Abstract
During T cell interaction with APC, CD28 is recruited to the central region (cSMAC) of the immunological synapse. CD28-mediated signaling through PI3K results in the recruitment of PKCθ to the cSMAC, activation of NF-κB, and upregulation of IL-2 transcription. However, the mechanism that mediates CD28 localization to the cSMAC and the functional consequences of CD28 localization to the cSMAC are not understood. In this report we show that CD28 recruitment and persistence at the immunological synapse requires TCR signals and CD80 engagement. Addition of mAb to either MHC class II or CD80 results in the rapid displacement of CD28 from the immunological synapse. Ligand binding is not sufficient for CD28 localization to the immunological synapse, as truncation of the cytosolic tail of CD28 disrupts synapse localization without effecting the ability of CD28 to bind CD80. Furthermore, a single point mutation in the CD28 cytosolic tail (tyrosine 188) interferes with the ability of CD28 to preferentially accumulate at the cSMAC. PKCθ distribution at the immunological synapse mirrors the distribution of tyrosine 188-mutated CD28, indicating that CD28 drives the localization of PKCθ even when CD28 is not localized to the cSMAC. Mutation of tyrosine 188 also results in diminished activation of NF-κB, suggesting that CD28-mediated localization of PKCθ to the cSMAC is important for efficient signal transduction. These data reinforce the importance of the interplay of signals between TCR and CD28 and suggest that CD28 signaling through PCKθ may be mediated through localization to the cSMAC region of the immunological synapse.
Keywords: T cells, costimulation, signal transduction
Introduction
T cell activation requires antigen-specific recognition in the context of MHC on the surface of an APC. The interaction with the APC provides the T cell with peptide-MHC ligands for the TCR as well as ligands for costimulatory receptors. Many surface receptors can provide costimulation in conjunction with the TCR, most notably among these is CD28. CD28 is the most potent and best described costimulatory molecule for T cells, participating in many aspects of T cell activation, survival, and differentiation (1-3). One of the best characterized function of CD28 is its ability to enhance IL-2 expression through at least two different mechanisms. CD28 promotes activation and nuclear translocation of transcription factors (NF-κB and AP-1) that will interact with the IL-2 enhancer and augment transcription (4). In addition, CD28 increases IL-2 mRNA stability by a mechanism that is not well characterized (1).
Despite the well-known functional importance of CD28, the biochemical signaling pathways induced downstream of CD28 are not clearly understood (2, 3). Part of the difficulty in identifying CD28-mediated signals is that one major function of CD28 is to amplify signals that can be initiated through the TCR. Profiling of protein signaling intermediates as well as changes in gene expression have suggested that CD28 costimulation functions primarily to modify TCR-mediated signaling pathways (5-7). In support of this, Lck, Itk, and PI3K, the kinases that can be recruited to the cytosolic tail of CD28 and have been associated with CD28 costimulation, can also be activated by TCR signaling in the absence of CD28 (8-14). Point mutations in the src homology domain-2 (SH2) and SH3 interaction motifs that recruit these signaling proteins to the CD28 cytosolic tail do not entirely reproduce the effects of CD28-deficiency, suggesting that additional pathways and/or functional redundancy within these pathways may be involved (15-20). For example, we have shown that mutation of the PI3K interaction site results in a failure of CD28 to enhance NF-κB activation and IL-2 transcription. However, this has little effect on IL-2 secretion, because this mutation does not inhibit the ability of CD28 to enhance IL-2 mRNA stability (21).
T cell recognition of antigen is associated with the formation of a tight cell:cell contact with APC, termed the immunological synapse (IS) (22, 23). Distinct membrane domains (supramolecular activation clusters or SMAC) are defined according to the distribution of the proteins recruited to and sorted within the IS. The most central cluster (cSMAC) is defined by the presence of TCR, a peripheral cluster (pSMAC) is defined by the distribution of the integrin LFA-1, and a more distal cluster (dSMAC) is defined by the localization of CD45 (24-26). Because many of the molecules associated with the cSMAC are involved in proximal T cell activation, it was initially thought that this region was the signaling compartment of the IS. However, the cSMAC is now thought to be the site for TCR downregulation (27, 28) and sustained TCR signaling is thought to be mediated by the constant recruitment of new TCR complexes that form along the outside of the pSMAC (29-32). According to this model, these TCR microclusters signal as they migrate through the pSMAC toward the cSMAC. TCR microclusters ultimately accumulate at the cSMAC, where they stop signaling, and are targeted for downregulation. In contrast, CD28 signaling has been indirectly associated with cSMAC localization. CD28 has been shown to be recruited to the IS and to accumulate at the cSMAC together with TCR (21, 33-35). CD28 is required for recruitment of protein kinase C-θ (PKCθ) to the cSMAC region (21, 34). In CD28-deficient T cells or T cells expressing a mutation in the PI3K interaction site, PKCθ is still recruited to the IS, but it is not focused into the cSMAC region. This results in a loss in PKCθ-dependent activation of NF-κB and upregulation of IL-2 transcription, suggesting that CD28-dependent activation of PI3K within the IS contributes to CD28 costimulation (21). CD28 engagement outside the IS (in trans) can still enhance IL-2 secretion, but PKCθ is not recruited to either the TCR or CD28 and IL-2 gene transcription is not upregulated. Rather, CD28 in trans enhances IL-2 secretion through the induction of IL-2 mRNA stabilization (36). These observations suggest that CD28 localization at the IS may be an important component for the transduction of some, but not all, aspects of CD28-mediated T cell costimulation.
Little is known about the molecular mechanisms involved in the recruitment of CD28 to the IS. CD28 distributes around the plasma membrane of non-polarized T cells, and within minutes of T cell:APC interaction, CD28 accumulates to the contact area, concentrating into the cSMAC. It has been shown that ligand interaction with CD80 or CD86 is important to promote CD28 recruitment to the IS (33, 35). But the signals that drive CD28 targeting to the cSMAC and the intracellular sorting motifs within CD28 that mediate this localization are not understood. In this report we have addressed both of these issues. We show that sustained signaling from both TCR and CD28 is required to maintain CD28 within the IS. Furthermore, we have identified a single point mutation at tyrosine 188 (Y188F) that diminishes CD28 localization to the cSMAC. The mislocalization of Y188F CD28 was mirrored by corresponding mislocalization of PKCθ and resulted in a reduction in NF-κB nuclear translocation. These observations support the model that CD28 costimulation may be controlled in part by localization of CD28 to the cSMAC region of the IS.
Materials and Methods
Cells and reagents
6132 Pro cell transfectants expressing class II (I-Ad) alone (ProAd) or in combination with CD80 (ProAd-B7), ICAM-1 (ProAd-ICAM), or both (ProAd-ICAM-B7) and purification of CD4-positive T cells from DO11.10 TCR-transgenic BALB/c mice have been previously described (21). All cell lines were maintained in DMEM (Invitrogen Life Technologies, Gaithersburg, MD) supplemented with 10% FCS, 2 mM glutamine, 0.1 mM nonessential amino acids, and 50 μM 2-ME. CD4+ T cells (either from CD28+/+ or CD28-deficient mice) were stimulated with 0.2 μg/ml OVA peptide (323-339) presented by irradiated syngeneic spleen cells and 20 U/ml human rIL-2. When noted, CD28-deficient T cells were activated and 1 day later transduced with retroviruses containing wild-type (WT) or mutated murine CD28 (16) or WT murine CD28 fused to Cyan Fluorescent Protein (CD28-CFP) (35). Additional mutations in CD28, where portions of the cytosolic tail were placed by alanine (ala scan) or where the entire cytosolic tail was deleted (ΔCT), were constructed by overlapping PCR, confirmed by DNA sequencing, and subcloned into the MIGR retroviral vector. All the viruses utilized (but not CD28-CFP) contain GFP expressed from an internal ribosome entry site (IRES) as a marker for transduced cells. Infection efficiency ranged from 10 to 60%, and cells were sorted to match the level of CD28 expression before each experiment. Antibodies against PKCθ (C-18) and NF-κB p65 (A) were purchased from Santa Cruz Biotechnology, antibodies against CD28 (37.51), CD80 (1G10) and MHC class I (34-2-12) were purchased from BD Pharmingen and fluorescently-labeled, species-specific, secondary Abs were obtained from Jackson ImmunoResearch Laboratories, West Grove, PA. Antibodies against CD28 (37.51), CD80 (1G10) and MHC class II (M5/114) were also purified from hybridoma supernatants by Protein A-sepharose. Soluble CD80 (rmB7-1/hFc) was purchased from R&D Systems (Minneapolis, MN).
Immunofluorescence microscopy
Peptide-pulsed APC (2 μg/ml OVA peptide) were centrifuged with T cells for 20 seconds at a relative centrifugal force of 2000 × g. The cell pellet was incubated for 5 min at 37°C, resuspended in complete media, and either incubated for various times or immediately plated on poly-L-lysine-coated coverslips. After plating, conjugates were incubated for 5 min at 37°C to allow cell binding to the poly-L-lysine. Cells were fixed in 3% (w/v) paraformaldehyde, permeabilized in 0.3% (v/v) Triton X-100, and stained. For NF-κB localization, the incubation time before plating on poly-L-lysine-coated coverslips was increased to 35 min, and nuclei were labeled with Hoechst stain after fixation and permeabilization. Samples were analyzed on a Zeiss Axiovert microscope controlled by SlideBook software (Intelligent Imaging Innovations, Denver, CO). Nearest-Neighbor deconvolution, digital analysis, and three-dimensional rendering were accomplished using SlideBook software. The threshold for the images was set using the APC (CD28 and PKCθ) or the T cell (MHC Class II) fluorescent intensity as nonspecific staining since these cells should not express the corresponding proteins. CD28 and PKCθ distribution within the IS was calculated on a midplane image by measuring the length of the cell:cell contact that contained CD28 or PKCθ and dividing it by the length of the entire interaction site between the T cell and the APC. NF-κB localization to the nucleus was quantified by defining a nuclear mask (Hoechst staining) and calculating the fluorescence intensity of NF-κB staining within the mask area.
Live cell microscopy
ProAd-ICAM-B7 were plated on Delta T Culture dishes (Bioptechs, Inc, Butler, PA) and incubated ON at 37°C. Antigen (2 μg/ml) was loaded for 2 hours and the dish was mounted on a heated stage maintained at 37°C. CD28-CFP-expressing T cells were added and allowed to interact with the APC. Conjugates were selected based on sustained recruitment of CD28 toward the APC. Images were collected every 21 sec for 5 minutes, 2 μg/ml control (αMHC class I) or blocking (either αCD80 or αMHC class II) mAbs were added and imaging was continued for an additional 5-20 minutes.
Calcium signaling
Calcium imaging of T cell:APC conjugates was done essentially as described (37). ProAd-ICAM-B7 cells were preincubated with 2.0 μg/ml OVA peptide for 2 h at 37°C and labeled with Alexa 633 (Molecular Probes, Gaithersburg, MD). T cells were loaded with 1 μM Indo-1AM (Molecular Probes) for 30 min at 37°C. T cells and APC were mixed at a 1:1 ratio in solution and run on an LSRII flow cytometer (BD Bioscience, San Diego, CA) to establish a baseline for intracellular calcium levels in the T cells. The T cell:APC mixture was then centrifuged for 15 s at a relative centrifugal force of 2000 × g at room temperature in a microfuge. The cell pellets were resuspended in warm medium and run on the flow cytometer at 37°C. T cell:APC conjugates were identified by dual Indo-1/Alexa 633 fluorescence, and intracellular calcium levels were determined by ratiometric analysis of Indo-1-Blue to Indo-1-Violet fluorescence using FlowJo software (Tree Star, Inc, Ashland, OR).
Results
CD28-ligand interactions are required to recruit and sustain CD28 at the IS
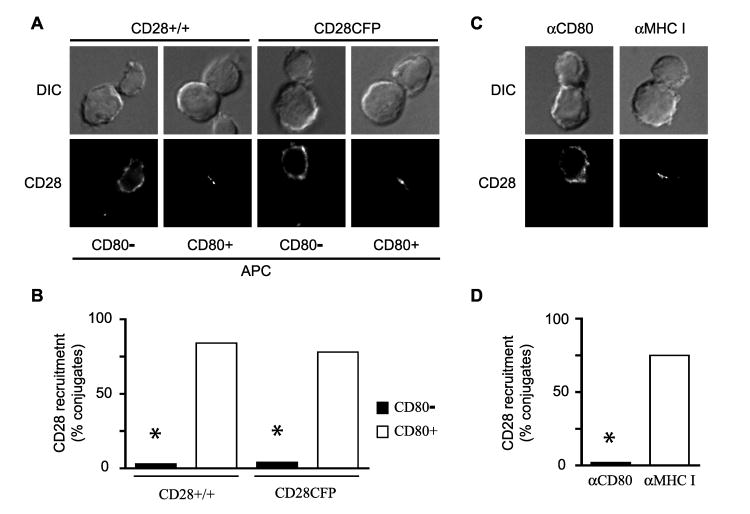
CD28 colocalizes with TCR at the cSMAC of the IS in a ligand-dependent manner (33, 35). The requirement for CD28 engagement with CD80 to recruit CD28 to the IS can be shown by interaction of T cells with APC that do and do not express CD80. Endogenous CD28, detected by αCD28 staining, is only recruited to the IS in conjunction with CD80-positive APC (Fig. 1A and B). Retroviral transduction of CD28-deficient T cells with a CD28-CFP fusion protein also demonstrates efficient recruitment of CD28 to the IS only with CD80-positive APC (Fig. 1A and B). Similarly, CD28 recruitment to the IS was disrupted when ligand-binding was blocked by the preincubation of APC with αCD80 mAb, while no effect was observed by the addition of a control αclass I mAb (Fig. 1C and D).
Figure 1. CD28 recruitment to the IS requires ligand binding.
A and B, Conjugates between DO11.10 TCR transgenic, CD4+ T cells (CD28+/+) or retrovirally transduced CD28KO T cells expressing a CD28-CFP fusion protein (CD28-CFP) and Ag-pulsed APC were analyzed base on their ability to recruit CD28 to the IS. CD80- APC are ProAd-ICAM cells that express MHC class II and ICAM-1, but not CD80 or CD86; while CD80+ APC are ProAd-ICAM-B7 cells that express MHC class II, ICAM-1 and CD80. CD28+/+ T cells were stained with αCD28 to label endogenous CD28, while localization of CD28-CFP was determined by CFP fluorescence. Representative images (A) and % of conjugates that recruit CD28 to the IS (B; n = 50 to 65 conjugates) from one experiment representative of two (CD28-CFP) or three (CD28 +/+) are shown. C and D, T cell conjugates with Ag-pulsed ProAd-ICAM-B7 APC were formed in the presence of 2 μg/ml mAb against CD80 (αCD80) or MHC class I (αMHC I) and analyzed for recruitment of CD28 to the IS. Representative images (C) and % of conjugates that recruit CD28 to the IS (D, n = 50 to 65 conjugates) from one experiment representative of three is shown. In all the images, the T cell is oriented toward the top in each panel. * p<0.0001 by two population proportion Z-student statistical test.
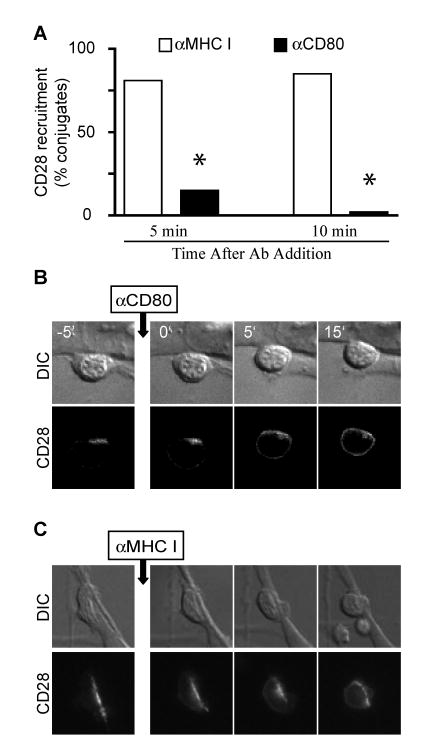
To determine whether continuous engagement with ligand is required to sustain CD28 at the IS, we allowed CD28 to be recruited at the IS and then added αCD80 mAb to block CD80-CD28 interactions (Fig. 2A). Within 5 minutes of αCD80 addition the majority of conjugates lack CD28 localization at the IS. Addition of a control αclass I mAb has no effect on CD28 accumulation at the IS. TCR signals remained intact when CD80-CD28 interactions were blocked, as this treatment does not inhibit calcium influx on T cells (data not shown). The rapid displacement of CD28 from the IS after αCD80 mAb addition can be visualized in real-time using T cells expressing the CD28-CFP chimera (Fig. 2B and Video 1 in Supplemental Data4). CD28-CFP-positive T cell:APC were imaged for 5 minutes to confirm that CD28 was stably recruited to the IS. Blocking αCD80 mAb was added and the conjugates were followed for an additional 15-20 minutes. About 5 minutes after mAb addition, CD28-CFP began to diffuse away from the APC and by 10 minutes was distributed evenly around the T cell surface. In contrast, CD28-CFP remained polarized toward the APC the entire period of time when αclass I mAb were added (Fig. 2C and Video 2 in Supplemental Data). These results demonstrated the crucial requirement of CD28-CD80 interactions not only to initially recruit, but also to maintain, CD28 within the IS.
Figure 2. CD28 ligand binding is required to sustain CD28 at the IS.
A, WT DO11.10 T cell conjugates with antigen-pulsed ProAd-ICAM-B7 APC were incubated 10 minutes to allow IS formation, 2 μg/ml of mAb against MHC class I or CD80 was added, and the cells were cultured for 5 or 10 minutes before fixation and microscopic analysis. Percentage of conjugates that recruit CD28 to the IS (n = 60 conjugates) are shown. * p<0.0001 by two population proportion Z-student statistical test. B and C, Conjugates between CD28-CFP-expressing T cells and Ag-pulsed ProAd-ICAM-B7 APC were selected based on CD28 recruitment at the IS. Live cell imaging was done for 5 minutes prior to addition of 2 μg/ml of anti-CD80 (B) or anti-MHC class I (C) and continued for 15 minutes after mAb addition. Representative images at various time points are shown; complete movie are shown in Videos 1 and 2 in Supplemental Data. The apparent change in morphology in the T cell following anti-class I addition, reflects the T cell crawling onto the top of the APC (see Video 2 in Supplemental Data).
CD28 requires constant TCR signal to persist at the IS
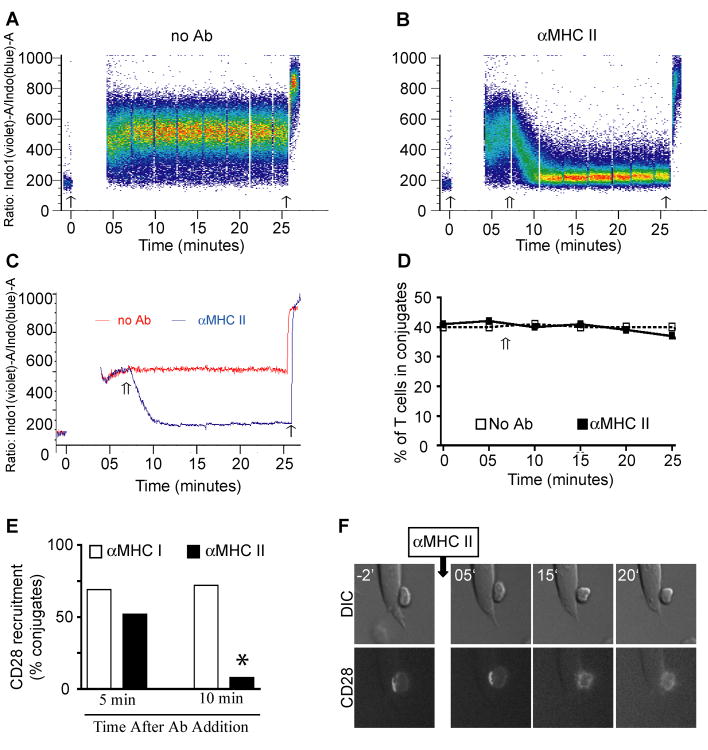
To determine if TCR engagement is required to sustain CD28 at the IS, TCR-peptide/class II interactions were blocked with the addition of αclass II blocking mAb. It has been shown that this treatment leads to the rapid inhibition of calcium influx, prevention of new TCR microcluster formation, and cessation of sustained TCR signaling (32, 38, 39). To confirm the efficacy of αclass II blocking, conjugates between indo-1-loaded CD4+ T cells and Alexa-633-labeled ProAd-ICAM-B7 were formed and the magnitude of the T cell calcium flux in T cell:APC conjugates was determined on the flow cytometer (Fig. 3A - C). Blocking amounts of αclass II mAb were added and conjugates were analyzed to simultaneously measure the effect of αclass II on intracellular calcium flux and on T cell:APC conjugate stability over time. After a lag of several minutes, calcium influx was rapidly inhibited in the entire population of T cells (Fig. 3B and C). In contrast, the majority of T cell:APC conjugates were stable for at least 15 minutes after the inhibition of calcium signaling (Fig. 3D). This provides a kinetic window where we could evaluate the requirement for sustained TCR signaling in CD28 localization to the IS without disrupting T cell:APC interactions. To determine whether CD28 remains recruited to the IS when TCR signals were blocked, T cells:ProAd-ICAM-B7 conjugates were formed in the presence of antigen, and incubated for 10 minutes to allow IS formation and CD28 recruitment toward the APC. Blocking antibodies for class II were added and conjugates were fixed 5 or 10 minutes later (Fig. 3E). As a negative control, conjugates were treated with αclass I mAb. CD28 remained recruited to the APC contact area in most of the conjugates 5 minutes after the addition of anti-class II mAb. However, after 10 minutes of TCR blocking, localization of CD28 to the IS was lost in most of the conjugates.
Figure 3. CD28 requires constant TCR signal to persist at the IS.
A - C, T cells were loaded with Indo-1-AM and mixed with antigen-pulsed, Alexa 633-labeled ProAd-ICAM-B7 APC. The cells were analyzed by flow cytometry to established a baseline of Indo-1 fluorescence and then briefly pelleted to induce T cell:APC conjugate formation (thin arrow on the left in A - C). The cells were resuspended, rapidly returned to the flow cytometer, and maintained at 37°C. T cell:APC conjugates were gated based on coincident Indo-1 and Alexa 633 fluorescence and the change in the ratio of violet to blue fluorescence of Indo-1, indicative of an increase in intracellular calcium, was monitored over time. Data are presented as dot plots of individual conjugates (A and B) and as median calcium response of the population (C). After 7 min anti-MHC class II was added to one sample (double arrow in B and C), which resulted in a rapid decline in calcium signaling within all the T cell:APC conjugates. Ionomycin was added at the end of the assay (thin arrow on the right in A - C) to assure that all T cells were effectively loaded with Indo-1. D, The samples in A and B were analyzed for the percentage of T cells that were in conjugates with APC over time in the presence and absence of anti-class II mAb. E, T cell:ProAd-ICAM-B7 conjugates were incubated 10 minutes to allow IS formation, 2 μg/ml of mAb against MHC class I or class II was added, and the cells were cultured for 5 or 10 minutes before fixation and microscopic analysis. Percentage of conjugates that recruit CD28 to the IS (n = 50 conjugates) are shown. This is one experiment representative of three. * p<0.0001 by two population proportion Z-student statistical test. F, Conjugates between CD28-CFP-expressing T cells and Ag-pulsed ProAd-ICAM-B7 APC were selected based on CD28 recruitment at the IS. Live cell imaging was done for 2 minutes prior to addition of 2 μg/ml of anti-MHC class II and continued for 20 minutes after mAb addition. Representative images at various time points are shown; the complete movie is shown in Video 3 in Supplemental Data.
To visualize CD28 displacement from the APC when TCR signals are blocked, we performed a live cell imaging analysis. CD28-CFP-expressing T cells were allowed to interact with Ag-pulsed APC for 10 minutes. Conjugates were selected for CD28 localization to the IS, blocking class II mAb was added, and CD28 localization was followed for another 20 minutes in the presence of the blocking mAb (Fig. 3F and Video 3 in Supplemental Data). CD28 remained polarized toward the APC for about 8 minutes and then rapidly diffused away, adopting a non-polarized distribution. Nevertheless, even after complete loss of CD28 polarization within the IS, T cell remained in close contact with the APC for at least 20 minutes after MHC class II blocking antibody was added. This indicates that cessation of TCR signaling results in displacement of CD28, well before complete dissolution of the IS. Taken together, these data indicate that steady state localization of CD28 at the IS depends on continuous CD28 ligand binding and TCR signaling.
Ligand binding is not sufficient to recruit CD28 to the IS
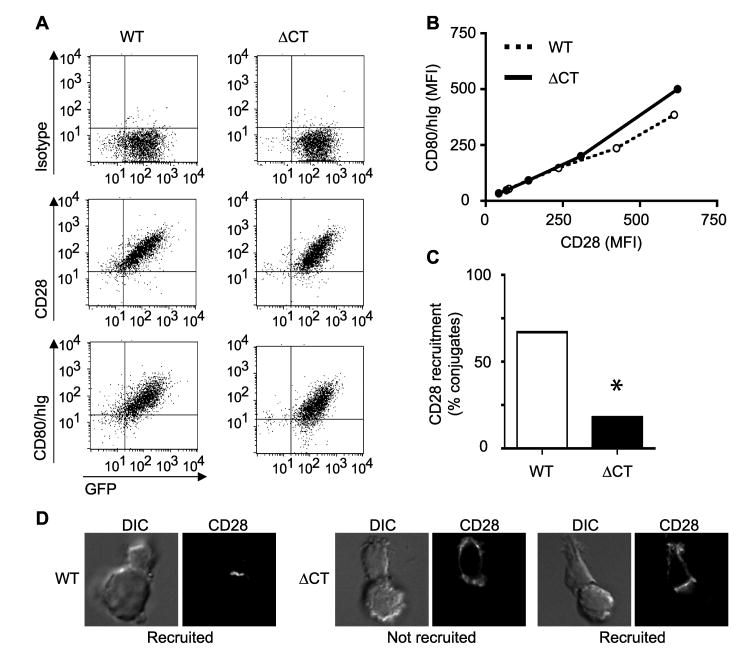
To identify the cis elements in CD28 that mediate localization to the IS, we first generated a truncation of CD28 that lacks the cytosolic tail (ΔCT) (for schematic see Fig 5A, below). The mutation does not effect cell surface expression of CD28. WT and ΔCT CD28 are expressed at equivalent levels at the cells surface following retroviral transduction into CD28-deficient T cells (Fig. 4A). To determine whether the CD28 cytosolic tail is required for recruitment to the IS, conjugates between retrovirally transduced T cells expressing either WT or ΔCT CD28 and Ag-pulsed ProAd-ICAM-B7 were analyzed by microscopy (Fig. 4, C and D). ΔCT CD28-expressing T cells showed a significantly reduced ability to recruit CD28 molecules toward the APC. Furthermore, even in those ΔCT-expressing T cells where CD28 was polarized toward the APC, significant CD28 was detected outside of the IS, a morphology that was not detected with cells expressing WT CD28 (Fig. 4D)
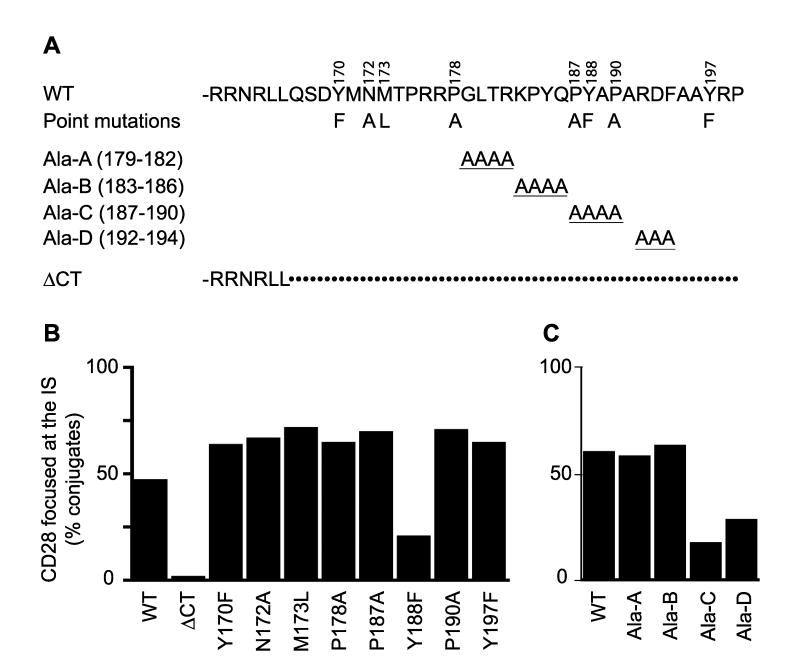
Figure 5. Mutation scan identifies a single site that controls CD28 focusing to the IS.
A, The sequence of the WT CD28 cytosolic tail is shown on the top line. The localization and amino acid substitution of single amino acid point mutations are shown above and below, respectively. Only the amino acid change is shown and all the individual mutations are included on a single line. The clustered alanine replacements (Ala-A through Ala-D) are shown on lines 3-6. Only the amino acid alterations are shown; all other positions are WT. The sequence of the cytosolic tail truncation (ΔCT) is shown on the bottom. In this case, only the sequence remaining in the truncated construct is shown. The remainder of the cytosolic tail (indicated by dots) is deleted. B and C, CD28-deficient T cells were retrovirally transduced with the mutants shown in A and T cell:ProAd-ICAM-B7 conjugates were screened for CD28 focusing at the IS. Focusing was defined as CD28 concentration within less than 60% of the IS area. Data are the percentage of conjugates that focus CD28 to the IS (n = 50).
Figure 4. Ligand binding is not sufficient to recruit CD28 to the IS.
CD28-deficient T cells were transduced with a retrovirus expressing WT CD28 or a truncated CD28 that lacks the cytosolic tail (ΔCT) in conjunction with an IRES-GFP cassette. A and B, T cells were sorted for GFP expression and then labeled with isotype control mAb, αCD28 mAb, or CD80/Ig fusion protein and levels of staining were compared with GFP expression. A, Dot plots of flow cytometric analyses are shown. B, Relative mean fluorescent intensity (MFI) of αCD28 and CD80/Ig binding, normalized to GFP, shows that for any given level of CD28 expression, WT and ΔCT CD28 binds equivalent levels of CD80/Ig. C and D, WT or ΔCT CD28 expressing T cell:APC conjugates were incubated for 15 minutes and analyzed by microscopy. Percentage of conjugates that recruit CD28 to the IS (n = 50) are shown (C). This is one experiment representative of two. * p<0.0001 by two population proportion Z-student statistical test. Images that represent the different phenotype observed between WT and ΔCT CD28 expressing T cells are shown (D). The T cell is oriented toward the top in each panel. Note that even in the ΔCT CD28 expressing T cells that were scored as CD28 recruitment to the IS, CD28 recruitment was often incomplete (image on right).
To confirm that the failure to recruit ΔCT CD28 to the IS was not secondary to a defect in ligand-binding, we tested the ability of ΔCT CD28 to bind CD80. WT or ΔCT CD28-expressing T cells were labeled with a soluble CD80/Ig fusion protein and analyzed by flow cytometry (Fig. 4A). Equivalent levels of CD28 were detected on WT and ΔCT-expressing T cells by labeling either with αCD28 mAb or with the soluble CD80/Ig fusion protein. Double staining with CD28 and CD80/Ig was not feasible, because the CD28 mAb utilized blocks the CD80 interaction site. Therefore, we utilized the IRES-GFP reporter cassette in the retroviral vector to normalize for levels of retroviral gene expression and then compared the cell surface labeling with αCD28 mAb and with the CD80/Ig fusion protein for a given level of GFP expression (Fig. 4B). WT and ΔCT CD28 bound equivalent levels of CD80/Ig fusion protein for any given amount of CD28 expression level. These results confirmed that the failure of ΔCT CD28 to localize at the IS was not due to expression levels or inability to interact with CD80, but was a direct result of the cytosolic tail deletion. Furthermore, CD28 localization to the IS is not mediated passively by binding to ligand expressed on the surface of the APC. Taken together these data indicate that CD28 ligand binding is necessary, but not sufficient, to target CD28 to the IS and additional signals from the TCR and cis elements in the CD28 cytosolic tail are also required.
Mutation of tyrosine at position 188 disrupts the ability of CD28 to focus at the IS
To identify the specific motif within the cytosolic tail of CD28 that mediates IS localization, we first analyzed a series of amino acids point mutations in suspected protein interaction motifs. We targeted the YMNM motif at positions 170-173, that upon tyrosine phosphorylation generates an SH2 binding site that has been shown to recruit PI3K and Grb-2 (8, 9, 40). We also targeted prolines at 178 and 187/190, that have been implicated in Itk and Lck SH3 domain interactions respectively (11-14), and two additional tyrosine residues at 188 and 197 (Fig 5A). Conjugates between CD28-deficient T cell expressing different point mutations and Ag-pulsed ProAd-ICAM-B7 were formed and analyzed by microscopy. Strikingly, only one of these mutations showed a significant defect in its ability to focus CD28 into the IS, a point mutation of the tyrosine at position 188 (Fig. 5B). To test the role of additional segments of the cytosolic tail, a series of clustered alanine replacements were generated (Fig 5A). Replacement of amino acids 179-182 and 183-186 with alanines had no effect on CD28 localization to the IS. In contrast, alanine substitutions of residues 187-190 (which contains Y188) and of residues 192-194 inhibited CD28 localization to the IS. These results indicate that residues 187-194, and especially Y188, are required for CD28 focusing within the IS.
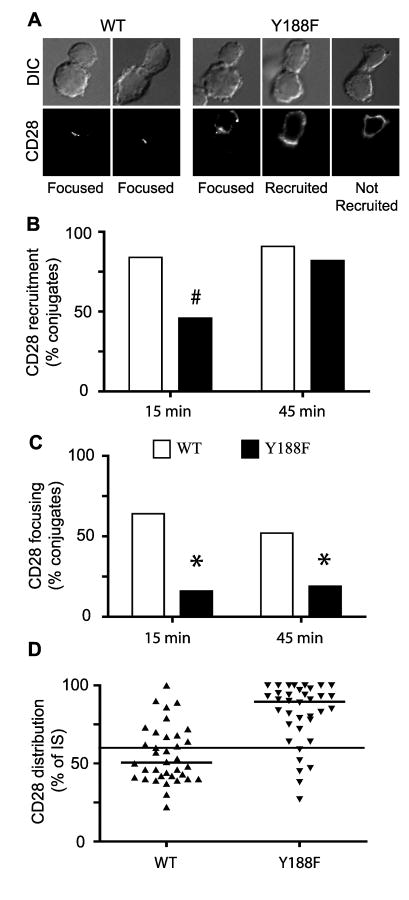
To more carefully address the role of the cytosolic tail on CD28 localization in the IS, we focused our analyses on the single amino acid mutation, Y188F (Fig. 6). T cells expressing Y188F CD28 showed a delay in the kinetics of CD28 accumulation at the IS compared with WT CD28 (Fig 6B). At 15 minutes there was a significant difference in the ability of WT and Y188F CD28 to be recruited at the IS, but by 45 minutes both WT and Y188F CD28 were recruited to the IS with similar frequency. However, even when Y188F was recruited to the IS, it was often incomplete and CD28 was detected outside of the IS (Fig. 6A). These results indicate that Y188 is required for efficient polarization of CD28 to the IS. A further defect in Y188 was detected when the morphology of CD28 localization at the IS was compared. WT CD28 was significantly more focused into the center of the IS (indicative of cSMAC localization) compared with Y188F CD28 at both time points evaluated (Fig 6, C and D). Mutation of Y188F did not interfere with the ability of CD28 to interact with CD80, as measured by CD80/Ig binding by flow cytometry (data not shown), suggesting that mislocalization within the IS is a direct consequence of the mutation of the tyrosine at position 188.
Figure 6. Mutation of tyrosine at position 188 disrupts the ability of CD28 to focus at the IS.
Conjugates between WT or Y188F CD28 expressing T cells and Ag-pulsed ProAd-ICAM-B7 were formed and analyzed for CD28 localization. A, Representative images of conjugates are shown. The T cell is oriented toward the top in each panel. For WT CD28 the majority of conjugates display efficient recruitment of CD28 to the IS and the majority of CD28 is focused into the cSMAC (as shown in the two examples). In contrast, in Y188F-expressing T cells, even when CD28 is recruited to the IS (the two images on the left), CD28 recruitment was often incomplete. B and C, Percentage of conjugates that recruit CD28 to the IS (B, n = 60) or that focus CD28 within the IS (C, n = 50) at 15 or 45 minutes after conjugate formation are shown. # p<0.001 by two population proportion Z-student statistical test. D, The distribution of CD28 within the IS was determined by the percentage of the IS that contained CD28. Individual T cells are shown and the thick line represent the population median (n = 34). The thin line at 60% represents the cutoff used for scoring in Panel C. One experiment representative of three is shown. The population distribution was compared using the nonparametric Mann-Whitney test (p<0.0001).
CD28 determines PKCθ localization within the IS
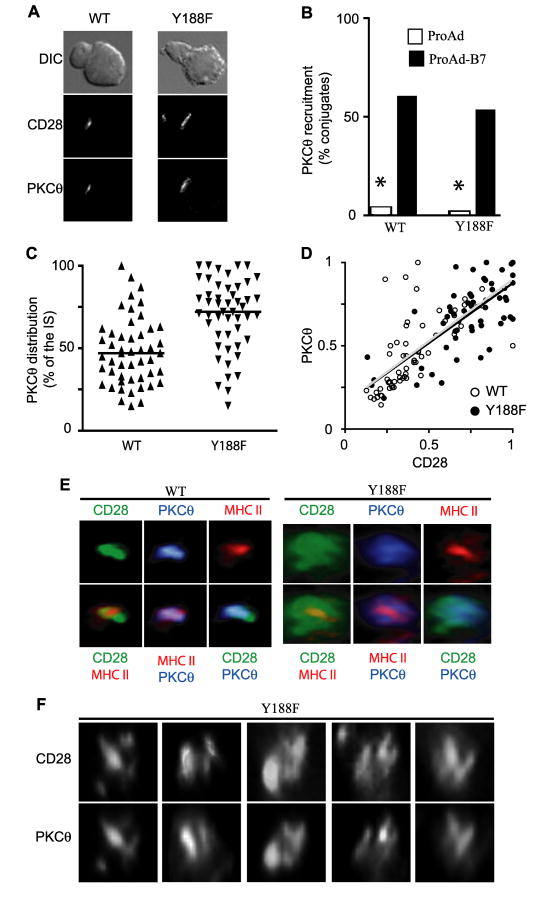
To evaluate signals downstream of CD28, we analyzed the localization of PKCθ. Costimulation through either LFA-1 or CD28 can recruit PKCθ to the IS, but CD28 costimulation is required to specifically localize PKCθ to the cSMAC and induce PKCθ-dependent activation of NF-κB (21). To determine how mislocalization of Y188F CD28 impacts on PKCθ, conjugates between CD28-deficient T cells expressing either WT or Y188F CD28 and Ag-pulsed APC were analyzed. To avoid LFA-1-mediated recruitment of PKCθ to the IS, the APC utilized did not express ICAM-1, and were either CD80-positive (ProAd-B7) or CD80-negative (ProAd). Y188F CD28 effectively retained it ability to recruit PKCθ to the IS with no significant differences with WT CD28 expressing T cells (Fig. 7, A and B). However, similar to CD28, PKCθ recruitment was delayed (data not shown) and PKCθ distribution at the IS was more diffuse in T cells expressing Y188F compared to WT CD28 (Fig 7C).
Figure 7. CD28 localization determines the distribution of PKCθ within the IS.
CD28-deficient T cells were retrovirally transduced with WT or Y188F CD28. A and B, T cell:APC conjugates with ProAd (fibroblast expressing MHC class II but not CD80) or ProAd-B7 (fibroblast expressing MHC class II and CD80) were analyzed for the ability to recruit CD28 and PKCθ to the IS. Representative images (A) and frequency of conjugates that have recruited PKCθ to the IS (B) are shown (n=50). * p<0.0001 by two population proportion Z-student statistical test. The T cell is oriented toward the top left in each panel. C and D, Mutation at Y188 in CD28 results in a failure to concentrate PKCθ in the cSMAC. C, The distribution of PKCθ within the IS is represented by the percentage of the IS that contains PKCθ (n = 50). Individual T cells are shown and lines represent the population median. The population distribution was compared using the nonparametric Mann-Whitney test (p<0.0001). D, Direct correlation between the relative distribution of CD28 and PKCθ within the IS is shown (n = 50). The open symbols represent individual T cells and the gray line represents the lineal regression for WT CD28 T cells, while the filled symbols represent individual T cells and the black line represents the lineal regression for Y188F-expressing T cells. Note the majority of WT T cells are clustered at the bottom left (where both CD28 and PKCθ are focused, while the majority of Y188F T cells are clustered in the upper right (where neither CD28 not PKCθ are focused). Strikingly, the linear regression lines for these two populations are essentially identical. E and F, Conjugates between WT or Y188F-expressing T cells with ProAdB7 APC were stained for CD28 (green), PKCθ (blue), and MHC class II (red). Three-dimensional reconstructions of the interaction site between the T cell and the APC of individual channels (top) and composites of two channels (bottom) are shown (E). Additional examples of 3-D reconstructions of the T cell:APC interaction site of Y188F-expressing T cells stained for CD28 (top row) and PKCθ (bottom row) are shown (F). Animated rotations of the 3-D representations shown in the first three panels are included in Videos 4-6 in Supplemental Data. Note that the distribution of CD28 and PKCθ are more diffuse in Y188F-expressing T cells and that the overall distribution of PKCθ mirrors the distribution of CD28 at the IS.
To determine if PKCθ localization at the IS correlates with CD28 localization, the distribution of both molecules was measured at the IS in each conjugate and these values were directly compared (Fig. 7D). In the majority of T cells expressing WT CD28, both CD28 and PKCθ were focused within the IS, while in the majority of T cells expressing Y188F, both CD28 and PKCθ were broadly distributed across the IS. Strikingly, the data sets are partially overlapping and the linear regression lines for these two populations are essentially identical, suggesting that the localization of CD28 is the primary determinant for the localization of PKCθ. To confirm that CD28 and PKCθ focusing in the IS represented localization to the cSMAC, T cell:APC conjugates were stained for CD28, PKCθ and MHC class II and analyzed by 3-dimensional reconstruction (Fig. 7E). Class II localization serves as a positive marker for the cSMAC region, because it labels the class II molecules that are juxtaposed to the TCR in the cSMAC. In T cells expressing WT CD28, both CD28 and PKCθ colocalized with class II in the cSMAC. In contrast, in T cells expressing Y188F, a cSMAC is found, as indicated by central and focused distribution of class II, but CD28 and PKCθ are broadly distributed across the IS. Y188F CD28 and PKCθ were not excluded from the cSMAC, but not preferentially concentrated in the cSMAC as in the majority of the WT CD28 T cell conjugates. In many conjugates, Y188F was not evenly distributed across the IS and formed multiple regions of higher concentration. Interestingly, in these cases PKCθ distribution mirrored CD28 localization; PKCθ and CD28 colocalized in these multiple foci (Fig. 7F and Videos 4-6 in Supplemental Data). These results further support the idea that CD28 determines the localization of PKCθ.
Mislocalization of Y188F CD28 correlates with reduced NF-κB nuclear translocation
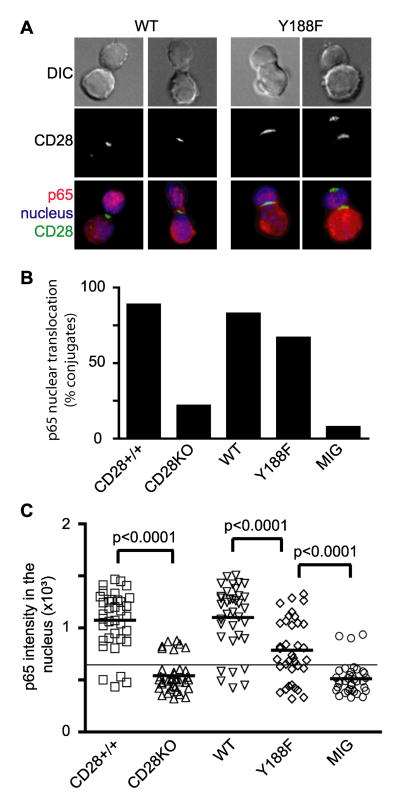
To evaluate signals downstream from CD28 and PKCθ we analyzed NF-κB nuclear translocation. WT or Y188F CD28 expressing T cell conjugates with Ag-pulsed ProAd-ICAM-B7 were analyzed at 45 minutes for nuclear translocation of NF-κB (Fig 8, A and B). No significant defect in the frequency of T cells that had activated NF-κB was noted in T cells expressing Y188F compared to WT CD28 (Fig. 8B). However, a difference in the magnitude of NF-κB translocation was apparent (Fig. 8A). To better quantify NF-κB nuclear translocation, we measured the intensity of NF-κB staining inside the nucleus of individual T cells (Fig. 8C). Y188F CD28 was sufficient to drive NF-κB nuclear translocation in the majority of T cells compared to CD28-deficient T cells, but the magnitude of NF-κB activation in T cells expressing Y188F was significantly decreased compared to WT T cells. Although it remains possible that this represents a decrease in CD28 signaling in Y188F, the correlation with Y188F and PKCθ mislocalization suggests that the disruption of cSMAC localization of CD28 may diminish CD28-mediated costimulation through PKCθ and NF-κB.
Figure 8. Mutation at Y188 diminishes the efficiency of NF-κB nuclear translocation.
CD28-deficient T cells were retrovirally transduced with WT CD28, Y188F CD28, or empty vector (MIG). CD28+/+ and CD28-deficient (CD28KO) T cells were included as controls. Conjugates with these T cells and Ag-pulsed ProAd-ICAM-B7 were stained for CD28 (green), NF-κB p65 (red), and the nucleus (blue) 45 minutes after conjugate formation. A, Representative images are shown. The T cell is oriented toward the top in each panel. B, Percentage of conjugates that translocated visually detectable levels of NF-κB into the nucleus (n = 40 conjugates). CD28+/+ vs CD28KO (p<0.0001), WT vs Y188F (p = 0.121), and Y188F vs MIG (p<0.0001) by two population proportion Z-student statistical test. C, The intensity of NF-κB staining in the nucleus of individual T cells is shown and the thick line represents the median of each population sample. The thin line across all the samples represents the limit of detection for visual identification of NF-κB translocation into the nucleus. Although Y188F CD28 did not impact on the frequency of T cells that activated NF-κB, the magnitude of NF-κB nuclear translocation was significantly reduced compared to T cells expressing WT CD28. One experiment representative of three is shown. The population distribution was compared using the nonparametric Mann-Whitney test; statistical significance between relevant samples is shown.
Discussion
The mechanisms that mediate CD28 localization to the cSMAC and the functional consequences of CD28 localization to the cSMAC are not understood. In this report we show that CD28 recruitment and persistence at the IS requires TCR signals, CD80 engagement, and sequences within the CD28 cytosolic tail. The requirement for both TCR and CD28 engagement indicates that there may be more cross-talk between TCR and CD28 signaling than would be predicted by the simple two signal model, where CD28 only provides costimulatory signals for TCR. Our data indicate that TCR signals are required to allow for CD28 ligand interaction, suggesting that TCR may also provide costimulatory functions to enhance CD28 signaling. We also show that a single point mutation at tyrosine 188 in the cytosolic tail disrupts the ability of CD28 to preferentially accumulate at the cSMAC. This provides the first indication of a cSMAC localization signal within CD28. Interestingly, PKCθ distribution at the IS mirrors CD28 localization, even when CD28 adopts a more diffuse distribution compared with TCR. This indicates that CD28 itself determines the location of PKCθ and there are no additional factors within the cSMAC that are required for PKCθ recruitment. Finally, mutation of Y188 diminishes CD28-dependent activation of NF-κB, suggesting that the colocalization of CD28 and PKCθ specifically to the cSMAC region is important for efficient signal transduction. Taken together these results provide new insight into the regulation of CD28 localization to the cSMAC and the functional consequences of specific protein organization within the IS.
TCR signals are essential to initiate the formation of the IS structure. We show here that, even after a mature IS has been formed, sustained TCR signaling is required to maintain CD28 localization at the IS. Addition of anti-class II mAb results in a rapid cessation of proximal TCR signaling following by the dispersion of CD28 from the IS. Both of these events occur well before T cell:APC conjugates are disrupted, indicating that availability of B7 ligand is insufficient to retain CD28 at the IS. This apparent TCR dependence for CD28-B7 interactions is reminiscent of the inside-out signaling associated with integrin activation (41). In this regard TCR signaling may have a direct effect on CD28 ligand binding, possibly through modulation of the cytosolic tail. Y188 can be phosphorylated upon T cell activation (42), and the conservative structural mutation (Y188F), supports a role for phosphorylation. Thus, it is possible that TCR-mediated phosphorylation of Y188 is required to efficiently recruit and/or sustain CD28 within the IS. So far, pY188 interacting proteins have not been clearly identified, although it has been suggested that phosphorylation of Y188 may create an SH2 docking site for Lck (43). Alternatively, CD28 localization at the IS may be dependent on TCR-induced changes in membrane domains. Although the biophysical properties of lipid rafts in living cell membranes and their functional importance in T cell activation remains controversial (44-46), Y188 has been implicated in the ability of CD28 to partition into detergent-resistant membrane fractions (47). Finally, Y188F may control CD28 interactions with specific adapter proteins that facilitate or promote cSMAC localization, such as Filamin-A, that can be associate with CD28 and impact on CD28-mediated lipid raft accumulation at the IS (48).
CD28 ligand binding is also required to recruit and sustain CD28 in the IS. CD28 is a disulfide-linked dimer and the conventional model would suggest that ligand binding transduces a conformational change through the dimer to alter the orientation of the cytosolic tails to allow for protein interactions that could mediate IS localization and/or signaling. However, ligand binding is not sufficient to induce CD28 localization to the IS and additional signals mediated through the TCR are required. This cross-talk between TCR and CD28 could be mediated through the inside-out model discussed above. Alternatively, CD28 ligand binding may function to retain CD28 in the IS. In this case, disruption of CD28 localization following the addition of anti-CD80 mAb might reflect the low affinity of CD28-CD80 interactions and the ability of this mAb to compete for CD28 binding. In the absence of available ligand the released CD28 would simply diffuse away from the IS. This possibility will favor a more dynamic model for CD28 localization at the IS, where the pool of CD28 at the cSMAC is constantly turning over and the steady state localization of CD28 at the IS depends on constant recruitment from the periphery.
It has recently been suggested that sustained TCR signaling is mediated through the continued formation of new TCR microclusters at the periphery of the IS (29-32). These microclusters mediate TCR signaling as they transit through the pSMAC en route to the cSMAC region. Addition of anti-class II mAb is thought to disrupt the formation of new microclusters and so inhibit sustained TCR signaling (32). TCR and CD28 have been shown to co-cluster in the immature IS, prior to the formation of an organized cSMAC region (49). It is possible that CD28 is also co-recruited with the TCR to the peripheral microclusters that continuously form after the mature IS has been established. In this case, anti-class II-mediated inhibition of new microcluster formation and sustained TCR signaling would result in a loss in the influx of CD28 to the cSMAC. In this microcluster model, the rapid dispersion of CD28 from the IS following the addition of anti-class II mAb would imply that CD28 retention in the cSMAC is transient. For example, CD28 may be rapidly internalized following TCR activation, ligand binding, and/or recruitment to the cSMAC region. This raises the possibility that CD28 follows a reciprocal pathway compared to CTLA-4. In resting activated T cells CTLA-4 resides primarily within intracellular compartments, due to rapid internalization. Upon T cell activation, tyrosine phosphorylation disrupts an internalization motif and redistributes CTLA-4 to the cell surface (50, 51). It is possible that TCR activation induces CD28 internalization. As long as sufficient antigenic peptide/MHC complexes remain on the cell surface, new TCR microclusters form, recruiting new CD28 molecules, and providing TCR signaling in the context of costimulation. Once the concentration of antigen drops below the threshold to drive new microcluster formation, new TCR/CD28 signaling would stop and the high concentration of CTLA-4 at the cell surface could inhibit any residual ongoing T cell activation.
The requirement for the cytosolic tail of CD28 implies that either ligand binding and/or TCR signals modify some molecular aspects of the tail that will facilitate CD28 recruitment at the IS. As discussed above, this could be a positive function of the cytosolic tail, mediated through phosphorylation of Y188, lipid raft association, and/or binding of an adaptor or cytoskeletal component. It has been shown that in resting T cells, CD28 mobility in the plasma membrane is reduced compared with other surface molecules suggesting that CD28 may be anchored to some cytoskeletal elements in the absence of TCR signals or ligand binding (33). Interestingly, deletion of the cytosolic tail increases mobility of CD28 in the plasma membrane. Thus, it is possible that this putative interaction in resting T cells may regulate CD28 recruitment to the cSMAC. Additional experiments to address the specific role of TCR signaling, ligand binding, and cytosolic tail sequences, including Y188, in the regulation of CD28 mobility in the plasma membrane will be required to address this issue.
One function of CD28 is to recruit PKCθ to the cSMAC (21, 34). We have shown that this is mediated through PI3K, and mutation of the PI3K interaction site on the cytosolic tail of CD28 results in a failure to recruit PKCθ, to translocate NF-κB to the nucleus, and to upregulate IL-2 transcription (21). This is consistent with the proposed role of PDK1 in PKCθ recruitment to the synapse (52). However, CD28 costimulation does not appear to generate a localized concentration of Phosphatidylinositol (3,4,5)-trisphosphate (the product of PI3K) within the cSMAC (C. Baker and J. Miller, unpublished data), suggesting that additional features of the cSMAC or CD28 signaling may be required for PKCθ recruitment. Consistent with this idea, we found that mislocalization of CD28 within the synapse by mutation of Y188 resulted in a corresponding mislocalization of PKCθ. A similar association of PKCθ with CD28 outside of the cSMAC regions was observed using transfected CHO cells as APC (53). These results suggest that there are no unique features to the cSMAC that allow for PKCθ recruitment; rather PKCθ recruitment appears to be an intrinsic function of CD28. No direct association between CD28 and PKCθ has been reported. The SH2 and SH2 domain docking sites on CD28 may recruit adaptor proteins that mediate PKCθ recruitment. For example, Filamin-A has been shown to localized with CD28 and PKCθ, and Filamin-A knock-down inhibits PKCθ recruitment to the IS (48, 54).
Activation of PKCθ results in the subsequent activation of NF-κB. Interestingly, the ability of CD28 to drive nuclear localization of NF-κB is reduced by mutation of Y188. It is possible that in addition to its effect on CD28 localization, Y188 may directly modulate some aspect of CD28 signaling that impacts on NF-κB activation. Alternatively, the correlation between CD28 and PKCθ mislocalization and diminished NF-κB activation suggests that the ability of Y188 to direct CD28 localization itself may account for the diminished signaling. Mutation of Y188 could effect CD28/TCR association in microclusters, which would have a secondary effect on CD28 cSMAC localization. Alternatively, CD28 signaling through PKCθ may be mediated at the cSMAC and reduced cSMAC localization of Y188F may account for reduced CD28 signaling.
Although the functional impact of CD28 on T cell activation can be clearly seen in CD28-deficient T cells, analysis of the specific elements in CD28 that mediate these functions have been difficult to define (15-20). This may be a result of both functional redundancy and in the ability of multiple elements within CD28 to synergize in the activation of specific signaling pathways and/or functional readouts of T cell activation. The interplay of TCR and CD28 in the localization of proteins within the immunological synapse, in the induction of specific signaling events, and in the functional activation/differentiation of T cells is more intricate than the original two-signal model of costimulation. Future studies will require both a molecular breakdown of signaling pathways along with sophisticated real time imaging studies to define the specific roles of TCR and CD28 in T cell activation.
Supplementary Material
Acknowledgments
The authors would like to thank Ryo Abe and Jim Allison for providing DNA constructs, Tim Bushnell in the URMC Flow Cytometry Core for help with the calcium assays, and Nathan Laniewski for help with cell sorting.
Footnotes
This work was supported by grant RO1-AI063418 from the National Institutes of Health to J. M. MSL was supported on National Institutes of Health Training Grant T32-AI007169.
Abbreviations used in this paper: cSMAC, central supramolecular activation cluster; pSMAC, peripheral supramolecular activation cluster; PKCθ, protein kinase C-θ; SH, src homology domain; IS, immunological synapse; WT, wild type; IRES, internal ribosomal entry site; CT, cytosolic tail; DIC, differential interference contrast; CFP, cyan fluorescent protein.
The online version of this article contains supplemental material.
Disclosures: The authors have no conflicting financial interests.
References
- 1.Lindsten T, June CH, Ledbetter JA, Stella G, Thompson CB. Regulation of lymphokine messenger RNA stability by a surface-mediated T cell activation pathway. Science. 1989;244:339–343. doi: 10.1126/science.2540528. [DOI] [PubMed] [Google Scholar]
- 2.Acuto O, Michel F. CD28-mediated co-stimulation; A quantitative support for TCR signaling. Nat Rev Immunol. 2003;3:939–951. doi: 10.1038/nri1248. [DOI] [PubMed] [Google Scholar]
- 3.Rudd C, Schneider H. Unifying concepts in CD28, ICOS and CTLA4 co-receptor signalling. Nat Rev Immunol. 2003;3:544–556. doi: 10.1038/nri1131. [DOI] [PubMed] [Google Scholar]
- 4.Fraser J, Irving B, Crabtree G, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 5.Diehn M, Alizadeh A, Rando O, Liu C, Stankunas K, Botstein D, Crabtree G, Brown P. Genomic expression programs and the integration of the CD28 costimulatory signal in T cell activation. Proc Natl Acad Sci USA. 2002;99:11796–11801. doi: 10.1073/pnas.092284399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michel F, Attal-Bonnefoy G, Mangino G, Mise-Omata S, Acuto O. CD28 as a molecular amplifier extending TCR ligation and signaling capabilities. Immunity. 2002;15:935–945. doi: 10.1016/s1074-7613(01)00244-8. [DOI] [PubMed] [Google Scholar]
- 7.Riley J, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson B, June C, Linsley P. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci USA. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pages F, Rageuneau M, Rottapel R, Truneh A, Nunes J, Imbert J, Olive D. Binding of phosphatidylinositol-3-OH kinase to CD28 is required for T-cell signalling. Nature. 1994;369:327–329. doi: 10.1038/369327a0. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y, Cefai D, Schneider H, Raab M, Nabavi N, Rudd C. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 10.Pages F, Ragueneau M, Klasen S, Battifora M, Couez D, Sweet R, Truneh A, Ward SG, Olive D. Two distinct intracytoplasmic regions of the T-cell adhesion molecule CD28 participate in phosphatidylinositol 3-kinase association. J Biol Chem. 1996;271:9403–9409. doi: 10.1074/jbc.271.16.9403. [DOI] [PubMed] [Google Scholar]
- 11.King P, Sadra A, Teng J, Liu X, Han A, Selvakumar A, August A, Dupont B. Analysis of CD28 cytoplasmic tail tyrosine residues as regulators and substrates for the protein tyrosine kinases, EMT and LCK. J Immunol. 1997;158 [PubMed] [Google Scholar]
- 12.Marengere L, Okkenhaug K, Clavreul A, Couez D, Gibson S, Mills G, Mak T, Rottapel R. The SH3 domain of Itk/Emt binds to proline-rich sequences in the cytoplasmic domain of the T cell costimulatory receptor CD28. J Immunol. 1997;159 [PubMed] [Google Scholar]
- 13.Holdorf AD, Green JM, Levin SD, Denny MF, Straus DB, Link V, Changelian PS, Allen PM, Shaw AS. Proline residues in CD28 and the Src homology (SH)3 domain of Lck are required for T cell costimulation. J Exp Med. 1999;190:375–384. doi: 10.1084/jem.190.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdorf AD, Lee KH, Burack WR, Allen PM, Shaw AS. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3:259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- 15.Burr J, Savage N, Messah G, Kimzey S, Shaw A, Arch R, Green J. Distinct motifs within CD28 regulate T cell proliferation and induction of Bcl-XL. J Immunol. 2001;166:5331–5335. doi: 10.4049/jimmunol.166.9.5331. [DOI] [PubMed] [Google Scholar]
- 16.Harada Y, Tokushima M, Matsumoto Y, Ogawa S, Otsuka M, Hayashi K, Weiss B, June C, Abe R. Critical requirement for the membrane-proximal tyrosine residue for CD28-mediated costimulation in vivo. J Immunol. 2001;166:3797–3803. doi: 10.4049/jimmunol.166.6.3797. [DOI] [PubMed] [Google Scholar]
- 17.Okkenhaug K, Wu L, Garza K, La Rose J, Khoo W, Odermatt B, Mak T, Ohashi P, Rottapel R. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat Immunol. 2001;2:325–332. doi: 10.1038/86327. [DOI] [PubMed] [Google Scholar]
- 18.Andres P, Howland K, Nirula A, Kane L, Barron L, Dresnek D, Sadra A, Imboden J, Weiss A, Abbas A. Distinct regions in the CD28 cytoplasmic domain are required for T helper type 2 differentiation. Nat Immunol. 2004;5:435–442. doi: 10.1038/ni1044. [DOI] [PubMed] [Google Scholar]
- 19.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 20.Friend LD, Shah DD, Deppong C, Lin J, Bricker TL, Juehne TI, Rose CM, Green JM. A dose-dependent requirement for the proline motif of CD28 in cellular and humoral immunity revealed by a targeted knockin mutant. J Exp Med. 2006;203:2121–2133. doi: 10.1084/jem.20052230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Lockhart M, Marin E, Graf B, Abe R, Harada Y, Sedwick CE, Miller J. CD28-mediated transcriptional and posttranscriptional regulation of IL-2 expression are controlled through different signaling pathways. J Immunol. 2004;173:7120–7124. doi: 10.4049/jimmunol.173.12.7120. [DOI] [PubMed] [Google Scholar]
- 22.Bromley SK, Burack WR, Johnson KG, Somersalo K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Ann Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 23.Huppa J, Davis M. T-cell-antigen recognition and the immunological synapse. Nat Rev Immunol. 2003;3:973–983. doi: 10.1038/nri1245. [DOI] [PubMed] [Google Scholar]
- 24.Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 25.Johnson K, Bromley S, Dustin M, Thomas M. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–10143. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freiberg B, Kupfer H, Maslanik W, Delli J, Kapplar J, Zaller D, Kupfer A. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, Dinner A, Tu C, Campi G, Raychaudhuri S, Varma R, Sims T, Burack W, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen P, Dustin M, Chakraborty A, Shaw A. The immunological synapse balances T cell receptor signaling and degredation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 28.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, Tokunaga M, Dustin ML, Saito T. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 31.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 32.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bromley S, Iaboni A, Davis S, Whitty A, Green J, Shaw A, Weiss A, Dustin M. The immunological synapse and CD28-CD80 interactions. Nat Immunol. 2001;2:1159–1166. doi: 10.1038/ni737. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Lo P, Zal T, Gascoigne N, Smith B, Levin S, Grey H. CD28 plays a critical role in the segregation of PKCθ within the immunological synapse. Proc Natl Acad Sci USA. 2002;99:9369–9373. doi: 10.1073/pnas.142298399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21:401–413. doi: 10.1016/j.immuni.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez-Lockhart M, Miller J. Engagement of CD28 outside of the immunological synapse results in upregulation of IL-2 mRNA stability, but not IL-2 transcription. J Immunol. 2006;176:4778–4784. doi: 10.4049/jimmunol.176.8.4778. [DOI] [PubMed] [Google Scholar]
- 37.Graf B, Bushnell T, Miller J. LFA-1-mediated T cell costimulation through increased localization of TCR/class II complexes to the central supramolecular activation cluster and exclusion of CD45 from the immunological synapse. J Immunol. 2007;179:1616–1624. doi: 10.4049/jimmunol.179.3.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valitutti S, Dessing S, Aktories K, Galletyi H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huppa J, Gleimer M, Sumen C, Davis M. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 40.Kim HH, Tharayil M, Rudd CE. Growth factor receptor-bound protein 2 SH2/SH3 domain binding to CD28 and its role in co-signaling. J Biol Chem. 1998;273:296–301. doi: 10.1074/jbc.273.1.296. [DOI] [PubMed] [Google Scholar]
- 41.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 42.Sadra A, Cinek T, Arellano JL, Shi J, Truitt KE, Imboden JB. Identification of tyrosine phosphorylation sites in the CD28 cytoplasmic domain and their role in the costimulation of Jurkat T cells. J Immunol. 1999;162:1966–1973. [PubMed] [Google Scholar]
- 43.Hofinger E, Sticht H. Multiple modes of interaction between Lck and CD28. J Immunol. 2005;174:3839–3840. doi: 10.4049/jimmunol.174.7.3839-a. [DOI] [PubMed] [Google Scholar]
- 44.Shaw AS. Lipid rafts: now you see them, now you don't. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 45.He HT, Marguet D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:525–530. doi: 10.1038/embor.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kenworthy AK. Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadra A, Cinek T, Imboden JB. Translocation of CD28 to lipid rafts and costimulation of IL-2. Proc Natl Acad Sci USA. 2004;101:11422–11427. doi: 10.1073/pnas.0403792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavano R, Contento RL, Baranda SJ, Soligo M, Tuosto L, Manes S, Viola A. CD28 interaction with filamin-A controls lipid raft accumulation at the T-cell immunologycal synapse. Nat Cell Biol. 2006;8(11):1270–1276. doi: 10.1038/ncb1492. [DOI] [PubMed] [Google Scholar]
- 49.Andres P, Howland K, Dresnek D, Edmondson S, Abbas A, Krummel M. CD28 signals in the immature immunological synapse. J Immunol. 2004;172:5880–5886. doi: 10.4049/jimmunol.172.10.5880. [DOI] [PubMed] [Google Scholar]
- 50.Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- 51.Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997;6:583–589. doi: 10.1016/s1074-7613(00)80346-5. [DOI] [PubMed] [Google Scholar]
- 52.Lee KY, D'Acquisto F, Hayden MS, Shim JH, Ghosh S. PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science. 2005;308:114–118. doi: 10.1126/science.1107107. [DOI] [PubMed] [Google Scholar]
- 53.Tseng SY, Liu M, Dustin ML. CD80 cytoplasmic domain controls localization of CD28, CTLA-4, and protein kinase Ctheta in the immunological synapse. J Immunol. 2005;175:7829–7836. doi: 10.4049/jimmunol.175.12.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hayashi K, Altman A. Filamin A is required for T cell activation mediated by protein kinase C-theta. J Immunol. 2006;177:1721–1728. doi: 10.4049/jimmunol.177.3.1721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.