Abstract
Background
Sleep disruption is a frequent occurrence in modern society. Whereas many studies have focused on the consequences of total sleep deprivation, few have investigated the condition of sleep disruption.
New Method
We disrupted sleep of mice during the light period for 9 consecutive days using an intermittently-rotating disc.
Results
Electroencephalogram (EEG) data demonstrated that non-rapid eye movement (NREM) sleep was severely fragmented and REM sleep was essentially abolished during the 12 h light period. During the dark period, when sleep was not disrupted, neither NREM sleep nor REM sleep times differed from control values. Analysis of the EEG revealed a trend for increased power in the peak frequency of the NREM EEG spectra during the dark period. The fragmentation protocol was not overly stressful as body weights and water consumption remained unchanged, and plasma corticosterone did not differ between mice subjected to 3 or 9 days of sleep disruption and home cage controls. However, mice subjected to 9 days of sleep disruption by this method responded to lipopolysaccharide with an exacerbated febrile response.
Comparison with existing methods
Existing methods to disrupt sleep of laboratory rodents often subject the animal to excessive locomotion, vibration, or sudden movements. This method does not suffer from any of these confounds.
Conclusions
This study demonstrates that prolonged sleep disruption of mice exacerbates febrile responses to lipopolysaccharide. This device provides a method to determine mechanisms by which chronic insufficient sleep contributes to the etiology of many pathologies, particularly those with an inflammatory component.
Keywords: rodent, LPS, stress, inflammation, fever
1.0 Introduction
Sleep loss is a common problem in our modern society. Data suggest that sleep loss contributes to the development of many health problems, including, but not limited to, mood disorders (Breslau, Roth et al., 1996;Ford and Kamerow, 1989;Neckelmann, Mykletun et al., 2007), metabolic diseases (Chaput, Brunet et al., 2006;Horne, 2011;Van Cauter E. and Knutson, 2008), immune dysfunction (Imeri and Opp, 2009;Irwin, 2002;Opp and Toth, 2003) and increased risk of cancer (Blask, 2009). Although the negative consequences of total sleep deprivation have been clearly demonstrated in humans and rodents (Borbely and Neuhaus, 1979;Carskadon, 2004;Downey and Bonnet, 1987;Everson, 1995;Everson, Bergmann et al., 1989;Knutson, Spiegel et al., 2007;Rechtschaffen, Gilliland et al., 1983;Soldatos and Paparrigopoulos, 2005), it has only recently become apparent that chronic sleep disruption or fragmentation is a common problem that can have an impact on recovery from illness (Irwin, 2002;Zager, Andersen et al., 2007), alertness (Rosekind, 2005) and cognitive abilities (Cohen and Albers, 1991;Stepanski, 2002).
There are many causes of sleep disruption, including but not limited to social behavior (Cain and Gradisar, 2010), shift work (Akerstedt, 1998) and medical conditions such as obstructive sleep apnea (Bandla and Gozal, 2000;Svanborg and Guilleminault, 1996), chronic pain (Moldofsky, 2001) and narcolepsy (Tafti, Rondouin et al., 1992;Zorick, Roehrs et al., 1986). Sleep disruption is characterized by brief arousals that occur throughout the night, often without reducing the total amount of time spent asleep (Bonnet and Arand, 2003). Sleep disruption results in decreased sleep efficiency, increased daytime sleepiness and cognitive impairment (Stepanski, 2002). For these and other reasons, recent efforts have been directed towards determining the negative consequences of chronic sleep disruption or fragmentation rather than total sleep deprivation, and several methods have been developed to mechanically disrupt sleep of rodents for prolonged periods (Ramesh, Kaushal et al., 2009;Sinton, Kovakkattu et al., 2009).
As briefly mentioned, a relationship between sleep and immune function exists and pro-inflammatory cytokines have been implicated in sleep regulation (Imeri and Opp, 2009;Irwin, 2002;Opp and Toth, 2003). Interestingly, the previously mentioned pathologies linked to disturbed sleep, including metabolic diseases, immune disorders and increased cancer risk, are also characterized by inflammation (Chung, Lee et al., 2011;Guess, Burch et al., 2009;Jun and Polotsky, 2009;Palma, Tiba et al., 2007). We report results obtained using an instrumentation platform that disrupts sleep of mice for prolonged periods. Such a method provides the means to determine mechanisms by which prolonged sleep fragmentation negatively impacts immune function, possibly via inflammation. In this study, we determined the impact of chronic sleep fragmentation during the light phase on subsequent nighttime behavior and on the febrile response to an immune challenge consisting of intraperitoneal administration of lipopolysaccharide (LPS).
2.0 Methods
2.1 Animals
Adult male C57BL/6J mice (~25 g at time of use; Jackson Laboratories, Bar Harbor, ME) were group housed until surgery and/or the beginning of the sleep fragmentation protocol. Mice that served as home cage controls were then placed individually in standard shoeboxes, and experimental mice were housed individually in sleep fragmentation devices. All mice were housed under a 12:12 light:dark cycle at 29±1°C with food and water provided ad libitum. Water consumption and body weights were measured daily 2 h after light onset. All procedures involving the use of animals were approved by the University of Washington IACUC in accordance with the US Department of Agriculture Animal Welfare Act and the National Institutes of Health policy on Humane Care and the Use of Laboratory Animals.
2.2 Sleep Fragmentation Device
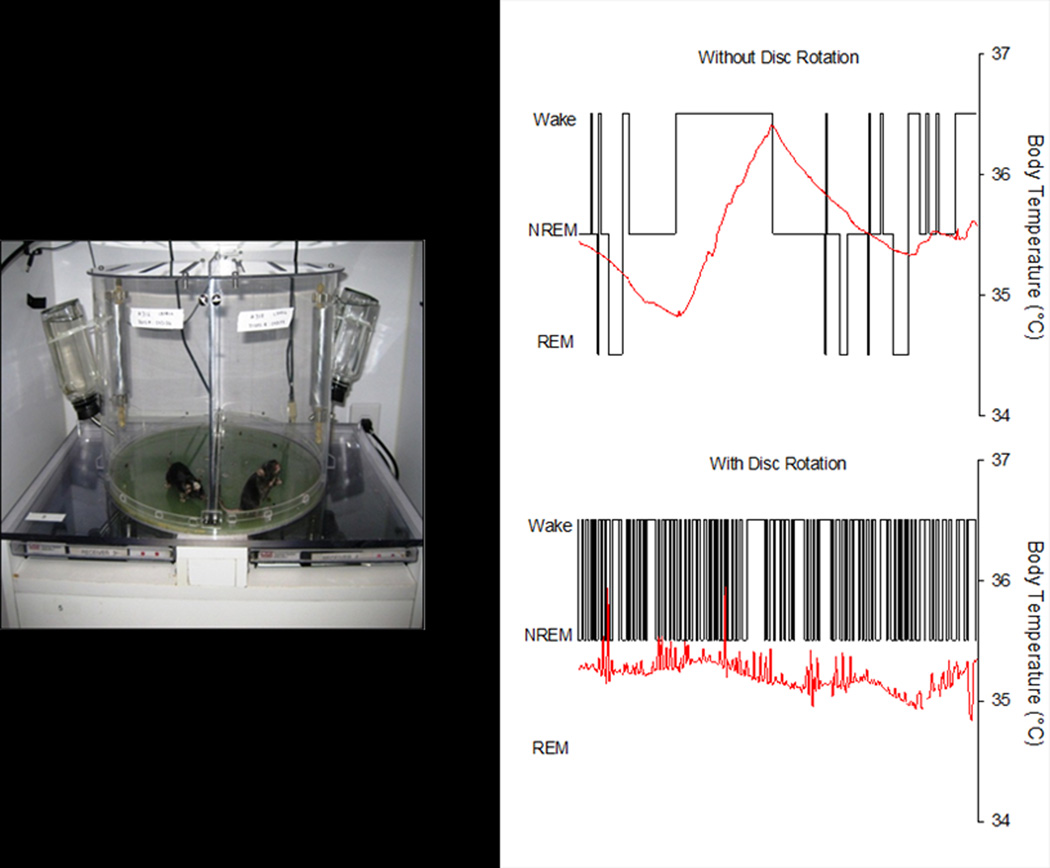
The device consists of a cylindrical Plexiglas® chamber divided into two separate compartments (Figure 1). The floor of the chamber is a disc that is programmed to rotate at specific intervals and durations as selected by the investigator (see later). The timing and direction of disc rotation is randomized to prevent behavioral adaptation by a rodent to disc movements. The disc rotates more than 180 degrees to ensure the mouse must move to avoid the center divider.
Figure 1. The sleep disruption device and protocol are effective.
Each sleep disruption device houses two mice and provides ad libitum access to food and water. Hypnograms depict consecutive 10s epochs from one hour in the same mouse when the disc was not rotating (top panel) and during sleep fragmentation (SF) when the disc was rotating (SF Day 9, bottom panel). Both hypnograms are from the fifth hour after light onset. Red lines depict core body temperature. The disruption of sleep is readily apparent. WAKE, wakefulness; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep.
2.3 Experiment 1: Effects of daytime sleep fragmentation on nighttime sleep, EEG power spectra, body weight and water consumption, and plasma corticosterone
This experiment consisted of two separate manipulations. Mice used to determine the impact of daytime sleep fragmentation on nighttime behavior, EEG spectra, and body weights and water consumption were used in a longitudinal protocol in which they served as their own controls. This part of the study was longitudinal in that data obtained during the habituation day (see later) were used as device control data for subsequent analysis and compared to data obtained during sleep fragmentation from the same mouse. A separate group of mice was used to determine effects on plasma corticosterone. This portion of the protocol was cross sectional, and animals were sacrificed at selected time points as detailed later.
Surgical procedures, recording protocol, and sleep-wake determination
Battery-operated biotelemetric transmitters [model # ETA-F10, Data Sciences International (DSI), St. Paul, MN] were surgically implanted in the peritoneal cavity under isoflurane anesthesia as previously described (Morrow and Opp, 2005a;Olivadoti, Weinberg et al., 2011). Briefly, insulated leads from the transmitter were passed subcutaneously to the skull, where they were attached to two small stainless steel screws implanted over the frontal and parietal brain cortices that served as electroencephalographic (EEG) recording electrodes. These telemetric devices also measure core body temperature, which was collected for each mouse throughout the duration of the protocol. Drinking water containing ibuprofen (0.2mg/mL) was provided 24 h before through 48 h after surgery. Mice were placed in a warm chamber until ambulatory, and allowed to recover for 21 days prior to the start of the experiment. Buprenorphine (0.01–0.05 mg/kg) was administered once at the time of surgery and one day following surgical procedures.
Transmitter signals were captured by a DSI receiver (RPC-1) located underneath each animal’s cage. Signals were sent to a DSI analog converter (ART Analog-8 CM). Cage activity, in home cages and on turntables, was detected using an infrared sensor housed in an observation unit that also contained a camera (BioBserve, GmbH, Bonn, Germany). All signals from the mice (EEG, body temperature, cage activity) were stored as binary files until later processing.
Undisturbed baseline recordings were obtained for 48 h while each animal was in its home cage. Mice were then placed in the sleep fragmentation device for two days of undisturbed recordings, followed by one day of habituation to the movement of the device (device control: DC). To habituate the mouse to the physical rotation of the disc, the disc was rotated with direction randomized for 8 sec once every 30 min for the 12 h light period. Following habituation to the device, the sleep fragmentation protocol began. Sleep fragmentation (SF) in this protocol consisted of an 8 second disc rotation once every 30 sec, on average, during the 12h light period for 9 consecutive days. Animals were allowed to freely behave during the intervening dark periods.
Arousal states were determined as previously described (Morrow and Opp, 2005b;Olivadoti, Weinberg et al., 2011), and classified as non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep or wakefulness (WAKE). Because the SF protocol used a disc rotation lasting 8 sec, arousal state determinations were made using a 4 sec epoch length. Artifact-free EEG epochs were subjected to fast Fourier transformation (FFT) to yield power spectra between 0.5 and 20 Hz in 0.5-Hz frequency bins. These artifact-free FFT spectra were matched to NREM, REM and WAKE epochs to obtain state-specific power spectra (Baracchi and Opp, 2008). Power in the delta (0.5 – 4.0 Hz) and theta (6.0 – 9.0) frequency bands within each hour was determined by averaging power density values from each epoch scored as NREMS, REMS or WAKE.
Body weights, water consumption and plasma corticosterone concentrations
To determine the impact of sleep fragmentation on generalized stress responses, body weights, water consumption and plasma corticosterone concentrations were determined from a total of 60 uninstrumented mice. Samples were analyzed from mice maintained in five different conditions: home cage control (n=12), device control (n=12), after 1 day of SF (n=12), after 3 days of SF (n=12), and after 9 days of SF (n=12). For all mice, blood was obtained via terminal orbital bleed, centrifuged (for 20 minutes at 4°C; 2,000 × g) and plasma collected. Plasma samples were stored at −80°C until assayed. Corticosterone concentrations from plasma samples were determined using an EIA kit (Enzo Life Sciences, Catalog # ADI-900-097, Farmingdale, NY) according to manufacturer guidelines.
2.4 Experiment 2: Impact of 9 days of sleep fragmentation on responses to an immune challenge
2.4.1 Surgical procedures and recording protocol
Battery-operated dataloggers (Mini SubCue Dataloggers, SubCue Dataloggers, Alberta, Canada) were surgically implanted into the peritoneal cavity. These devices record only core body temperature. After surgery, mice were placed in a warmed cage until ambulatory. Drinking water containing ibuprofen (0.2mg/mL) was provided 24 hours before through 48 hours after surgery. Mice were allowed to recover 10 – 14 days before the start of the experiments.
Mice were randomized into one of three conditions
home cage controls (n=8), device controls (n=6) or 9 days SF (n=6). Home cage control mice were housed in standard shoeboxes, and device control animals were housed in the disruption devices for the same duration, but the disc did not rotate. All animals were injected with 0.4 mg/kg lipopolysaccharide (LPS; Escherichia coli serotype 0111:B4, Sigma-Aldrich, St. Louis, MO) dissolved in 0.2 mL pyrogen-free saline (PFS, vehicle). Injections were given at light onset of the day immediately following the last day of the protocol, i.e., 10 days after the start of the protocol. As such, for the SF group, there was one intervening 12 h dark period during which the mice were able to freely behave. Body temperatures were continuously recorded for 96 h following the LPS injection. To control for the effects of the injection per se on body temperature, after the 96 h period all mice were injected with 0.2 mL vehicle, and body temperature recorded for an additional 24 h.
2.5 Statistical Analyses
Where appropriate, repeated measures ANOVA was used to determine the impact of manipulation on measures of interest (body temperature, NREM sleep, REM sleep and daily changes in body weights and water intake). Post hoc evaluation was performed using Tukey’s HSD test. All statistical values listed in the results section for repeated measures ANOVA reflect significant differences in time × group interaction effects, unless otherwise noted.
For EEG spectral analyses, paired T-tests were used to determine if the peak frequency for a given manipulation and behavioral state (0.5 – 4 Hz, NREM) differed between device control and SF conditions. For NREM delta power during the dark period, we require that each mouse spend at least a total of 5 min in NREM sleep before EEG spectral values for that animal are included for that hour in subsequent analysis. We use this criterion to reduce a potential for a relatively few epochs to exert a disproportionate effect on outcome measures for EEG spectral analyses. Based on this criterion, some hours during the dark period did not contain enough NREM sleep for analysis, and thus NREM delta power could not be analyzed using repeated measures because data were lacking for some animals. For this reason, one-way ANOVA was performed using the average NREM delta power across the 12h dark period.
Plasma corticosterone values were analyzed across conditions using a one-way ANOVA with Tukey’s HSD multiple comparisons.
3.0 Results
3.1 Experiment 1: Effects of daytime sleep fragmentation on nighttime sleep, power spectra, body weight and water consumption, and plasma corticosterone
Body weights and water intake
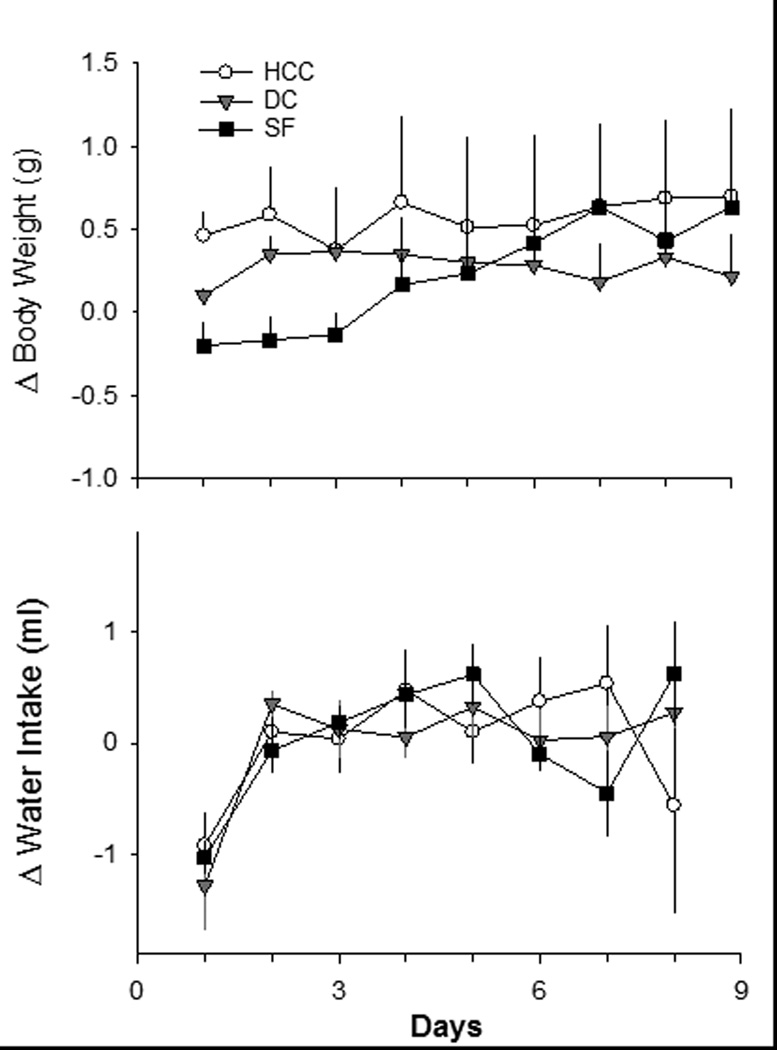
No significant differences in daily body weight change or daily water intake were detected among mice in the home cage control, device control or SF groups across the 9 days of the sleep fragmentation protocol (Figure 2). Based on visual inspection, we divided the body weight data into tertiles based on 3-day blocks. During Day 1 – Day 3, there was a non-significant trend for attenuated weight gain in mice subjected to SF as compared to home cage control mice [repeated measures ANOVA between subjects effect: F(1,2)= 2.844, p=0.086]. There was no trend for differences in body weight among groups from Day 4 – Day 9 of the protocol.
Figure 2. Daily changes in body weight and water intake are not altered by sleep fragmentation.
Body weights and water consumption were recorded from groups of mice housed for 9 days as home cage controls (HCC; open circles, n=8) or device controls (DC; gray triangles, n=6). A separate group of mice was subjected to 9 days of sleep fragmentation (SF; black squares, n=6) mice. Values are the mean ± SEM. No statistically significant differences were revealed among groups.
Plasma Corticosterone
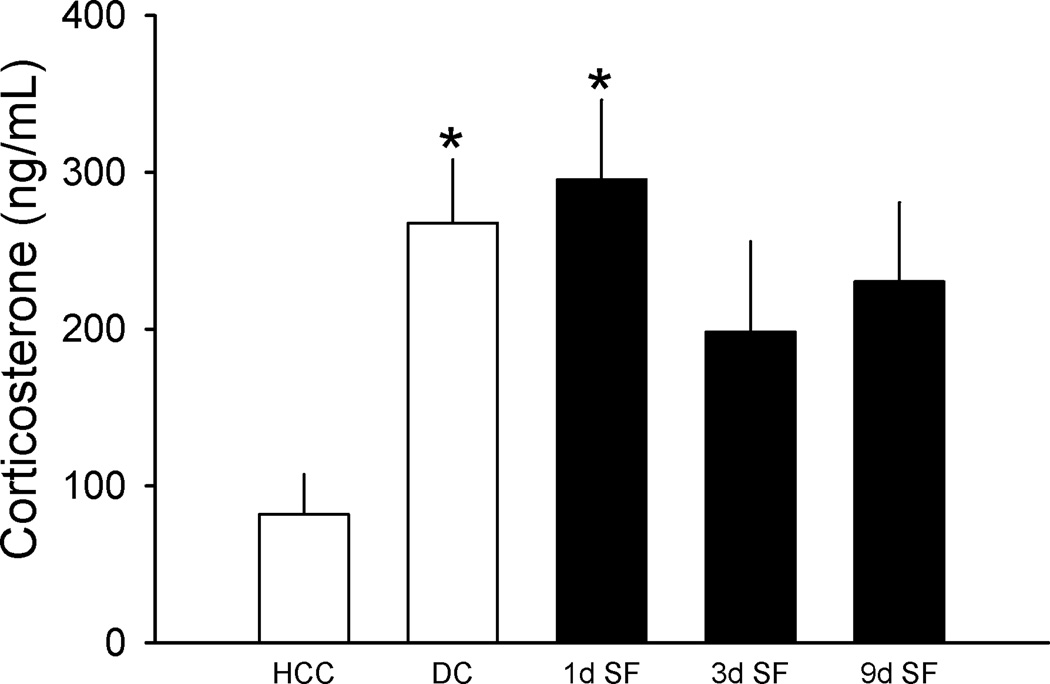
Plasma corticosterone differed among samples obtained from mice in this experiment [F(4,55)=3.479, p=0.013); Figure 3]. Post-hoc comparisons revealed that relative to samples from home cage control mice, plasma corticosterone was elevated in samples obtained from device control mice and in samples from mice subjected to SF for 1 day. Plasma corticosterone concentrations in samples from mice subjected to 3 or 9 days of SF did not differ significantly from samples obtained from home cage control mice, and corticosterone concentrations did not differ among samples from the device control mice or mice subjected to SF for 1, 3 or 9 days.
Figure 3. Sleep fragmentation only modestly elevates plasma corticosterone.
Plasma corticosterone concentrations were determined by ELISA from samples collected from home cage control mice (HCC, open bar), device control mice (DC, open bar), or mice subjected to sleep fragmentation for 1, 3 or 9 days (SF, filled bars). Separate groups of mice (n=12/group) were used for each condition. *=p<0.05 vs. home cage control.
Sleep-wake behavior, EEG spectra, and body temperature
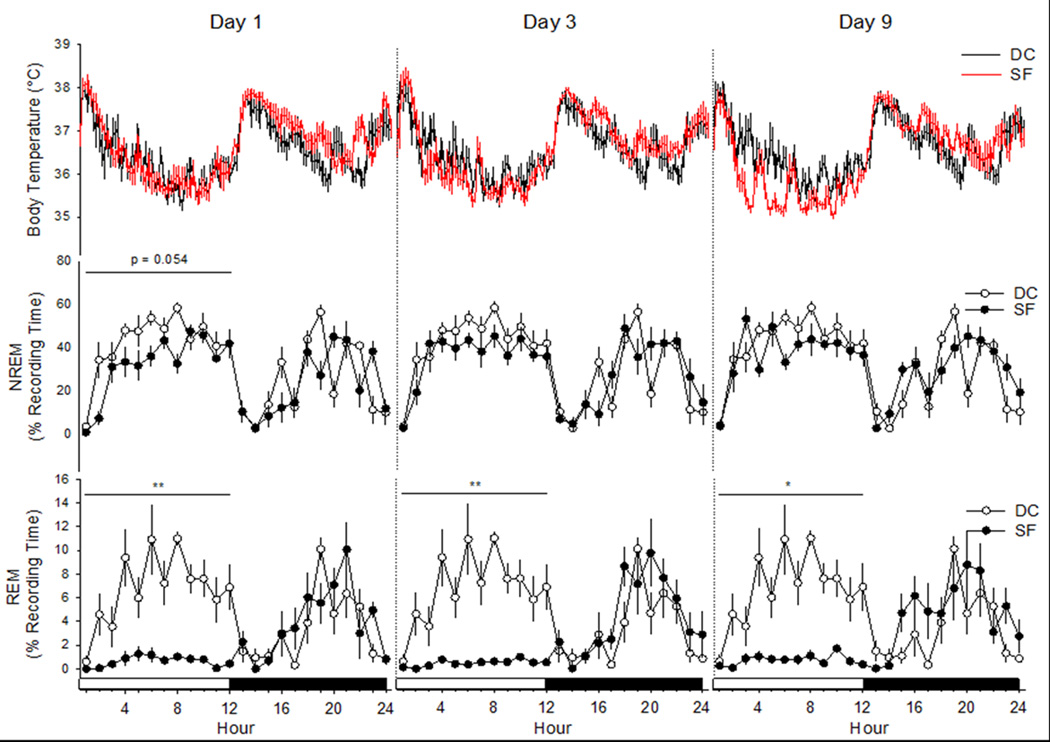
Analyses of sleep-wake behavior revealed a differential impact of SF on NREM and REM sleep (Figure 4; Table 1). There was a non-significant trend for a reduction in NREM sleep during the light periods across this protocol [F(3,20)=2.638, p=0.078]. Post-hoc analyses indicated that relative to values obtained during the device control day, there was a trend for NREM sleep to be reduced on the first day of SF (Day 1) that nearly achieved statistical significance (p=0.054). NREM sleep time during the dark periods, when there was no disc rotation, did not differ across protocol days [F(3,20)=1.196, p=0.337]. There was a non-significant trend for a reduction in total 24 h NREM sleep time across the recording days of this protocol [F(3,20)=2.505, p=0.088].
Figure 4. Sleep fragmentation during the light period does not alter body temperature or sleep during the subsequent dark period.
Body temperature of mice is not altered during the sleep disruption protocol (top panels). There is a tendency for non-rapid eye movements (NREM) sleep to be reduced during the light period fragmentation on day 1 that did not achieve statistical significance (middle panels). Rapid eye movements (REM) sleep is essentially abolished by sleep fragmentation on this device and protocol (bottom panel). Depicted are the mean ± SEM values obtained from n= 6 mice during the device control day (DC), and from the same mice after 1, 3, and 9 days of sleep fragmentation (SF). Control values were obtained from mice housed in the sleep fragmentation devices without disc rotation, and as such each mouse served as its own control. *=p<0.05 and **=p<0.01 vs. device control.
Table 1.
Distribution of NREM and REM sleep of mice during control conditions and nine days of sleep fragmentation
| Light Period | Dark Period | Total 24h | |
|---|---|---|---|
| NREM Sleep | |||
| Device | 42.1 ± 2.3 | 24.5 ± 2.5 | 33.3 ± 1.8 |
| Day 1 | 32.1 ± 2.1# | 22.6 ± 2.4 | 27.3 ± 1.6 |
| Day 3 | 35.4 ± 2.0 | 26.0 ± 2.5 | 30.7 ± 1.7 |
| Day 9 | 36.6 ± 2.2 | 28.1 ± 2.2 | 32.4 ± 1.5 |
| REM Sleep | |||
| Device | 6.8 ± 0.6 | 3.3 ± 0.5 | 5.0 ± 0.4 |
| Day 1 | 0.6 ± 0.1** | 3.9 ± 0.5 | 2.3 ± 0.3** |
| Day 3 | 0.5 ± 0.1** | 4.4 ± 0.6 | 2.4 ± 0.3** |
| Day 9 | 0.7 ± 0.1** | 4.6 ± 0.6 | 2.7 ± 0.3* |
| Total Sleep (NREM + REM) | |||
| Device | 48.9 ± 2.7 | 27.7 ± 2.9 | 38.3 ± 2.2 |
| Day 1 | 32.7 ± 2.1** | 26.5 ± 2.8 | 29.6 ± 1.8* |
| Day 3 | 35.8 ± 2.0** | 30.4 ± 3.0 | 33.1 ± 1.8 |
| Day 9 | 37.3 ± 2.1** | 32.7 ± 2.6 | 35.0 ± 1.7 |
Values are mean ± SEM percentage of recording time during the 12 h light and dark periods and the total 24 h period spent in non-rapid eye movement (NREM) sleep, rapid eye movement (REM) sleep, and total sleep (NREM + REM). Recordings were obtained from mice (n=6) housed in the sleep fragmentation device without disk rotation (Device Control) and from the same mice when subjected to disc rotation during the light period for 9 days (Day 1, 3, 9). Data were analyzed using a one-way ANOVA and Tukey’s HSD post hoc multiple comparisons.
Differences from Device control values: # p = 0.054;
p < 0.05;
p < 0.01.
In contrast to NREM sleep, REM sleep was significantly reduced by this SF protocol (Figure 4; Table 1). REM sleep was essentially abolished during the light periods of the SF protocol, and was significantly reduced when compared to the device control condition [F(3,20)=36.168, p=0.000]. Post-hoc comparisons indicate that relative to device control values, REM sleep was significantly reduced during each of the SF days analyzed. During the dark periods, when there was no disc rotation, time spent in REM sleep did not differ from device control values [F(3,20)=0.685, p=0.572]. However, total 24 h REM sleep time was reduced during each of the SF days relative to device control values [F(3,20)=6.393, p=0.003].
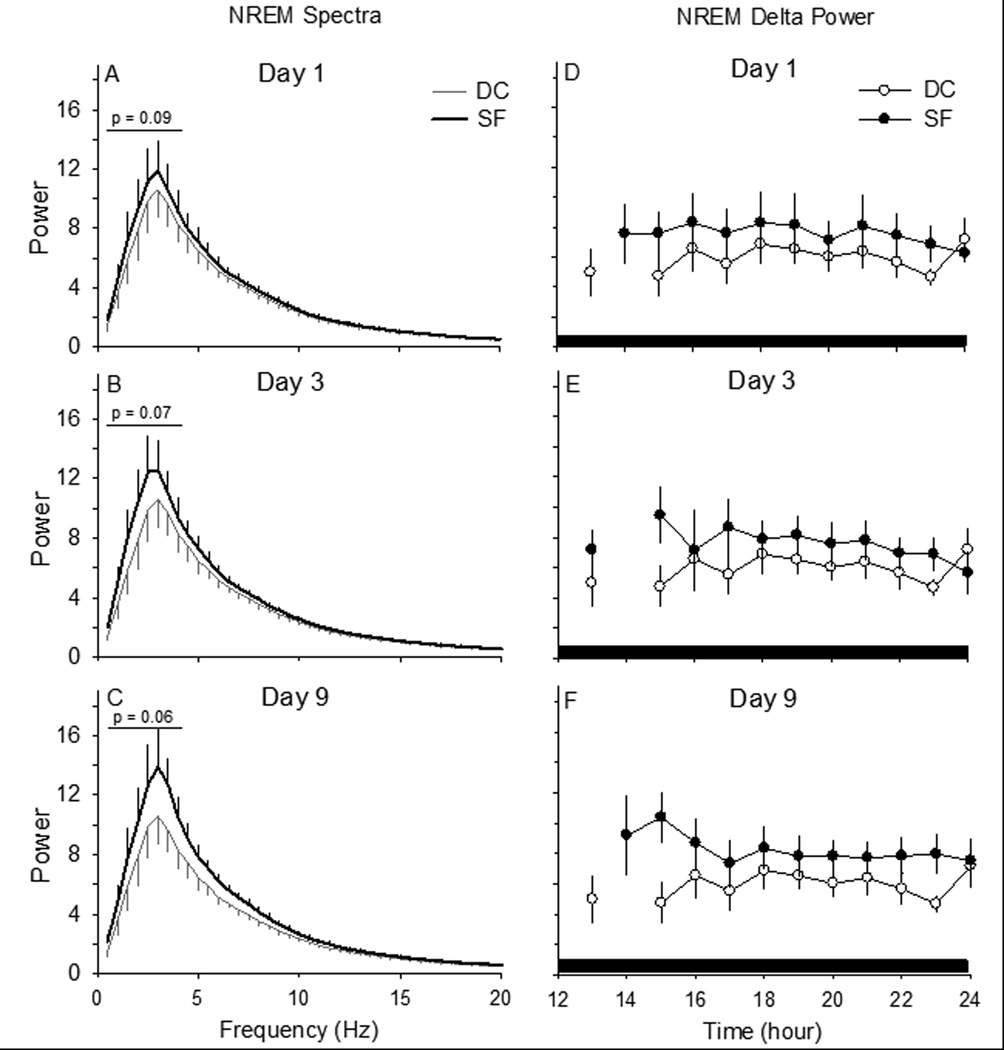
We did not analyze EEG spectra during the light period because the SF protocol results in very short NREM sleep bouts. As a consequence, the build-up in delta power that normally occurs through the progression of a single NREM sleep bout (Franken, Chollet et al., 2001;Franken, Tobler et al., 1991) is artificially truncated by the programmed disruption. During the intervening dark periods, when no sleep disruption occurred, spectral analyses of power in the peak frequency of the delta frequency band (0.5 – 4.0 Hz) revealed a trend to increase during the 9 day protocol, but this effect did not reach statistical significance (Figure 5 Panels A – C: Paired T-test: DC vs. Day 1 SF: t=−2.108, p=0.089; DC vs. Day 3 SF: t=−2.361, p=0.065; DC vs. Day 9 SF: t=−2.465, p=0.057). During the 9 days of the protocol, NREM delta power during the 12 h dark period significantly increased on day 1 of SF and there is a tendency for an increase during day 3 and day 9 of SF compared to DC (Figure 5 Panels D – F: Paired T-test: DC vs. Day 1 SF: t=2.697, p<0.05; DC vs. Day 3 SF: t=−2.341, p=0.066; DC vs. Day 9 SF: t=−2.524, p=0.053).
Figure 5. Sleep pressure may accumulate during prolonged sleep fragmentation even if sleep time is not altered.
(Panels A – C) Spectral analyses conducted on artifact-free electroencephalogram epochs of non-rapid eye movements (NREM) sleep during the 12-h dark period, when there is no disc rotation, suggest accumulating sleep pressure. The magnitude of the peak spectral power in the delta frequency band (0.5 – 4.0 Hz) tends to increase across days of sleep fragmentation (SF). (Panels D – F) Hourly averages for NREM delta power after the first day of sleep fragmentation were significantly greater as compared to values from the same animals when housed as device controls (DC), without disc rotation. Values are means ± SEM for n=6 mice.
Sleep fragmentation did not significantly impact core body temperatures of mice (Figure 4). One-way ANOVA of average body temperatures during the 12 h light periods, 12 hour dark periods, or across 24 h periods did not reveal any differences among the four conditions (Device Control, SF Days 1, 3 and 9). Activity-dependent changes in body temperature are apparent during some time points, such as the light period of SF Day 9. However, these deviations in body temperature were not of sufficient magnitude or duration so as to result in a statistically significant alteration in body temperatures during the SF protocol.
3.2 Experiment 2: Impact of 9 days of sleep fragmentation on responses to an immune challenge
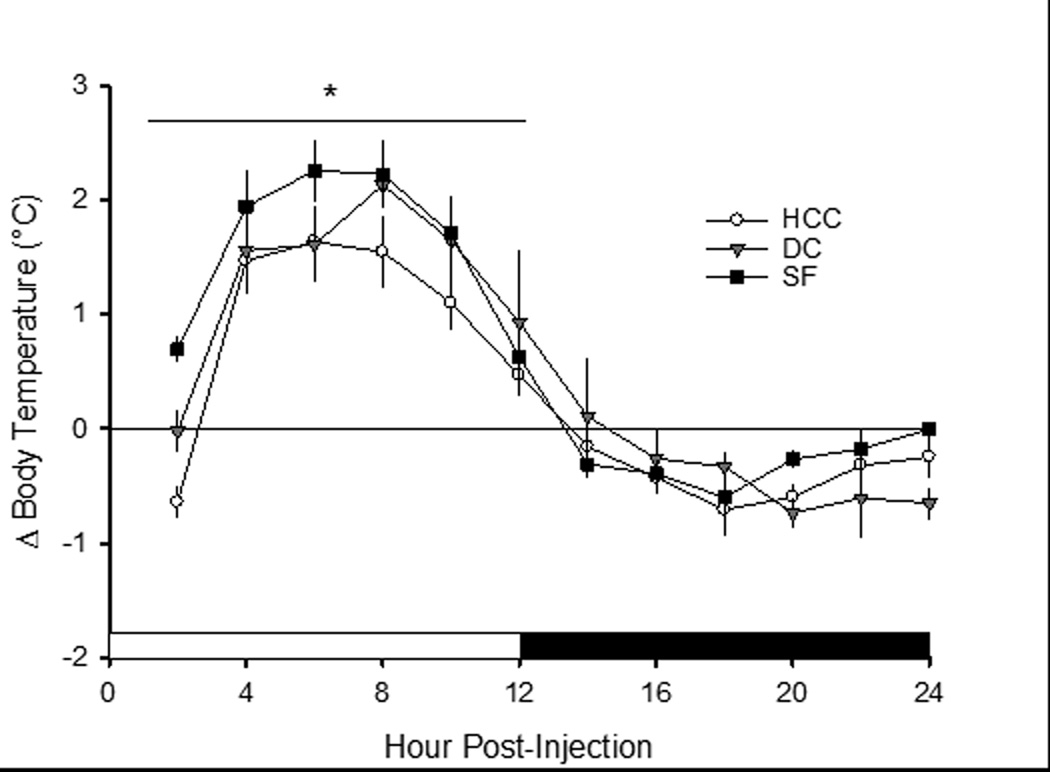
Intraperitoneal injection of LPS induced a febrile response in all mice, irrespective of group condition (Figure 6). Data were analyzed and are presented as difference scores: core body temperature values for each mouse obtained after injection of vehicle (PFS) were subtracted from the body temperature values obtained from the same mouse during the initial 24 h after injection of LPS. During the 12-hour light period after injections, there was a significant difference between groups [F(22,165)=1.961, p=0.009], as SF mice displayed a greater increase in body temperature after LPS challenge as compared to device and home cage control groups.
Figure 6. Sleep fragmentation exacerbates febrile responses to immune challenge.
Body temperature responses (°C) of mice to lipopolysaccharide (LPS) administration differ when housed as home cage controls (HCC; open circles, n=7), device controls (DC; grey triangles, n=5) or subject to sleep fragmentation (SF; filled squares, n=6) mice. All mice were injected with LPS on day 10 of the protocol (see text), and recordings continued for 96 h. The same mice were then injected with vehicle (pyrogen-free saline) to control for the potentially confounding effects of the injection procedure itself. As such, each mouse served as its own control. Difference scores (Δ) were calculated by subtracting values obtained after vehicle injection from those obtained after LPS administration. Mice in the SF group were subjected to disc rotation during the light period for a total of 9 consecutive days, whereas mice in the DC group were housed on non-rotating disks and HCC mice remained in their home cages for the same amount of time. Values are the means ± SEM. *=p<0.05 among groups.
4.0 Discussion
This study demonstrates that the device and protocol used are effective in disrupting sleep of mice in a manner that is apparently not physiologically stressful as determined by corticosterone concentrations. Fragmenting daytime sleep of mice using this device and protocol has little impact on subsequent nighttime sleep-wake behavior, which is an unexpected observation. Many studies demonstrate that one 6 h period of total sleep deprivation during the light period results in significant increases in the amount of time mice spend in NREM and REM sleep during the subsequent dark period (Baracchi and Opp, 2008;Franken, Malafosse et al., 1999;Huber, Deboer et al., 2000;Morrow and Opp, 2005b). The amount of time spent in NREM sleep is only modestly impacted during the light period when the disc is rotating in this protocol. As such, NREM sleep is minimally reduced, which may explain the lack of a NREM sleep rebound during the intervening undisturbed dark periods. It is possible that sleep pressure increases during the course of this protocol, as suggested by a trend for increased EEG NREM delta power during the dark periods. However, as with time spent in NREM sleep, the change in EEG NREM delta power during periods of spontaneous behavior is not of the magnitude typically observed after a period of total sleep deprivation (Easton, Meerlo et al., 2004;Huber, Deboer et al., 2000;McKenna, Tartar et al., 2007;Morrow and Opp, 2005b;Opp and Krueger, 1994a).
In contrast to the minimal impact on NREM sleep, this protocol essentially eliminates REM sleep during light periods. REM sleep during disc rotations is abolished, likely due to the fact that the inter-rotation interval is too short to allow mice to enter this sleep stage. Although daytime REM sleep is eliminated, there is no nighttime REM sleep rebound and total REM sleep amounts across the 24 h period remains less than during undisturbed baseline periods. Furthermore, there are no changes in the theta frequency band of the EEG spectra (data not shown), such as would indicate an increase in REM sleep pressure. Whether longer periods of daytime sleep fragmentation by this method would result in rebounds of NREM and/or REM sleep during subsequent dark periods remains to be determined.
The duration of time freely behaving mice spend in sleep stages during the dark period is not altered dramatically, but other facets of sleep are impacted by this protocol. Power in the delta frequency band (0.5 – 4.0 Hz) during NREM sleep is generally accepted as an index of sleep depth or sleep pressure (Benington and Heller, 1994;Brunner, Dijk et al., 1993;Chemelli, Willie et al., 1999;Franken, Dijk et al., 1991;Machado, Hipolide et al., 2004). However, the duration of NREM sleep and the mechanisms responsible for manifestation of the cortical EEG are regulated independently, and there are conditions under which these two parameters are dissociated [reviewed in (Davis, Clinton et al., 2011)]. Although the trend for increases in NREM spectral power across the 9 days of sleep fragmentation in this protocol did not achieve statistical significance, these data suggest that sleep pressure accumulates, in spite of the fact that differences in time spent in NREM sleep does not statistically differ between manipulations.
Although REM sleep is reduced, and spectral properties of the EEG may be altered, sleep fragmentation by this device and protocol does not alter body weight or food intake. Changes in body weight of rodents during conditions of reduced sleep opportunity have previously been reported. However, previous studies either used long periods of total sleep deprivation [e.g., (Bergmann, Kushida et al., 1989)], severe sleep restriction for up to 20 h per day [e.g.,(Barf, Meerlo et al., 2010)] or selective REM sleep deprivation [e.g., (Hipolide, Suchecki et al., 2006;Koban, Sita et al., 2008)]. In addition, the methods commonly used to deprive rodents of sleep [gentle handling (Franken, Dijk et al., 1991;Franken, Tobler et al., 1993;Morrow and Opp, 2005b), disk-over-water (Bergmann, Kushida et al., 1989), a continuously rotating drum (Barf, Meerlo et al., 2010), inverted flower pot (Hipolide, Suchecki et al., 2006;Koban, Sita et al., 2008)], are designed to eliminate all sleep or to selectively reduce REM sleep. Although mice are deprived of REM sleep during the light period, sleep disruption by the protocol used in this study does not affect total sleep time during the subsequent dark period. Furthermore, after the first day on the device, 24 h total sleep time is not reduced. The general lack of reduction in total sleep time may explain the lack of impact on body weight and water intake.
This protocol does not appear to be overly stressful based on the lack of an effect on nighttime sleep, body weights, water consumption and body temperature. This conclusion is strengthened by data demonstrating that plasma corticosterone is only modestly altered by sleep fragmentation using this technique. There is an initial increase in plasma corticosterone the first day mice are placed on the device, and during the first day of disc rotation. These effects may be explained by the fact the mice are in a novel environment, which is known to elevate corticosterone in mice (Hennessy, 1991). However, after 3 and 9 days of sleep fragmentation, plasma corticosterone does not statistically differ from values obtained from mice housed in their home cages.
Finally, our new data demonstrate that sleep fragmentation alters responses to immune challenge, even if the protocol does not dramatically alter sleep-wake behavior. We (Opp and Krueger, 1994a;Opp and Krueger, 1994b) and others (Everson, 1993;Everson, 2005;Everson and Toth, 2000;Toth, 1995;Zager, Andersen et al., 2007) have demonstrated adverse effects of sleep loss on immune function of rodents. Studies also demonstrate that sleep loss impairs immune function in human volunteers [reviewed (Born, Lange et al., 1997;Marshall and Born, 2002;Opp, Born et al., 2007)]. In this study, mice challenged with LPS after 9 days of sleep fragmentation respond with a fever of greater magnitude than control mice. The fever recorded after LPS in this study do not differ among groups with respect to timing or duration as reported previously (Morrow and Opp, 2005a), but the increased magnitude of the peak temperature response suggests that prolonged sleep fragmentation by this method exacerbates some aspects of responses to immune challenge. The increase in the magnitude of the peak febrile response is also of interest because the LPS challenge was administered 12 h after the end of sleep fragmentation. This observation suggests that the impact of sleep fragmentation by this device and protocol are not ameliorated by a short period (12 h) of undisturbed sleep opportunity. How long such an effect of sleep fragmentation on immune challenge may last is not known, and requires additional investigation. At present, mechanisms by which prolonged sleep fragmentation contributes to altered responsiveness to immune challenge are not well understood. The device and protocol of sleep fragmentation reported in this study allows us to begin to determine the neuroanatomic and biochemical substrates that link sleep fragmentation to immune status under conditions when amounts of sleep per se do not differ substantially from normal.
We consider several strengths of using this approach to fragment sleep of mice. Within the context of public health, many medical conditions and societal constraints result in sleep disruption without large decrements in total sleep time. For example, sleep disorders such as sleep apnea (Svanborg and Guilleminault, 1996), narcolepsy (van den Hoed, Kraemer et al., 1981), and restless legs syndrome (Montplaisir, Boucher et al., 1998), generally do not result in reductions in total sleep time. These medical conditions are characterized by inflammation (Kornum, Kawashima et al., 2011;Shamsuzzaman, Gersh et al., 2003;Weinstock, Walters et al., 2012), which may be induced by sleep fragmentation per se (Bryant, Trinder et al., 2004;Krueger, Obal et al., 2001;Mullington, Haack et al., 2009;Opp and Toth, 2003). Additionally, shift work (Akerstedt, 1998), caregiving (Happe and Berger, 2002;McCurry, Logsdon et al., 2007) or the care of a newborn (Hunter, Rychnovsky et al., 2009) disrupt sleep but rarely result in total sleep deprivation. These demands are often associated with fragmented NREM sleep, as well as a significant loss of REM sleep (McKenna, Tartar et al., 2007;Stepanski, 2002). Studies demonstrate that sleep restriction of human volunteers for periods as short as four hours induces inflammation (Irwin, Wang et al., 2006). Although additional studies are required, the observation that short periods of sleep restriction induce inflammation in humans provides a public health context within which future research efforts should be directed.
While the protocol we used in this study lasted 9 days, the software controlling the disc allows the investigator complete freedom to program the timing and duration of disc rotation. For example, the duration of the sleep fragmentation protocol could be shortened or lengthened, or the intensity of the fragmentation could be made more or less severe by changing the duration and frequency of disc rotations. The device and protocol have been designed to disrupt sleep of mice in an unpredictable fashion. Randomization of rotation direction and inter-rotation interval prevent behavioral anticipation and adaptation of the mice to sleep fragmentation. As such, the device and protocol potentially remain effective in disrupting sleep for a protocol of any length. Because the disc rotates only slightly more than 180 degrees and then stops, there is no confound of locomotor activity. Observations with a video monitoring system reveal that mice awaken and move when the disc rotation starts. When the disc is not rotating and mice are awake, they engage in general cage activity, such as grooming, eating and drinking. Therefore, it is not necessary to include an additional control manipulation to account for locomotor activity, such as necessary when a treadmill or a continuously rotating disc or drum is used to deprive an animal of total sleep (Barf, Meerlo et al., 2010;Leenaars, Dematteis et al., 2011;McKenna, Tartar et al., 2007). In addition to the software features that control the disc rotation schedule, the motor and drive system are extremely quiet, and the stepper motor provides a very smooth disc rotation. After the first few rotations during habituation, there is no “startle response”, as would be indicated by the mouse “flinching” when the disc rotation starts or stops. The quiet and smooth nature of this device eliminates the potential confounds associated with methods that use laboratory shakers to physically vibrate mice or sweeper bars that jostle mice (Ramesh, Kaushal et al., 2009;Sinton, Kovakkattu et al., 2009).
Few, if any published studies of which we are aware include disruption of rodent sleep for prolonged periods. Therefore, it is difficult to assess other methods with respect to the impact of perceived stress in response to the sleep fragmentation method itself. Similarly, few studies using mechanical means to disrupt sleep of mice report the impact of the method on spectral properties of the EEG (Ramesh, Kaushal et al., 2009;Sinton, Kovakkattu et al., 2009). The device we use houses two mice simultaneously, and as such animals are not isolated during the fragmentation protocol. This approach also doubles the number of animals that can be manipulated within the same apparatus and footprint. Finally, although a telemetry system may be incorporated, as in this study, animals may also be implanted with cranial headpieces because the enclosure accommodates tethers to connect the mouse to a recording system.
As with all methods and protocols to disturb or deprive laboratory animals of sleep, there are some limitations to this approach. This system was designed only to disrupt sleep, and it is effective in doing so. No capabilities have been incorporated that would allow feedback from the animal to the system such that a mouse could be deprived of a specific stage of sleep, as in the classic rat studies of Rechtschaffen (Rechtschaffen and Bergmann, 1995;Rechtschaffen, Gilliland et al., 1983). We do not consider this a limitation due to the nature of our studies, although other investigators may desire this capability. There is an advantage to housing two mice on the same physical apparatus in terms of animal density and elimination of social isolation. However, because the floor space is shared by two animals, it is not possible to determine food intake by an individual animal. During sleep fragmentation mice often “play” with their food, and there is food waste, which gets mixed between mice when the disc rotates. It is conceivable that a liquid diet could be used to allow assessment of caloric intake by each individual animal, but we have not yet used this approach.
In summary, the device and protocol used are effective in fragmenting sleep of mice. This initial study demonstrates biologic responses of mice to sleep fragmentation that validate this approach as a method by which sleep can be reliably disrupted for prolonged periods. This system is capable of mimicking in rodents the sleep disruption or fragmentation that occurs in many human conditions. Because the disc rotations are unpredictable, this system provides a means to conduct continuous long-term experiments without behavioral adaptation by the subjects, despite potentially increased sleep pressure. This initial study demonstrates that sleep disruption of mice exacerbates febrile responses to LPS, an observation that provides impetus for future experiments aimed at furthering our understanding of mechanisms by which chronic insufficient sleep contributes to inflammatory disease.
Highlights.
The method used is effective in fragmenting sleep of mice for prolonged periods without behavioral adaptation.
Although NREM sleep is disrupted and REM sleep is abolished by this method, there is no compensatory increase in sleep during periods without fragmentation.
Water intake and body weight are unchanged by this protocol, and corticosterone is only modestly elevated.
After prolonged sleep fragmentation, febrile responses to immune challenge are exacerbated.
Acknowledgements
We thank Ms. Ashley Ingiosi for her support and assistance with this study. Dr. Tom Gardiner and Dr. Linda Toth, Southern Illinois University School of Medicine, designed and developed the sleep disruption devices and controlling software. This work was funded by National Institutes of Health grant AG041827, and by the Department of Anesthesiology & Pain Medicine of the University of Washington.
Footnotes
Conflict of interest
The authors state they have no conflicts of interest.
Reference List
- Akerstedt T. Shift work and disturbed sleep/wakefulness. Sleep Med. Rev. 1998;2:117–128. doi: 10.1016/s1087-0792(98)90004-1. [DOI] [PubMed] [Google Scholar]
- Bandla HP, Gozal D. Dynamic changes in EEG spectra during obstructive apnea in children. Pediatr. Pulmonol. 2000;29:359–365. doi: 10.1002/(sici)1099-0496(200005)29:5<359::aid-ppul4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Baracchi F, Opp MR. Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1. Brain Behav. Immun. 2008;22:982–993. doi: 10.1016/j.bbi.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barf RP, Meerlo P, Scheurink AJ. Chronic sleep disturbance impairs glucose homeostasis in rats. Int. J. Endocrinol. 2010;2010:819414. doi: 10.1155/2010/819414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benington JH, Heller HC. Does the function of REM sleep concern non-REM sleep or waking? Prog. Neurobiol. 1994;44:433–449. doi: 10.1016/0301-0082(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat: II. Methodology. Sleep. 1989;12:5–12. doi: 10.1093/sleep/12.1.5. [DOI] [PubMed] [Google Scholar]
- Blask DE. Melatonin, sleep disturbance and cancer risk. Sleep Med. Rev. 2009;13:257–264. doi: 10.1016/j.smrv.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003;7:297–310. doi: 10.1053/smrv.2001.0245. [DOI] [PubMed] [Google Scholar]
- Borbely AA, Neuhaus HU. Sleep-deprivation: effects on sleep and EEG in the rat. J. comp. physiol. Psychol. 1979;133:71–87. [Google Scholar]
- Born J, Lange T, Hansen K, Molle M, Fehm HL. Effects of sleep and circadian rhythm on human circulating immune cells. J. Immunol. 1997;158:4454–4464. [PubMed] [Google Scholar]
- Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol. Psychiatry. 1996;39:411–418. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- Brunner DP, Dijk DJ, Borbely AA. Repeated partial sleep deprivation progressively changes in EEG during sleep and wakefulness. Sleep. 1993;16:100–113. doi: 10.1093/sleep/16.2.100. [DOI] [PubMed] [Google Scholar]
- Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat. Rev. Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- Cain N, Gradisar M. Electronic media use and sleep in school-aged children and adolescents: A review. Sleep Med. 2010;11:735–742. doi: 10.1016/j.sleep.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Carskadon MA. Sleep deprivation: health consequences and societal impact. Med. Clin. North Am. 2004;88:767–776. doi: 10.1016/j.mcna.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the 'Quebec en Forme' Project. Int. J. Obes. (Lond) 2006;30:1080–1085. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chung HY, Lee EK, Choi YJ, Kim JM, Kim DH, Zou Y, Kim CH, Lee J, Kim HS, Kim ND, Jung JH, Yu BP. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J. Dent. Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Albers HE. Disruption of human circadian and cognitive regulation following a discrete hypothalamic lesion: a case study. Neurology. 1991;41:726–729. doi: 10.1212/wnl.41.5.726. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM. Delta wave power: an independent sleep phenotype or epiphenomenon? J. Clin. Sleep Med. 2011;7:S16–S18. doi: 10.5664/JCSM.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey R, Bonnet MH. Performance during frequent sleep disruption. Sleep. 1987;10:354–363. doi: 10.1093/sleep/10.4.354. [DOI] [PubMed] [Google Scholar]
- Easton A, Meerlo P, Bergmann B, Turek FW. The suprachiasmatic nucleus regulates sleep timing and amount in mice. Sleep. 2004;27:1307–1318. doi: 10.1093/sleep/27.7.1307. [DOI] [PubMed] [Google Scholar]
- Everson CA. Sustained sleep deprivation impairs host defense. Am J. Physiol. 1993;265:R1148–R1154. doi: 10.1152/ajpregu.1993.265.5.R1148. [DOI] [PubMed] [Google Scholar]
- Everson CA. Functional consequences of sustained sleep deprivation in the rat. Behav. Brain Res. 1995;69:43–54. doi: 10.1016/0166-4328(95)00009-i. [DOI] [PubMed] [Google Scholar]
- Everson CA. Clinical assessment of blood leukocytes, serum cytokines, and serum immunoglobulins as responses to sleep deprivation in laboratory rats. Am J. Physiol Regul. Integr. Comp Physiol. 2005;289:R1054–R1063. doi: 10.1152/ajpregu.00021.2005. [DOI] [PubMed] [Google Scholar]
- Everson CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: III. Total sleep deprivation. Sleep. 1989;12:13–21. doi: 10.1093/sleep/12.1.13. [DOI] [PubMed] [Google Scholar]
- Everson CA, Toth LA. Systemic bacterial invasion induced by sleep deprivation. Am J. Physiol Regul. Integr. Comp Physiol. 2000;278:R905–R916. doi: 10.1152/ajpregu.2000.278.4.R905. [DOI] [PubMed] [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Franken P, Chollet D, Tafti M. The homeostatic regulation of sleep need is under genetic control. The Journal of Neuroscience. 2001;21:2610–2621. doi: 10.1523/JNEUROSCI.21-08-02610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franken P, Dijk D-J, Tobler I, Borbély AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am. J. Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- Franken P, Malafosse A, Tafti M. Genetic determinants of sleep regulation in inbred mice. Sleep. 1999;22:155–169. [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbély AA. Sleep homeostasis in the rat: simulation of the time course of EEG slow-wave activity. Neurosci. Lett. 1991;130:141–144. doi: 10.1016/0304-3940(91)90382-4. [DOI] [PubMed] [Google Scholar]
- Franken P, Tobler I, Borbely AA. Effects of 12-h sleep deprivation and of 12-h cold exposure on sleep regulation and cortical temperature in the rat. Physiol Behav. 1993;54:885–894. doi: 10.1016/0031-9384(93)90297-s. [DOI] [PubMed] [Google Scholar]
- Guess J, Burch JB, Ogoussan K, Armstead CA, Zhang H, Wagner S, Hebert JR, Wood P, Youngstedt SD, Hofseth LJ, Singh UP, Xie D, Hrushesky WJ. Circadian disruption, Per3, and human cytokine secretion. Integr. Cancer Ther. 2009;8:329–336. doi: 10.1177/1534735409352029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe S, Berger K. The association between caregiver burden and sleep disturbances in partners of patients with Parkinson's disease. Age Ageing. 2002;31:349–354. doi: 10.1093/ageing/31.5.349. [DOI] [PubMed] [Google Scholar]
- Hennessy MB. Sensitization of the plasma corticosterone response to novel environments. Physiol Behav. 1991;50:1175–1179. doi: 10.1016/0031-9384(91)90579-d. [DOI] [PubMed] [Google Scholar]
- Hipolide DC, Suchecki D, Pimentel de Carvalho PA, Chiconelli FE, Tufik S, Luz J. Paradoxical sleep deprivation and sleep recovery: effects on the hypothalamic-pituitary-adrenal axis activity, energy balance and body composition of rats. J. Neuroendocrinol. 2006;18:231–238. doi: 10.1111/j.1365-2826.2006.01412.x. [DOI] [PubMed] [Google Scholar]
- Horne J. Obesity and short sleep: unlikely bedfellows? Obes. Rev. 2011;12:e84–e94. doi: 10.1111/j.1467-789X.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- Huber R, Deboer T, Tobler I. Effects of sleep deprivation on sleep and sleep EEG in three mouse strains: empirical data and simulations. Brain Res. 2000;857:8–19. doi: 10.1016/s0006-8993(99)02248-9. [DOI] [PubMed] [Google Scholar]
- Hunter LP, Rychnovsky JD, Yount SM. A selective review of maternal sleep characteristics in the postpartum period. J Obstet. Gynecol. Neonatal Nurs. 2009;38:60–68. doi: 10.1111/j.1552-6909.2008.00309.x. [DOI] [PubMed] [Google Scholar]
- Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav. Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch. Intern. Med. 2006;166:1756–1762. doi: 10.1001/archinte.166.16.1756. [DOI] [PubMed] [Google Scholar]
- Jun J, Polotsky VY. Metabolic consequences of sleep–disordered breathing. ILAR. J. 2009;50:289–306. doi: 10.1093/ilar.50.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med. Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban M, Sita LV, Le WW, Hoffman GE. Sleep deprivation of rats: the hyperphagic response is real. Sleep. 2008;31:927–933. [PMC free article] [PubMed] [Google Scholar]
- Kornum BR, Kawashima M, Faraco J, Lin L, Rico TJ, Hesselson S, Axtell RC, Kuipers H, Weiner K, Hamacher A, Kassack MU, Han F, Knudsen S, Li J, Dong X, Winkelmann J, Plazzi G, Nevsimalova S, Hong SC, Honda Y, Honda M, Hogl B, Ton TG, Montplaisir J, Bourgin P, Kemlink D, Huang YS, Warby S, Einen M, Eshragh JL, Miyagawa T, Desautels A, Ruppert E, Hesla PE, Poli F, Pizza F, Frauscher B, Jeong JH, Lee SP, Strohl KP, Longstreth WT, Jr, Kvale M, Dobrovolna M, Ohayon MM, Nepom GT, Wichmann HE, Rouleau GA, Gieger C, Levinson DF, Gejman PV, Meitinger T, Peppard P, Young T, Jennum P, Steinman L, Tokunaga K, Kwok PY, Risch N, Hallmayer J, Mignot E. Common variants in P2RY11 are associated with narcolepsy. Nat. Genet. 2011;43:66–71. doi: 10.1038/ng.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger JM, Obal FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad. Sci. 2001;933:211–221. doi: 10.1111/j.1749-6632.2001.tb05826.x. [DOI] [PubMed] [Google Scholar]
- Leenaars CH, Dematteis M, Joosten RN, Eggels L, Sandberg H, Schirris M, Feenstra MG, Van Someren EJ. A new automated method for rat sleep deprivation with minimal confounding effects on corticosterone and locomotor activity. J. Neurosci. Methods. 2011;196:107–117. doi: 10.1016/j.jneumeth.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Machado RB, Hipolide DC, Benedito-Silva AA, Tufik S. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51. doi: 10.1016/j.brainres.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Marshall L, Born J. Brain-immune interactions in sleep. Int. Rev. Neurobiol. 2002;52:93–131. doi: 10.1016/s0074-7742(02)52007-9. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Teri L, Vitiello MV. Sleep disturbances in caregivers of persons with dementia: contributing factors and treatment implications. Sleep Med. Rev. 2007;11:143–153. doi: 10.1016/j.smrv.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JT, Tartar JL, Ward CP, Thakkar MM, Cordeira JW, McCarley RW, Strecker RE. Sleep fragmentation elevates behavioral, electrographic and neurochemical measures of sleepiness. Neuroscience. 2007;146:1462–1473. doi: 10.1016/j.neuroscience.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldofsky H. Sleep and pain. Sleep Med. Rev. 2001;5:385–396. doi: 10.1053/smrv.2001.0179. [DOI] [PubMed] [Google Scholar]
- Montplaisir J, Boucher S, Nicolas A, Lesperance P, Gosselin A, Rompre P, Lavigne G. Immobilization tests and periodic leg movements in sleep for the diagnosis of restless leg syndrome. Mov Disord. 1998;13:324–329. doi: 10.1002/mds.870130220. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin 6-deficient mice. Brain Behav. Immun. 2005a;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Sleep-wake behavior and responses of interleukin 6-deficient mice to sleep deprivation. Brain Behav. Immun. 2005b;19:28–39. doi: 10.1016/j.bbi.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog. Cardiovasc. Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckelmann D, Mykletun A, Dahl AA. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–880. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivadoti MD, Weinberg JB, Toth LA, Opp MR. Sleep and fatigue in mice infected with murine gammaherpesvirus 68. Brain Behav. Immun. 2011;25:696–705. doi: 10.1016/j.bbi.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Anti-interleukin 1β reduces sleep and sleep rebound after sleep deprivation in rats. Am J. Physiol. 1994a;266:R688–R695. doi: 10.1152/ajpregu.1994.266.3.R688. [DOI] [PubMed] [Google Scholar]
- Opp MR, Krueger JM. Interleukin1 is involved in responses to sleep deprivation in the rabbit. Brain Res. 1994b;639:57–65. doi: 10.1016/0006-8993(94)91764-7. [DOI] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Neural-immune interactions in the regulation of sleep. Front Biosci. 2003;8:d768–d779. doi: 10.2741/1061. [DOI] [PubMed] [Google Scholar]
- Opp M, Born J, Irwin M. Sleep and the immune system. In: Ader R, editor. Psychoneuroimmunology. Burlington, MA: Elseveir Academic Press; 2007. pp. 579–618. [Google Scholar]
- Palma BD, Tiba PA, Machado RB, Tufik S, Suchecki D. [Immune outcomes of sleep disorders: the hypothalamic-pituitary-adrenal axis as a modulatory factor] Rev. Bras. Psiquiatr. 2007;29(Suppl 1):S33–S38. doi: 10.1590/s1516-44462007000500007. [DOI] [PubMed] [Google Scholar]
- Ramesh V, Kaushal N, Gozal D. Sleep fragmentation differentially modifies EEG delta power during slow wave sleep in socially isolated and paired mice. Sleep Science. 2009;2:64–75. [Google Scholar]
- Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav. Brain Res. 1995;69:55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–184. doi: 10.1126/science.6857280. [DOI] [PubMed] [Google Scholar]
- Rosekind MR. Underestimating the societal costs of impaired alertness: safety, health and productivity risks. Sleep Med. 2005;6(Suppl 1):S21–S25. doi: 10.1016/s1389-9457(05)80005-x. [DOI] [PubMed] [Google Scholar]
- Shamsuzzaman AS, Gersh BJ, Somers VK. Obstructive sleep apnea: implications for cardiac and vascular disease. JAMA. 2003;290:1906–1914. doi: 10.1001/jama.290.14.1906. [DOI] [PubMed] [Google Scholar]
- Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J. Neurosci. Methods. 2009;184:71–78. doi: 10.1016/j.jneumeth.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Soldatos CR, Paparrigopoulos TJ. Sleep physiology and pathology: pertinence to psychiatry. Int. Rev. Psychiatry. 2005;17:213–228. doi: 10.1080/09540260500104565. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ. The effect of sleep fragmentation on daytime function. Sleep. 2002;25:268–276. doi: 10.1093/sleep/25.3.268. [DOI] [PubMed] [Google Scholar]
- Svanborg E, Guilleminault C. EEG frequency changes during sleep apneas. Sleep. 1996;19:248–254. [PubMed] [Google Scholar]
- Tafti M, Rondouin G, Besset A, Billiard M. Sleep deprivation in narcoleptic subjects: effect on sleep stages and EEG power density. Electroencephalogr. Clin. Neurophysiol. 1992;83:339–349. doi: 10.1016/0013-4694(92)90069-t. [DOI] [PubMed] [Google Scholar]
- Toth LA. Sleep, sleep deprivation and infectious disease: studies in animals. Advan. Neuroimmunol. 1995;5:79–92. doi: 10.1016/0960-5428(94)00045-p. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Knutson KL. Sleep and the epidemic of obesity in children and adults. Eur. J Endocrinol. 2008;159(Suppl 1):S59–S66. doi: 10.1530/EJE-08-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Hoed J, Kraemer H, Guilleminault C, Zarcone VP, Jr, Miles LE, Dement WC, Mitler MM. Disorders of excessive daytime somnolence: polygraphic and clinical data for 100 patients. Sleep. 1981;4:23–37. doi: 10.1093/sleep/4.1.23. [DOI] [PubMed] [Google Scholar]
- Weinstock LB, Walters AS, Paueksakon P. Restless legs syndrome--theoretical roles of inflammatory and immune mechanisms. Sleep Med. Rev. 2012;16:341–354. doi: 10.1016/j.smrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Zager A, Andersen ML, Ruiz FS, Antunes IB, Tufik S. Effects of acute and chronic sleep loss on immune modulation of rats. Am J. Physiol Regul. Integr. Comp Physiol. 2007;293:R504–R509. doi: 10.1152/ajpregu.00105.2007. [DOI] [PubMed] [Google Scholar]
- Zorick F, Roehrs T, Wittig R, Lamphere J, Sicklesteel J, Roth T. Sleep-wake abnormalities in narcolepsy. Sleep. 1986;9:189–193. doi: 10.1093/sleep/9.1.189. [DOI] [PubMed] [Google Scholar]