Introduction
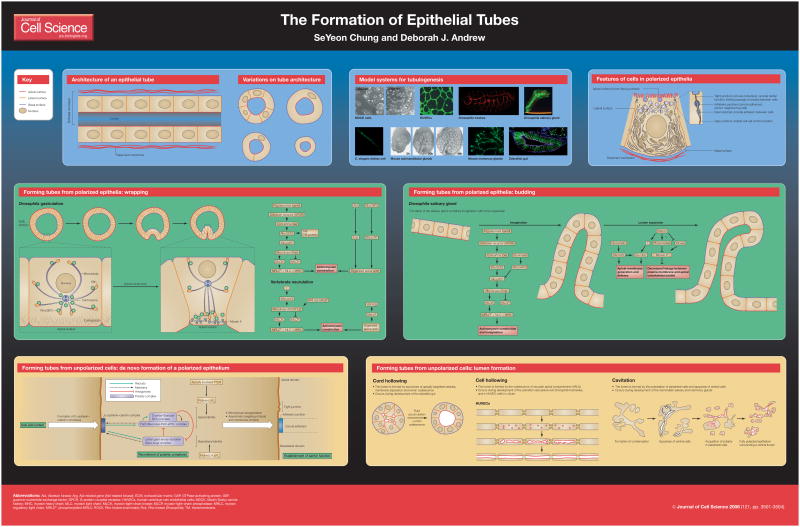
Tubular organs are the thoroughfares through which the gases and liquids that sustain life are transported. Such organs also often serve as factories for the synthesis and delivery of the hormones and enzymes that are essential for tissue homeostasis and energy metabolism. Examples of tubular organs in mammals include the blood vessels, kidneys, lungs, pancreas, and the mammary, salivary and lacrimal glands, as well as the heart and central nervous system at early developmental stages. Several model systems have been developed to study the molecular and cellular events required to organize cells into functional tubular organs (see poster panel ‘Model systems for tubulogenesis’); these include Madin-Darby canine kidney (MDCK) cells and human umbilical vein endothelial cells (HUVECs), both of which can form tubes when grown in 3D matrices in the presence of appropriate signals (Brouns et al., 2000) (O’Brien et al., 2002). Other model systems include the Drosophila melanogaster trachea and salivary gland (Kerman et al., 2006), the single-cell kidney of Caenorhabditis elegans (Buechner, 2002), and the zebrafish gut and vasculature (Ober et al., 2003; Weinstein, 2002). In addition, recent developments in the ex vivo culture of the mammalian salivary and mammary glands, in combination with RNAi knockdown, small-molecule inhibitors and antibody-based blocking experiments, are providing exciting new information about the molecules and mechanisms that drive tube formation, growth and elaboration (Hinck and Silberstein, 2005; Patel et al., 2006; Sternlicht et al., 2006). An understanding of the molecular and cellular events that underlie tube formation and maintenance is key to human health, as many of the most common human diseases, including atherosclerosis and kidney disease, arise from defects in tubular architecture. Here, we focus on the earliest stages of tube formation in several model systems, studies of which reveal some common underlying mechanisms (Hogan and Kolodziej, 2002; Lubarsky and Krasnow, 2003). We describe the details of epithelial-tube architecture and discuss how tubular organs initially form from either polarized or unpolarized cells.
Architecture of cellular tubes
Tubular organs vary considerably in size and shape but all tubes, whether they are highly intricate networks of branched tubes or simple cylinders, comprise a polarized epithelium that surrounds a shared central lumen (see poster panel ‘Architecture of an epithelial tube’). The apical (or free) surface of each epithelial cell is ‘in’ (faces the lumen), whereas the basal surface is ‘out’ (faces surrounding tissues). The lateral surfaces of each cell contact neighboring cells via a set of specialized junctions [adherens junctions, tight junctions and (in invertebrates) septate junctions] that anchor cells to one another, provide a barrier function, and block the movement of integral membrane proteins between the different cell surfaces; this enables specialized membrane functions at the different surfaces and allows the vectorial delivery of ions and secretory vesicles to the membrane. The formation of tubes from epithelia that are already polarized, such as the mammalian neural tube, lung and liver, and the D. melanogaster salivary gland and trachea, typically occurs through two related mechanisms – wrapping and budding – and requires only a change in cell shape and/or organization (see poster panels ‘Forming tubes from polarized epithelia: wrapping’ and ‘Forming tubes from polarized epithelia: budding’). By contrast, the formation of tubes from unpolarized groups of cells, which occurs during the development of the mammalian vasculature, mammary gland, kidney, salivary gland and pancreas, first requires the establishment of a polarized epithelium that has distinct apical and basolateral cellular domains (see poster panel ‘Forming tubes from unpolarized cells: de novo formation of a polarized epithelium’). As the cells acquire polarity, they form tubes by one of three general mechanisms: cord hollowing, cell hollowing or cavitation (see poster panel ‘Forming tubes from unpolarized cells: lumen formation’).
Formation of tubes from polarized epithelia
Wrapping and budding – the two pathways by which polarized epithelia form tubes –are mechanistically similar processes, both of which are driven by apical constriction (Lubarsky and Krasnow, 2003). Wrapping occurs when cells within an epithelial sheet undergo coordinated apical constriction, which causes the inward curving or bending of the sheet of cells. This continues until the edges of the sheet meet and seal to form a tubular structure that lies parallel to the plane of the epithelium. The best-known examples of wrapping are neurulation in vertebrates and mesoderm invagination during gastrulation in D. melanogaster. Cells that form tubes by wrapping often first adopt a columnar morphology giving rise to an epithelial placode (a plate-like thickening) of cells that are elongated along the apical-basal axis. Subsequently, actinomyosin-driven contraction of the apical cell surface in a subset of these cells changes their shapes from columnar to pyramidal, which drives the bending and internalization of the placode.
In contrast to wrapping, budding occurs as cells within an epithelium invaginate through apical constriction and subsequently extend in a direction that is orthogonal to the plane of the original epithelium (Lubarsky and Krasnow, 2003); the tube often remains contiguous with the surrounding epithelium. Orthogonal extension of the tube can occur through additional cell recruitment, cell division and/or cell elongation. Budding is the mechanism of tube formation in the D. melanogaster salivary gland and trachea (Kerman et al., 2006), and is the mechanism by which the branches of the mammalian salivary gland and lung are formed (Hogan and Kolodziej, 2002). During branching morphogenesis, new buds form from existing branches and the new lumena remain contiguous with the original lumen.
Molecular mechanisms of epithelial invagination
Many of the molecular events that drive apical constriction in wrapping and budding have been described (see poster). During gastrulation in D. melanogaster, the transcription factor Twist is expressed in the mesodermal primordium and regulates the expression of two key factors for invagination: Folded gastrulation (Fog), an apically secreted ligand, and T48, an apically localized transmembrane protein (Barrett et al., 1997; Dawes-Hoang et al., 2005; Hacker and Perrimon, 1998; Kolsch et al., 2007). Fog binds to an unknown G-protein-coupled receptor that activates Concertina (Cta), a Gα subunit. Cta and T48 recruit adherens-junction components and the downstream cytosolic effector RhoGEF2 to sites of apical constriction (Rogers et al., 2004). RhoGEF2 travels to the cortex through its association with the microtubule plus-end tracking protein EB1. Activated Concertina is proposed to drive localized dissociation of RhoGEF2 from the plus ends of microtubules in the apical domain. Apical RhoGEF2 drives Rho into its active GTP-bound form, activating the downstream effector Rho kinase (Rok). Active Rok leads to the activation and recruitment of non-muscle myosin to the apical membrane. In parallel, RhoGEF2 and the Abelson (Abl) tyrosine kinase recruit and organize filamentous actin at the apical surface (Fox and Peifer, 2007). Contraction of myosin leads to apical constriction because the actinomyosin cytoskeleton is tethered to the apical adherens junctions.
poster.
Similar to gastrulation in D. melanogaster, vertebrate neurulation requires the activation of Rho kinases at the apical surface. In this case, however, the actin-binding adherens-junction protein Shroom3 binds directly to the Rho kinases ROCK1 and ROCK2, which leads to local activation and accumulation of nonmuscle myosin, again through Rok-dependent phosphorylation of myosin (Haigo et al., 2003; Nishimura and Takeichi, 2008). As in the D. melanogaster mesoderm, the mammalian Abl homologues Abl and Arg organize actin at the apical surface of the invaginating neural plate (Koleske et al., 1998). p190RhoGAP has also been implicated in neural-tube closure, during which it might downregulate apical actin accumulation and/or Rho activation (Brouns et al., 2000).
Similar to tubes that form through wrapping, compartmentalization of Rho regulators is key to the morphogenesis of tubes that form through budding. An excellent example of this is the D. melanogaster salivary gland, in which defects in invagination are observed when genes that also affect mesodermal invagination are mutated, including fog, rhoGEF2, rho1 and rok. Similarly, mutations in genes that encode salivary-gland-expressed RhoGAPs disrupt invagination (Kolesnikov and Beckendorf, 2007; Nikolaidou and Barrett, 2004; Xu et al., 2008).
Live imaging of D. melanogaster tracheal-tube invagination in wild-type flies and EGF-pathway mutants has revealed that, although apical constriction initiates invagination, oriented cell divisions and cell intercalation also contribute (Nishimura et al., 2007). Most tracheal cells invaginate in EGF-pathway mutants (Llimargas and Casanova, 1999; Wappner et al., 1997); without EGF-pathway activity, however, cells fail to select a single focus for apical constriction, to coordinate cell rearrangements, and to delay mitosis until the cells are in a position where their oriented divisions contribute to internalization (Nishimura et al., 2007).
Lumen expansion
Tube formation through epithelial budding requires that the apical surface expand following invagination to enable lumen growth. In the D. melanogaster salivary gland, this process is regulated by the transcription factors Huckebein (Hkb) and Ribbon (Rib). Hkb upregulates the expression of Klarsicht (Klar), a protein that mediates delivery of apical-membrane vesicles, and stabilizes Crumbs (Crb), a protein that is implicated in apical-membrane expansion in several developmental contexts (Myat and Andrew, 2002). In both the salivary gland and the trachea, Rib upregulates Crb expression and deactivates moesin, an ezrin-radixin-moesin (ERM) family member that, in its active form, links the apical membrane to the underlying actin cytoskeleton (Kerman et al., 2008). The increased genesis and delivery of apical membrane and decreased linkage of this membrane to the underlying cytoskeleton facilitates lumenal expansion during tube elongation.
Formation of tubes from unpolarized condensations
Establishing apical-basal polarity
Many tubular organs begin as condensations or cords of unpolarized mesenchymal cells, which must first polarize to form epithelial tubes (Martin-Belmonte and Mostov, 2008; Nelson, 2003). A key step in mesenchymal-to-epithelial transitions is the upregulation of the transmembrane protein E-cadherin and the formation of the E-cadherin–catenin junction. The homotypic interactions of the extracellular domains of E-cadherin between neighbors anchors cells within the epithelium. The E-cadherin junctional complex, which recruits several other protein complexes (see below) that are involved in establishing and maintaining overall apical-basal polarity, develops into the mature adherens junction, which physically separates the apical (lumen-facing) membrane domain from the basolateral membrane domain. Adherens junctions link the apical plasma membrane to the apical actin network via the cytosolic proteins α-catenin, β-catenin (Armadillo in D. melanogaster) and γ-catenin (also known as plakoglobin); they therefore organize the actin belt that surrounds the apical surface of epithelial cells (the zonula adherens).
Differential distributions of the phosphatidylinositides phosphatidylinositol (4,5)-bisphosphate (Ptd(4,5)P2) and phosphatidylinositol (3,4,5)-trisphosphate (PtdIns(3,4,5)P3), as well as interactions among three interdependent protein complexes – the Crumbs-Stardust-PATJ complex, the Par3 (Bazooka)-Par6-aPKC complex and the Lethal-giant-larvae–Scribble–Discs-large complex (Nelson, 2003) are key to the apical-basal polarity of epithelia (see poster panel ‘Forming tubes from unpolarized cells: de novo formation of a polarized epithelium’). In mammalian epithelia, PtdIns(4,5)P2 is highly enriched in the apical domain, whereas PtdIns(3,4,5)P3 is restricted to the basolateral domain; this occurs because PTEN, the enzyme that converts PtdIns(3,4,5)P3 to PtdIns(4,5)P2, is localized apically. The three protein complexes, working in part through a mutual exclusion mechanism, establish and maintain polarity. The link between the polarity complexes and phosphoinositide distribution is not fully understood (see Martin-Belmonte and Mostov, 2008), but it is clear that the correct assembly of these protein complexes and the differential distribution of PtdIns(4,5)P2 and PtdIns(3,4,5)P3 are both required for the asymmetric targeting of other lipids and membrane proteins. Moreover, the polarity complexes are required for the localization and assembly of additional cell-cell junctions, including the apical tight junctions in vertebrates and the lateral septate junctions in invertebrates, which provide barrier function to the epithelium and maintain ionic homeostasis. Additionally, studies of MDCK cells in 3D culture indicate that interactions between cells and the extracellular matrix (ECM) contribute to the overall direction of polarity (O’Brien et al., 2002; Yu et al., 2005). Interestingly, reversal of polarity in this system requires Rho and the same downstream effectors (Rho-kinase and myosin) as drive invagination in polarized epithelia (Yu et al., 2008). Thus, by utilizing the same molecular machinery for both processes, tube formation from unpolarized primordia might link the acquisition of cell polarity to the cell-shape changes that are required for tube construction.
Mechanism of lumen establishment
It is clear that de novo formation of a lumen is key to the development of tubes that are formed from unpolarized cells. Lumena can form by several mechanisms, which include cord hollowing, cell hollowing and cavitation (Lubarsky and Krasnow, 2003) (see poster panel ‘Forming tubes from unpolarized cells: lumen formation’). During cord hollowing, which occurs during formation of the zebrafish gut, cells form primitive cell-cell junctions and acquire apical-basal polarity de novo; concomitantly, they form several small apical lumena that eventually coalesce into a single common lumen. In the zebrafish gut, formation of this shared large lumen requires the tight junction protein Claudin15 as well as the gut-expressed Na+/K+-ATPases. These proteins regulate paracellular ion transport to drive the accumulation of lumenal fluid, which in turn provides a force for the coalescence of individual lumena (Bagnat et al., 2007). Cell hollowing, a mechanism that has been proposed to occur in HUVECs in culture and during the formation of the fine branches of the insect tracheae (tracheoles), involves the formation of specialized organelles that are known as vacuolar apical compartments (VACs) (Lubarsky and Krasnow, 2003). These vesicular structures, which are thought to form through endocytosis of the plasma membrane, fuse with each other to produce intracellular lumena, and ultimately with the plasma membrane to connect with the lumena of distal and proximal cells within the tube. VAC-like structures might also contribute to lumen establishment in MDCK cells and to tube expansion in the D. melanogaster salivary gland and trachea, although the ‘VACs’ in these cells are usually smaller and shorter-lived than those seen in HUVECs (Jiang et al., 2007; Kakihara et al., 2008; Kerman et al., 2008; Martin-Belmonte and Mostov, 2008; Myat and Andrew, 2002). Whether, and how much, cell hollowing contributes to tube formation in vivo is unclear; one study suggests that cell hollowing has a major role in formation of the zebrafish vasculature (Kamei et al., 2006), whereas a more recent study (in which individual endothelial cells were marked) suggests that cord hollowing is the dominant mechanism (Blum et al., 2008). In any case, the distinctions between cord hollowing and cell hollowing are subtle, and are largely limited to whether the lumen forms and expands extracellularly and is shared among adjacent neighboring cells (cord hollowing), or forms and expands entirely within a single cell (cell hollowing). Finally, cavitation (or canalization) is the mechanism by which tubular organs such as the mammalian salivary gland, which begin as a cord or rod of cells, form; tubes form as cells in the center of the rod or cord die by apoptosis and cells in the periphery become polarized (Melnick and Jaskoll, 2000). Both cavitation and cord hollowing occur in MDCK cells, in which the choice of mechanism appears to depend on how quickly cells within the cord or cyst become polarized (Martin-Belmonte et al., 2008).
Conclusions and perspectives
Recent studies of model systems have revealed a number of shared mechanisms for tube formation. For example, both mechanisms of tube formation in polarized cells – wrapping and budding – are driven by the process of apical constriction, which is mediated by the apical localization and activation of an actinomyosin network that is linked to adherens junctions. Because all tubes are formed from polarized epithelium, mesenchymal-to-epithelial transitions are necessary to advance unpolarized tube primordia to the same starting point as polarized epithelial tube primordia. All mechanisms of tube formation require that lumenal space be created and expanded. In cells that are already polarized, lumena form from existing apical surfaces that are internalized as a consequence of cell shape change and reorganization. In unpolarized primordia, lumena form de novo, often concurrently with cell polarization. Nonetheless, lumenal expansion in both types of systems involves related organellar structures that share common protein components. This suggests that the maintenance of tube architecture involves the same molecular and cellular components that are required to form tubes during development; such commonalities should provide fertile ground for the improvement of therapies for human diseases that arise from defects in tube homeostasis.
Acknowledgments
We thank Cathrin Brisken, Rebecca Newbury, Alan Cheshire and Bilal Kernan for the use of unpublished images. The image of HUVECs is reproduced courtesy of Becton Dickinson and Company, and shows tubule formation by BD™ HUVEC-2 cells cultured on BD Matrigel™ Matrix, using the BD Biocoat™ Angiogenesis System: Endothelial Cell Tube Formation. The images of MDCK cells, mouse salivary gland and zebrafish gut are reproduced from Birchmeier and colleagues (Birchmeier et al., 2003), Patel and colleagues (Patel et al., 2006) and Bagnat and colleagues (Bagnat et al., 2007) respectively, with permission.
References
- Bagnat M, Cheung ID, Mostov KE, Stainier DY. Genetic control of single lumen formation in the zebrafish gut. Nat Cell Biol. 2007;9:954–960. doi: 10.1038/ncb1621. [DOI] [PubMed] [Google Scholar]
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 1997;91:905–915. doi: 10.1016/s0092-8674(00)80482-1. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Blum Y, Belting HG, Ellertsdottir E, Herwig L, Lüders F, Affolter M. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev Biol. 2008;316:312–322. doi: 10.1016/j.ydbio.2008.01.038. [DOI] [PubMed] [Google Scholar]
- Brouns MR, Matheson SF, Hu KQ, Delalle I, Caviness VS, Silver J, Bronson RT, Settleman J. The adhesion signaling molecule p190 RhoGAP is required for morphogenetic processes in neural development. Development. 2000;127:4891–4903. doi: 10.1242/dev.127.22.4891. [DOI] [PubMed] [Google Scholar]
- Buechner M. Tubes and the single C. elegans excretory cell. Trends Cell Biol. 2002;12:479–484. doi: 10.1016/s0962-8924(02)02364-4. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Christiansen AE, Phelps CB, Brand AH, Wieschaus EF. folded gastrulation, cell shape change and the control of myosin localization. Development. 2005;132:4165–4178. doi: 10.1242/dev.01938. [DOI] [PubMed] [Google Scholar]
- Fox DT, Peifer M. Abelson kinase (Abl) and RhoGEF2 regulate actin organization during cell constriction in Drosophila. Development. 2007;134:567–578. doi: 10.1242/dev.02748. [DOI] [PubMed] [Google Scholar]
- Hacker U, Perrimon N. DRhoGEF2 encodes a member of the Dbl family of oncogenes and controls cell shape changes during gastrulation in Drosophila. Genes Dev. 1998;12:274–284. doi: 10.1101/gad.12.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 2005;7:245–251. doi: 10.1186/bcr1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL, Kolodziej PA. Organogenesis: molecular mechanisms of tubulogenesis. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- Jiang L, Rogers SL, Crews ST. The Drosophila Dead end Arf-like3 GTPase controls vesicle trafficking during tracheal fusion cell morphogenesis. Dev Biol. 2007;311:487–499. doi: 10.1016/j.ydbio.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakihara K, Shinmyozu K, Kato K, Wada H, Hayashi S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 small GTPase in Drosophila. Mech Dev. 2008;125:325–336. doi: 10.1016/j.mod.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Kamei M, Saunders WB, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Andrew DJ. From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis. Differentiation. 2006;74:326–348. doi: 10.1111/j.1432-0436.2006.00095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerman BE, Cheshire AM, Myat MM, Andrew DJ. Ribbon modulates apical membrane during tube elongation through Crumbs and Moesin. Dev Biol. 2008;320:278–288. doi: 10.1016/j.ydbio.2008.05.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koleske AJ, Gifford AM, Scott ML, Nee M, Bronson RT, Miczek KA, Baltimore D. Essential roles for the Abl and Arg tyrosine kinases in neurulation. Neuron. 1998;21:1259–1272. doi: 10.1016/s0896-6273(00)80646-7. [DOI] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev Biol. 2007;307:53–61. doi: 10.1016/j.ydbio.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science. 2007;315:384–386. doi: 10.1126/science.1134833. [DOI] [PubMed] [Google Scholar]
- Llimargas M, Casanova J. EGF signalling regulates cell invagination as well as cell migration during formation of tracheal system in Drosophila. Dev Genes Evol. 1999;209:174–179. doi: 10.1007/s004270050241. [DOI] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA. Tube morphogenesis: making and shaping biological tubes. Cell. 2003;112:19–28. doi: 10.1016/s0092-8674(02)01283-7. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald A, Werb Z, Alonso MA, Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnick M, Jaskoll T. Mouse submandibular gland morphogenesis: a paradigm for embryonic signal processing. Crit Rev Oral Biol Med. 2000;11:199–215. doi: 10.1177/10454411000110020401. [DOI] [PubMed] [Google Scholar]
- Myat MM, Andrew DJ. Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell. 2002;111:879–891. doi: 10.1016/s0092-8674(02)01140-6. [DOI] [PubMed] [Google Scholar]
- Nelson WJ. Adaptation of core mechanisms to generate cell polarity. Nature. 2003;422:766–774. doi: 10.1038/nature01602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidou KK, Barrett K. A Rho GTPase signaling pathway is used reiteratively in epithelial folding and potentially selects the outcome of Rho activation. Curr Biol. 2004;14:1822–1826. doi: 10.1016/j.cub.2004.09.080. [DOI] [PubMed] [Google Scholar]
- Nishimura M, Inoue Y, Hayashi S. A wave of EGFR signaling determines cell alignment and intercalation in the Drosophila tracheal placode. Development. 2007;134:4273–4282. doi: 10.1242/dev.010397. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- Ober EA, Field HA, Stainier DY. From endoderm formation to liver and pancreas development in zebrafish. Mech Dev. 2003;120:5–18. doi: 10.1016/s0925-4773(02)00327-1. [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE. Opinion: building epithelial architecture: insights from three-dimensional culture models. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
- Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Hacker U, Turck C, Vale RD. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr Biol. 2004;14:1827–1833. doi: 10.1016/j.cub.2004.09.078. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Kouros-Mehr H, Lu P, Werb Z. Hormonal and local control of mammary branching morphogenesis. Differentiation. 2006;74:365–381. doi: 10.1111/j.1432-0436.2006.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wappner P, Gabay L, Shilo BZ. Interactions between the EGF receptor and DPP pathways establish distinct cell fates in the tracheal placodes. Development. 1997;124:4707–4716. doi: 10.1242/dev.124.22.4707. [DOI] [PubMed] [Google Scholar]
- Weinstein BM. Plumbing the mysteries of vascular development using the zebrafish. Semin Cell Dev Biol. 2002;13:515–522. doi: 10.1016/s1084952102001052. [DOI] [PubMed] [Google Scholar]
- Xu N, Keung B, Myat MM. Rho GTPase controls invagination and cohesive migration of the Drosophila salivary gland through Crumbs and Rho-kinase. Dev Biol. 2008;321:88–100. doi: 10.1016/j.ydbio.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep. 2008;9:923–929. doi: 10.1038/embor.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]