Abstract
Airway obstruction is a common cause of poor performance in horses. Structural abnormalities (insufficient length, rigidity) can be a cause for the obstruction. Currently, there are a few effective clinical options for reconstruction of the equine larynx. A regenerative medicine approach to reconstruction may provide the capability to stabilize laryngeal structures and to encourage restoration of site-appropriate, functional, and host-derived tissue. The purpose of this study was the histopathological evaluation of (1) decellularization of equine (horse) laryngeal cartilages (epiglottis and arytenoids); (2) the host response to decellularized laryngeal cartilages implanted subcutaneously in a donkey model as a test of biocompatibility; and (3) the use of decellularized laryngeal cartilages in a clinically relevant pilot study in the horse larynx. Equine laryngeal cartilages were found to be sufficiently decellularized and were subsequently implanted subcutaneously in donkeys to test biocompatibility. After 4 weeks, the implanted cartilage was harvested. In the subcutaneous model, the samples did not elicit a rejection or foreign body type reaction and were judged suitable for implantation in a clinically relevant equine model. Implants were placed in the upper airway (arytenoids and epiglottis) of one horse. At 4 weeks, the implants were observed to remodel rapidly and were replaced by dense connective tissue with signs of new hyaline cartilage formation in the arytenoids and by connective tissue containing glandular structures and an epithelial covering in the epiglottis. The results of the present study demonstrate the feasibility of a scaffold-based regenerative medicine approach to reconstruction of the equine upper airway; however, further studies investigating long-term integration, formation of new cartilage, and mechanical properties are needed.
Introduction
During exercise, the airflow through the equine larynx is unique as peak flow ranges from 60 to 80 L/s during the expiratory and inspiratory cycle, leading to minute volumes of approximately 1800 L/min.1,2 Large airway pressure gradients are needed to drive this large volume of air such that during inspiration at exercise, clinically normal horses can experience subatmospheric pressures reaching as low as −18 to −37 mmHg.3–5 This negative pressure can result in dynamic pharyngeal or/and laryngeal collapse.6–11 Increased compliance of the upper airway is seen with neuromuscular dysfunction/disease and structurally weak or flaccid upper airway cartilages, contributing to airway collapse.12 Palatal instability and particularly dorsal displacement of the soft palate (DDSP) have been reported to occur in 10–20% of racehorses.13,14 Chondritis is a structural abnormality of the arytenoid cartilage secondary to infection and inflammation, which may also lead to laryngeal collapse and obstruction.15 The current standard therapy for this condition is removal of the affected cartilage segments, which can lead to collapse of the unsupported soft tissue structure and loss of airway patency. Optimal surgical treatments for this dynamic upper airway problem are still under investigation.16 In one study, an injection of a Teflon material into the submucosal space of the epiglottis has been used to restore structural integrity to the epiglottic cartilage. However, results were poor because this treatment does not improve the length of the abnormal epiglottic cartilage and is associated with a significant chronic inflammatory response.17,18
The clinical target of this study is to develop a novel treatment for palatal instability and DDSP associated with structural abnormality of the epiglottic or arytenoid cartilage (i.e., length, deformity, and flaccidity). A regenerative medicine approach to reconstruction of airway cartilages may provide the capability to stabilize these laryngeal structures. Regenerative medicine and tissue engineering approaches utilize scaffold-based strategies, with or without cell seeding, to induce and restore normal structure and function.19–21 Biological scaffold materials derived from extracellular matrix (ECM) have been shown to support the constructive remodeling of a number of tissues and organs in both preclinical and clinical applications, including the airway.22 That is, implantation of acellular tissue-based matrices has been demonstrated to promote the formation of site-appropriate, functional, host tissues.23 Decellularization of intact laryngeal tissues has recently been described.24 The results of these studies have shown that it is possible to remove potentially immunogenic cellular constituents from tissues while retaining scaffold material which is both structurally and mechanically similar to native larynx.25,26 Similar approaches have been applied to the human trachea, and cell-seeded tracheal matrices have been shown to be clinically effective tracheal replacements.27,28
To explore the feasibility of this approach in the laryngeal cartilages of the horse, two studies were carried out. In the first study, a decellurized matrix was implanted in a subcutaneous model to examine the host response. In a second study, the decellularized matrix was implanted into the larynx of one horse, replacing the rostral with two thirds of the epiglottic cartilage and a section of the body of the arytenoid cartilage. The goals of these studies were three-fold: (1) to evaluate the decellularization of clinically relevant equine laryngeal cartilages, including the epiglottis and arytenoids; (2) to evaluate the host tissue response to decellularized laryngeal cartilage in a donkey model of subcutaneous implantation to assess biocompatibility; and (3) to assess the incorporation of decellularized laryngeal cartilages implanted into the upper airway of a horse.
Materials and Methods
Preparation of equine laryngeal ECM
Equine tissues were harvested from adult horses (3–5 years old) immediately after euthanasia for reasons unrelated to the airway. The epiglottis and arytenoids were separated from the remaining larynx by sharp dissection. The surrounding, noncartilaginous tissues, including the mucosal surfaces, were removed by sharp dissection, leaving only cartilage. Tissues were frozen at −80°C for at least 24 h and remained frozen until further treatment. Samples were thawed and decellularized using the method described by Remlinger, et al.24 Briefly, tissues were placed into a flask containing de-ionized water (diH2O) on a shaker for 1 h at room temperature. The diH2O was decanted, and the samples were placed into a flask containing 3% Triton-X 100 (a nonionic surfactant) solution on a shaker for 48 h at 4°C. The Triton-X was decanted and replaced with fresh solution at 24 h. After Triton-X treatment, samples were rinsed repeatedly twice in phosphate-buffered saline (PBS) and twice in ddH2O for 15 min per rinse. The samples were then further decellularized and sterilized by immersion in a solution of 4% ethanol and 0.1% peracetic acid on a shaker for 2 h at room temperature. The peracetic acid treatment was followed by two PBS (pH 7.4) washes and two water washes of 15 min each at room temperature. After decellularization, samples were lyophilized for 48 h at a temperature of −30°C or lower until dry. Lyophilized samples were packaged individually and terminally sterilized by exposure to ethylene oxide gas.
Evaluation of decellularization
Portions of the decellularized sample were fixed in formalin, embedded in paraffin, then sectioned at five microns, and affixed to glass slides. Effectiveness of decellularization was assessed qualitatively by hematoxylin and eosin staining (H&E), and the quantity and length of remaining DNA was assessed using a PicoGreen DNA assay and agarose gel electrophoresis as previously described.29 Briefly, total DNA content was collected using phenol/chloroform extraction. The amount of remaining DNA was quantified by PicoGreen assay as per the manufacturer's protocols. The base pair (bp) length of remaining DNA was assessed by agarose gel electrophoresis.
Qualitative histologic analysis was performed to determine the presence, distribution, and relative quantity of constitutive components of the cartilaginous tissues. Briefly, hematoxylin and eosin, safranin O, and Verhoeff's elastic stain were used to examine overall tissue structure, maintenance of glycosaminoglycan (GAG) content, and elastin organization, respectively. All results were compared with native tissues as a control.
Surgical procedures
Subcutaneous implantation model
A subcutaneous implantation model was used to assess the host tissue response to the decellularized scaffold materials. Ten donkeys in good physical condition as determined by physical examination were used. All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the National Institutes of Health. All animal procedures complied with the guidelines provided by the Institutional Animal Care and Use Committee of Ross University School of Veterinary Medicine and the Cornell University College of Veterinary Medicine.
Under general anesthesia and after aseptic preparation of the cervical area, a 10 cm incision was made through the skin and a pocket was created subcutaneously by blunt dissection. A one-centimeter cube-sized equine laryngeal ECM was placed into the pocket. The subcutaneous tissue was closed with 2-0 polydioxanone suture in a continuous pattern. The skin was closed with 2-0 nylon suture with a simple interrupted mattress pattern. Postoperatively, the animals received trimethoprim-sulfadiazine (15 mg/kg q12h) for 3 days, and phenylbutazone (2 mg/kg q24h) orally on the day of surgery and the next day. After 30 days, the implanted tissue was harvested with a small amount of the surrounding native tissue under general anesthesia and the donkey recovered. After suture removal, the donkeys were released to pasture.
Histologic evaluation
One month after implantation, the implantation sites were removed with a small amount of the surrounding native tissue and fixed in 10% neutral buffered formalin. Sections were then embedded in paraffin. Five-micron sections were stained with hematoxylin and eosin for evaluation of tissue reaction and implant morphology. Additional slides were stained by safranin O and Verhoff's elastic stain techniques for evaluation of GAG and elastin content. Histologic reviewers were blinded to sample origin, and a subjective assessment of biopsy specimens was performed. Four to five, randomly selected, roughly 0.5 cm square areas were selected for cell count evaluation in samples harvested from the subcutaneous implantation study. At least five hundred cells were classified and counted for each of the randomly selected areas. Categories of evaluation included acute inflammatory cells (number of neutrophils and number of eosinophils), chronic inflammatory cells (number of lymphocytes, plasma cells, macrophages, and multinucleated giant cells), and number of spindle cells. Counts were performed at two different laboratories, and the mean data were reported.
Laryngeal implantation model
A pilot study was performed to assess the integration of decellularized constructs within the equine upper airway. One horse was used to explore the technical feasibility of implanting decellurized cartilage in a high motion and nonsterile environment. Decellularized scaffold materials were placed into two separate locations within the equine larynx: (1) decellularized epiglottis as replacement after resection of the rostral with two thirds of the native epiglottis, (2) submucosal placement of decellularized arytenoid into the body of the arytenoid cartilage. A 4 year-old thoroughbred gelding with normal upper airway cartilage as evidenced by endoscopy and laryngeal ultrasound was used.
General anesthesia in dorsal recumbency was maintained by administering an isoflurane and oxygen mixture via an endotracheal tube placed in mid-cervical tracheostomy, which was performed to allow for surgical intra-laryngeal manipulations. Under aseptic conditions, a 10 cm ventral midline skin incision was made and the incision was bluntly extended between the sternohyoid muscles and the cricothyroid membrane. A routine laryngotomy was performed, and the native epiglottic cartilage was retroverted into the larynx. Two stay sutures with 2-0 poliglecaprone were placed at the left and right base of the epiglottis. The lateral attachment of the aryepiglottic folds on the epiglottis was incised, and epiglottic cartilage at the caudal junction of the caudal 1/3 and rostral 2/3 was transected and removed by sharply separating the ary-epiglottic membrane from the epiglottic cartilage; no effort was made to preserve the epiglottic mucosa, as it is tightly adhered and cannot be preserved in clinical applications. The decellularized cartilage (rehydrated for 30 min in saline) was then placed and sutured to the remaining base of the epiglottic cartilage using four 2-0 polypropylene sutures. The left and right ary-epiglottic membrane were sutured to the edge of the epiglottis using 3-0 poliglecaprone in a simple continuous pattern. A decellularized porcine urinary bladder matrix (UBM), prepared as previously described,30,31 was placed on the dorsal surface of the epiglottis and then sutured to the periphery of the cartilage using 3-0 poliglecaprone to provide a basement membrane-like surface covering the implant as a barrier to infection and as a substrate for epithelial cell growth.
A U-shaped incision was performed over the medial surface of the left arytenoids, and the mucosa was reflected and removed: a 1×1×0.5 cm section of the arytenoid within the body was removed and a decellularized left arytenoid section was placed into the area. The decellularized bladder mucosal matrix was then sutured to the periphery of the cartilage to cover its surface using 3-0 poliglecaprone. The same procedure was repeated on the right arytenoid.
Perioperatively, the horse was administered gentamicin (6.6 mg/kg q24h, intravenously), procaine penicillin (22,000 IU/kg q12h, intramuscularly), and phenylbutazone (2 mg/kg q12h, intravenously) and maintained on a soft diet for 10 days (wet hay and bran mash added in equal parts). Clinical status was evaluated daily. Video laryngoscopy was performed on postoperative days 1, 7, 14, 21, and 28 to document the laryngeal function, macroscopic appearance, and healing process. Tracheostomy, evaluated and cleaned daily, was maintained patent through a self-retaining tube until confirmation of normal laryngeal function and airway patency. Laryngeal ultrasound was performed bilaterally through the caudo-lateral window to assess the arytenoids cartilages in both longitudinal and transverse views as previously described.32 Four weeks after surgery, the horse was subjected to euthanasia by sodium pentobarbital overdose injection. The larynx was collected and fixed in formaldehyde 10% solution for histo-pathologic evaluation as described earlier.
Results
Decellularization of equine tissues
Macroscopically, the samples were observed to maintain the tissue-specific shape and size of the native tissue (Fig. 1). Histologically, the samples were observed to maintain the native tissue structure and a large degree of the constituent GAG and elastin content. Samples were characterized by an acellular perichondrial surface consisting of dense, well-aligned, primarily collagenous, connective tissue surrounding the elastic cartilage portion of the epiglottis consisting of large amounts of GAG and elastin as evidenced by safranin red O and Verhoeff's elastic stain, respectively. Little of the epithelial surface remained, having been stripped during the decellularization process. The glandular structures observed in the native tissues were found to be decellularized, leaving acellular spaces within the decellularized tissues. In some samples, these spaces were observed to run from the perichondrial surface well into the cartilaginous portion of the tissues. Representative histologic images of native and decellularized tissues can be observed in Figure 2.
FIG. 1.
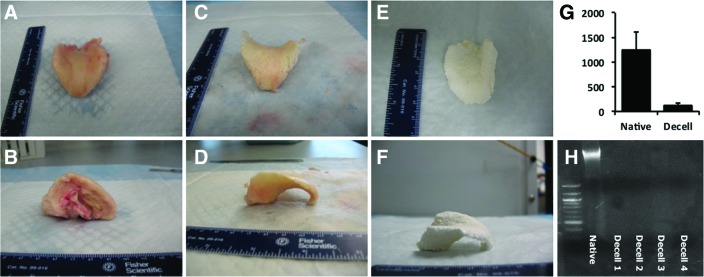
Gross morphologic view of native (A, B), decellularized (C, D), and lyophilized (E, F) tissues. The three-dimensional structure is mostly retained. PicoGreen assay (G) and agarose gel electrophoresis (H) confirm decellularization. Color images available online at www.liebertpub.com/tea
FIG. 2.
Histologic sections of native equine epiglottis (A–C) and decellularized epiglottis (D–F). (A, D) Hematoxylin and eosin, (B, E) safranin O (red=glycosaminoglycan), and (C, F) Verhoeff's elastic stain (black=elastin). Note glandular structures (G), perichondrium (arrow), and elastic cartilage (E). Magnification=4×objective, scale bar=300 μm. Inset magnification=20× objective, scale bar=100 μm. Color images available online at www.liebertpub.com/tea
A few intact nuclei were observed at high magnification, leaving acellular lacunae within the cartilaginous potion of the samples. PicoGreen analysis of dsDNA content of the native (n=2) and decellularized (n=4) tissues indicated a content of 1249.45±358.07 ng DNA/mg tissue and 114.78±56.76 ng/mg, respectively, representing an average 91% reduction in DNA content. Agarose gel electrophoresis demonstrated no visible DNA fragments within samples after phenol/chloroform isolation. Results of the analysis of decellularization can be found in Figure 1.
Subcutaneous implantation model
Core body temperature was normal in all donkeys throughout the study. The implants were well tolerated, and the donkeys could freely move their necks. The location where the implants were placed under the skin could be seen and palpated in all donkeys. All but one had moderate swelling, which peaked at days 4 or 5 and then, decreased within 5 days thereafter. Harvest of the cartilage implants was easily accomplished, as a dense mass was apparent on palpation. Tissue surrounding the implants appeared grossly normal without inflammation or infection.
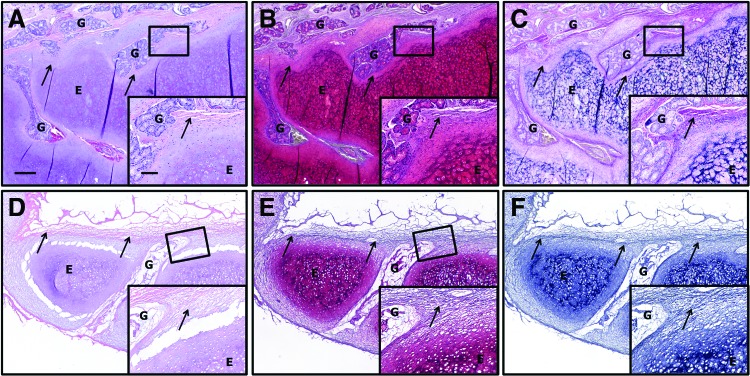
Histologically, the subcutaneously implanted samples were largely intact at 4 weeks post-implantation. Distinct areas representing the perichondrium, elastic cartilage, and glandular structures could be observed. However, levels of GAG and elastin were observed to be qualitatively lower than in the preimplantation material by histologic observation. The area representing the perichondrial surface of the implant was found to be well invested by a mix of mononuclear and spindle-shaped cells. A few cells were observed to infiltrate the bulk of the elastic cartilage; however, a number of cells near the periphery of the implant were observed as occupying space within lacunae, suggestive of chondrocyte phenotype. The glandular structures of the implant were invaded by large populations of mononuclear and spindle-shaped cells.
The cellular reaction at the interface with the sample was characterized by the presence of predominantly macrophages and spindle-shaped cells of unknown phenotype as observed by a quantitative histopathologic analysis. Results of the quantitative analysis are presented in Table 1. Implants were surrounded by a loose network of highly cellular collagenous connective tissue and adipose tissue. Extensive neovascularization was observed around the implant. This response is characteristic of the early constructive remodeling type response observed in other decellularized tissues in a number of other studies and, of note, did not resemble either a rejection type or foreign body reaction type response (Fig. 3).
Table 1.
Quantification of Cellular Reaction at the Implant Interface
| Cell type | Percentage |
|---|---|
| Neutrophil | 1.02±1.68 |
| Eosinophil | 10.09±5.73 |
| Macrophage | 18.46±11.15 |
| Lymphocyte | 6.6±3.07 |
| Plasma | 3.84±1.44 |
| Multinucleated giant | 0.33±0.26 |
| Spindle shaped | 59.67±13.65 |
FIG. 3.
Histologic sections of decellularized epiglottis implanted subcutaneously in donkeys for 1 month. Note the maintenance of structures. A few cells were seen to invade the bulk of the scaffold; however, invasion was observed in the glands. No foreign body reaction or fibrotic encapsulation was observed. Some glycosaminoglycan and the majority of the elastin remain intact. (A) Hematoxylin and eosin, (B) safranin O (red=glycosaminoglycan), and (C) Verhoeff's elastic stain (black=elastin). Note glandular structures (G), perichondrium (arrow), and elastic cartilage (E). Magnification=4× objective, scale bar=300 μm. Inset magnification=20× objective, scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Laryngeal implantation model
The horse recovered uneventfully from surgery. Clinical status was evaluated daily. Throughout the 4 weeks postoperatively, the horse ate and drank normally, exhibited no signs of coughing or aspiration, and transient swelling of head and neck near the surgical site resolved during the first week postsurgery. Endoscopy on day 1 revealed marked edema/swelling of the ary-epiglottic fold and epiglottis, causing obstruction of the glottis. In contrast, arytenoid swelling was mild and laryngeal abduction and adduction movements were preserved, during both breathing and swallowing. The swelling progressively decreased over the next week, and the tracheostomy tube was removed on day 21. On week 4, endoscopy revealed the arytenoids very mildly enlarged and the arytenoid scaffolds being covered by a macroscopically healthy layer of mucosa, slightly protruding from the luminal surface of the cartilages, likely due to implants having a slightly larger size than the defect site at the time of implantation (Fig. 4).
FIG. 4.
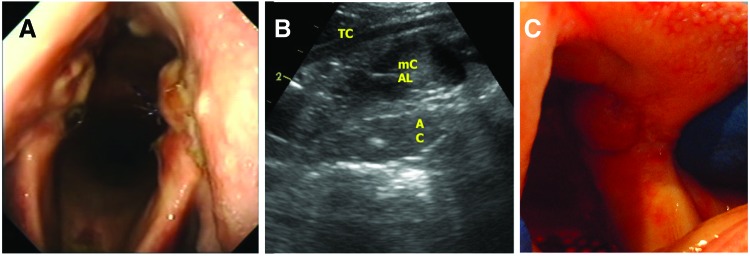
Endoscopic picture of the arytenoid cartilages 4 weeks after implantation (A), ultrasound picture showing mildly enlarged arytenoid cartilage (AC) within the implant site (B), normal intrinsic laryngeal musculature (mCAL) and thyroid cartilage (TC), and post-mortem view of the mucosal covering of implant (C). Color images available online at www.liebertpub.com/tea
Laryngeal ultrasound performed on week 4 confirmed the thickening of both arytenoids with a localized irregular shape of the medial and lateral borders, indicative of the implantation area. Heterogeneous echogenicity of the cartilage body, with hyperechoic spots in the site of the scaffold implantation, showed the variability in tissue density (Fig. 4). Ultrasonographically, a definite boundary discriminating native cartilage and implanted scaffold was not identified. Compared with the presurgical findings, no changes in the appearance of the intrinsic laryngeal muscles were observed.
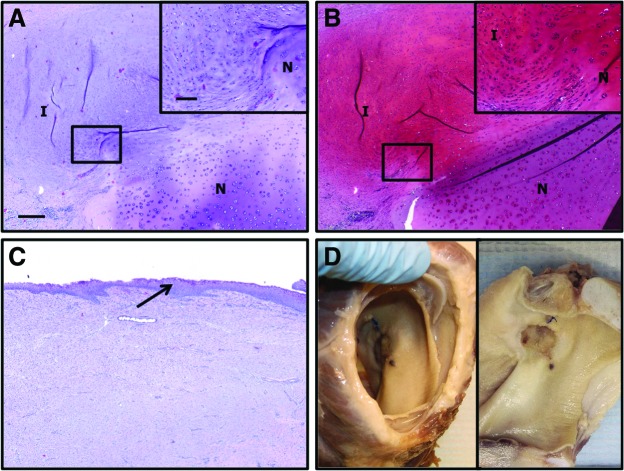
It was not possible to distinguish the original implants from newly formed tissues at the time of harvest. Histologically, the sample implanted in the epiglottis of the horse was observed to be completely degraded and replaced by newly deposited tissue at 4 weeks post-implantation. The newly formed tissue was characterized by the presence of a largely intact mucosal epithelial covering and a subjacent submucosa consisting of highly organized connective tissues and blood vessels (Fig. 5). Glandular structures were observed within the newly formed tissues. The samples were well integrated with native tissues; however, little to no evidence of new elastic cartilage formation was observed within the remodeled epiglottic implant.
FIG. 5.
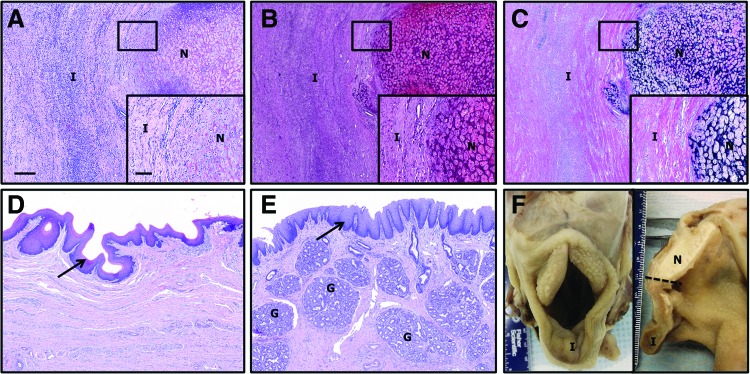
Histologic sections of implanted epiglottis at 1 month post-implantation. The native (N)/implant (I) interface is shown (A–C). The implanted samples were well integrated with the native tissue, and some areas of new cartilage formation were observed. Evidence of epithelialization (arrows, D, E) and glandular formation (G) were observed within the newly formed tissues. Gross morphologic view of intact (left) and bisected larynx (right) at euthanasia is shown (F). (A, D, E) Hematoxylin and eosin, (B) safranin O (red=glycosaminoglycan), and (C) Verhoeff's elastic stain (black=elastin). Magnification=4× objective, scale bar=300 μm. Inset magnification=20× objective, scale bar=100 μm. Color images available online at www.liebertpub.com/tea
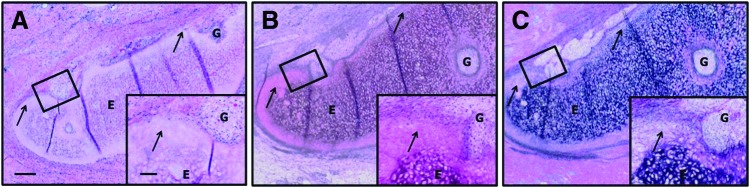
Samples implanted within the arytenoid location were also fully degraded and replaced by newly formed tissues at 4 weeks post-implantation (Fig. 6). The newly formed tissue was well integrated with native tissue and consisted of an epithelial covering and subjacent connective tissue. Evidence of new hyaline cartilage formation was observed at the interface of the implant with native tissue. The area of new cartilage formation was observed as an outgrowth of neighboring cells from native tissue adjacent to the implant and was characterized by the presence of chondrocyte-like cells within lacunae, locally high levels of GAG, and little or no elastin (Fig. 6). Overlying stratified squamous epithelium was hyperplastic with extensive rete peg formation, and glandular hyperplasia/metaplasia was marked within the underlying mucosal connective tissues of some regions.
FIG. 6.
Histologic sections of implanted arytenoid at 1 month post-implantation. The native (N)/implant (I) interface is shown (A, B). The implanted samples were well integrated with the native tissue, and some areas of new cartilage formation were observed. Evidence of epithelialization (arrow, C) was within the newly formed tissues. Gross morphologic view of intact (left) and bisected larynx (right) at euthanasia is shown (D). (A, C) Hematoxylin and eosin, and (B) safranin O (red=glycosaminoglycan). No positive staining was observed for Verhoeff's elastic stain (not shown). Magnification=4× objective, scale bar=300 μm. Inset magnification=20× objective, scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Discussion
The present study demonstrates that it is possible to decellularize intact cartilaginous components of the equine upper airway, and that these decellularized constructs are biocompatibible and may be capable of promoting the formation of new functional host tissues within the equine larynx. Donkeys were readily available, less costly, and provided a model for evaluation of the potential for rejection and foreign body type responses to the decellularized matrices. Host rejection of xenograft and allograft implants is a direct consequence of the recognition of residual cells within the tissues. Therefore, thorough cell removal is critical to avoid inflammatory or rejection type responses.33 The residual amount of double-stranded DNA is one indicator of the effectiveness of the decellularization process. In the present study, the decellularized scaffolds were shown to have 114.78±56.76 ng dsDNA/mg scaffold dry weight. When comparing the ds-DNA content before and after decellularization, this results in an average of 91% DNA reduction. These levels of DNA are comparable or less than those found in many commercially available, FDA-approved ECM materials.29 While the presence of large amounts of cellular debris and DNA within an ECM scaffold material has been demonstrated to have deleterious effects on its ability to remodel,34 the consequences of small amounts of DNA, such as those reported in the present study, remain unknown. Deleterious effects on remodeling were not observed in the present study. The goal of an ideal tissue decellularization protocol is effective for cell removal, but this should be balanced against preserving advantageous aspects of the ECM scaffold, including-tissue specific biochemical composition, ultrastructure, and mechanical behavior. Generally, a 100% reduction in DNA content is not achievable without significantly compromising the ECM scaffold.
Each of the mechanical, chemical, or enzymatic steps included in a decellularization procedure may have a deleterious effect on the ECM. For example, glutaraldehyde fixation of heart valves has been demonstrated to result in GAG loss with impacts on mechanical durability.35 Similarly, Triton-X, which was utilized in the present study, has been reported to have mixed effects on GAG content.20 For example, chemical-enzymatic decellularization of tracheas resulted in GAG reduction.36 When TritonX-100 was specifically used for decellularization, a noticeable effect on GAG content was not detected.37,38 Based on these results, a decellularization method consisting primarily of TritonX-100 washes and a peracetic acid wash was selected for the present studies. The results suggest that this method produced sufficient decellularization to avoid rejection or foreign body type reactions on implantation.
A wide variety of decellularization processes have been suggested, each with differing effects on resultant tissue structure and composition. These protocols should be tailored to the specific tissue of interest with the maintenance of important components assessed in each case. For example, a human decellularized larynx was recently able to retain its mechanical stability under stress-strain tests.25 The decellularization protocol selected for the present study was based on the results of Remlinger, et al.,24 who demonstrated the protocol to be effective for decellularization of tracheal tissues. Qualitative histologic staining in the present study showed that compared with the native tissue, the preservation of a significant portion of the GAG and elastin constituents was achieved.
In the present study, the progressive reduction in local swelling in the first week postimplantation can be interpreted as an indicator of toleration of the ECM and is clinical evidence that the decellularization process removed a sufficient portion of the cellular constituents to avoid an adverse response. Microscopically, the subcutaneous implants were surrounded by a cellular population that consisted predominantly of macrophages and spindle-shaped cells. While these results may have multiple interpretations—including fibrous encapsulation—these results are consistent with a number of other studies of ECM implants at time points of 1 month or less. Briefly, the persistence of an inflammatory cell population within the site of implantation has been classically associated with downstream granulation tissue formation and encapsulation.39 However, macrophages have also been shown to be an important and essential participant in the constructive remodeling process that occurs after ECM scaffold implantation.34,40,41 ECM-based scaffold materials have been shown to recruit a wide variety of cells, including progenitor cells and local tissue-derived cells.42–45 However, the exact phenotype and origin of the cells observed in the present study are unknown.
A response which was distinct from that observed subcutaneously was found when the same implants were placed into the equine larynx. Excessive swelling was noted surrounding the epiglottic cartilage but not the arytenoids. This response is unsurprising, as the aryepiglottic fold is known to be susceptible to swelling after surgical manipulation of the equine upper airway. This also suggests that the epiglottis and arytenoids, though both are airway tissues, may provide different tissue remodeling environments. Histologically, the implants were observed to degrade and the original implants were no longer distinguishable from newly formed tissues from a gross morphologic perspective. Remodeling of the implanted scaffolds in the epiglottis and arytenoids was characterized by epithelial formation development of an organized submucosal tissue. In the epiglottis, the submucosa was observed to contain glandular structures. In the arytenoids, multifocal stimulation of cells with the formation of new hyaline cartilage within the implant was observed. These results indicate that remodeling in the laryngeal location results in the formation of tissue-specific structures which are indicative of an early constructive remodeling response.
The present pilot study was limited to the observation of one horse over only 4 weeks, and there are a number of points that should be assessed in future studies. First, the present study did not include an assessment of the cellular infiltrate in the equine model due to low animal number. Second, it is not possible in the present study to separate the contributions of the decellularized cartilage and the UBM matrices. Future studies could include decellularization with mucosal surfaces intact, obviating the need for an additional matrix material. Additional studies that investigate the mechanical properties of the remodeled tissues over time are needed to determine the viability of this approach in the long term. Future studies may investigate the consequences of seeding the decellularized constructs with a bone marrow-derived cell population. Such methods have been used by others to promote tissue formation and have resulted in clinical success in tracheal applications.27 Lastly, it may be important to investigate the effects of source tissue age on remodeling outcomes. Older horses may have a more dense or fibrous cartilage and may exhibit localized areas of mineralization. In addition, a recent study demonstrated that source tissues from younger animals produced improved remodeling when compared with those harvested from older animals when used to produce decellularized constructs.46,47
To our knowledge, this is the first report of replacing the epiglottis with ECM in the horse. While the present study was performed in horse and reconstruction of the epiglottis represents a primarily equine application, we believe that the horse may be an ideal translational model for tissue engineering of the larynx due to extensive characterization of the cartilaginous and neuroanatomical structures of the equine upper airway, the ability to assess airway function without sedation, and the presence of naturally occurring inflammation and degeneration.
Acknowledgments
The authors would like to acknowledge Caroline Seitz for assistance in data collection and animal care. This work was funded by an intramural grant from Ross University.
Disclosure Statement
No competing financial interests exist.
References
- 1.Butler P.J., Woakes A.J., Smale K., Roberts C.A., Hillidge C.J., Snow D.H., and Marlin D.J.Respiratory and cardiovascular adjustments during exercise of increasing intensity and during recovery in thoroughbred racehorses. J Exp Biol 179,159, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Tetens J., Derksen F.J., Stick J.A., Lloyd J.W., and Robinson N.E.Efficacy of prosthetic laryngoplasty with and without bilateral ventriculocordectomy as treatments for laryngeal hemiplegia in horses. Am J Vet Res 57,1668, 1996 [PubMed] [Google Scholar]
- 3.Holcombe S.J., and Ducharme N.G.Upper airway function of normal horses during exercise. In: Hinchcliff K.W., Kaneps A.J., and Geor R.J., eds. Equine Sports Medicine and Surgery. Basic and Clinical Sciences of the Equine Athlete. Edinburgh: W.B. Saunders, p. 541, 2004 [Google Scholar]
- 4.Derksen F.J., Robinson N.E., and Holcombe S.J.The upper airway as a conduit for air flow. In: Auer J.A., and Stick J.A., eds. Equine Surgery. Philadelphia: W.B. Saunders, p. 307, 1999 [Google Scholar]
- 5.Ducharme N.G., Hackett R.P., Ainsworth D.M., Erb H.N., and Shannon K.J.Repeatability and normal values for measurement of pharyngeal and tracheal pressures in exercising horses. Am J Vet Res 55,368, 1994 [PubMed] [Google Scholar]
- 6.Ducharme N.G., and Hackett R.P.The value of surgical treatment of laryngeal hemiplegia in horses. Compend Contin Educ Pract Vet 13,472, 1991 [Google Scholar]
- 7.Williams J.W., Pascoe J.R., Meagher D.M., and Hornof W.J.Effects of left recurrent laryngeal neurectomy, prosthetic laryngoplasty, and subtotal arytenoidectomy on upper airway pressure during maximal exertion. Vet Surg 19,136, 1990 [DOI] [PubMed] [Google Scholar]
- 8.Jannson N., Ducharme N.G., Hackett R.P., and Mohammed H.O.An in vitro comparison of cordopexy, cordopexy and laryngoplasty, and laryngoplasty for treatment of equine laryngeal hemiplegia. Vet Surg 29,326, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Dart A.J., Dowling B.A., and Smith C.L.Upper airway dysfunction associated with collapse of the apex of the corniculate process of the left arytenoid cartilage during exercise in15 horses. Vet Surg 34,543, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Holcombe S.J., Rodriguez K., Lane J., and Caron J.P.Cricothyroid muscle function and vocal fold stability in exercising horses. Vet Surg 35,495, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Derksen F.J., Stick J.A., Scott E.A., Robinson N.E., and Slocombe R.F.Effect of laryngeal hemiplegia and laryngoplasty on airway flow mechanics in exercising horses. Am J Vet Res 47,16, 1986 [PubMed] [Google Scholar]
- 12.Barakzai S.Z., and Hawkes C.S.Dorsal displacement of the soft palate and palatal instability. Review article. Equine Vet Educ 22,253, 2010 [Google Scholar]
- 13.Lane J.G., Blandon B., Little D.R., Naylor J.R., and Franklin S.H.Dynamic obstructions of the equine upper respiratory tract. Part I: Observations during high-speed treadmill endoscopy of 600 Thoroughbred racehorses. Equine Vet J 38,393, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Pollock P.J., Reardon R.J., Parkin T.D., Johnson M.S., Tate J., and Love S.Dynamic respiratory endoscopy in 67 Thoroughbred racehorses training under normal ridden exercise conditions. Equine Vet J 41,137, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Davenport C.L., and Parente E.J.Disorders of the larynx. Vet Clin North Am Equine Pract 19,169, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Greet T.R., and Dixon P.M.“Flapping like a sail in the breeze”: the history of equine soft palate displacement and its treatment. Equine Vet J 37,386, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Tulleners E., Mann P., and Raker C.W.Epiglottic augmentation in the horse. Vet Surg 19,181, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Tulleners E., Stick J.A., Leitch M., Trumble T.N., and Wilkerson J.P.Epiglottic augmentation for the treatment of dorsal displacement of the soft palate in racehorses: 59 cases (1985–1994). JAVMA 211,1022, 1997 [PubMed] [Google Scholar]
- 19.Bonassar L.J., and Vacanti C.A.Tissue engineering: the first decade and beyond. J Cell Biochem Suppl 30/31,297, 1998 [PubMed] [Google Scholar]
- 20.Gilbert T.W., Sellaro T.L., and Badylak S.F.Decellularization of tissues and organs. Biomaterials 27,3675, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Badylak S.F., Brown B.N., Gilbert T.W., Daly K.A., Huber A., and Turner N.J.Biologic scaffolds for constructive tissue remodeling. Biomaterials 32,316, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Badylak S.F., Freytes D.O., and Gilbert T.W.Extracellular matrix as a biological scaffold material: structure and function. Rev Acta Biomater 5,1, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Spievack A., Ringel R.L., Simmons-Byrd A., and Badylak S.F.Extracellular matrix as a scaffold for laryngeal reconstruction. Ann Otol Rhinol Laryngol 112,428, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Remlinger N.T., Czajka C.A., Juhas M.E., Vorp D.A., Stolz D.B., Badylak S.F., Gilbert S., and Gilbert T.W.Hydrated exenogenic decellularized matrix as a scaffold for tracheal reconstruction. Biomaterials 31,3520, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Baiguera S., Gonfiotti A., Jaus M., Comin C.E., Paglierani M., Del Gaudio C., Bianco A., Ribatti D., and Macchiarini P.Development of bioengineered human larynx. Biomaterials 32,4433, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Ringel R.L., Kahane J.C., Hillsamer P.J., Lee A.S., and Badylak S.F.The application of tissue engineering procedures to repair the larynx. J Speech Lang Hear Res 49,194, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Macchiarini P., Jungbluth P., Go T., Asnaghi A., Rees L.e., Cogan T.A., Dodson A., Martorell J., Bellini S., Parnigotto P.P., Dickinson S.C., Hollander A.P., Mantero S., Conconi M.T., and Birchall M.A.Clinical transplantation of a tissue-engineered airway. Lancet 372,2023, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Go T., Jungbluth P., Baiguero S., Asnaghi A., Martorell J., Ostertag H., Mantero S., Birchall M., Bader A., and Macchiararini P.Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorarac Cardiovasc Surg 139,437, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Gilbert T.W., Freund J., and Badylak S.F.Quatification of DNA in biologic scaffold materials. J Surg Res 152,135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown B.N., Lindberg K., Reing J., Stolz D.B., and Badylak S.F.The basement membrane component of biologic scaffolds derived from extracellular matrix. Tissue Eng 12,519, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Brennan E.P., Reing J., Chew D., Myers-Irvin J.M., Young E.J., and Badylak S.F.Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng 12,2949, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmers H.J., Cheetham J., Yeager A.E., and Ducharme N.G.Ultrasonography of the equine larynx. Vet Radiol Ultrasound 47,476, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Crapo P.M., Gilbert T.W., and Badylak S.F.An overview if tissue and whole organ decellularization processes. Rev Biomater 32,3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown B.N., Valentin J.E., Stwart-Akers A.M., McCabe G.P., and Badylak S.F.Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30,1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovekamp J.J., Simionescua D.T., Mercuria J.J., Zubiateb B., Sacks M.S., and Vyavaharea N.R.Stability and function of glycosaminoglycans in porcine bioprosthetic heart valves. Biomaterials 27,1507, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Partington L., Mordan N.J., Mason C., Knowles J.C., Kim H-W., Lowdell M.W., Birchall M.A., and Wall I.B.Biochemical changes caused by decellularization may compromise mechanical integrity of tracheal scaffolds. Acta Biomater 9,5251, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Reing J.E., Brown B.N., Daly K.A., Frend J.M., Gilbert T.W., Hsiong S.X., Huber A., Kullas K.E., Tottey S., Wolf M.T., and Badylak S.F.The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31,8626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown B.N., Freund J.M., Han L., Rubin P.J., Reing J.E., Jeffries E.M., Wolf M.T., Tottey S., Barnes C.A., Ratner B.D., and Badylak S.F.Comparison of three methods for the derivation of a biologic scaffold composed of adipose tissue extracellular matrix. Tissue Eng 17,411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson J.M., Rodriguez A., and Chang D.T.Foreign body reaction to biomaterials. Semin Immunol 20,86, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F.Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8,978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentin J.E., Stewart-Akers A.M., Gilbert T.W., and Badylak S.F.Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng 15,1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agrawal V., et al. Recruitment of progenitor cells by an extracellular matrix cryptic peptide in a mouse model of digit amputation. Tissue Eng Part A 17,2435, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan E.P., et al. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med 2,491, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vorotnikova E., et al. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 29,690, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Beattie A.J., et al. Chemoattraction of progenitor cells by remodeling extracellular matrix scaffolds. Tissue Eng Part A 15,1119, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sicari B.M., et al. The effect of source animal age upon the in vivo remodeling characteristics of an extracellular matrix scaffold. Biomaterials 33,5524, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tottey S., et al. The effect of source animal age upon extracellular matrix scaffold properties. Biomaterials 32,128, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]