Abstract
Background: Homozygous loss-of-function mutations in the FOXE1 gene have been reported in several patients with partial or complete Bamforth–Lazarus syndrome: congenital hypothyroidism (CH) with thyroid dysgenesis (usually athyreosis), cleft palate, spiky hair, with or without choanal atresia, and bifid epiglottis. Here, our objective was to evaluate potential functional consequences of a FOXE1 mutation in a patient with a similar clinical phenotype.
Methods: FOXE1 was sequenced in eight patients with thyroid dysgenesis and cleft palate. Transient transfection was performed in HEK293 cells using the thyroglobulin (TG) and thyroid peroxidase (TPO) promoters in luciferase reporter plasmids to assess the functional impact of the FOXE1 mutations. Primary human thyrocytes transfected with wild type and mutant FOXE1 served to assess the impact of the mutation on endogenous TG and TPO expression.
Results: We identified and characterized the function of a new homozygous FOXE1 missense mutation (p.R73S) in a boy with a typical phenotype (athyreosis, cleft palate, and partial choanal atresia). This new mutation located within the forkhead domain was inherited from the heterozygous healthy consanguineous parents. In vitro functional studies in HEK293 cells showed that this mutant gene enhanced the activity of the TG and TPO gene promoters (1.5-fold and 1.7-fold respectively vs. wild type FOXE1; p<0.05), unlike the five mutations previously reported in Bamforth–Lazarus syndrome. The gain-of-function effect of the FOXE1-p.R73S mutant gene was confirmed by an increase in endogenous TG production in primary human thyrocytes.
Conclusion: We identified a new homozygous FOXE1 mutation responsible for enhanced expression of the TG and TPO genes in a boy whose phenotype is similar to that reported previously in patients with loss-of-function FOXE1 mutations. This finding further delineates the role for FOXE1 in both thyroid and palate development, and shows that enhanced gene activity should be considered among the mechanisms underlying Bamforth–Lazarus syndrome.
Introduction
Forkhead box E1/thyroid transcription factor 2 (FOXE1, also known as TTF2 or TITF2) is a forkhead/winged helix-domain containing protein (1). FOXE1 was initially characterized as a thyroid-specific transcription factor that binds to both the thyroglobulin (TG) and thyroid peroxidase (TPO) gene promoters, two thyroid differentiation markers (2,3). During development, FOXE1 is expressed in the thyroid and oropharyngeal epithelium of humans and mice, and in the thymus of humans only (4,5). FOXE1 is also expressed in the adult human thyroid, as well as in the hair follicles and prepubertal testis (6).
Homozygous Foxe1-null mice exhibit thyroid agenesis or ectopia and cleft palate (7). In humans, Bamforth–Lazarus syndrome is a rare inherited condition characterized chiefly by congenital hypothyroidism (CH) due to thyroid dysgenesis (usually agenesis), cleft palate, spiky hair, with or without choanal atresia, and bifid epiglottis (OMIM access number 241850). To date, five missense FOXE1 mutations have been reported in patients with Bamforth–Lazarus syndrome (8–12). These mutations were typically inherited from heterozygous carrier parents who were usually consanguineous, although a uniparental isodisomy mechanism was reported recently in a nonconsanguineous family (9). In all reported cases of Bamforth–Lazarus syndrome caused by FOXE1 mutations, the mutations affected the forkhead domain of the protein, whose activity was decreased or abolished.
Here, we describe the identification and characterization of a new homozygous missense FOXE1 mutation in a patient born from consanguineous parents and exhibiting features similar to those previously reported in FOXE1-mutated patients. In contrast to previously reported mutations, this new mutation results in increased transcriptional activity of the mutant protein on the TG and TPO promoters. Thus, two different molecular mechanisms, gain and loss of function, are associated with the same phenotype (Bamforth–Lazarus syndrome).
Patients and Methods
The study complied with French law on medical research and was approved by our local ethics committee. The parents gave their written informed consent for the inclusion of their children in the study and for the genetic tests required for the study.
Patients
We sequenced the FOXE1 gene in eight patients with thyroid dysgenesis (four with athyreosis, one with an ectopic gland, and three with an orthotopic gland) and cleft palate. Two patients had congenital heart defects, and two were born from consanguineous parents.
DNA sequencing and multiplex ligation-dependent probe amplification
Genomic DNA was isolated from whole blood. The entire coding exon of FOXE1 (NM_004473.3) was amplified by PCR, and DNA sequencing was performed (ABI Prism 377 automatic sequencer; Life Technologies Corporation, Carlsbad, CA; primer sequences and PCR conditions available on request). No mutations were found in the PAX8 or NKX2-1 exonic regions. We screened 100 normal individuals for the identified FOXE1 alterations. Multiplex ligation-dependent probe amplification (MLPA) was performed using the SALSA MLPA KIT P319A1 thyroid kit (MRC-Holland, Amsterdam, The Netherlands) as previously described (13).
Functional characterization of the new FOXE1 mutation
For the functional analysis, the p.R73S mutant identified in one of the eight patients was generated from a wild type FOXE1 cDNA template as described previously, using the Stratagene Quikchange® kit (Agilent Technologies, Santa Clara, CA) (14). Transient co-transfection assays with wild type and p.R73S mutant FOXE1 were performed as described previously (14), using the two natural human TPO and TG gene promoters upstream of the luciferase gene (kindly provided by S. Refetoff and G. Vassart) in nonthyroid cells (HEK293, ATCC, CRL-1573). Cells were plated at 2×105/well in a 24-well plate 24 h before transfection. We used lipofectamine to co-transfect cells transiently with 520 ng of reporter plasmid containing TG or TPO, 260 ng of pFlag, or wild type or mutated FOXE1. We chose to co-transfect FOXE1 with PAX8 and NKX2-1 because interactions between these transcription factors have been demonstrated (15), and their simultaneous expression is unique to thyroid follicular cells (5). Normalization of transfection efficiency was done using 150 ng of plasmid Bosβ-galactosidase [kindly provided by K.C. Chatterjee (11)]. After 48 h, cells were harvested, and luciferase and β-galactosidase assays were performed. The Mann–Whitney U test was used to evaluate differences in transcriptional activities. Statistical analyses were performed using Statview v5.0 statistical package (SAS Institute, Cary, NC). Bar graphs represent mean±standard error of the mean (SEM) of four independent experiments in triplicate, as indicated in the figure legends. Western blot was performed as previously described (14).
Normal human thyroid tissue specimens were collected at the Institut Gustave Roussy, Villejuif, France, in accordance with local and national ethical requirements. Informed consent was obtained from all donors. Primary human thyrocytes cultured as previously described (16) were transfected using XtremeGENE HP DNA, as recommended by the manufacturer (Roche Diagnostics, Meylan, France).
EMSA was performed as previously described (17) using biotinylated labeled TG (5′-GAG GGA GCT CCT TTT GAC CAG CAG AGA AAA CAG GAT GGG GCA C-3′) and TPO 5′-TGT TCC CAA CAG TAA CTC ATC ATT GAG CCA TGG AAT CC-3′) probes derived from the corresponding human promoters, as recommended by the manufacturer. For competition incubations, a 100-fold excess of cold probe was used. An unrelated protein (EBNA; Pierce Antibodies, Rockford, IL) was used to establish the specificity of the TPO and TG DNA sequences (data not shown).
Results
Among our eight patients with thyroid dysgenesis and cleft palate, one had a previously unreported FOXE1 mutation, c.217C>A, p.R73S. Importantly, MLPA revealed no instances of FOXE1 deletion in our cohort.
Patient having the new mutation
The patient with the new mutation was a boy born at full term, after an uneventful pregnancy, to first cousins of Egyptian origin. At birth, he was noted to have partial choanal atresia on the right side and cleft palate, which was repaired at the age of two months. At one month of age, excessive sleepiness and poor feeding were noted. Serum total thyroxine (T4) was undetectable (<0.02 ng/dL), and thyrotropin (TSH) was markedly elevated (>100 μU/mL). Thyroid ultrasound findings were consistent with athyreosis, and thyroid scintigraphy showed no tracer uptake along the normal thyroid migration trajectory. Thyroxine therapy was started. Both parents and the patient's brother were euthyroid with normal thyroid gland morphology and no birth defects detected by otorhinolaryngological investigations.
FOXE1 gene sequencing
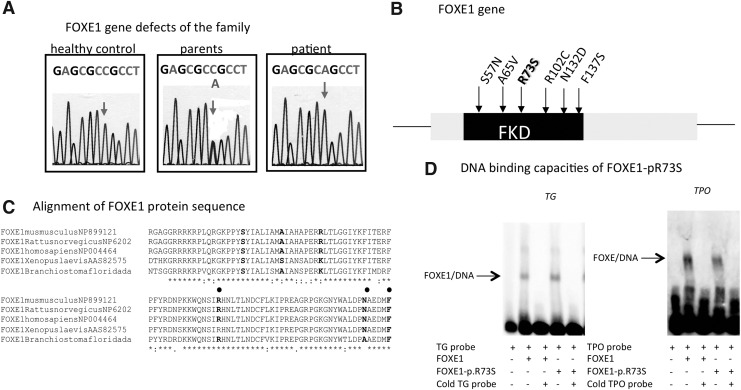
Sequencing of FOXE1 amplified from the patient's DNA showed a biallelic, single-nucleotide substitution (c.217C>A) responsible for an arginine-to-serine change at codon 73 (p.R73S, numbering relative to NP_004464) within the forkhead DNA binding domain of the predicted protein sequence (Fig. 1A and B). Both consanguineous parents were heterozygous for this FOXE1 mutation, suggesting autosomal recessive inheritance. DNA from the unaffected sibling was not available for analysis. Alignment of amino acid sequences within forkhead domains of a number of proteins indicated marked cross-species conservation of the residue corresponding to arginine 73 in FOXE1 (Fig. 1C), suggesting functional significance of this amino acid.
FIG. 1.
Identification and analysis of FOXE1 mutations. (A) FOXE1 gene defects: chromatograms showing the heterozygous or homozygous FOXE1 mutations. (B) FOXE1 gene: position of the mutations identified in previous studies and in our cohort. (C) Alignment of the forkhead domain (FKD) sequence of human FOXE1 with sequences from other species, indicating marked conservation (arrow) of the different mutations (spot). (D) DNA-binding capacities of FOXE1-p.R73S to the human TG and TPO promoters; 2 μg of nuclear extract from HEK293 cells transfected with wild type or mutated FOXE1 were incubated with biotinylated TG and TPO probes with (+) or without (−) a competitor (cold probe).
Functional characterization of the FOXE1-p.R73S mutant
EMSA showed no difference in binding to the TG and TPO promoters between wild type FOXE1 and FOXE1-pR73S (Fig. 1D).
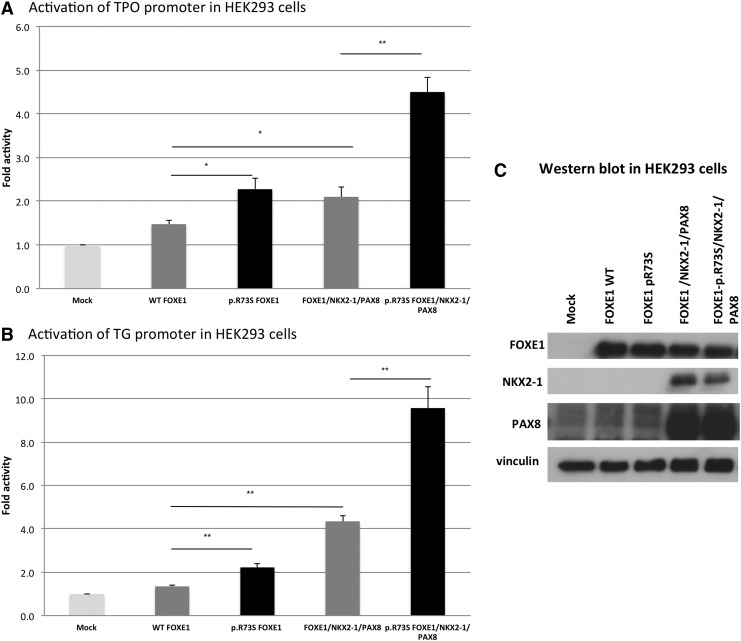
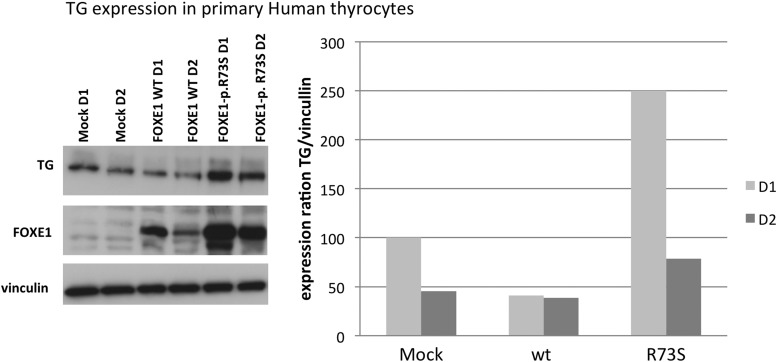
FOXE1-p.R73S mutant co-transfection in HEK293 cells resulted in enhanced transcriptional activity of both the TG and the TPO promoters compared to the wild type expression vector (1.7-fold with the TG promoter and 1.5-fold with the TPO promoter, p<0.05; Fig. 2A and B). As previously described, the transcriptional activity of wild type FOXE1 was significantly higher in the thyroidal context (i.e., using human PAX8- and NKX2-1-expressing vectors) than when co-transfected alone. This transcriptional activity was markedly increased with mutant FOXE1 (2.2-fold compared to the wild type gene, for both the TG and the TPO promoters, p<0.05). Western blot confirmed that the amounts of wild type FOXE1 and mutated FOXE1 proteins were identical when the genes were transfected alone or with PAX8 and NKX2-1 in HEK293 cells (Fig. 2C). Moreover, FOXE-p.R73S transfection in primary human thyrocytes confirmed the increase in endogenous TG transcriptional activity in the thyroid cells (Fig. 3), whereas TPO transcriptional activity was not increased (data not shown). In primary human thyrocytes, the mutant FOXE1 protein was expressed at a higher level than the wild type FOXE1 protein after transfection with the same amounts of plasmid. These results were found in five independent experiments.
FIG. 2.
Functional activity of wild type (WT) versus mutant FOXE1 on TPO (A) and TG (B) luciferase reporter constructs. Promoter constructs were co-transfected in HEK293 cells with the empty pFlag vector or the expression vector for WT or mutated FOXE1. Effects on transcriptional activity of mutant FOXE1 co-expressed with PAX8 and NKX2-1. Luciferase activities are expressed as the fold increase relative to the activity obtained with the empty vector and assigned a value of 1. Results are the mean±standard error of the mean of four independent experiments performed in triplicate. *p<0.05, **p<0.01 (Mann–Whitney U test). (C) Expression of FOXE1 WT or mutant proteins when transfected alone or with PAX8 and NKX2-1 proteins in HEK293 cells, as assessed by Western blot. pFlag was the empty vector.
FIG. 3.
Expression of FOXE1 WT or mutant proteins, TG, and vinculin in primary human thyrocytes after one (D1) or two days (D2) of transfection, as assessed by Western blot. Expression ratios are shown in graphical form. These results were representative of five experiments in primary human thyrocytes.
Discussion
We have identified the first FOXE1 mutation leading to increased activity associated with CH and cleft palate. Interestingly, this homozygous missense gain-of-function mutation resulted in the same phenotype as previously described loss-of-function FOXE1 mutations, suggesting that both mechanisms can induce CH.
All the FOXE1 mutations identified so far produce proteins with decreased activity. In contrast, the new mutation identified here in a patient with Bamforth–Lazarus syndrome enhanced transcriptional activity as studied in a similar cellular context to that used previously (8–11). Thus, functional studies showed a marked increase in mutant FOXE1 protein activity in a nonthyroidal context, a simulated thyroidal context (with NKX2-1 and PAX8), and a true thyroidal context (primary human thyrocytes). In HEK293 cells, mutant FOXE1 resulted in overactivation of the TG and TPO promoters. Moreover, in primary human thyrocytes, mutant FOXE1 was overexpressed and resulted in TG promoter overactivation. However, we were unable to identify the exact mechanism responsible for overactivation. Increased binding is one possibility, but EMSA may not be well suited to demonstrate it (data not shown). We can only speculate that FOXE1 DNA interaction become more efficient in TG transactivation.
In the primary thyrocytes, a FOXE1-pR73S induced activity increase was shown but not in the HEK293 cells. We can hypothesized different interactions with FOXE1 could be modifying the FOXE1 expression by stabilization in primary thyrocytes and thus make it resistant to proteasomal degradation.
Moreover, the FOXE1 gain of function due to the mutation could be leading to the thyroid bud degeneration by apoptosis. In fact, the link between Pax8 and apoptosis was clearly shown. However, this link between FOXE1 and apoptosis remains speculative (18–20).
Cleft palate has been reported in both Foxe1 null mice and mice with Foxe1 overexpression (7,21). Unfortunately, the thyroid phenotype of these mice was either not studied or not reported (personal communication from the authors) (21). Nevertheless, the findings in this mouse model support our observation of impaired palate and thyroid development in a patient with a FOXE1 gain-of-function mutation.
In several other diseases, transcription factor mutations resulting in loss or gain of function have been shown to produce similar phenotypes. Missense mutations in the human T Box transcription factor gene (TBX1) gene have been identified in patients exhibiting the same phenotype as those with 22q11.2 deletion (22). Zweier et al. hypothesized that TBX1 missense mutations may alter the transcriptional activity of the TBX1 protein, possibly through stabilization of the protein–protein or protein–DNA interaction than that of increased 22q11.2 dosage (22). Moreover, in mice, both over- and underexpression of Tbx1 resulted in a phenotype similar to that in humans (23). HESX1 mutations were usually identified as leading to mutant proteins with decreased activity, but mutations that enhance DNA binding resulting in increasing interaction with PROP1 have been also found in patients harboring congenital pituitary disorder (24). In other species, Armstrong et al., working on amyotrophic lateral sclerosis, submitted results which implicate both a gain of toxic function following expression of mutant (but not wild type) human fused in sarcoma (FUS) and a loss of function following antisense morpholino knockdown of zebrafish Fus generate locomotor impairment by reducing presynaptic function at the neuromuscular junctions (25). Moreover, another example of such a gain- or loss-of-function was the Triplo-lethal locus (Tpl) of Drosophila melanogaster, which causes lethality when it is present in either three copies or one copy (26,27).
In conclusion, we report the sixth missense FOXE1 mutation and provide first evidence that a FOXE1 gain-of-function mutation can result in the same phenotype as that caused in humans by FOXE1 loss-of-function mutations. Enhanced transcriptional activity should be considered among the mechanisms capable of producing thyroid and other developmental abnormalities.
Acknowledgments
We thank Prof. Heba Elsedfy for her assistance in investigating the family. We are grateful to Dr. Cartigny (Hôpital Jeanne de Flandre, Lille, France), Prof. Boudailliez (Hôpital Nord, Amiens, France), Dr. Loeuille (Centre Hospitalier, Dunkerque, France), Prof. Bost (Centre Hospitalier, Grenoble, France), Dr. Cabrol (Hôpital Trousseau, Paris, France), Prof. Léger (Hôpital Robert Debré, Paris, France), and Prof. Leheup (Hôpital Brabois, Vandoeuvre les Nancy, France) for providing us with specimens from their patients for FOXE1 gene analysis. We gratefully acknowledge Lucy Whitehouse of inScience Communications, a Wolters Kluwer business, who provided assistance with English language editing; this assistance was funded by Pfizer.
A.C. was supported by CIFRE in collaboration with HRA Pharma and by the French Ministère de l'Education Nationale et de la Recherche. M.P. received a Collaborative Research Unit Grant from the European Society for Paediatric Endocrinology (ESPE). This study was supported in part by grants from Sandoz France, Electricité de France (RB 200605), and Merck-Serono France.
Author Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as jeopardizing the impartiality of the reported research.
References
- 1.Civitareale D, Saiardi A, Falasca P.1994Purification and characterization of thyroid transcription factor 2. Biochem J 304:981–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aza-Blanc P, Di Lauro R, Santisteban P.1993Identification of a cis-regulatory element and a thyroid-specific nuclear factor mediating the hormonal regulation of rat thyroid peroxidase promoter activity. Mol Endocrinol 7:1297–1306 [DOI] [PubMed] [Google Scholar]
- 3.Civitareale D, Lonigro R, Sinclair AJ, Di Lauro R.1989A thyroid-specific nuclear protein essential for tissue-specific expression of the thyroglobulin promoter. EMBO J 8:2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dathan N, Parlato R, Rosica A, De Felice M, Di Lauro R.2002Distribution of the TITF2/FOXE1 gene product is consistent with an important role in the development of foregut endoderm, palate, and hair. Dev Dyn 224:450–456 [DOI] [PubMed] [Google Scholar]
- 5.Trueba SS, Auge J, Mattei G, Etchevers H, Martinovic J, Czernichow P, Vekemans M, Polak M, Attie-Bitach T.2005PAX8, TITF1, and FOXE1 gene expression patterns during human development: new insights into human thyroid development and thyroid dysgenesis-associated malformations. Clin Endocrinol Metab 90:455–462 [DOI] [PubMed] [Google Scholar]
- 6.Sequeira M, Al-Khafaji F, Park S, Lewis MD, Wheeler MH, Chatterjee VK, Jasani B, Ludgate M.2003Production and application of polyclonal antibody to human thyroid transcription factor 2 reveals thyroid transcription factor 2 protein expression in adult thyroid and hair follicles and prepubertal testis. Thyroid 13:927–932 [DOI] [PubMed] [Google Scholar]
- 7.De Felice M, Ovitt C, Biffali , Rodriguez-Mallon A, Arra C, Anastassiadis K, Macchia PE, Mattei MG, Mariano A, Schöler H, Macchia V, Di Lauro R.1998A mouse model for hereditary thyroid dysgenesis and cleft palate. Nat Genet 19:395–398 [DOI] [PubMed] [Google Scholar]
- 8.Baris I, Arisoy AE, Smith A, Agostini M, Mitchell CS, Park SM, Halefoglu AM, Zengin E, Chatterjee VK, Battaloglu E.2006A novel missense mutation in human TTF-2 (FKHL15) gene associated with congenital hypothyroidism but not athyreosis. J Clin Endocrinol Metab 91:4183–4187 [DOI] [PubMed] [Google Scholar]
- 9.Castanet M, Mallya U, Agostini M, Schoenmakers E, Mitchell C, Demuth S, Raymond FL, Schwabe J, Gurnell M, Chatterjee VK.2010Maternal isodisomy for chromosome 9 causing homozygosity for a novel FOXE1 mutation in syndromic congenital hypothyroidism. J Clin Endocrinol Metab 95:4031–4036 [DOI] [PubMed] [Google Scholar]
- 10.Castanet M, Park SM, Smith A, Bost M, Leger J, Lyonnet S, Pelet A, Czernichow P, Chatterjee K, Polak M.2002A novel loss-of-function mutation in TTF-2 is associated with congenital hypothyroidism, thyroid agenesis and cleft palate. Hum Mol Genet 11:2051–2059 [DOI] [PubMed] [Google Scholar]
- 11.Clifton-Bligh RJ, Wentworth JM, Heinz P, Crisp MS, John R, Lazarus JH, Ludgate M, Chatterjee VK.1998Mutation of the gene encoding human TTF-2 associated with thyroid agenesis, cleft palate and choanal atresia. Nat Genet 19:399–401 [DOI] [PubMed] [Google Scholar]
- 12.Kang IN, Musa M, Harun F, Junit SM. 2010Characterization of mutations in the FOXE1 gene in a cohort of unrelated Malaysian patients with congenital hypothyroidism and thyroid dysgenesis. Biochem Genet 48:141–151 [DOI] [PubMed] [Google Scholar]
- 13.Teissier R, Guillot L, Carré A, Morandini M, Stuckens C, Ythier H, Munnich A, Szinnai G, de Blic J, Clement A, Leger J, Castanet M, Epaud R, Polak M.2012Multiplex ligation-dependent probe amplification improves the detection rate of NKX2.1 mutations in patients affected by brain-lung-thyroid syndrome. Horm Res Paediatr 77:146–151 [DOI] [PubMed] [Google Scholar]
- 14.Carre A, Castanet M, Sura-Trueba S, Szinnai G, Van Vliet G, Trochet D, Amiel J, Leger J, Czernichow P, Scotet V, Polak M.2007Polymorphic length of FOXE1 alanine stretch: evidence for genetic susceptibility to thyroid dysgenesis. Hum Genet 122:467–476 [DOI] [PubMed] [Google Scholar]
- 15.Di Palma T, Nitsch R, Mascia A, Nitsch L, Di Lauro R, Zannini M.2003The paired domain-containing factor Pax8 and the homeodomain-containing factor TTF-1 directly interact and synergistically activate transcription. J Biol Chem 278:3395–3402 [DOI] [PubMed] [Google Scholar]
- 16.Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C.2010Intracellular expression of ROS-generating NADPH oxidase NOX4 in normal and cancer thyroid tissues. Endocr Relat Cancer 17:27–37 [DOI] [PubMed] [Google Scholar]
- 17.Guillot L, Carré A, Szinnai G, Castanet M, Tron E, Jaubert F, Broutin I, Counil F, Feldmann D, Clement A, Polak M, Epaud R.2010NKX2-1 mutations leading to surfactant protein promoter dysregulation cause interstitial lung disease in “brain-lung-thyroid syndrome.” Hum Mutat 31:E1146–E1162 [DOI] [PubMed] [Google Scholar]
- 18.Fagman H, Amendola E, Parrillo L, Zoppoli P, Marotta P, Scarfò M, De Luca P, de Carvalho DP, Ceccarelli M, De Felice M, Di Lauro R.2011Gene expression profiling at early organogenesis reveals both common and diverse mechanisms in foregut patterning. Dev Biol 359:163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porreca I, De Felice E, Fagman H, Di Lauro R, Sordino P.2012Zebrafish Bcl2l is a survival factor in thyroid development. Dev Biol 366:142–152 [DOI] [PubMed] [Google Scholar]
- 20.Di Palma T, Filippone MG, Pierantoni GM, Fusco A, Soddu S, Zannini M.2013Pax8 has a critical role in epithelial cell survival and proliferation. Cell Death Dis 18:e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng T, Shi JY, Wu M, Wang Y, Li L, Liu Y, Zheng Q, Huang L, Shi B.2012Overexpression of mouse TTF-2 gene causes cleft palate. J Cell Mol Med 16:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zweier C, Sticht H, Aydin-Yaylagul I, Campbell CE, Rauch A.2007Human TBX1 missense mutations cause gain of function resulting in the same phenotype as 22q11.2 deletions. Am J Hum Genet 80:510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funke B, Epstein JA, Kochilas LK, Lu MM, Pandita RK, Liao J, Bauerndistel R, Schüler T, Schorle H, Brown MC, Adams J, Morrow BE.2001Mice overexpressing genes from the 22q11 region deleted in velo-cardio-facial syndrome/DiGeorge syndrome have middle and inner ear defects. Hum Mol Genet 10:2549–2556 [DOI] [PubMed] [Google Scholar]
- 24.Cohen RN, Cohen LE, Botero D, Yu C, Sagar A, Jurkiewicz M, Radovick S.2003Enhanced repression by HESX1 as a cause of hypopituitarism and septooptic dysplasia. J Clin Endocrinol Metab 88:4832–4839 [DOI] [PubMed] [Google Scholar]
- 25.Armstrong GA, Drapeau P.2013Loss and gain of FUS function impair neuromuscular synaptic transmission in a genetic model of ALS. Hum Mol Genet 22:4282–4292 [DOI] [PubMed] [Google Scholar]
- 26.Denell RE.1976The genetic analysis of a uniquely dose-sensitive chromosomal region of Drosophila melanogaster. Genetics 84:193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindsley DL, Sandler L, Baker BS, Carpenter AT, Denell RE, Hall JC, Jacobs PA, Miklos GL, Davis BK, Gethmann RC, Hardy RW, Steven AH, Miller M, Nozawa H, Parry DM, Gould-Somero M, Gould-Somero M.1972Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics 71:157–184 [DOI] [PMC free article] [PubMed] [Google Scholar]