Abstract
Objective
Boswellic acid is a plant-derived molecule with putative anti-inflammatory effects. This study was performed to determine whether oral or topical administration of boswellic acid can attenuate joint damage in a mouse model of osteoarthritis (OA).
Methods
Levels of boswellic acid were measured in the blood and synovium of mice treated with oral or topical boswellic acid. OA was generated by surgical destabilization of the medial meniscus (DMM). Therapy with oral or topical boswellic acid was initiated one day after surgery and continued for 12 weeks, when knees were harvested and scored histologically for degree of cartilage loss, osteophyte formation, and synovitis. Microdissected OA synovium was stimulated with IL-1β or lipopolysaccharide (LPS) in the presence or absence of boswellic acid and cytokine production by quantitative polymerase chain reaction (PCR) or multiplex enzyme linked immunoabsorbant assay (ELISA).
Results
Topical treatment resulted in synovial concentrations of boswellic acid 2–6-fold higher than that measured in plasma. Cartilage loss was significantly reduced in mice treated with oral or topical boswellic acid compared with vehicle control (P < 0.01 for both oral and topical therapies). Likewise, treatment with either oral boswellic acid or boswellic acid ointment reduced of synovitis (P = 0.006 and 0.025, respectively) and osteophyte formation (P = 0.009 and 0.030, respectively). In vitro, boswellic acid was able to inhibit IL-1β and TLR4 mediated induction of several inflammatory mediators from OA synovial explant tissue.
Conclusions
Significant synovial concentration and therapeutic efficacy can be achieved with topical boswellic acid treatment. These findings suggest that boswellic acid has potential as a disease-modifying agent in OA.
Keywords: Osteoarthritis, Disease modifying anti-osteoarthritis drugs, Topical therapy summary
Introduction
80% of the population has radiographic evidence of osteoarthritis (OA) by age 65, and over 60% of those have symptoms of OA1. Current treatments are limited to behavioral interventions, symptomatic medical management, and ultimately, joint replacement surgery. Therapies with potential to slow or halt disease progression are urgently needed. Given that OA is a chronic, nonfatal disease that occurs in an older population with medical and physical comorbidities, such disease-modifying therapies must display a stringent safety profile.
One approach to improving a drug's safety profile is to limit exposure by topical application at or near sites of pathology as has been demonstrated successfully to limit systemic corticosteroid exposure using transdermal or inhaled administration. In the treatment of OA, several agents have been used topically, including analgesics (e.g., salicylates, capsaicin), and indomethacin2,3.
Boswellia resin, usually derived from the plant Boswellia serrata, has been used since biblical times as a natural anti-inflammatory therapeutic in traditional Indian Ayurvedic medicine and traditional Chinese medicine4. Findings from small clinical trials suggest that oral Boswellia is efficacious in the treatment of both OA5,6 as well as rheumatoid arthritis (RA) several other inflammatory conditions (Reviewed in Ref.4).
Boswellic acids, especially acetyl-11-keto-β-boswellic acid are potent inhibitors of 5-lipoxygenase (5-LO), an enzyme that catalyzes the generation of leukotrienes including LTB47; a molecule strongly implicated in OA-associated inflammation8. Additionally, boswellic acid can inhibit toll-like receptor (TLR)-mediated activation of monocytes, suppressing LPS-induced production of nitric oxide, IL-1β, and TNFα9,10. Finally, derivatives of boswellic acid have been demonstrated to suppress IL-β induced apoptosis of chondrocytes as well as TNFα induced production of MMP3 by synovial fibroblasts11 thus demonstrating clear therapeutic potential for the treatment of OA.
To date, there have been few studies of boswellic acid in animal models of OA and, to our knowledge no study has assessed the efficacy of topically therapy. In this study, we used a well-established mouse model of OA to evaluate and compare the therapeutic efficacy of topical and oral boswellic acid preparations in treating post-traumatic OA.
Methods
Animals
20-week-old male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and treated according to the Guidelines for Animal Care of the US National Institutes of Health and Stanford University. All animal experiments were performed under protocols approved by the Stanford Committee of Animal Research.
Surgical mouse model of OA
Mouse OA was generated according to the destabilization of the medial meniscus (DMM) model, which results in articular cartilage loss and synovitis similar to that observed in human OA12,13. In the DMM model, the anterior cruciate ligament (ACL) and medial meniscotibial ligament (MML) of the mouse are severed under microscopy, and the mice are sacrificed 12 weeks after surgery. We utilized four groups of eight mice (oral boswellic acid, topical boswellic acid ointment or cream, or vehicle control ointment). This experiment was replicated once with 14 mice per group providing eight mice for histology and allowing an addition six mice for harvesting of synovial tissue to allow quantitation of boswellic acid (n = 3) as well as inflammatory cytokines (n = 3) in each treatment group. All animals were housed with other mice in their treatment groups however, with the exception of orally dosed, mice, handing was identical between topical treatment and control groups.
Treatment of mouse OA
Starting one day after surgery, we mice were administered either oral (10 mg/kg) or topical boswellic acid cream or ointment twice daily for 12 weeks. Control mice received topical treatment with the formulation ointment base without boswellic acid. For topical application of boswellic acid, we shaved the right stifle joint mice and applied approximately 25 μl of cream or ointment to the joint. Boswellic acid cream and ointment were compounded as described in Supplemental materials.
Evaluation of tissue and plasma levels of boswellic acid
Plasma was obtained by tail-vein bleeding, and synovial tissue was microdissected from the stifle joint. Plasma or tissue samples were precipitated with acetonitrile, and level of beta-boswellic acid were evaluated by liquid chromatography/mass spectrometry (LC/MS) at Climax Laboratories, Inc. (San Jose, CA). The LC/MS analysis was conducted by using Shimazu 10 A HPLC system (Shimadzu Scientific Instruments, Inc.) with ACE C18, 50 × 2.1 HPLC column and ABSciex API-4000 Mass Spectrometer (ABSciex Corp) with Electrospray Ionization (ESI) and negative Multiple Reaction Monitoring (MRM) Scan. A gradient elution was used in separating the test compound with a mobile phase A (0.1% formic acid in 5 mM of NH4AC) and B (0.1% formic acid in acetonitrile).
Scoring of cartilage degeneration, osteophyte formation, and synovitis in mouse OA
Limbs were removed and knees decalcified, paraffin-embedded, and cut into 4-μm-thick sections and stained with Safranin-O. Cartilage degeneration, osteophyte formation and synovitis scores were calculated as previously described13. Scores for synovitis were recorded for the femoral-medial and the tibial-medial condyles on the operated side of the joint, and the scores for the two regions were summed.
Stimulation of murine OA synovium
3 months after DMM induction, synovium was microdissected from an additional group of untreated mice and roughly equal pieces (matched by weight) were stimulated in triplicate with LPS in the presence or absence of 140 ug/ml boswellic acid. Tissue was lysed and levels of inflammatory cytokines were measured in supernatants using a multiplex bead-based cytokine assay on the Luminex platform (Millipore) or after extracting RNA for quantitative PCR with results normalized to expression of gapdh expression.
Statistical analysis
Cartilage degeneration, osteophyte formation, and synovitis scores as well in vitro stimulation assays were analyzed with a two-tailed unpaired t test to compare means of treatment groups. For in vitro assays, minimal skew of triplicate results was confirmed by eye and for murine studies a Gaussian distribution was confirmed using the D'Agastino-Pearson normality test (GraphPad Prism.) Data are presented as mean ±95% confidence intervals (CI).
Results
Levels of boswellic acid in plasma and synovium after oral or topical administration
Table I demonstrates that topical administration resulted in tissue levels of boswellic acid 2–6 times higher than plasma levels, suggesting local absorption of boswellic acid rather than systemic absorption with local recirculation and deposition. As expected, topical administration resulted in lower plasma levels of boswellic acid than did oral administration and the lipid-based topical formulation (boswellic acid ointment) achieved significantly higher synovial levels than the aqueous topical formulation (boswellic acid cream). Although the plasma levels of boswellic acid were only slightly higher with the lipid-based formulation than with the aqueous formulation, the synovial:plasma ratio (6.64:1.87) was significantly higher with the lipid-based formulation. Given the observation that lipid-based boswellic acid ointment achieves higher relative synovial tissue levels than boswellic acid cream, this formulation was used as the topical comparator in the reported murine treatment studies.
Table I. Boswellic acid levels in synovial tissue and plasma after oral or topical administration.
| Boswellic acid | 1 h after treatment | ||
|---|---|---|---|
|
|
|||
| Oral (n = 3 mice) | Ointment (n = 3 mice) | Cream (n = 3 mice) | |
| Synovial tissue level* (ng/g) | 854 ± 479 | 6,462 ± 1,452 | 1,052 ± 269 |
| Plasma level (ng/mL) | 7,350 ± 3,531 | 973 ± 320 | 563 ± 164 |
Synovium was microdissected from three mice and independently assayed for levels of boswellic acid as normalized by weight. Results represent average concentration ±95% CI.
Oral or topical boswellic acid attenuates mouse OA
At 12 weeks, we assessed the effect of the various treatments on cartilage erosion, osteophyte formation, and synovitis. Analysis of Safranin-O-stained tissues showed that both the oral and the ointment preparation significantly attenuated articular cartilage erosion [Fig. 1(A)–(F)] with only a non-statistically significant a trend toward increased efficacy in the oral treatment group [Fig. 1(G)]. In addition, both the oral and ointment preparations significantly reduced knee synovitis and osteophyte formation (P < 0.01 for each assessment, data not shown).
Fig. 1.
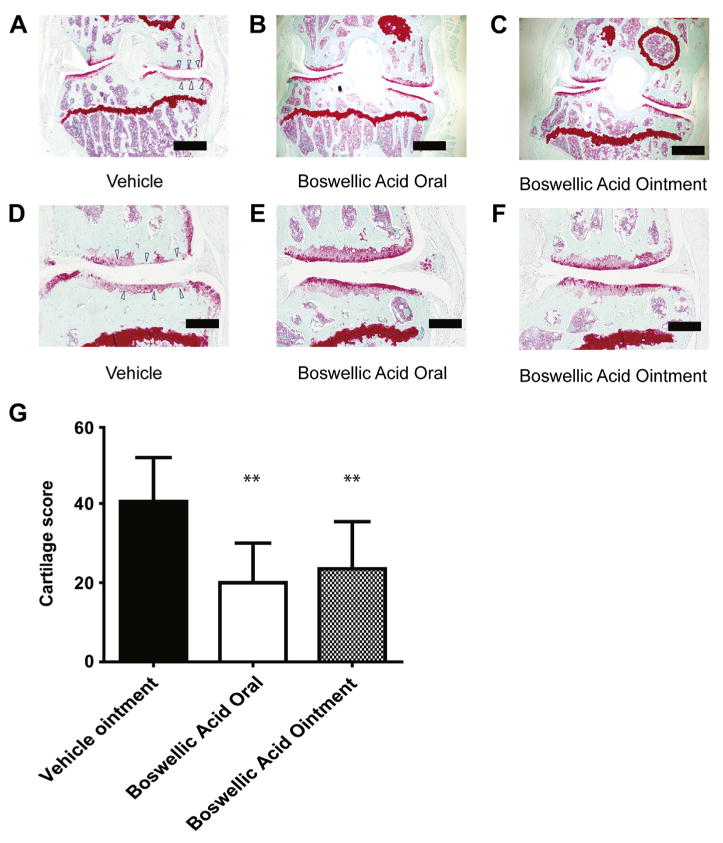
Oral or topical boswellic acid reduces cartilage erosion in a mouse model of OA. Mice were surgically induced to develop OA via DMM, and then treated with vehicle, oral boswellic acid, or boswellic acid ointment for 12 weeks after surgery. (A–F) Representative Safranin-O-stained sections of the medial region of the mouse stifle joints at 12 weeks. Scale bar: low-magnification (top) images, 500 μM; high-magnification (bottom) images, 125 μm. (G) Quantification of cartilage loss in mice treated with vehicle ointment, oral, or topical boswellic acid ointment (n = 8 mice per group). Data are shown as the mean + 95% CI. **P < 0.01 by Student's t-test. Results shown are representative of two independent experiments.
Boswellic acid inhibits IL-1β and TLR4 induced synovial activation
Induction of synovial inflammation by several innate immune ligands has been implicated in the pathogenesis of human and murine OA14,15. Fig. 2(A) demonstrates a reduction in level of several inflammatory cytokines in synovium of mice treated with topical boswellic acid ointment compared with vehicle control ointment. Using OA synovium from untreated mice, we demonstrate the ability of boswellic acid to directly inhibit IL-1β induced production of several inflammatory mediators from OA synovial tissue at both the transcriptional [Fig. 2(B)] and protein level [Fig. 2(C)]. Similarly, we confirm the ability of boswellic acid to inhibit TLR4 induced cytokine production from OA synovial tissue after exposure to LPS [Fig. 2(D)].
Fig. 2.
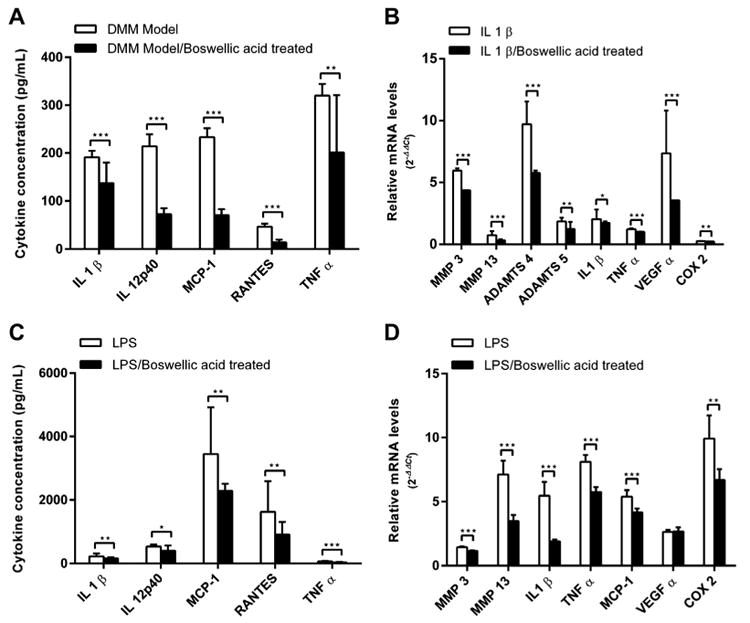
Boswellic acid inhibits IL-1β and TLR4 induced cytokine production from OA synovium. Mice were surgically induced to develop OA via DMM and treated with topical Boswellic acid ointment or vehicle control (n = 3 mice per treatment group) and at 4 months, OA synovial tissue was microdissected and tissue lysates directly assayed for levels of inflammatory cytokines (A). Alternatively, mice were surgically induced to develop OA and left untreated and at 4 months, OA synovial tissue was microdissected and stimulated with IL-1β (B) or LPS (C, D) with or without Boswellic acid pre-treatment and levels of a panel of inflammatory mediators measured by quantitative PCR or by multiplex immunoassay. All experiments were performed in triplicate. Data are shown as the mean + 95% CI. *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired t-test. LPS, lipopolysaccharide; IL, interleukin; MCP, monocyte chemotactic protein; RANTES, regulated on activation normal T cell expressed and secreted; TNF, tumor necrosis factor; MMP, matrix metalloproteinase; ADAMTs, a disintegrin and metalloproteinase with thrombospondin motifs; VEGF, vascular endothelial growth factor; COX, cyclooxygenase.
Discussion
Current treatments for OA are purely symptomatic—there are no approved disease-modifying therapies. Moreover, most oral medications are plagued by off-target effects and toxicities; in fact, the most recent guidelines for the management of OA-associated pain recommend topical rather than oral NSAIDs whenever possible for people over age 75 years3. In this study we assessed the ability of both oral and topical boswellic acid to modify structural progression in a post-traumatic model of OA.
We show that boswellic acid reduces cartilage loss, osteophyte formation, and synovitis in this model and that topical administration is similarly efficacious to oral administration and provide evidence to implicate inhibition of both IL-1β and TLR signaling as potential mechanisms mediating this protective effect. Notably, another well-studied mechanism by which boswellic acid may inhibit inflammation is though inhibition of the 5-LO pathway. Previous attempts to inhibit this pathway using have also demonstrated the potential to provide disease modifying therapy for patients with OA16 including two proof-of-concept studies utilizing orally administered derivatives of boswellic acid5,17.
Previous studies of topical NSAIDs, specifically topical diclofenac, have shown that drugs can be delivered directly to the affected joint, resulting in a greater drug concentration in synovial tissues than in the blood2; we found that this was also the case with topically administered boswellic acid. Although we cannot exclude the possibility that boswellic acid is being locally concentrated in the joint after systemic absorption, we believe that the demonstration of significantly higher concentration of boswellic acid in the joint tissue compared with the blood supports the hypothesis that the compound is acting locally and is relatively restricted to the targeted joint.
Given the immunomodulatory functions of boswellic acid, our findings suggest that boswellic acid has potential as a novel, targeted, anti-inflammatory therapy that attenuates joint damage in OA. The most well-described mechanism of action ascribed to boswellic acid is inhibition of the 5-LO pathway7. This pathway involves the conversion of arachidonic acid to leukotrienes, including LTB. Levels of LTB4 are abnormally high in OA synovial fluid, and animal studies suggest that LTB4 has a critical role in OA. In addition to preventing LTB4-induced cytokine production, boswellic acid has been suggested to inhibit cytokine mediated cartilage loss11 TLR4-mediated cytokine production in human monocytes9. Given the role of the innate immune system14, including specific endogenous TLR agonists, in driving OA-associated inflammation18, our data suggest that cytokine and/or TLR4 inhibition may be another mechanism by which boswellic acid exerts its protective effects and further identifies the TLR4 pathway as a potential target for disease modifying anti-osteoarthritis drug (DMOAD) therapy.
One limitation of this study is the administration of a Boswellia serrata extract. Though the major component of this extract is Boswellic acids, the presence of other undefined molecules could also contribute tothe therapeutic efficacy observed in this study and thus future studies will benefit from use of a more pure formulation of specific Boswellic acids both to confirm efficacy and to provide a regulatory path for human trials. Another limitation is the use of a mouse model of traumatic OA in which disease is induced in a controlled manner and therapy initiated at the time of the initial joint injury. However, despite the inherent limitation of such controlled animal experiments, the DMM model appears highly relevant to human OA as demonstrated by both histopathologic and mechanistic similarities12. Similarly, our ex vivo studies utilize a non-physiologic TLR4 agonist, bacterial LPS. However, our parallel studies have obtained similar results using endogenous TLR4 ligands previously identified from RA and OA synovial fluid (data not shown). Another limitation of this study is the observation that, despite higher levels of boswellic acid in synovium from mice treated with topical therapy compared with oral therapy, disease was abrogated to a greater degree in the orally treated mice. Though we have no clear explanation for this observation, it is possible that there are unmeasured metabolites after or administration. We hypothesize that although topical therapy may suppress synovial inflammation, oral therapy may better inhibit intra-articular and cartilaginous inflammation due to its wider availability.
Given the overall safety of Boswellia and its boswellic acid derivatives in small clinical studies in individuals with OA, and a presumed decrement in risk with topical therapy, boswellic acid could potentially be used for the prolonged durations likely required to prevent and/or abrogate the progression of post-traumatic knee OA. Human studies of oral and topical boswellic acid are needed to better define the pharmacokinetics properties and, ultimately, evaluate the safety and therapeutic efficacy in both the treatment of pain and prevention of structural progression in human OA.
Supplementary Material
Acknowledgments
These studies were supported by a Rehabilitation Research and Development Merit Award from the Department of Veterans Affairs and US National Heart, Lung, and Blood Institute Proteomics Center contract N01 HV 28183 (W.H.R.). JS is the recipient of a Veteran Affairs Career Development Award and an Arthritis Foundation Innovative Research Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Author contributions: Conception and study design: QW, XP, WHR, JS; Performed and analyzed experimental data: QW, HHW, CAW, LJL, JS; Wrote and edited the manuscript; QW, XP, HHW, CAW, LJL, WHR, JS.
Conflict of interest: The authors declare no conflict of interest with respect to this manuscript.
Supplementary data: Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2013.10.012.
Contributor Information
W.H. Robinson, Email: wrobins@stanford.edu.
J. Sokolove, Email: sokolove@stanford.edu.
References
- 1.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354(8):841–8. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 2.Banning M. Topical diclofenac: clinical effectiveness and current uses in osteoarthritis of the knee and soft tissue injuries. Expert Opin Pharmacother. 2008;9(16):2921–9. doi: 10.1517/14656566.9.16.2921. [DOI] [PubMed] [Google Scholar]
- 3.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64(4):455–74. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui MZ. Boswellia serrata, a potential antiinflammatory agent: an overview. Indian J Pharm Sci. 2011;73(3):255–61. doi: 10.4103/0250-474X.93507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta K, Alluri KV, Satish AR, Mishra S, Golakoti T, Sarma KV, et al. A double blind, randomized, placebo controlled study of the efficacy and safety of 5-Loxin for treatment of osteoarthritis of the knee. Arthritis Res Ther. 2008;10(4):R85. doi: 10.1186/ar2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulkarni RR, Patki PS, Jog VP, Gandage SG, Patwardhan B. Treatment of osteoarthritis with aherbomineral formulation: adouble-blind, placebo-controlled, cross-over study. J Ethnopharmacol. 1991;33(1–2):91–5. doi: 10.1016/0378-8741(91)90167-c. [DOI] [PubMed] [Google Scholar]
- 7.Safayhi H, Mack T, Sabieraj J, Anazodo MI, Subramanian LR, Ammon HP. Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther. 1992;261(3):1143–6. [PubMed] [Google Scholar]
- 8.Pelletier JP, Boileau C, Boily M, Brunet J, Mineau F, Geng C, et al. The protective effect of licofelone on experimental osteoarthritis is correlated with the downregulation of gene expression and protein synthesis of several major cartilage catabolic factors: MMP-13, cathepsin K and aggrecanases. Arthritis Res Ther. 2005;7(5):R1091–102. doi: 10.1186/ar1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Syrovets T, Buchele B, Krauss C, Laumonnier Y, Simmet T. Acetyl-boswellic acids inhibit lipopolysaccharide-mediated TNF-alpha induction in monocytes by direct interaction with IkappaB kinases. J Immunol. 2005;174(1):498–506. doi: 10.4049/jimmunol.174.1.498. [DOI] [PubMed] [Google Scholar]
- 10.Sengupta K, Trimurtulu G, Marasetti AK, Tummala T, Ravada SR, Krishnaraju AV, et al. Inhibition of TNF production and blocking of mitogen-activated protein Kinase/NF B activation in lipopolysaccharide-induced Thp-1 human monocytes by 3-O-Acetyl-11-Keto- -Boswellic acid. J Food Lipids. 2009;16(3):325–44. [Google Scholar]
- 11.Sengupta K, Kolla JN, Krishnaraju AV, Yalamanchili N, Rao CV, Golakoti T, et al. Cellular and molecular mechanisms of anti-inflammatory effect of Aflapin: a novel Boswellia serrata extract. Mol Cell Biochem. 2011;354(1–2):189–97. doi: 10.1007/s11010-011-0818-1. [DOI] [PubMed] [Google Scholar]
- 12.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15(9):1061–9. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Rozelle AL, Lepus CM, Scanzello CR, Song JJ, Larsen DM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17(12):1674–9. doi: 10.1038/nm.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20(5):565–72. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 15.Schelbergen RF, Blom AB, van den Bosch MH, Sloetjes A, Abdollahi-Roodsaz S, Schreurs BW, et al. Alarmins S100A8 and S100A9 elicit a catabolic effect in human osteoarthritic chondrocytes that is dependent on Toll-like receptor 4. Arthritis Rheum. 2012;64(5):1477–87. doi: 10.1002/art.33495. [DOI] [PubMed] [Google Scholar]
- 16.Raynauld JP, Martel-Pelletier J, Bias P, Laufer S, Haraoui B, Choquette D, et al. Protective effects of licofelone, a 5-lipoxygenase and cyclo-oxygenase inhibitor, versus naproxen on cartilage loss in knee osteoarthritis: a first multicentre clinical trial using quantitative MRI. Ann Rheum Dis. 2009;68(6):938–47. doi: 10.1136/ard.2008.088732. [DOI] [PubMed] [Google Scholar]
- 17.Vishal AA, Mishra A, Raychaudhuri SP. A double blind, randomized, placebo controlled clinical study evaluates the early efficacy of aflapin in subjects with osteoarthritis of knee. Int J Med Sci. 2011;8(7):615–22. doi: 10.7150/ijms.8.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Lent PL, Blom AB, Schelbergen RF, Sloetjes A, Lafeber FP, Lems WF, et al. Active involvement of alarmins S100A8 and S100A9 in the regulation of synovial activation and joint destruction during mouse and human osteoarthritis. Arthritis Rheum. 2012;64(5):1466–76. doi: 10.1002/art.34315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


