and Neuroscience, Brigham Young University, Provo, Utah.
Peter J. Molfese, Haskins Laboratories, New Haven, Connecticut
R.M.P. Fearon, Division of Psychology & Language Sciences, University College London, London, United Kingdom
Linda C. Mayes, Yale Child Study Center, Yale School of Medicine, New Haven, Connecticut
REFERENCES
- Casey BJ, Getz S, Galvan A. The adolescent brain. Developmental Review. 2008;28(1):62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: Changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45(4):468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Bailey CA, Mayes LC. Bringing in the negative reinforcements: The avoidance feedback-related negativity. Neuroreport. 2009;20(17):1513–1517. doi: 10.1097/WNR.0b013e32832ff2f5. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Crutcher C, Bailey CA, Lejuez CW, Mayes LC. Risk-taking and the feedback negativity response to loss among at-risk adolescents. Developmental Neuroscience. 2009;31(1–2):137–148. doi: 10.1159/000207501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppinger B, Mock B, Kray J. Developmental differences in learning and error processing: Evidence from ERPs. Psychophysiology. 2009;46(5):1043–1053. doi: 10.1111/j.1469-8986.2009.00838.x. [DOI] [PubMed] [Google Scholar]
- Fein G, Chang M. Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naive alcoholics. Drug and Alcohol Dependence. 2008;92(1–3):141–148. doi: 10.1016/j.drugalcdep.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferree TC, Eriksen KJ, Tucker DM. Regional head tissue conductivity estimation for improved EEG analysis. Ieee Transactions on Biomedical Engineering. 2000;47(12):1584–1592. doi: 10.1109/10.887939. [DOI] [PubMed] [Google Scholar]
- Friedman D, Putnam L, Sutton S. Longitudinal and cross-sectional comparisons of young children’s cognitive ERPs and behavior in a picture-matching task: Preliminary findings. International Journal of Psychophysiology. 1990;8(3):213–221. doi: 10.1016/0167-8760(90)90013-4. [DOI] [PubMed] [Google Scholar]
- Fukushima H, Hiraki K. Perceiving an opponent’s loss: Gender-related differences in the medial-frontal negativity. Social Cognitive and Affective Neuroscience. 2006;1(2):149–157. doi: 10.1093/scan/nsl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. Medial prefrontal cortex and rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21(2):527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24(2):95–112. [Google Scholar]
- Hämmerer D, Li SC, Müller V, Lindenberger U. Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience. 2011;23(3):579–592. doi: 10.1162/jocn.2010.21475. [DOI] [PubMed] [Google Scholar]
- Handy TC. Event-related potentials: A methods handbook. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Hermans EJ, Bos PA, Ossewaarde L, Ramsey NF, Fernandez G, van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. Neuroimage. 2010;52(1):277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Watson CL, Kesler SR, Bettinger KE, Reiss AL. Gender differences in themesocorticolimbic system during computer game-play. Journal of Psychiatric Research. 2008;42(4):253–258. doi: 10.1016/j.jpsychires.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Baker TE, Kerns KA, Muller U. Electrophysiological evidence of atypical motivation and reward processing in children with attention-deficit hyperactivity disorder. Neuropsychologia. 2008;46(8):2234–2242. doi: 10.1016/j.neuropsychologia.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH, Nieuwenhuis S. Medial prefrontal cortex and error potentials. Science. 2002;296(5573):1610–1611. doi: 10.1126/science.296.5573.1610. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, Cohen JD. Errors in reward prediction are reflected in the event-related brain potential. Neuroreport. 2003;14(18):2481–2484. doi: 10.1097/00001756-200312190-00037. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Spaeth SB, Lee JH, Ogden LA, Klibanski A, Whitfield-Gabrieli S, Goldstein JM. Stress response circuitry hypoactivation related to hormonal dysfunction in women with major depression. Journal of Affective Disorders. 2011;131(1–3):379–387. doi: 10.1016/j.jad.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone SJ, Barry RJ, Anderson JW, Coyle SF. Age-related changes in child and adolescent event-related potential component morphology, amplitude and latency to standard and target stimuli in an auditory oddball task. International Journal of Psychophysiology. 1996;24(3):223–238. doi: 10.1016/s0167-8760(96)00065-7. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110(6):1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Rangaswamy M, Chorlian DB, Manz N, Tang Y, Pandey AK, Porjesz B. Theta oscillations during the processing of monetary loss and gain: A perspective on gender and impulsivity. Brain Research. 2008;1235:45–62. doi: 10.1016/j.brainres.2008.06.051. …. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. Journal of Affective Disorders. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Luu P, Shane M, Pratt NL, Tucker DM. Corticolimbic mechanisms in the control of trial and error learning. Brain Research. 2009;1247:100–113. doi: 10.1016/j.brainres.2008.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science. 2003;14(1):47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Marceau K, Ram N, Houts RM, Grimm KJ, Susman EJ. Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology. 2011;47(5):1389–1409. doi: 10.1037/a0023838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, Coles MGH. Event-related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience. 1997;9(6):788–798. doi: 10.1162/jocn.1997.9.6.788. [DOI] [PubMed] [Google Scholar]
- Muller SV, Moller J, Rodriguez-Fornells A, Munte TF. Brain potentials related to self-generated and external information used for performance monitoring. Clinical Neurophysiology. 2005;116(1):63–74. doi: 10.1016/j.clinph.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Collins P, Lang AR, Bernat EM. Alcohol impairs brain reactivity to explicit loss feedback. Psychopharmacology. 2011;218(2):419–428. doi: 10.1007/s00213-011-2323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg SA, Christie GJ, Tata MS. Problem gamblers exhibit reward hypersensitivity in medial frontal cortex during gambling. Neuropsychologia. 2011;49(13):3768–3775. doi: 10.1016/j.neuropsychologia.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: A 128-channel EEG study. Human Brain Mapping. 2006;27(3):185–201. doi: 10.1002/hbm.20172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polezzi D, Sartori G, Rumiati R, Vidotto G, Daum I. Brain correlates of risky decision-making. Neuroimage. 2010;49(2):1886–1894. doi: 10.1016/j.neuroimage.2009.08.068. [DOI] [PubMed] [Google Scholar]
- Poulsen C, Luu P, Crane SM, Quiring J, Tucker DM. Frontolimbic activity and cognitive bias in major depression. Journal of Abnormal Psychology. 2009;118(3):494–506. doi: 10.1037/a0015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: From affect to decision-making. Progress in Neurobiology. 2008;86(3):216–244. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Dzyundzyak A, Segalowitz SJ. Age, sex and individual differences in punishment sensitivity: Factors influencing the feedback-related negativity. Psychophysiology. 2011;48(11):1481–1489. doi: 10.1111/j.1469-8986.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- Santesso DL, Steele KT, Bogdan R, Holmes AJ, Deveney CM, Meites TM, Pizzagalli DA. Enhanced negative feedback responses in remitted depression. NeuroReport. 2008;19(10):1045–1048. doi: 10.1097/WNR.0b013e3283036e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual Review of Neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Willoughby T, Reker DL, Campbell K, Chalmers H, Rose-Krasnor L. Adolescent peer interaction and trait surgency weaken medial prefrontal cortex responses to failure. Social Cognitive and Affective Neuroscience. 2012;7(1):115–124. doi: 10.1093/scan/nsq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Grunder G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4(2):158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, McCarthy MM. Sexual differentiation of the brain and ADHD: What is a sex difference in prevalence telling us? Current Topics in Behavioral Neurosciences. 2010;9:341–360. doi: 10.1007/7854_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York, NY: Psychological Corporation; 1999. [Google Scholar]
- Zottoli TM, Grose-Fifer J. The feedback-related negativity (FRN) in adolescents. Psychophysiology. 2011;49(3):413–420. doi: 10.1111/j.1469-8986.2011.01312.x. [DOI] [PubMed] [Google Scholar]