Abstract
The first comprehensive study on the prevalence of canine vector-borne pathogens (Anaplasma phagocytophilum, Borrelia burgdorferi, Ehrlichia canis, and Dirofilaria immitis) was carried in Hungary because, except for babesiosis and dirofilariosis caused by Dirofilaria repens, there were no data on their regional distribution and prevalence. In 2011 and 2012, 1305 blood samples were collected from randomly selected, apparently healthy pet dogs in 167 localities of 19 counties of Hungary. All sera samples from dogs were screened for simultaneous qualitative detection of circulating antibodies to E. canis and B. burgdorferi sensu lato and A. phagocytophilum and D. immitis antigen using SNAP® 4Dx (IDEXX Laboratories). Overall, 170 dogs (13.0%; 95% confidence interval [CI] 11–15) were serologically positive to one or more of the tested pathogens. A. phagocytophilum was the most prevalent pathogen detected in 102 dogs by antibody titers (7.9%, 95% CI 6.5–9.5), followed by D. immitis (2.4%, 95% CI 1.0–4.0, n=64) and B. burgdorferi (0.4%, 95% CI 0.0–1.1, n=11) out of 1305 tested dogs. The least prevalent infection was with E. canis, with only two positive dogs (0.16%, 95% CI 0.03–0.6). Co-infection was found in eight dogs (0.61%, 95% CI 0.29–1.21), of which seven were seropositive to two pathogens (five with A. phagocytophilum and D. immitis, two with A. phagocytophilum and B. burgdorferi). One dog was serologically positive to three pathogens (A. phagocytophilum, B. burgdorferi, and D. immitis). Purebred and crossbred animals did not show significantly different levels of seropositivity. There was no significant association between the gender and the results of diagnostic testing. Logistic regression analysis showed a higher chance of seropositivity in the older dogs.
Key Words: : Canine vector-borne diseases, Anaplasma phagocytophilum, Borrelia burgdorferi, Dirofilaria immitis, Ehrlichia canis, In-clinic ELISA test
Introduction
Vectors and vector-borne pathogens (VBPs) affect animals and human health worldwide. These VBPs can cause symptomless or serious even lethal infections in domestic dogs, and some of these disease agents (e.g., Borrelia burgdorferi, Anaplasma phagocytophilum, Dirofilaria immitis) have zoonotic importance because dogs potentially serve as sentinels for human infection (Day 2011). The detection of canine VBPs (CVBPs) is helpful in the management of the affected animals because these pathogens alone and especially the co-infections are a constant challenge for practicing veterinarians (De Tommasi et al. 2013). In addition, the epidemiological data of the vector-borne infections are also useful for public health authorities.
Ixodes ricinus is the most common and well-known important vector of many disease agents in Europe. A. phagocytophilum transmitted transstadially by mainly I. ricinus is the causative agent of human granulocytic ehrlichiosis (anaplasmosis) and many domestic animals. In dogs, infection may result in a mild to severe acute illness with fever, lameness, anorexia, lethargy, and thrombocytopenia (Alleman and Wamsley 2008). Although humans are only involved as incidental, dead-end hosts, the affected persons have nonspecific symptoms (Karlsson et al. 2001). The prevalence of anaplasmosis has not been studied in dogs or wild canids in Hungary. The rickettsia was only detected with PCR in I. ricinus ticks collected from red foxes (Sréter et al. 2004) and in the environment (Egyed et al. 2012).
I. ricinus also transmits B. burgdorferi sensu lato (s.l.), the agent of Lyme borreliosis, to humans and animals. So far, only B. burgdorferi sensu stricto has been found to be pathogenic in dogs after experimental infections (Krupka and Straubinger 2010). Relatively few infected dogs demonstrate clinical signs (Littman et al. 2006). In Hungary, except for one study (Kapiller et al. 1995), no data have been available concerning the occurrence of Lyme borreliosis in dogs.
Ehrlichia canis, a causative agent of canine monocytic ehrlichiosis (CME), is transmitted by Rhipicephalus sanguineus group. CME ranges from subclinical infection to fatal illness. The clinical signs of acute ehrlichiosis are nonspecific (Harrus and Waner 2011). Regarding the occurrrence of E. canis in Hungary, there has been only a report about its occurrence when this rickettsia was found in Dermacentor marginatus nymphs collected from dogs and in Ixodes canisuga larvae removed from red foxes in south of the country (Hornok et al. 2013).
Heartworm disease is a cosmopolitan parasitic infection of domestic and wild carnivores caused by D. immitis transmitted by mosquitoes and is a potentially fatal condition in dogs (McCall et al. 2008). D. immitis very rarely causes pulmonary dirofilariosis in humans (Irwin and Jefferies 2004). To date, the geographical distribution and the prevalence of D. immitis are not well known in Hungary. The first autochthonous canine heartworm infection was reported a few years ago (Jacsó et al. 2009).
The aims of the present study were to: (1) Investigate the seroprevalence of three tick-borne infections and one mosquito-borne infection in dogs caused by A. phagocytophilum, B. burgdorferi, E. canis, and D. immitis, respectively; (2) determine the risk factors (age, sex, and breed) associated with the presence of antibodies or antigen.
Materials and Methods
Animals and sample collection
In 2011 and 2012, blood samples were collected from 1305 randomly selected apparently healthy pet dogs in 167 localities of 19 counties of Hungary. None of these animals had been vaccinated against Lyme borreliosis. All dogs were aged between 8 months and 15 years (average=4.34±3.18 years). There were more purebred (817/1305; 62.6%) than mixed-breed (488/1305; 37.4%) dogs. The purebred dogs belonged to 113 breeds, of which Hungarian Vizsla (69) and German Shepherds (58) were the most prevalent. In all, 670 were male (607 total, 63 neutered) and 635 were female (511 total, 124 neutered). A 2-mL blood sample was drawn from the cephalic vein of each dog using tubes without anticoagulant. Serum was collected following centrifugation of clotted blood and was stored at [[[minus sign]]20[[[degree sign]]]C until further processing. Information was recorded on the animals' gender, age, and breed. The owners of the dogs enrolled were previously informed about the study protocol.
Serology assay
All serum samples from dogs were screened for simultaneous qualitative detection of circulating antibodies, both immunoglobulin G (IgG) and IgM, to E. canis, B. burgdorferi s.l. and A. phagocytophilum and D. immitis antigen using an in-clinic enzyme-linked immunosorbent assay (ELISA) SNAP® 4Dx (IDEXX Laboratories, Hoofddorp, The Netherlands), according to the manufacturer's directions. The membrane matrix of the test is impregnated with synthetic peptide from the major surface protein p44/MSP2 of A. phagocytophilum, C6 peptide derived from the IR6 region within the Borrelia membrane protein VlsE, peptides p30 and p30-1 from the outer membrane of E. canis, and antibodies against specific antigens of D. immitis (Philipp et al. 2001, O'Connor et al. 2004). The sensitivity (Se) and specificity (Sp) are as follows: 84% Se and 97% Sp for D. immitis; 94.4% Se and 99.5% Sp for B. burgdorferi s.l.; 95.7% Se and 100% Sp for E. canis; and 99.1% Se and 100% Sp for A. phagocytophilum (Chandrashekar et al. 2010).
Statistical analysis
The relationship of seropositivity and age was analyzed by logistic regression. Dogs were classified into different categories to assess the significance of several risk factors (variables)—gender and breed (pure and mixed). The association of seropositivity and these classes was tested by the Fisher exact test. In the estimation of prevalence, the confidence interval was obtained by Sterne method. All statistical analysis was performed using the R-environment.
Results
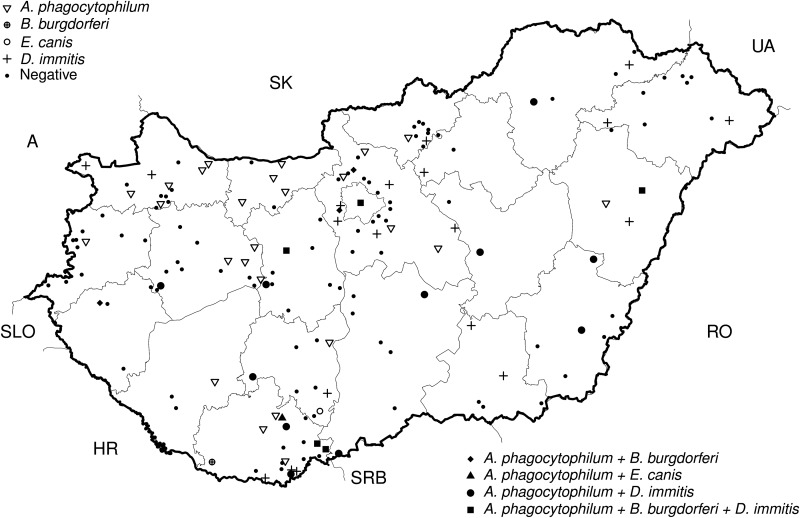
Overall, 170 dogs (13.0%; 95% confidence interval [CI] 11–15) were serologically positive to one or more of the tested pathogens. The seropostive animals occurred throughout Hungary, in all counties (Fig. 1).
FIG. 1.
Geographical distribution of seropositive dogs infected with vector-borne pathogens in Hungary.
A. phagocytophilum was the most prevalent pathogen and was detected in 102 dogs by antibody titers (7.9%, 95% CI 6.5–9.5), followed by D. immitis (2.4%, 95% CI 1.0–4.0, n=64) and B. burgdorferi (0.4%, 95% CI 0.0–1.1, n=11). The least prevalent infection was with E. canis, found only in two positive dogs (0.16%, 95% CI 0.03–0.6) that were restricted to two localities in southern Hungary.
Logistic regression showed the higher chance of seropositivity in the elder dogs (odds ratio [OR]=1.057, 95% CI 1.007–1.109, p=0.025). No significant (p=0.13) association was between the pure or mixed breeds, as well as the gender and the results of diagnostic test. The breed did not influence the seroprevalence of any tested pathogens.
Co-infection was found in eight dogs (0.61%, 95% CI 0.29–1.21) of which seven were seropositive to two pathogens (five with A. phagocytophilum and D. immitis [0.38%, 95% CI 0.15–0.90], two with A. phagocytophilum and B. burgdorferi [0.15%, 95% CI 0.03–0.56]). One dog was serologically positive to three pathogens (A. phagocytophilum, B. burgdorferi, and D. immitis) (0.08%, 95% CI 0.004–0.441).
Discussion
Except for babesiosis (Földvári et al. 2005, Hornok et al. 2006) and dirofilariosis caused by Dirofilaria repens (Fok 2007), there are no comprehensive data available on the regional distribution and prevalence of CVBPs. For this reason, the results of these point-of-care assays are especially valuable from the epidemiological point of view for veterinarians as well as public health authorities.
In the present study, 170 (13.0%) out of 1305 dogs were serologically positive to one or more of the tested pathogens. Although dogs represent an accidental host, A. phagocytophilum was the most prevalent pathogen detected in 102 dogs (7.9%). This rickettsial infection has the widest geographical distribution in the country, which is related to the presence of the competent vector (Széll et al. 2006). To the best of our knowledge, these are the first data regarding the occurrence of Anaplasma infection in the local dogs. It is known that serological cross-reactivities could occur between A. phagocytophilum and other related species (A. platys, E. ewingii, and E. chaffeensis) (Greig and Armstrong 2006). However, these pathogens and their tick vectors are not endemic in Hungary. Lower percentages (2.72–5.5%) were reported from France (Pantchev et al. 2009), Romania (Mircean et al. 2012), and Spain (Miró et al. 2013). In other countries, such as Germany (Kohn et al. 2011), the United States (Beall et al. 2008), and Korea (Lim et al. 2010), much higher seropositivity (18.8–43.2%) was found amongst the local dogs. The seroprevalence of this rickettsial infection in dogs has a very wide range worldwide, even between the different regions of a country (Kohn et al. 2011, Miró et al. 2013). The likely reasons are related to the many factors, e.g., the occurrence of the vector I. ricinus and the natural reservoirs (e.g., small mammals and deer) of A. phagocytophilum. I. ricinus ticks collected from red foxes (Sréter et al. 2004) and from field (Egyed et al. 2012) were PCR-positive for A. phagocytophilum, but based on a larger representative tick population data should be collected about the prevalence of A. phagocytophilum in Hungary.
Before this study, Kapiller et al. (1995) reported dogs having lameness caused by borreliae. Later Borrelia spp. could be detected with PCR in six (5.6%) of 108 I. ricinus samples removed from dogs, and sequencing revealed the highest similarity with B. afzelii and B. garinii (Földvári et al. 2007). Surprisingly, almost 10 times more dogs were seropositive for A. phagocytophilum (n=102) than for B. burgdorferi (n=11). It might be possible that fewer ticks harbored B. burgdorferi that infested the dogs of this survey. The low number of seropositive animals can also be explained with the assay because the SNAP® 4Dx Kit only detects the antibodies against B. burgdorferi during active infection (Liang et al. 1999). Bhide et al. (2004) reported that IgG antibodies generally start emerging about 1–2 months past infection or reinfection and can persist up to 1–1.5 years in dogs. Aside from this study, no reliable information is available regarding whether dogs can be infected for life with persistence of anti-B. burgdorferi antibodies or not. In some countries such as in Spain (Miró et al. 2013), France (Pantchev et al. 2009), and Romania (Mircean et al. 2012), very low (0.33–0.6%) seroprevalences similar to our result were found. However, in other parts of Europe where I. ricinus is also common, much higher seroactivity occurred in the local dogs to B. burgdorferi (Kybicová et al. 2009, Pantchev et al. 2009). Differences in seroprevalence rates can arise from variability in tick densities or the proportion of infected ticks. In a recent Hungarian study, the minimum prevalence of B. burgdorferi s.l. was low (2.5%) in pooled samples of questing I. ricinus and five Borrelia species (B. afzelii, B. burgdorferi sensu stricto, B. garinii, B. lusitaniae, and B. valaisiana) were identified (Egyed et al. 2012). So far there is no evidence of human infection with B. burgdorferi resulting from contact with dogs. If dogs would take home infested ticks that attach to humans in such cases, dogs would be merely carriers of the vectors. Similar to Spanish (Miró et al. 2013) and Romanian (Mircean et al. 2012) data, no relationship was found between seroreactivity to B. burgdorferi and gender or age in our study; however, the chance of seropositivity increases with the age of dog. Spanish scientists also reported a higher prevalence of B. burgdorferi in older animals (Inmaculada et al. 2008) that could be the result of higher opportunities to be infected throughout their lives.
It is not suprising that only two dogs (0.16%) were seropositive to E. canis, which lived in two localities in southern Hungary. It is known that the occurrance of this pathogen is largely dependent on the distribution of its vector Rh. sanguineus, which is not endemic in the country (Hornok and Farkas 2005). It is unclear how these two dogs became seropositive for E. canis. The animals never left their localities and no Rh. sanguineus occurred around them. It might be possible that other tick species could also transmit E. canis. Not far from these areas, D. marginatus nymphs and I. canisuga larvae from red foxes revealed the presence of E. canis. On the basis of these findings, Dermacentor spp. may also be potential or alternative vectors (Hornok et al. 2013). Further studies are needed to confirm this hypothesis as well as whether red foxes can play a reservoir role in the epidemiology of canine ehrlichiosis or not.
D. repens can occur in dogs throughout the country (Fok et al. 2007). The detection of D. immitis antigen in 64 dogs (2.4%) was unexpected because autochthonous heartworm infection had been diagnosed only in a local dog before this study (Jacsó et al. 2009). However, it was assumed that the prevalence of this nematode infection would increase in the near future because of the suitable climate for geographical distribution of mosquito vectors as well as the movement of infected dogs from endemic areas to Hungary. The findings of this study also confirm that dirofilariosis caused by both Dirofilaria species has been spreading throughout previously uninfected European areas, in particular in Eastern Europe (Genchi et al. 2005). There was no significant association between the gender and the results of diagnostic test. The prevalence rates increased with the age of the animals as in other studies (Lim et al. 2010) because older dogs have more time and more opportunities to become infected with heartworms. On the basis of the first epidemiological results, probably more dogs could be infected with D. immitis or both nematode species in the future.
In this study, co-infection was found only in eight dogs (0.61%), of which three were seropositive to A. phagocytophilum and B. burgdorferi. Six dogs became infected with tick- and moquito-borne borne pathogens (A. phagocytophilum, B. burgdorferi, and D. immitis), of which one animal was seroactive to all these agents. These findings indicate that the local veterinarians in small animal clinics should also pay more attention and they should use more methods because co-infections by different VBPs can cause more severe clinical and pathological disorders than one agent alone (De Tommasi et al. 2013).
This is the first comprehensive study carried out in Hungary on the prevalence of CVBD agents in relation to their geographical areas. Further epidemiological surveillance is required to map the regional risk of these and other CVBPs. This would useful for veterinarians as well as for public health authorities concerning the disease agents of zoonotic importance.
Acknowledgments
We thank Bayer Hungaria Ltd. for supplying the IDEXX kits. This study was partially funded by European Union grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee ascEDENext171 (www.edenext.eu). The contents of this publication are the sole responsibility of the authors and do not necessarily reflect the views of the European Commission.
The authors are indebted to the veterinarians and pet owners participating in this study.
Author Disclosure Statement
No author has had any financial or personal relationship with people or organizations that could inappropriately influence their work.
References
- Alleman AR, Wamsley HL. An update on anaplasmosis in dogs. Vet Med 2008; 103:212–220 [Google Scholar]
- Beall MJ, Chandrashekar R, Eberts MD, Cyr KE, et al. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector-Borne Zoonotic Dis 2008; 8:455–464 [DOI] [PubMed] [Google Scholar]
- Bhide M, Travnicek M, Curlik J, Stefancıková A. The importance of dogs in eco-epidemiology of Lyme borreliosis: A review. Vet Med Czech 2004; 49:135–142 [Google Scholar]
- Chandrashekar R, Mainville CA, Beall MJ, Connor T, et al. Performance of a commercially available in-clinic ELISA for the detection of antibodies against Anaplasma phagocytophilum, Ehrlichia canis, Borrelia burgdorferi and Dirofilaria immitis antigen in dogs. Am J Vet Res 2010; 71:1443–1450 [DOI] [PubMed] [Google Scholar]
- Day MJ. One health: The importance of companion animal vector-borne diseases. Parasit Vectors 2011; 4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tommasi AS, Otranto D, Dantas-Torres F, Capelli G, et al. Are vector-borne pathogen co-infections complicating the clinical presentation in dogs? Parasit Vectors 2013; 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egyed L, Élő P, Sréter-Lancz ZS, Széll Z, et al. Seasonal activity and tick-borne pathogen infection rates of Ixodes ricinus ticks in Hungary. Ticks Tick-Borne Dis 2012; 3: 90–94 [DOI] [PubMed] [Google Scholar]
- Földvári G, Hell É, Farkas R. Babesia canis canis in dogs from Hungary: Detection by PCR and sequencing. Vet Parasitol 2005; 127:221–226 [DOI] [PubMed] [Google Scholar]
- Földvári G, Márialigeti M, Solymosi N, Lukács N, et al. Hard ticks infesting dogs in Hungary and their infection with Babesia and Borrelia species. Parasitol Res 2007; 101(Suppl):25–3417252272 [Google Scholar]
- Fok É. The importance of dirofilariosis in carnivores, humans in Hungary, past, present. In: Genchi C, Rinaldi L, Cringoli G, eds. Dirofilaria immitis and D. Repens in Dog and Cat and Human Infections. Naples, Italy: Rolando Editore, 2007:182–188 [Google Scholar]
- Genchi C, Rinaldi L, Cascone C, et al. Is heartworm disease really spreading in Europe? Vet Parasitol 2005; 133:137–148 [DOI] [PubMed] [Google Scholar]
- Greig B, Armstrong PJ. Canine granulocytotropic anaplasmosis (A. phagocytophilum infection) In: Green CE. Infectious Diseases of Dog, Cat, 3rd ed. St. Louis, MO: Saunders Elsevier, 2006:219–224 [Google Scholar]
- Harrus S, Waner T. Diagnosis of canine monocytotropic ehrlichiosis (Ehrlichia canis): An overview. Vet J 2011; 187:292–296 [DOI] [PubMed] [Google Scholar]
- Hornok S, Farkas R. First autochthonous infestation of dogs with Rhipicephalus sanguineus (Acari: Ixodidae) in Hungary: Case report and review of current knowledge on this tick species. Magy Állatorv Lapja 2005; 127:623–629. [In Hungarian]. [Google Scholar]
- Hornok S, Edelhofer R, Farkas R. Seroprevalence of canine babesiosis in Hungary suggesting breed predisposition. Parasit Res 2006; 99:638–642 [DOI] [PubMed] [Google Scholar]
- Hornok S, Fuente J, Horváth G, Nieuwenhuijs H, et al. Molecular evidence of Ehrlichia canis and Rickettsia massiliae in ixodid ticks of carnivores from South Hungary. Acta Vet Hung 2013; 61:42–50 [DOI] [PubMed] [Google Scholar]
- Inmaculada A, Tesouro MA, Kakoma I, Sainz Á. Serological Reactivity to Ehrlichia canis, Anaplasma phagocytophilum, Neorickettsia risticii, Borrelia burgdorferi and Rickettsia conorii in dogs from northwestern Spain. Vector Borne Zoonotic Dis 2008; 8:797–804 [DOI] [PubMed] [Google Scholar]
- Irwin PJ, Jefferies R. Arthropod-transmitted diseases of companion animals in Southeast Asia. Trends Parasitol 2004; 20:27–34 [DOI] [PubMed] [Google Scholar]
- Jacsó O, Mándoki M, Majoros G, Pétsch M, et al. First autochthonous Dirofilaria immitis (Leidy, 1856) infection in a dog in Hungary. Helminthologia 2009; 46:159–161 [Google Scholar]
- Kapiller Z, Bózsik B, Bognár Cs, Lakatos B. Lyme borreliosis in dogs. Kisállatorvoslás 1995; 6:303–306[in Hungarian with English abstract]. [Google Scholar]
- Karlsson U, Bjöersdorff A, Massung RF, Christensson B. Human granulocytic ehrlichiosis—a clinical case in Sweden. Scand J Infect Dis 2001; 33:73–74 [DOI] [PubMed] [Google Scholar]
- Kohn B, Silaghi C, Galke D, Arndt G, et al. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci 2011; 91:71–76 [DOI] [PubMed] [Google Scholar]
- Krupka I, Straubinger RK. Lyme borreliosis in dogs and cats: Background, diagnosis, treatment and prevention of infections with Borrelia burgdorferi sensu stricto. Vet Clin Small Anim 2010; 40:1103–1119 [DOI] [PubMed] [Google Scholar]
- Kybicová K, Schánilec P, Hulínská D, Uherková L, et al. Detection of Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in dogs in the Czech Republic. Vector-Borne Zoonotic Dis 2009; 9:655–661 [DOI] [PubMed] [Google Scholar]
- Liang FT, Steere AC, Marques AR, Johnson BJ, et al. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J Clin Microbiol 1999; 37:3990–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Irwin PJ, Lee S, Oh MH, et al. Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasit Vectors 2010; 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman MP, Goldstein RE, Labato MA, et al. Small animal consensus statement on Lyme disease in dogs: Diagnosis, treatment, and prevention. J Vet Intern Med 2006; 20:422–434 [DOI] [PubMed] [Google Scholar]
- McCall JW, Genchi C, Kramer LH, Guerrero J, et al. Heartworm disease in animals and humans. Adv Parasitol 2008; 66:193–285 [DOI] [PubMed] [Google Scholar]
- Mircean V, Dumitrache MO, Györke A, Pantchev N, et al. Seroprevalence and geographic distribution of Dirofilaria immitis and tick-borne infections (Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato, and Ehrlichia canis) in dogs from Romania. Vector Borne Zoonotic Dis 2012; 12:595–604 [DOI] [PubMed] [Google Scholar]
- Miró G, Montoya A. Roura X, Gálvez R, et al. Seropositivity rates for agents of canine vector-borne diseases in Spain: A multicentre study. Parasit Vectors 2013; 6:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor TP, Esty KJ, Hanscom JL, Shields P, et al. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin Diagn Lab Immun 2004; 11:458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantchev N, Schaper R, Limousin S, Norden N, et al. Occurrence of Dirofilaria immitis and tick-borne infections caused by Anaplasma phagocytophilum, Borrelia burgdorferi sensu lato and Ehrlichia canis in domestic dogs in France: Results of a countrywide serologic survey. Parasitol Res 2009; 105:S101–S113 [DOI] [PubMed] [Google Scholar]
- Philipp MT, Bowers LC, Fawcett PT, Jacobs MB, et al. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J Infect Dis 2001; 184:870–878 [DOI] [PubMed] [Google Scholar]
- Sréter T, Sréter-Lancz ZS, Széll Z, Kálmán D. Anaplasma phagocytophilum: An emerging tick-borne pathogen in Hungary and Central Eastern Europe. Ann Trop Med Parasitol 2004; 98:401–405 [DOI] [PubMed] [Google Scholar]
- Széll Z, Sréter-Lancz ZS, Márialigeti K, Sréter T. Temporal distribution of Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna in Hungary. Vet Parasitol 2006; 141:377–379 [DOI] [PubMed] [Google Scholar]