Abstract
We have previously demonstrated that octacalcium phosphate (OCP) collagen composite (OCP/collagen) promotes bone regeneration in a critical-sized bone defect of a rodent or canine model. This study was designed to investigate the bone regeneration of OCP/collagen in human bone defect as a first clinical trial. Two patients who had a radicular cyst or apical periodontitis consented to participate in our clinical study, and OCP/collagen was implanted into the defects after operation. Radiographic examination showed effective bone healing in each bone defect at 3 or 6 months. Likewise, computed tomography value significantly increased after implantation. Postoperative wound healing was uneventful, and neither infection nor allergic reaction against OCP/collagen was observed for the entire period. This study demonstrated that OCP/collagen would be safely used and enhanced bone regeneration in human bone defects. To reinforce the efficacy of OCP/collagen as a bone substitute material, it should be compared with other suitable comparators in the future.
Introduction
Reconstruction of large bone defects caused by extirpation of tumor and cyst is an important issue in the field of orthopedic and oral surgery.1 Autologous bone includes osteoprogenitor cells, collagenous bone matrix, bone mineral, and growth factors, such as bone morphogenetic proteins.2 This composition in autologous bone should promote the induction of new bone within bone defects if it is transplanted. However, there are problems associated with the use of autologous bone in terms of donor site pain, morbidity, infection, extra blood loss, and higher cost due to longer operation.3
Synthetic calcium phosphate has been widely used as a bone substitute of autologous bone, such as sintered ceramics of hydroxyaptite (HA), β-tricalcium phosphate (β-TCP), and calcium phosphate-based cements to form HA because it has no limitation in terms of availability, excellent osteoconductivity, and relatively uniform quality regardless of the production lot provided by manufacturers.4–6 However, it is widely accepted that its bone regenerative properties are still inferior to those of autologous bone.7
Octacalcium phosphate (OCP) has been suggested to be a precursor of biological apatite, such as dentin, enamel, and bone. Indeed, the presence of OCP has been demonstrated in these types of biological apatite.8–10 Our previous study indicated the superior osteoconductivity of synthetic OCP compared with that of amorphous calcium phosphate, dicalcium phosphate, Ca-deficient HA, and HA if implanted into the subperiosteal area of the calvaria of mice.11 In addition, it has been confirmed that bone regeneration of synthetic OCP is higher than that of HA or β-TCP ceramic in vivo,12 that synthetic OCP promotes osteoblastic cell differentiation while OCP is converted into HA in vitro,13 and that the osteogenic effect is dependent on the amount of OCP.14
Although OCP possesses many desirable properties as a bone substitute, it cannot be molded using sintering processes because of its intrinsic crystal structure. To resolve the disadvantages, including the improvement of handling, OCP combined with atelocollagen (OCP/collagen) was prepared.15 OCP/collagen significantly enhances bone regeneration more than OCP alone, collagen alone, and HA or β-TCP ceramic combined with collagen in rat calvarial bone defect,15,16 and bone formation by OCP/collagen increases with the amount of OCP in the OCP/collagen.17 Aiming at clinical application, bone regeneration by OCP/collagen was examined in canine models, and implantation of OCP/collagen significantly enhanced bone regeneration more than those of β-TCP, collagen, and untreated control at a critical-sized calvarial bone defect, a tooth extraction socket, or an alveolar cleft model.18–21
To the best of our knowledge, the present report is the first on the clinical evaluation of OCP/collagen. This new bone regenerative material was used for filling bone defects after cystectomy. The clinical evaluation of the two patients was conducted according to Japanese ethical guidelines and regulations. The first objective of this study was to investigate the safety of OCP/collagen for clinical use. The second objective was to investigate the efficacy of this material if implanted into a cyst cavity. Radiographic examination of the implanted sites was performed, and the findings were compared to those of the surrounding host bone up to 6 months after implantation.
Materials and Protocols of the Clinical Trial
Preparation of OCP/collagen
OCP was prepared by mixing calcium acetate hydrate solution and sodium phosphate monobasic solution as described previously,11 and using sterile-filtered water during the whole preparation. The precipitates were washed several times with sterile-filtered water and then lyophilized. Sieved OCP granules (particle sizes 300–500 μm) obtained from the dried OCP were sterilized by heating at 120°C for 2 h in dry condition. Our previous study showed that such heating does not affect physical properties, such as the crystalline structure or specific surface area of OCP granules, although it was reported that increasing the temperature above 100°C induced collapse of the OCP structure because of dehydration.22 Powder X-ray diffraction (XRD) patterns of the obtained OCP were recorded using step-scanning at 0.05-degree intervals from 3.00 to 60.00 degrees, with Cu Kα X-rays on a diffractometer (Mini Flex; Rigaku Electrical Co., Tokyo, Japan) at 30 kV and 15 mA. The range of 2θ included the primary peak (100) reflection of OCP around 4.7 degrees. Collagen was prepared from NMP collagen PS (Nippon Meat Packers, Tsukuba, Ibaraki, Japan), and a lyophilized powder of pepsin-digested atelocollagen was isolated from porcine dermis. NMP collagen PS was dissolved in sterile-filtered water and adjusted to a final concentration of 3% at pH 7.4. OCP/collagen was prepared from NMP collagen PS and OCP granules. OCP was added to the concentrated collagen and mixed.15 The weight percentage of OCP in OCP/collagen was 77%. This OCP/collagen mixture was then lyophilized and disks were molded (9 mm diameter, 1 mm thick) (Fig. 1). The molded OCP/collagen underwent dehydrothermal treatment (150°C, 24 h) in a Vacuum Drying Oven, DP32 (Yamato Scientific, Tokyo, Japan). The clinical batches were prepared aseptically. Two pieces of the molded OCP/collagen were placed in a sterilized microcentrifuge tube (509-GRD-SC; Quality Scientific Plastics, San Diego, CA), and the OCP/collagen-containing tube was then packed with Fisherbrand® instant sealing sterilization pouch (9×13 cm; Fisher Scientific, Pittsburgh, PA). The packed OCP/collagen was subsequently sterilized using electron beam irradiation (5 kGy) to make it ready-to-use. After sterilization, the XRD pattern derived from OCP/collagen indicated a collapsed and reduced primary (100) peak with a shift from 4.7 to 5.3 degrees at 2θ, as previously reported.15
FIG. 1.

A picture of OCP/collagen disks. OCP/collagen disk is 9 mm in diameter and 1 mm thick. OCP, octacalcium phosphate.
Design of the clinical trials
This study is a part of the clinical study of “Bone regenerative therapy by OCP collagen composites,” which was registered as JPRN-UMIN000004655 in the University Hospital Medical Information Network in Japan (UMIN) and International Clinical Trials Registry Platform Search Portal of the World Health Organization. The protocol of the clinical trial was submitted and approved by the research ethics committee of the Tohoku University Graduate School of Dentistry under reference number 20–27. The principal investigator and promoter was Prof. Shinji Kamakura (DDS, PhD), and this trial was performed at the Department of Oral and Maxillofacial Surgery, Tohoku University Hospital, Sendai, Japan.
This clinical trial is a single-arm nonrandomized intervention study, and 10 cases of tooth extraction socket and cyst cavity were studied. All patients signed a formal consent form and were finally included in the study. The aim of the first clinical evaluation in this study was to demonstrate the safety, by clinical examination and recording of adverse events, of using OCP/collagen to fill these defects. The second clinical evaluation focused on bone regeneration in these defects by radiographic examination. This study reported two cases of cyst cavity after implantation of OCP/collagen.
Clinical, laboratory, and radiographic examination
Dental radiography and clinical examination were performed before cystectomy, and 1 and 7 days, and 1, 3, and 6 months after OCP/collagen implantation. A computed tomography (CT) was made before the cystectomy and 3 or 6 months after implantation. Then, the value of CT was measured at the center of the bone defect. The clinical examination involved the observation of general and local conditions, including inflammatory symptoms in the operative site. In addition, laboratory examination was carried out before cystectomy, and 1 day, and 1, 3, and 6 months after implantation. This comprised factors such as peripheral blood figures, liver and kidney function, and urinalysis.
Case Reports
The first case (patient A) was a 37-year-old man and the second one (patient B) was a 23-year-old woman. Both patients consulted our hospital for the examination of radiolucent areas of anterior maxillary regions. Their medical histories contained nothing of relevance, and both patients were clinically diagnosed with radicular cyst of anterior maxilla by radiographic examination (Fig. 2a, b). The radiolucent figure of patient A was about 8 mm in diameter at the apical region of the left maxillary lateral incisor and that of patient B was about 5×5×10 mm at the apical region of the left maxillary central incisor. Under local anesthesia, 2% lidocaine hydrochloride with 1:80,000 epinephrine was injected into anterior maxillary region, and both patients underwent cystectomy (Fig. 3a, b). After irrigation, OCP/collagen disks were implanted in the defect. Four or six disks of OCP/collagen were used in the defect of each patient (Fig. 3c, d). Then, ablated gingiva and periosteum were repositioned and sutured with absorbable suture (4-0 Coated Vicryl™; Ethicon, Inc., Irvine, CA). Both cases were diagnosed as radicular cyst by postoperative pathological examination.
FIG. 2.
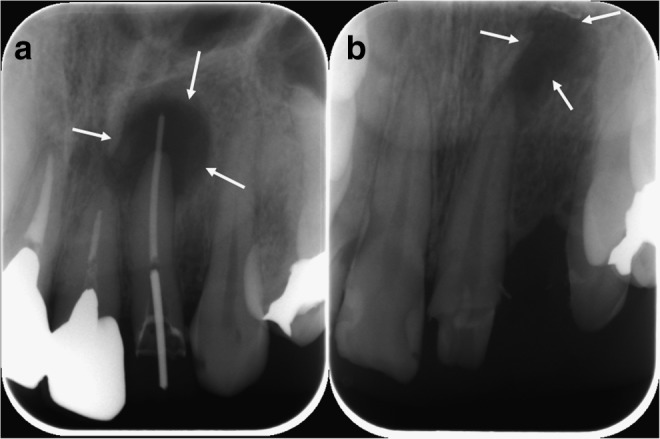
X-ray pictures of patients. (a) Patient A: A radicular cyst at the apex of the left maxillary lateral incisor. (b) Patient B: A radicular cyst at the apex of the left maxillary central incisor. Arrows show the margin of the bone defect.
FIG. 3.
Pictures of cystectomy and OCP/collagen disk implantation. Cystectomy of (a) patient A and (b) patient B. Arrows show the margins of these defects. Implantation of OCP/collagen disks at the defects of (c) patient A and (d) patient B. Dot arrows show OCP/collagen disks in these defects. Color images available online at www.liebertpub.com/tea
In both cases, postoperative wound healing was satisfactory, and there was no postoperative infection or allergic reaction at the operative site during the observation period. The height or width of alveolar bone was maintained until 6 months after OCP/collagen implantation (Fig. 4). Laboratory examination revealed no abnormal findings except that a slight increase of C-reactive protein was observed. In radiographic examination after the implantation of OCP/collagen, there was little radiopacity in the defect 1 day after the implantation (Fig. 5a, b). The radiopacity in the defect increased slightly at 3 months (Fig. 5c, d) and was further improved 6 months after the operation (Fig. 5e, f ). Examination by CT scan showed that the defect of patient A or B was dotted with hard tissue-like bone at 3 months after implantation (Fig. 6c, d). At 6 months after OCP/collagen implantation, the density of the hard tissue was increased and the hard tissue spread in the defect (Fig. 6e, f ). This hard tissue arose from the center of the defect as well as its margin.
FIG. 4.

Pictures of operative region in patient B at 6 months after OCP/collagen implantation. There was temporary crown restoration. Color images available online at www.liebertpub.com/tea
FIG. 5.
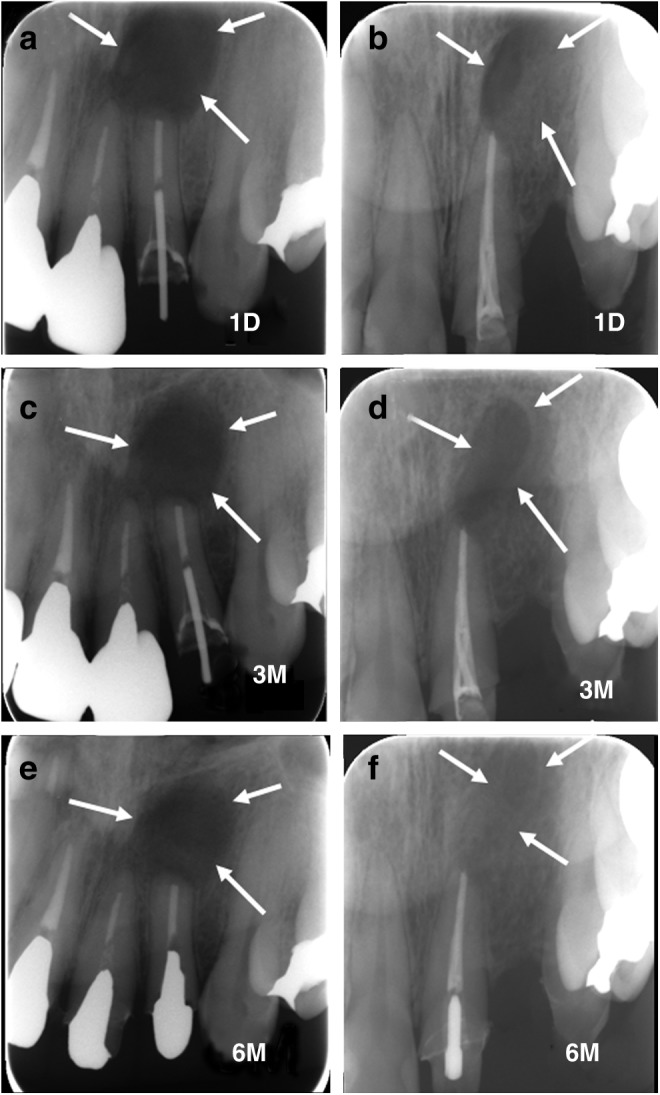
X-ray pictures. (a) At 1 day after cystectomy in patient A and (b) patient B. (c) At 3 months after cystectomy in patient A and (d) patient B. (e) At 6 months after cystectomy in patient A and (f ) patient B. Arrows show the margins of the bone defects.
FIG. 6.
Computed tomography pictures of horizontal plane. (a) Before cystectomy in patient A. The size was almost 8 mm in diameter. (b) Before cystectomy in patient B. The size was almost 5×5×10 mm. (c) At 3 months after cystectomy in patient A and (d) patient B. (e) At 6 months after cystectomy in patient A and (f ) patient B.
The CT values of the radicular cysts before implantation were 21 Hounsfield units (HU) in patient A and 23 HU in patient B. The CT values of patient A were 327 and 527 HU at 3 and 6 months after OCP/collagen implantation, respectively. Similarly, those of patient B were 316 and 602 HU. The CT values increased significantly at 3 or 6 months compared with those before implantation (Table 1).
Table 1.
Computed Tomography Value at Bone Defect
| Cyst (before cystectomy), HU | 3 months | 6 months | |
|---|---|---|---|
| Patient A | 21 | 327 | 527 |
| Patient B | 23 | 316 | 602 |
Computed tomography value was measured at the center of the defect.
HU, hounsfield unit.
Discussion
When OCP/collagen was implanted into bone defects after cystectomy, no postoperative infection or allergic symptom was observed for the entire period. In addition, no serious side effects were observed in this investigation. Laboratory examination showed no serious abnormal results in each patient, although C-reactive protein increased 1 day after OCP/collagen implantation as well as usual radicular cystectomy and recovered to its normal level at 7 days (data not shown). Although these patients complained of pain in the operative site for a few days, this symptom was controlled with a painkiller. In addition, postoperative wound swelling seemed to be limited compared with the operative stress. At 3 months after OCP/collagen implantation, the implanted site became hard on palpation. These results suggest that OCP/collagen would be safe to use if implanted into adult patients after cystectomy.
In radiographic examination, the radiopacity in the bone defect after OCP/collagen implantation increased with time. In addition, CT examination in the defect indicated dotted radiopacity at 3 months, which had increased and spread in the defect at 6 months. If OCP/collagen were implanted into a bone defect, the implanted OCP/collagen indicated radiopacity at several weeks after implantation, whereas OCP/collagen itself had little radiopacity.14,15 Previous studies confirmed that the implanted OCP/collagen focuses and enhances bone regeneration and it is converted to apatite crystals as determined by XRD or Fourier transform infrared spectroscopy.15,16 The increase of radiopacity in the bone defect originated from apatitic conversion from OCP and bone formation,15 with most of it being dependent on bone formation.14,16,21 Therefore, the increased radiopacity in these cases would be involved in new bone formation. This means that the implanted OCP/collagen would enhance bone formation in human bone defects.
The CT values in the defects of both patients were increased from 21 to 23 HU (before implantation) through 327–316 HU (3 months) to 527–602 HU (6 months). As CT value is proportional to bone mineral density, cancellous bone around the bone defect showed a change from ∼200 to 600 HU, whereas cortical bone around it changed from ∼600 to 1600 HU in the present cases. In addition, that of OCP or OCP/collagen changed from 130 to 140 HU. This suggests that new bone derived from OCP/collagen implantation was almost the same as cancellous bone at 6 months.
At 6 months after OCP/collagen implantation, dental radiography of patient B in the treated defect was more radiopaque than that of patient A, whereas the difference of radiopacity on CT or CT value at the center of the defect was less noticeable. The size of the defect or the amount of the implanted material might be related to the difference between the patients. Although this study remains obscure what would be elicited these difference, it should be examined in the future. Although this study indicated that the implantation of OCP/collagen would regenerate new bone in defects after cystectomy, no comparison of bone regeneration was performed between OCP/collagen-treated cases and control cases treated with cystectomy but no implantation. This should be considered a limitation of this study, and there is a need for comparative study between OCP/collagen-treated cases and control cases to be performed to clarify the bone regenerative properties of OCP/collagen in the future.
In this study, we confirmed that OCP/collagen disk had good handling performance with softness like that of a sponge and styptic characterization by collagen. Because OCP/collagen itself has little radiopacity, it should be easy for clinicians to confirm bone regeneration in bone defects using X-ray examination. These properties convey some advantages to OCP/collagen in clinical use compared with other bone substitute materials.
In this clinical study, OCP/collagen was used in small bone defects created by cystectomy. However, our previous study demonstrated that OCP/collagen implantation enhanced bone healing at tooth extraction socket, alveolar cleft model, and mandibular bone defect in a canine model.19–21,23 Therefore, OCP/collagen should be able to enhance bone regeneration in large bone defects. Studies to elucidate the bone regenerative properties of OCP/collagen in other bone defects or large bone defects should be performed in humans.
Conclusion
This study demonstrated that OCP/collagen would be safely used and enhanced bone regeneration in human bone defects. To reinforce the efficacy of OCP/collagen as a bone substitute material, it should be compared with other suitable comparators in the future.
Acknowledgments
This study was supported in part by Grants-in-aid for Scientific Research on Priority Areas (17076001) and Scientific Research (B) (19390490, 23390450) from the Ministry of Education, Science, Sports and Culture of Japan.
Disclosure Statement
The authors (S.K. and O.S.) obtained a patent of OCP/Col in Japan (#5046511).
References
- 1.Petite H., Viateau V., Bensaid W., Meunier A., de Pollak C., Bourguignon M., Oudina K., Sedel L., and Guillemin G.Tissue-engineered bone regeneration. Nat Biotechnol 18, 959, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Wozney J.M., Rosen V., Celeste A.J., Mitsock L.M., Whitters M.J., Kriz R.W., Hewick R.M., and Wang E.A.Novel regulators of bone formation: molecular clones and activities. Science 242, 1528, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Geiger M., Li R.H., and Friess W.Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev 55, 1613, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bucholz R.W.Nonallograft osteoconductive bone graft substitutes. Clin Orthop Relat Res 395, 44, 2002 [DOI] [PubMed] [Google Scholar]
- 5.LeGeros R.Z.Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395, 81, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Ogose A., Kondo N., Umezu H., Hotta T., Kawashima H., Tokunaga K., Ito T., Kudo N., Hoshino M., Gu W., and Endo N.Histological assessment in grafts of highly purified beta-tricalcium phosphate (OSferion) in human bones. Biomaterials 27, 1542, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Lichte P., Pape H.C., Pufe T., Kobbe P., and Fischer H.Scaffolds for bone healing: concepts, materials and evidence. Injury 42, 569, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Bodier-Houlle P., Steuer P., Voegel J.C., and Cuisinier F.J.First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallogr D Biol Crystallogr 54, 1377, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Tohda H., Yamada M., Yamaguchi Y., and Yanagisawa T.High-resolution electron microscopical observations of initial enamel crystals. J Electron Microsc 46, 97, 1997 [Google Scholar]
- 10.Crane N.J., Popescu V., Morris M.D., Steenhuis P., and Ignelzi M.A., Jr.Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone 39, 434, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Suzuki O., Nakamura M., Miyasaka Y., Kagayama M., and Sakurai M.Bone formation on synthetic precursors of hydroxyapatite. Tohoku J Exp Med 164, 37, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Kamakura S., Sasano Y., Shimizu T., Hatori K., Suzuki O., Kagayama M., and Motegi K.Implanted octacalcium phosphate is more resorbable than beta-tricalcium phosphate and hydroxyapatite. J Biomed Mater Res 59, 29, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Suzuki O., Kamakura S., Katagiri T., Nakamura M., Zhao B., Honda Y., and Kamijo R.Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 27, 2671, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Anada T., Kumagai T., Honda Y., Masuda T., Kamijo R., Kamakura S., Yoshihara N., Kuriyagawa T., Shimauchi H., and Suzuki O.Dose-dependent osteogenic effect of octacalcium phosphate on mouse bone marrow stromal cells. Tissue Eng Part A 14, 965, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Kamakura S., Sasaki K., Honda Y., Anada T., and Suzuki O.Octacalcium phosphate combined with collagen orthotopically enhances bone regeneration. J Biomed Mater Res B Appl Biomater 79, 210, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Kamakura S., Sasaki K., Homma T., Honda Y., Anada T., Echigo S., and Suzuki O.The primacy of octacalcium phosphate collagen composites in bone regeneration. J Biomed Mater Res A 83, 725, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Kawai T., Anada T., Honda Y., Kamakura S., Matsui K., Matsui A., Sasaki K., Morimoto S., Echigo S., and Suzuki O.Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng Part A 15, 23, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Kawai T., Matsui K., Iibuchi S., Anada T., Honda Y., Sasaki K., Kamakura S., Suzuki O., and Echigo S.Reconstruction of critical-sized bone defect in dog skull by octacalcium phosphate combined with collagen. Clin Implant Dent Relat Res 13, 112, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Iibuchi S., Matsui K., Kawai T., Sasaki K., Suzuki O., Kamakura S., and Echigo S.Octacalcium phosphate (OCP) collagen composites enhance bone healing in a dog tooth extraction socket model. Int J Oral Maxillofac Surg 39, 161, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Matsui K., Matsui A., Handa T., Kawai T., Suzuki O., Kamakura S., and Echigo S.Bone regeneration by octacalcium phosphate collagen composites in a dog alveolar cleft model. Int J Oral Maxillofac Surg 39, 1218, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Tanuma Y., Matsui K., Kawai T., Matsui A., Suzuki O., Kamakura S., and Echigo S.Comparison of bone regeneration between octacalcium phosphate/collagen composite and beta-tricalcium phosphate in canine calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol 115, 9, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Suzuki O., Nakamura M., Miyasaka Y., Kagayama M., and Sakurai M.Maclura pomifera agglutinin-binding glycoconjugates on converted apatite from synthetic octacalcium phosphate implanted into subperiosteal region of mouse calvaria. Bone Miner 20, 151, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Miura K., Matsui K., Kawai T., Kato Y., Matsui A., Suzuki O., Kamakura S., and Echigo S.Octacalcium phosphate (OCP) collagen composites with titanium mesh facilitate alveolar augmentation in canine mandibular bone defects. J Oral Maxillofac Surg 41, 1161, 2012 [DOI] [PubMed] [Google Scholar]




