Abstract
Vitis thunbergii Sieb. et Zucc. var. taiwaniana Lu (VT) is an indigenous plant in Taiwan that is traditionally used for promoting joint health. In this study, we used in vitro primary human chondrocytes (PHCs) and two in vivo animal models to evaluate the anti-inflammatory effects of VT on arthritis. Results showed that the water extract of the stems and roots from VT (VT-SR) was rich in flavones and phenols with 1.1 mg/g of resveratrol, 6.7 mg/g of hopeaphenol, and 5.1 mg/g of (+)-ɛ-viniferin. VT-SR significantly scavenged DPPH radicals and inhibited prostaglandin E2 (PGE2) production in lipopolysaccharide (LPS)-induced PHCs without exhibiting significant cytotoxicity. In in vivo models, the VT-SR (500 mg/kg) significantly decreased serum PGE2 and knee 2-18F-fluoro-2-deoxy-D-glucose (18F-FDG) levels in LPS-induced acute inflammatory arthritis in rabbits. In addition, dietary supplementation with VT-SR for 28 days significantly alleviated type II collagenase-induced rat osteoarthritis with improvements in weight bearing and range of motion tests. In conclusion, our results suggest that the VT-SR is a good candidate for developing dietary supplements to prevent joint deterioration and inhibit inflammation.
Key Words: : dietary supplement, 18F-FDG uptake, inflammatory arthritis, type II collagenase, Vitis thunbergiivar.taiwaniana
Introduction
The Vitis genus (grape), which contains about 60 species, is a common food with numerous phytonutrients and is well documented for its health-promoting functions throughout the world.1 People usually use the seeds, leaves, and skins of these species as dietary supplements. A study by Huang et al. indicated that the root extract of Vitis amurensis displayed significant anti-inflammatory effects against leukotriene B4 biosynthesis.2 Vitis vinifera (grapevine) exhibited a broad spectrum of health-promoting effects, such as cardioprotective, neuroprotective, anti-inflammatory, and antioxidative activities.3 Phytochemicals in grapes are strongly associated with the prevention of oxidative stress-related diseases. Grapes are rich in flavones and phenols, such as quercetin, resveratrol, myricetin, gallic acid, (+)-catechin, (−)-epicatechin, and (−)-epicatechin-3-O-gallate. Evidence has shown that low concentrations of (+)-catechin and gallic acid exert cellular protective effects against oxidative DNA damage.3 Quercetin and resveratrol significantly alleviated inflammatory responses and inflammation-related arthritis.4,5 Vitis thunbergii Sieb. et Zucc. var. taiwaniana Lu (VT) is native to Taiwan and is widely used to prepare foods. Ethnopharmacological uses of VT include treating stomachache, jaundice, hepatitis, and arthritis.6 In Peng et al.'s study, VT displayed antimicrobial activity against gram-positive bacteria.7 Systolic and diastolic blood pressures of spontaneously hypertensive rats were obviously reduced after administration of VT.8 In addition, VT significantly decreased the inducible nitric oxide (iNO) synthase protein expression and NO production in vitro and delayed hippocampus neuronal cell loss in vivo.9
Inflammation-associated arthritis is a common age-related disease. Characteristics of this typical arthritis are inflammatory cell recruitment, synovial joint inflammation, and articular cartilage degeneration.10 In the United States, inflammation-related arthritis is estimated to affect about 27 million adults.11 The pathogenesis of inflammatory arthritis involves a combination of biochemical mediators, mechanical stress, matrix metalloproteinases, and reactive oxygen species (ROS). ROS play key roles in the formation of inflammatory arthritis, by directly causing chondrocyte depletion, extracellular matrix (ECM) breakdown, and cartilage degradation.12 The present therapeutic target for treating arthritis is still pain and symptom relief, but results remain unsatisfactory. For complex inflammatory arthritis, about 30% of patients search for alternative modalities to relieve arthritis symptoms. For example, the anti-arthritic effects of dietary supplements containing ginger (Zingiber officinale) and turmeric (Curcuma longa) extracts are well documented. Inflammation-related arthritis syndromes can be relieved through the inhibition of cyclooxygenase (COX)-2 and blocking of prostaglandin E2 (PGE2) production. All of this evidence suggests that certain plant extracts are suitable for developing dietary supplements for treating arthritis.
Our previous study showed that resveratrol significantly alleviated lipopolysaccharide (LPS)-induced inflammatory arthritis in vitro and in vivo. In addition, resveratrol in VT had synergistic PGE2-inhibitory effects with other oligostilbenes, such as (+)-ɛ-viniferin, in LPS-induced primary human chondrocytes.4 Hence, the goal of this study was to determine the anti-inflammatory knee damage-associated anti-arthritic effects of VT. Different fractions of the VT water extract were screened for antioxidative and anti-inflammatory effects in vitro. In an in vivo assay, the anti-inflammatory properties of VT extract were evaluated by a 2-18F-fluoro-2-deoxy-D-glucose (18F-FDG) micro–positron emission tomographic (PET) imaging system, an incapacitance test, and a 24-hour video tracking system.
Materials and Methods
Chemicals and reagents
Various chemicals, including dimethyl sulfoxide (DMSO), LPS, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), gallic acid, catechin, and 1,1-diphenyl-2-picrylhydrazyl (DPPH), were purchased from Sigma (St. Louis, MO, USA). For cell culture, Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), antibiotics, glutamine, and other cell culture materials were purchased from Gibco (Grand Island, NY, USA). Acetonitrile and trifluoroacetic acid (TFA) used for the high-performance liquid chromatographic (HPLC) analysis were of chromatographic grade and were purchased from J.T. Baker (Phillipsburg, NJ, USA).
Primary human chondrocytes (PHCs)
PHCs were obtained from Dr. Ming-Shium Hsieh's laboratory.4 PHCs were incubated at 37°C in a humidified atmosphere of 5% CO2 using DMEM and confirmed by a Safranin O solution (data not shown). Experiments were performed with cells of about the third passage.
Preparation of VT extracts
Vitis thunbergii Sieb. et Zucc. var. taiwaniana Lu (VT) was purchased from an herb store in Hualien and identified by Chi-Luan Wen (Taiwan Seed Improvement and Propagation Station, Taichung, Taiwan). A voucher specimen (VT-005) was deposited in the Department of Microbiology, Immunology, and Biopharmaceuticals, College of Life Sciences, National Chiayi University. In this study, leaves and branches (VT-LB) and stems and roots (VT-SR) from VT were used to prepare water extracts. Samples were immersed in deionized water and boiled for at least 30 minutes until half of the original amount was left. Aqueous solutions were then filtered, and vacuum freeze-dried. The above preparative procedures were stipulated by the Department of Chinese Medicine and Pharmacy, Ministry of Health and Welfare, Taipei, Taiwan. Respective yield rates of VT-LB and VT-SR were 39.38% and 39.20%.
Determination of total phenols and total flavones
Contents of total phenols and total flavones in the VT-LB and VT-SR were determined by modification of methods in a previous study.13 First, each extract was reacted with Folin-Ciocalteu reagent and then a 7.5% aqueous Na2CO3 solution at 50°C for 5 min, and the absorbance at 600 nm was measured with a μQuant spectrophotometer (BioTek, Winooski, VT, USA). The gallic acid equivalent (μg gallic acid/mg extract) was used to calculate the total phenol content. Second, the total flavone content was measured using the vanillin assay. Each extract was reacted with vanillin reagent (vanillin in 80% H2SO4) for 15 minutes at room temperature, and the absorbance at 530 nm was determined. The catechin equivalent (μg catechin/mg extract) was used to calculate the total flavone content.
Extraction and isolation
The dried root of VT (2.2 kg) was pulverized and refluxed with 10 L methanol for 2 h (twice). After filtering, the filtrates were combined and concentrated with a rotary evaporator below 42°C to obtain the methanol extract (233.6 g). Fifty grams of root methanol extract was chromatographed on silica gel column (10 cm i.d.×20 cm) and then eluted with chloroform-methanol (10:1→10:2→10:3→10:4→10:5, 10 L for each composition). The chloroform-methanol (10:3) fraction (7.95 g) was re-chromatographed through a Sephadex LH-20 column (2.5 cm i.d.×52 cm), followed by elution with methanol and then purified with a preparative reversed-phase HPLC column (Tosoh ODS-80Ts, i.d. 21.5 mm i.d.×300 mm, 5 μm) eluted with 31% methanol at a flow rate of 10.0 mL/min to obtain hopeaphenol (102 mg).
The fresh stem of VT (1.2 kg) was sliced and refluxed with 6 L of methanol for 2 h (twice). After filtering, the filtrates were combined and concentrated with a rotary evaporator below 42°C to obtain methanol extract (65.6 g). The methanol extract of the stem (16.85 g) was dissolved in methanol and chromatographed on a Sephadex LH-20 column (2.5 cm i.d.×51 cm) eluted with methanol to yielded fraction A-X (100 mL/fraction). Fraction F (1.164 g) and fraction G (835.5 mg) were further purified with LiChroprep RP-18 column (2.5 cm i.d.×57 cm) eluted with 0.05% trifluoroacetic acid-acetonitrile (70:30) to give resveratrol (11 mg) and (+)-ɛ-viniferin (352 mg) respectively.
The purity of resveratrol, hopeaphenol, and (+)-ɛ-viniferin were calculated (98.1%, 96.4%, and 95.9% respectively) by relative area percentage from an HPLC chromatogram at 280 nm.
Substance marker analysis of the VT-SR
Resveratrol, hopeaphenol, and (+)-ɛ-viniferin were used to evaluate the quality of the VT-SR. The HPLC equipment included an SCL-10Avp System Controller, an SPD-M10A Diode Array Detector, an LC-10ATvp Liquid Chromatograph Pump, an SIL-10Avp Auto Injector, FCV-10Avp Flow-Channel Selection Valves (Shimadzu, Tokyo, Japan), and an ERC-3415 Degasser (ERC, Altegolfsheim, Regensburg, Germany). A LiChrospher 100 RP-18e column (4 mm i.d.×250 mm, 5 μm), and an acetonitrile-water system was used as the mobile phase in the gradient mode as follows: acetonitrile: 0–50 min, 5–55%; 50–60 min, 55%; 60–70 min, 5%. The flow rate was 1 mL/min, and the column temperature was maintained at 40°C. We used a UV wavelength of 280 nm to detect amounts of resveratrol, hopeaphenol, and (+)-ɛ-viniferin.
Preparation of calibration curve
Resveratrol, hopeaphenol, and (+)-ɛ-viniferin were accurately weighed, dissolved, and serially diluted with methanol to give a concentration in the range of 15.6–500 μg/mL. Calibration curves were plotted after linear regression of the peak areas.
Antioxidative assay
The DPPH-scavenging assay protocol followed that of a previous study.14 The VT extract was dissolved in distilled water and serially diluted into different concentrations. Samples were individually mixed with a DPPH ethanolic solution (1 mM) at room temperature for 15 minutes. α-tocopherol was used as a positive control. The absorbance was measured at 530 nm with an enzyme-linked immunosorbent assay (ELISA) spectrophotometer.
Anti-inflammatory effects of the VT-LB and VT-SR
PHCs were seeded on a 96-well plate at 105 cells/mL for one day. We co-treated the VT-LB and VT-SR with LPS (1 μg/mL) for 18 h.15 After 18 h of incubation, PHC viability was tested by an MTT assay, and the culture supernatant was collected for PGE2 detection with a commercial assay kit (Enzo Life Sciences, Farmingdale, NY, USA).
Animals
Male Wistar rats weighing about 250–300 g and New Zealand white rabbits weighing about 1.5–2.5 kg were used in the in vivo model. They were maintained at 21±2°C with food and water ad libitum, and kept on a regular light–dark cycle. Animals used in this experiment were cared for according to the Ethical Regulations on Animal Research of Taipei Medical University (approval no.: LAC-97-0005).
LPS induction of acute inflammatory arthritis in rabbits
LPS was used as an inducer to simulate acute inflammatory arthritis in rabbits. LPS (10 ng in 100 μL of phosphate-buffered saline [PBS]) was injected into the rabbits' right knee joint on day 0. The test group received oral administration of the VT-SR (500 mg/kg) on days 0, 2, 4, and 6, while the control group received the vehicle. Serum was collected from an ear vein on days 0, 2, 4, and 6. 18F-FDG microPET was performed on day 1 (16 h after the LPS injection) and day 7. The experimental procedure is summarized in Figure 1A. The anti-arthritic effect of the VT-SR was assessed with 18F-FDG microPET and simultaneous assessment of serum PGE2 concentrations. The assay protocol and data calculation of standard uptake value (SUV), the most popular quantitation parameter of PET, were modified from those of our previous study.4 We used a Concorde microPET R4 scanner (Concorde Microsystems, Knoxville, TN, USA) for the 18F-FDG-PET analysis. Rabbits received an average of 38.0 MBq of FDG by an intravenous bolus injection into one of the ear veins. A 1200-second emission PET scan was done two hours after FDG administration. We reconstructed the PET images through Fourier rebinning and ordered-subset expectation maximization (OSEM) using default corrections for radioactive decay, dead time, and attenuation provided by the vendor. To evaluate the arthritis level, the SUV was determined. ASIPro VM™ analysis software was used to draw the regions of interest (ROIs) and to determine the SUVs.
FIG. 1.
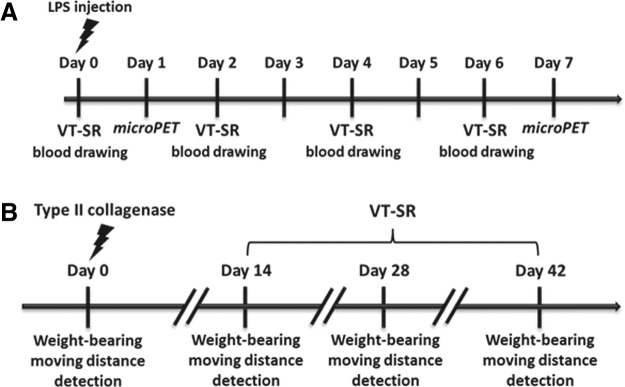
Experimental procedure of lipopolysaccharide (LPS)-induced acute inflammatory arthritis in rabbits (A) and type II collagenase-induced chronic inflammatory arthritis in rats (B).
Type II collagenase induction of osteoarthritis in rats
Type II collagenase was used as an inducer of osteoarthritis (OA) because it causes knee cartilage to degenerate. In this study, we used two different animal models—an incapacitance test and a 24-hour video tracking system—to measure the anti-arthritic effects of VT extracts against type II collagenase-induced OA.16,17 Type II collagenase (4 mg/kg) was directly injected into the right knee of Wistar rats under anesthesia with Zoletil and xylazine. On day 0, baseline levels of rat weight-bearing tolerance and distances moved were respectively detected by an incapacitance meter and a 24-hour video tracking system. An incapacitance tester with a dual-channel weight averager (Linton Instrumentation, Norfolk, United Kingdom) was used to evaluate changes in the rats' weight-bearing tolerance. Rats were carefully placed into the measuring chamber. The weight-bearing force exerted by the hind limb was averaged over a three-second period. The weight distribution ratio was calculated as the right hind limb weight/left hind limb weight. Each data point was the mean of three duplicate readings. The ratio of hind paw weight distributions between the right and left limbs was used to assess the progress of OA.18 Moreover, because the pain level was positively correlated with the arthritis level, we used a 24-hour video tracking system to determine the pain levels of the experimental animals.13,19 Moving distances of different experimental animals were monitored with a Multi-Cage Locomotion Monitor by TrackMot 2 Analysis V5.45 (Diagnostic & Research Instruments, TaoYuan, Taiwan). After 14 days of induction with type II collagenase, the VT-SR (500 mg/kg) and the positive control, glucosamine sulfate (150 mg/kg), were orally administrated for another 28 days, while the rat weight-bearing and moving distance results were measured on days 14, 28, and 42 (Fig. 1B).
Statistical analysis
Data are presented as the mean and standard deviation (SD). Data were analyzed by Student's t-test and one-way analysis of variance (ANOVA) using SPSS version 12 software (SPSS, Inc., Chicago, IL, USA).
Results
Phytochemical contents of the VT-LB and VT-SR
The total phenol and total flavone contents of VT-LB and VT-SR were respectively determined as gallic acid equivalents and catechin equivalents. As summarized in Table 1, the total phenol and total flavone contents in the VT-SR were higher than those in the VT-LB. The total phenol and total flavone contents in the VT-SR were 67.5±1.4 μg gallic acid/mg and 25.4±0.7 μg catechin/mg respectively. In addition, we used three different components of VT, resveratrol, hopeaphenol, and (+)-ɛ-viniferin to assess the quality of the VT-SR. An HPLC profile of the VT-SR is shown in Figure 2. The calibration equations and correlation coefficients of resveratrol, hopeaphenol, and (+)-ɛ-viniferin revealed linear relationships between the peak areas and concentrations (Table 2). The respective retention times and contents of resveratrol, hopeaphenol, and (+)-ɛ-viniferin in VT-SR were 30.0 min (1.1 mg/g), 35.1 min (6.7 mg/g), and 40.9 min (5.1 mg/g).
Table 1.
Total Phenols and Flavones, and the Antioxidative Effects of Vitis thunbergii var. taiwaniana Extracts
| Sample | Total phenolsa(μg gallic acid/mg) | Total flavonesb(μg catechin/mg) | DPPH-scavengingIC50 value (μg/mL) |
|---|---|---|---|
| VT-LB | 38.9±0.3 | 13.8±0.1 | 170.7±14.9 |
| VT-SR | 67.5±1.4 | 25.4±0.7 | 88.3±17.1 |
| α-Tocopherol | — | — | 64.9±1.2 |
Gallic acid equivalents (μg gallic acid/mg extract) were used to calculate the total phenol content.
Catechin equivalents (μg catechin/mg extract) were used to calculate the total flavone content.
VT, Vitis thunbergii var. taiwaniana; LB, leaves and branches; SR, stems and roots; IC50, 50% inhibitory concentration.
FIG. 2.

High-performance liquid chromatography (HPLC) fingerprints of resveratrol, hopeaphenol, and (+)-ɛ-viniferin in the water extract of stems and roots of Vitis thunbergii var. taiwaniana (VT-SR). The respective retention times of resveratrol, hopeaphenol, and (+)-ɛ-viniferin were 30.0, 35.1, and 40.9 minutes.
Table 2.
Calibration Equations of Resveratrol, Hopeaphenol, and (+)-ɛ-Viniferin
| Compound | Linear equation | R2 | Conc. range (μg/mL) |
|---|---|---|---|
| Resveratrol | Y=39648X – 28476 | 0.9999 | 15.6–500 |
| Hopeaphenol | Y=7857X – 3747 | 0.9999 | 15.6–500 |
| (+)-ɛ-Viniferin | Y=15429X – 24840 | 0.9996 | 15.6–500 |
Antioxidative effects of the VT-LB and VT-SR
ROS, including superoxide anions (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH), play crucial roles in disease formation. As shown in Table 1, the VT-SR displayed stronger antioxidative effects than did the VT-LB with a 50% inhibitory concentration (IC50) value of 88.3±17.1 μg/mL.
PGE2 inhibitory effects of the VT-SR in the LPS-induced PHC model
As mentioned above, VT-SR displayed stronger antioxidative effects than VT-LB (Table 1). In addition, both the VT-LB and VT-SR were used to evaluate the cytotoxicity and PGE2-inhibitory effects in an LPS-induced PHC model. As shown in Table 3, the VT-SR showed stronger PGE2-inhibitory effects than the VT-LB without significant cytotoxicity at 100 μg/mL. Thus, the VT-SR was used in the following in vivo anti-inflammatory arthritis assays.
Table 3.
Inhibition of Prostaglandin E2 Production by Vitis thunbergii var. taiwaniana Extracts in Lipopolysaccharide-Induced Primary Human Chondrocytes
| PHCs | ||
|---|---|---|
| Parts | Cell viabilitya(%) | PGE2 inhibition (%) |
| VT-LB | 88.74±1.53 | 32.50±6.32 |
| VT-SR | 94.33±0.73 | 66.76±1.82 |
| Indomethacinb | 95.15±0.14 | 93.74±0.61 |
Concentrations of test samples were 100 μg/mL.
Indomethacin was used as a positive control at a concentration of 10 μM.
PHC, primary human chondrocyte; PGE2, prostaglandin E2.
Inhibitory effects of the VT-SR on acute LPS-induced inflammatory arthritis in rabbits
In the microPET analysis, brighter areas on coronal and transverse views of the knee joints suggested a worse arthritis status. Figure 3A shows that the sham group showed a weak microPET signal, indicating a noninflammatory status. In the control group, brighter areas were found in the LPS-injected knee joint in transverse and coronal views. Administration of the VT-SR (500 mg/kg) led to weaker microPET signals than in the control group. In the quantitative analysis, the SUV of the control group on day 7 was significantly higher than that on day 1. After administration of the VT-SR, the SUV on day 7 was obviously lower than that of the control group, indicating significant anti-arthritic effects (Table 4). Moreover, rabbit serum was collected on days 0, 2, 4, and 6 to measure PGE2 levels. Figure 3B shows that the highest serum PGE2 levels were found in the control group on days 2, 4, and 6. The VT-SR significantly reduced LPS-induced serum PGE2 levels, suggesting the potential in vivo anti-inflammatory arthritic effects.
FIG. 3.
Typical positron emission tomography (PET) image volume of a pair of rabbit's knees on day 7 (A) and inhibitory effects of the water extract of stems and roots of Vitis thunbergii var. taiwaniana (VT-SR) on serum prostaglandin E2 (PGE2) of rabbits with lipopolysaccharide (LPS)-induced osteoarthritis (B). Note that the anterior (A, head)–posterior (P, tail) and right (R)–left (L) labels are reversed with respect to the actual imaging orientations. Sham, no LPS induction; control, injection of LPS in the right knee; VT-SR, oral administration of VT-SR (500 mg/kg) before the LPS injection. *P<.05; **P<.005, compared to the control group, n=3. Color images available online at www.liebertpub.com/jmf
Table 4.
Quantitative Analysis of the microPET Results
| Variation of the maximal SUV | ||
|---|---|---|
| Group | Day 1 | Day 7 |
| Control | 0.69±0.12 | 1.49±0.50a |
| VT-SR | 0.42±0.12 | 0.55±0.07b |
Day 7 of control compared to day 1, P<.005.
Day 7 of the VT-SR compared to day 7 of the control, P<.05.
microPET, micro–positron emission tomography; SUV, standard uptake value; VT-SR, water extract of the stems and roots of Vitis thunbergii var. taiwaniana.
Inhibitory effects of the VT-SR on type II collagenase-induced rat OA
Type II collagenase was directly injected into the rat knee to induce rat OA, and an incapacitance test and 24-hour video tracking systems were used to observe the pain condition and motion range of the rats. As shown in Figure 4A, the weight bearing significantly changed from days 14 to 42, suggesting that OA was obviously induced by the type II collagenase injection. The VT-SR (500 mg/kg) eased OA from day 28 and displayed obvious changes in rat weight bearing on day 42. Our positive control—glucosamine sulfate—significantly eased OA from days 28 to 42. As to the arthritis-induced motion loss level, type II collagenase significantly reduced the rats' moving distance from day 14 (Fig. 4B). After administration of the VT-SR (500 mg/kg) from day 14, the moving distance of rats treated with VT-SR was significantly higher than that of the control group on days 28 and 42, suggesting an obvious reduction in arthritis-induced pain. There was no significant loss of body weight in any rats during the 28 days of administration (data not shown). Glucosamine sulfate also played an effective role in reducing the moving distance. Based on the just-described positive data, dietary supplementation with the VT-SR for 28 days significantly alleviated knee pain and increased the range of motion without producing significant toxicity.
FIG. 4.
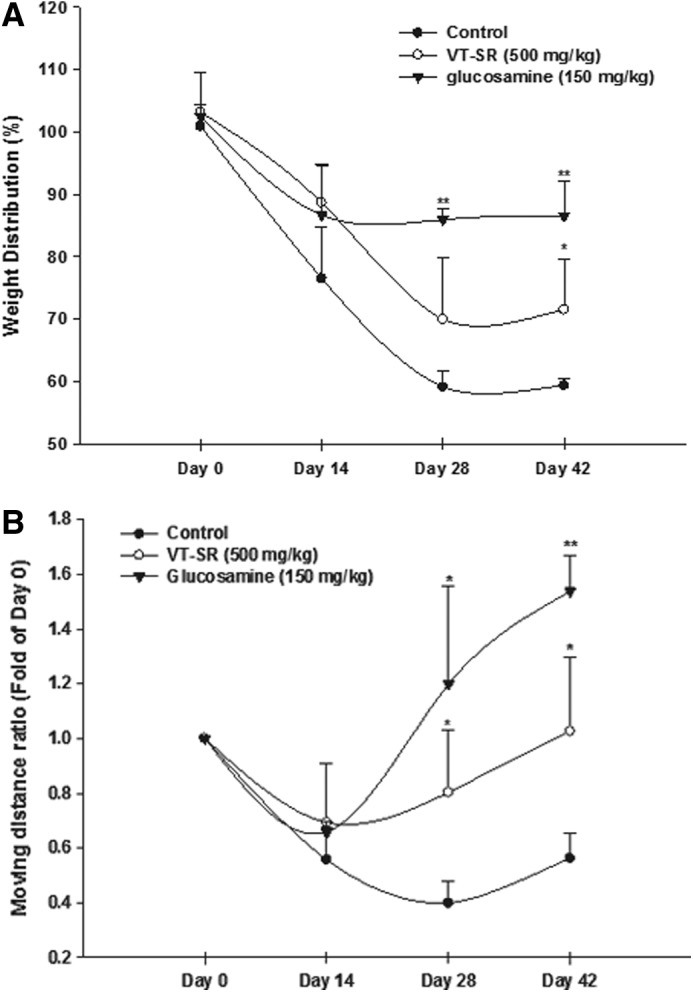
Influence of the water extract of stems and roots of Vitis thunbergii var. taiwaniana (VT-SR) on type II collagenase-reduced rat weight-bearing (A) and moving distance ratio (B). Control, injection of type II collagenase in the right knee; VT-SR, oral administration of VT-SR (500 mg/kg); glucosamine, oral administration of glucosamine sulfate (150 mg/kg) before LPS induction. The weight distribution ratio was calculated as the right hind limb weight divided by the left hind limb weight. *P<.05; **P<0.005, compared to the control group, n=3.
Discussion
VT is a plant indigenous to Taiwan with well-documented health-promoting effects. Polyphenols are abundant micronutrients in plant foods, and their contributions to human health are well documented.20,21 A review by Marjan et al. demonstrated that grapes are rich in polyphenols, such as gallic acid, catechin, epicatechin, quercetin, and resveratrol.3 Our previous study showed that resveratrol significantly alleviated LPS-induced acute inflammatory arthritis in rats and had synergistic effects with other oligostilbenes, such as vitisin and (+)-ɛ-viniferin. Resveratrol is a well-known grape polyphenol with antioxidative and cardiovascular-promoting effects.22,23 Evidence shows that resveratrol possesses various anti-inflammatory activities, such as decreasing neuroinflammation, chronic inflammation, and cytokine-mediated inflammations.24,25 In addition, hopeaphenol and (+)-ɛ-viniferin, resveratrol derivatives in VT, were also reported to have anti-inflammatory effects.2,26 Studies have demonstrated that two components are neuroprotective and improve vascular function.27,28 These studies indicated that VT has potential for use in anti-arthritic supplements. The goal of this study was to determine the anti-inflammatory effects against arthritis of the VT extract in vitro and in vivo.
In the pathogenesis of arthritis, increasing ROS are well correlated with disease formation. ROS play crucial roles in inhibiting cartilage matrix synthesis, inducing chondrocyte apoptosis, and breaking down the cartilage matrix.29 The VT-SR, with abundant polyphenols, displayed significant antioxidative effects. Comparing the phytochemical contents and antioxidative effects, the VT-SR had significant free radical-scavenging activities due to its abundant total phenol and total flavone contents. A study by Sharma et al. demonstrated that inhibition of ROS and inflammatory mediators can be used to treat inflammatory arthritis.30 The in vitro anti-inflammatory effects were assessed. We used PHCs, instead of the commonly used human chondrosarcoma cell line, SW1353, to evaluate PGE2-inhibitory effects against LPS-induced chondrocyte inflammation. PHCs, originally cultured from patients' cartilage in an orthopedist's laboratory, can reflect true human physical conditions.
In this study, the dry weights of the leaf, branch, stem, and root of VT were small amounts. To increase the extraction yield rate and avoid the limited content of each VT extract, leaves and branches (VT-LB) and stems and roots (VT-SR) from VT were used to prepare water extracts. The PGE2-inhibitory effect of the VT-SR was stronger than that of the VT-SB in an LPS-induced PHC model (Table 3). Therefore, the VT-SR was used to evaluate the anti-inflammatory arthritic effects.
In evaluating anti-arthritis agents, hind-paw thickness, histological observations of joints, and an arthritis index are commonly used methods.31,32 However, these models do not directly reflect the continuous pathological process and require many experimental animals. In this study, we used an 18F-FDG microPET imaging system to evaluate acute inflammatory arthritis and an incapacitance test and 24-hour video tracking systems to observe chronic inflammatory arthritis. This is the first study to evaluate the anti-arthritic effects by combining three continuous pathological models. First, the microPET imaging system was helpful in measuring acute inflammatory arthritis in our previous study.4 SUV values calculated by the ASIPro VM™ analysis software were used to quantify the parameter in the microPET image. As shown in Figure 3, the VT-SR obviously decreased the LPS-induced inflammatory arthritic status in the microPET analysis and lowered the serum PGE2 concentration. This study provides a good platform to evaluate the anti-arthritic effects of dietary supplements or botanical drugs. After screening the in vitro anti-inflammatory effects of the candidate drugs, three continuous pathological models including microPET imaging, incapacitance test, and 24-hour video tracking systems can be useful to assess the in vivo and real-time anti-arthritic functions.
Dietary supplements are well documented for treating OA. Dietary supplements include herbal medicines, natural medicines, and alternative medicines and have nearly $20 billion in U.S. sales annually. Due to the increasingly aging population, there are approximately 27 million adults with OA in the United States alone.11 About 30% of OA patients have used dietary supplements to alleviate their conditions in the United States.33 In this study, we used an intra-articular injection of type II collagenase to induce experimental OA in rats. Degradation of the α1-chain in type II collagen plays an important role in cartilage destruction and OA.34 After that, destruction of the extracellular matrix with subchondral bone exposure, subchondral bone sclerosis, and osteophyte formation are seen.35 Clinical symptoms of OA include pain, inflammation, and a limited range of motion.36 Administration of the VT-SR at 500 mg/kg for 28 days significantly ameliorated type II collagenase-induced inflammation and pain levels in the rat knee (Fig. 4). However, the real mechanism of VT-SR against LPS- or type II collagenase-induced OA will be the subject of further investigation.
In conclusion, the VT-SR, which contains abundant resveratrol derivatives, displayed significant anti-inflammatory arthritic effects and has potential as a therapeutic dietary supplement for treating inflammatory arthritis.
Acknowledgments
The authors would like to thank the New Taipei City Hospital (grant no. TPCH99-a05) for financially supporting this study. The microPET technology was supported by the Molecular and Genetic Imaging Core/National Research Program for Genomic Medicine at National Yang-Ming University.
Author Disclosure Statement
No competing financial interests.
References
- 1.Myles S, Chia JM, Hurwitz B, et al. : Rapid genomic characterization of the genus Vitis. PLoS One 2011;5:e8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang KS, Lin M, Cheng GF: Anti-inflammatory tetramers of resveratrol from the roots of Vitis amurensis and the conformations of the seven-membered ring in some oligostilbenes. Phytochemistry 2001;58:357–362 [DOI] [PubMed] [Google Scholar]
- 3.Nassiri-Asl M, Hosseinzadeh H: Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother Res 2009;23:1197–1204 [DOI] [PubMed] [Google Scholar]
- 4.Wang KT, Chen LG, Tseng SH, Huang JS, Hsieh MS, Wang CC: Anti-inflammatory effects of resveratrol and oligostilbenes from Vitis thunbergii var. taiwaniana against lipopolysaccharide-induced arthritis. J Agric Food Chem 2011;59:3649–3656 [DOI] [PubMed] [Google Scholar]
- 5.Yang ZG, Jia LN, Shen Y, Ohmura A, Kitanaka S: Inhibitory effects of constituents from Euphorbia lunulata on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells. Molecules 2011;16:8305–8318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen LG, Wang CC: Preparative separation of oligostilbenes from Vitis thunbergii var. taiwaniana by centrifugal partition chromatography followed by Sephadex LH-20 column chromatography. Sep Purif Technol 2009;66:65–70 [Google Scholar]
- 7.Peng SC, Cheng CY, Sheu F, Su CH: The antimicrobial activity of heyneanol A extracted from the root of Taiwanese wild grape. J Appl Microbiol 2008;105:485–491 [DOI] [PubMed] [Google Scholar]
- 8.Huang CY, Wen CL, Lu YL, Lin YS, Chen LG, Hou WC: Antihypertensive activities of extracts from tissue cultures of Vitis thunbergii var. taiwaniana. Bot Stud 2010;51:317–324 [Google Scholar]
- 9.Wang CK, Chen LG, Wen CL, et al. : Neuroprotective activity of Vitis thunbergii var. taiwaniana extracts in vitro and in vivo. J Med Food 2010;13:170–178 [DOI] [PubMed] [Google Scholar]
- 10.Shahab U, Ahmad S, Moinuddin , et al. : Hydroxyl radical modification of collagen type II increases its arthritogenicity and immunogenicity. PLoS One 2012;7:e31199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouladbakhsh J: Complementary and alternative modalities to relieve osteoarthritis symptoms. Am J Nurs 2012;112:S44–S51 [DOI] [PubMed] [Google Scholar]
- 12.Henrotin Y, Kurz B, Aigner T: Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage 2005;13:643–654 [DOI] [PubMed] [Google Scholar]
- 13.Kerio LC, Wachira FN, Wanyoko JK, Rotich MK: Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem 2013;136:1405–1413 [DOI] [PubMed] [Google Scholar]
- 14.Balbir-Gurman A, Fuhrman B, Braun-Moscovici Y, Markovits D, Aviram M: Consumption of pomegranate decreases serum oxidative stress and reduces disease activity in patients with active rheumatoid arthritis: a pilot study. Isr Med Assoc J 2011;13:474–479 [PubMed] [Google Scholar]
- 15.Hsieh MS, Wang KT, Tseng SH, Lee CJ, Chen CH, Wang CC: Using 18F-FDG microPET imaging to measure the inhibitory effects of Clematis chinensis Osbeck on the pro-inflammatory and degradative mediators associated with inflammatory arthritis J Ethnopharmacol 2011;136:511–517 [DOI] [PubMed] [Google Scholar]
- 16.Drevemo S, Roepstorff L, Kallings P, Johnston CJ: Application of TrackEye in equine locomotion research. Acta Anat (Basel) 1993;146:137–140 [DOI] [PubMed] [Google Scholar]
- 17.Allen KD, Shamji MF, Mata BA, et al. : Kinematic and dynamic gait compensations in a rat model of lumbar radiculopathy and the effects of tumor necrosis factor-alpha antagonism. Arthritis Res Ther 2011;13:R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bove SE, Calcaterra SL, Brooker RM, et al. : Weight bearing as a measure of disease progression and efficacy of anti-inflammatory compounds in a model of monosodium iodoacetate-induced osteoarthritis. Osteoarthritis Cartilage 2003;11:821–830 [DOI] [PubMed] [Google Scholar]
- 19.Hansen BD: Assessment of pain in dogs: veterinary clinical studies. ILAR J 2003;44:197–205 [DOI] [PubMed] [Google Scholar]
- 20.Manach C, Williamson G, Morand C, Scalbert A, Remesy C: Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005;81:230S–242S [DOI] [PubMed] [Google Scholar]
- 21.Sies H: Polyphenols and health: update and perspectives. Arch Biochem Biophys 2010;501:2–5 [DOI] [PubMed] [Google Scholar]
- 22.Vang O, Ahmad N, Baile CA, et al. : What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS One 2011;6:e19881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakata R, Takahashi S, Inoue H: Recent advances in the study on resveratrol. Biol Pharm Bull 2012;35:273–279 [DOI] [PubMed] [Google Scholar]
- 24.Gatson JW, Liu MM, Abdelfattah K, et al. : Resveratrol decreases inflammation in the brain of mice with mild traumatic brain injury. J Trauma Acute Care Surg 2013;74:470–474 [DOI] [PubMed] [Google Scholar]
- 25.Chachay VS, Kirkpatrick CM, Hickman IJ, Ferguson M, Prins JB, Martin JH: Resveratrol-pills to replace a healthy diet? Br J Clin Pharmacol 2011;72:27–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Jayaprakasam B, Seeram NP, Olson LK, DeWitt D, Nair MG: Insulin secretion and cyclooxygenase enzyme inhibition by cabernet sauvignon grape skin compounds. J Agric Food Chem 2004;52:228–233 [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Jeong HY, Lee HK, et al. : Neuroprotection of the leaf and stem of Vitis amurensis and their active compounds against ischemic brain damage in rats and excitotoxicity in cultured neurons. Phytomedicine 2012;19:150–159 [DOI] [PubMed] [Google Scholar]
- 28.Zghonda N, Yoshida S, Ezaki S, et al. : ɛ-Viniferin is more effective than its monomer resveratrol in improving the functions of vascular endothelial cells and the heart. Biosci Biotechnol Biochem 2012;76:954–960 [DOI] [PubMed] [Google Scholar]
- 29.Woo YJ, Joo YB, Jung YO, et al. : Grape seed proanthocyanidin extract ameliorates monosodium iodoacetate-induced osteoarthritis. Exp Mol Med 2011;43:561–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma S, Sahu D, Das HR, Sharma D: Amelioration of collagen-induced arthritis by Salix nigra bark extract via suppression of pro-inflammatory cytokines and oxidative stress. Food Chem Toxicol 2011;49:3395–3406 [DOI] [PubMed] [Google Scholar]
- 31.Kubo N, Matsuda H, Tanka M, et al. : Studies on Scutellariae Radix. VII. Anti-arthritic and anti-inflammatory actions of methanolic extract and flavonoid components from Scutellariae Radix. Chem Pharm Bull (Tokyo) 1984;32:2724–2729 [DOI] [PubMed] [Google Scholar]
- 32.Kim HS, Kim AR, Lee JM, et al. : A mixture of Trachelospermi caulis and Moutan cortex radicis extracts suppresses collagen-induced arthritis in mice by inhibiting NF-κB and AP-1. J Pharm Pharmacol 2012;64:420–429 [DOI] [PubMed] [Google Scholar]
- 33.Gregory PJ, Sperry M, Wilson AF: Dietary supplements for osteoarthritis. Am Fam Physician 2008;77:177–184 [PubMed] [Google Scholar]
- 34.Price JS, Chambers MG, Poole AR, Fradin A, Mason RM: Comparison of collagenase-cleaved articular cartilage collagen in mice in the naturally occurring STR/ort model of osteoarthritis and in collagen-induced arthritis. Osteoarthritis Cartilage 2002;10:172–179 [DOI] [PubMed] [Google Scholar]
- 35.Yeh TT, Wen ZH, Lee HS, Lee CH, Yang Z, Jean YH: Intra-articular injection of collagenase induced experimental osteoarthritis of the lumbar facet joint in rats. Eur Spine J 2008;17:734–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmed S: Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res Ther 2010;12:208. [DOI] [PMC free article] [PubMed] [Google Scholar]



