Abstract
The bark of Prunus yedoensis is used in antitussive medicines and in oral herbal formulations for inflammatory skin disorders. In the present study, we explored whether P. yedoensis bark extract (PYE) and its solvent partitioned fractions could modulate lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α and interleukin (IL)-6 in vivo and in vitro. In addition, we examined the effect of PYE extract and its fractions on LPS-induced NF-κB and mitogen-activated protein kinase (MAPK) signaling in mouse peritoneal macrophages. Oral treatment of PYE decreased serum levels of TNF-α and IL-6 in LPS injected mice. PYE inhibited LPS-induced TNF-α and IL-6 in macrophages at the transcriptional level and also suppressed LPS-induced IκBα degradation and MAPK activation in vitro. Among the fractions, the chloroform fraction, which contains genistein, naringenin, sakuranetin, prunetin, and amygdalin, showed inhibitory effects at much lower concentrations than the water and ethyl acetate fractions. Taken together, our results indicate that PYE was able to inhibit LPS-induced expression of TNF-α and IL-6, the latter of which was more prominent. The effects of PYE on inflammatory cytokine synthesis may involve modulation of NF-κB and MAPK activation.
Key Words: : cytokine, inflammation, macrophages, Prunus yedoensis bark
Introduction
Prunus yedoensis belongs to the Rosaceae family. The bark from several Prunus spp. comprise a major ingredient of antitussive medicines.1 In addition, P. yedoensis is a component of herbal formulations for inflammatory skin disorders. The leaves, bark, and fruit of P. yedoensis have been demonstrated to have antioxidant properties.2 Further, a recent study showed that leaf extracts of P. yedoensis stimulate glucose uptake in insulin sensitive skeletal muscle cells.3 Likewise, extracts from the bark of P. yedoensis have been shown to reduce the expression of various inflammatory chemokines in human keratinocytes.4
Inflammation is a protective physiological response for the removal of noxious substances. However, when inflammation is excessive, damage to the host tissue cannot be healed and chronic inflammatory disorders, including arthritis, autoimmune disease, and vascular disease, can occur. Macrophages are one of the major players in the first-line inflammatory responses, and mediate their biological functions by producing cytokines, eicosanoids, proteases, reactive oxygen species, and nitrogen intermediates. The major inflammatory cytokines produced by macrophages are tumor necrosis factor (TNF)-α, interleukin (IL)-1, and IL-6, all of which stimulate the upregulation of adhesion molecules in endothelial cells to promote the recruitment of circulating white blood cells and convert the vascular surface to a procoagulant state to attract platelets.5 Systemic responses to such inflammatory cytokines include fever, stimulation of white blood cell production in bone marrow, and the synthesis of acute phase proteins in the liver.5 At the cellular level, inflammatory cytokines are produced via NF-κB and mitogen-activated protein kinase (MAPK) pathways that act through autocrine and paracrine signaling mechanisms.6,7
Conventional approaches to anti-inflammatory therapy such as nonsteroidal anti-inflammatory drugs and glucocorticoids work by blocking the synthesis of eicosanoids. More recently, inflammatory cytokines have been targeted, and anti-TNF-α and anti-IL-1 drugs are now available. However, the majority of these anticytokine drugs are administered either subcutaneously or intravenously. In the present study, we explored whether oral administration of P. yedoensis bark extract (PYE) can modulate inflammatory cytokines in lipopolysaccharide (LPS)-injected mice. In addition, we examined the effect of PYE and its solvent partitioned fractions on TNF-α and IL-6 gene expression and NF-κB and MAPK signaling in LPS-stimulated mouse peritoneal macrophages.
Materials and Methods
Preparation of PYE
P. yedoensis bark was purchased from Dongwoodang Co. Ltd. (Yeongchen, South Korea) and verified by Professor Choi of the Department of Herbology at Kyung Hee University. The bark was pulverized and extracted three times in 30% aqueous ethanol under heating mantle-reflux. The extract was then evaporated and lyophilized. The yield of PYE was 10.52%. Next, 1 g of the lyophilized extract was subjected to fractionation by solvent partitioning with chloroform, ethyl acetate, and water. The resulting fractions were condensed and lyophilized. The yields of water, ethyl acetate, and chloroform fractions were 47.8%, 44.5%, and 2.3% respectively.
High performance liquid chromatography
Analyses were performed using a reversed-phase high performance liquid chromatography (HPLC) system (Agilent model 1200 series; Hewlett Packard, Palo Alto, CA, USA) with a Symmetry C18 column (5 μm×4.6 mm×250 mm; Waters, Milford, MA, USA) and a photodiode array detector. Chromatography was performed at room temperature at a flow rate of 0.5 mL/min, and 10 μL was analyzed for 110 min. The mobile phase consisted of 0.1% formic acid (A) and acetonitrile (B) in a ratio specified by the following binary gradient with linear interpolation: 0 min 20% B, 60 min 30% B, 70 min 60% B, and 100 min 70% B. The column eluent was monitored at a wavelength of UV 280 nm. Naringenin, genistein, prunetin, sakuranetin, and amygdalin were purchased from Sigma (St. Louis, MO, USA).
Animals
Eight-week-old male BALB/c mice were purchased from the Korean branch of Taconic, SamTaco (Osan, South Korea) and fed rodent chow and water ad libitum in a temperature- and humidity-controlled pathogen-free animal facility at the Medical Center of Kyung Hee University Hospital. Mice were maintained in accordance with the Guide for the Care and Use of Laboratory Animals issued by the U.S. National Research Council (1996), and the study protocol (KHMC-IACUC12-006) was approved by the Kyung Hee University Medical Center Institutional Animal Care and Use committee.
In vivo LPS injection
PYE was given orally to mice at doses of 10, 50, or 250 mg/kg body weight for seven days. Control mice received an equal volume of phosphate buffered saline (PBS) during the experimental period. On day 7, LPS (serotype 055:B5, 1.3 mg/kg; Sigma) was injected intraperitoneally 1 h before serum sampling. Dexamethasone (5 mg/kg; Sigma) was used as a reference drug and intraperitoneally administered 16 h before LPS stimulation, as previously described.8 Blood was obtained by cardiac puncture and centrifuged. The obtained serum samples were stored at −20°C until assayed.
Cytokine measurement
Levels of TNF-α and IL-6 from serum were measured by enzyme-linked immunosorbant assay (ELISA) according to the manufacturer's protocol (BD Pharmingen, San Diego, CA, USA).
Isolation and culture of peritoneal macrophages
Mice were injected intraperitoneally with 2 mL of sterile thioglycollate medium (BD, Spork, MD, USA), and macrophages were collected three days later by peritoneal gavage with cold Dulbecco's modified Eagle's medium (DMEM). After centrifugation, cells were resuspended in DMEM with 10% fetal bovine serum and incubated for 3 h at 37°C with 5% CO2. Nonadherent cells were subsequently removed.
Lactate dehydrogenase release assay
Cells were seeded in 48-well plates and incubated with PYE or its fractions for 24 h. Supernatants were collected and lactate dehydrogenase (LDH) levels were measured with the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega, Madison, WI, USA) according to the manufacturer's instructions. Extracellular LDH content was presented as optical density using a 96-well microplate reader (Molecular Devices, Sunnyvale, CA, USA).
cDNA preparation and real-time polymerase chain reaction
Cells were seeded in six-well plates and pretreated with PYE or its fractions for 1 h, followed by stimulation with 100 ng/mL LPS for 4 h. Total RNA was isolated using an RNeasy Mini Kit (Qiagen, Hilden, Germany), and cDNA was reverse-transcribed using Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Diluted cDNA was mixed with Power SYBR Green PCR Master mix (Applied Biosystems, Foster City, CA, USA) and 2 pmol of primers for TNF-α, IL-6, or Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following forward and reverse primer sequences were used: TNF-α, forward: 5′-ATG ATC GCG GAC GTG GAA-3′ and reverse: 5′-AGG GCC TGG AGT TCT GGA A-3′; IL-6, forward: 5′-AGG ATA CCA CTC CCA ACA GAC CT-3′ and reverse: 5′-GGA ACT CAC CTT TCT GGT TAC A-3′; GAPDH, forward: 5′-GGC ATG GAC TGT GGT CAT GA-3′ and reverse: 5′-TTC ACC ACC ATG GAG AAG GC-3′. Amplification of cDNA was performed in triplicate using a StepOne real-time polymerase chain reaction (PCR) system (Applied Biosystems). After an initial heat denaturation at 95°C for 10 min, PCR conditions were set at 95°C for 15 sec and 60°C for 1 min for 40 cycles. For each PCR, a corresponding mRNA sample without RT was included as a negative control. Quantification of each cDNA copy number was determined according to the manufacturer's protocol. GAPDH was used as an endogenous control.
Western blot analysis
Cells were seeded in six-well plates and pretreated with PYE or its fractions for 1 h and then stimulated with LPS for 30 min. Total cell extracts were prepared by resuspending the cells in lysis buffer (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 20 mM NaF; 0.5% NP-40; and 1% Triton X-100) containing a phosphatase inhibitor (Sigma) and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein concentrations of lysates were determined by Bradford assay. Cell extracts were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to polyvinylidene fluoride membranes. Membranes were blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 h, followed by incubation with IκBα, tubulin (Santa Cruz Biotechnology, CA, USA), phospho-JNK, phospho-ERK1/2, and phospho-p38 diluted in 5% skim milk in TBST overnight at 4°C. Blots were then washed with TBST and incubated for 1 h with an antirabbit horseradish peroxidase-conjugated antibody. Immunoreactive bands were developed using the Enhanced Chemiluminescence System (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom).
Statistical analysis
Statistical evaluation was carried out by one-way analysis of variance followed by Dunnet's post hoc test. Calculations were carried out using SPSS v20 (IBM Corp., Amonk, NY, USA). P-values<.05 were considered significant.
Results
HPLC analysis
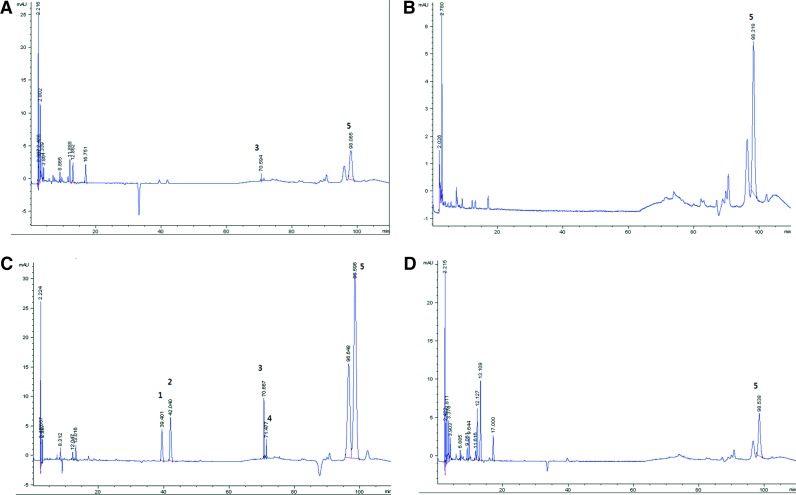
The HPLC chromatograms of PYE and its water, ethyl acetate, and chloroform fractions are shown in Figure 1. The peaks identified in our analysis were genistein, naringenin, sakuranetin, prunetin, and amygdalin. Genistein, naringenin, sakuranetin, and prunetin were identified in the chloroform fraction, while amygdalin was present in all these fractions.
FIG. 1.
High performance liquid chromatography (HPLC) chromatograms of Prunus yedoensis bark extract (PYE) and its water, ethyl acetate, and chloroform fractions. 1, genistein; 2, naringenin; 3, sakuranetin; 4, prunetin; 5, amygdalin. (A) PYE, (B) water fraction, (C) chloroform fraction, and (D) ethyl acetate fraction. Color images available online at www.liebertpub.com/jmf
Effect of PYE on serum levels of TNF-α and IL-6 after LPS injection in mice
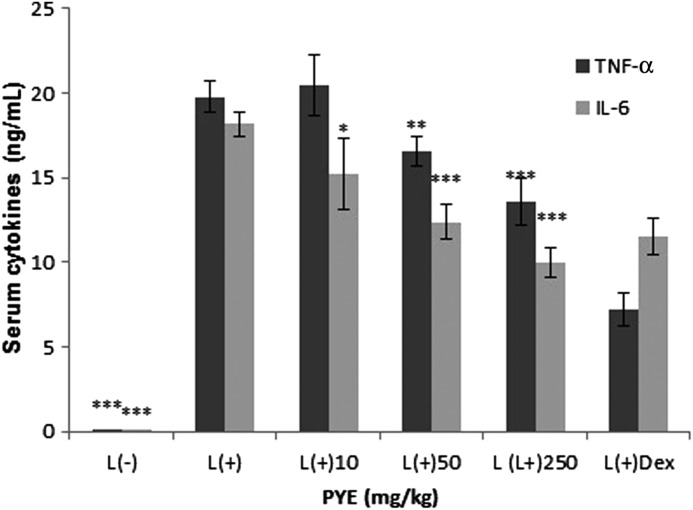
PYE was given orally to mice at doses of 10, 50, and 250 mg/kg for seven consecutive days, followed by intraperitoneal injection of a sublethal dose of LPS (1.3 mg/kg).9 Peak serum release of TNF-α and IL-6 are reported to occur at 1 h and 2 h after LPS injection respectively. Thus, we obtained serum 1 h after LPS challenge. Serum levels of TNF-α were significantly inhibited in the 250 mg/kg and dexamethasone-treated groups, and IL-6 was significantly lower in the 50 mg/kg and 250 mg/kg groups and the dexamethasone-treated group (Fig. 2). Our results showed that IL-6 was more responsive to PYE treatment than TNF-α.
FIG. 2.
Effect of PYE on systemic inflammatory responses. PYE (10, 50, and 250 mg/kg) was administered orally to mice for seven days before intraperitoneal injection of lipopolysaccharide (LPS; 1.3 mg/kg). Dexamethasone (Dex) was intraperitoneally injected 16 h before LPS stimulation. Serum was obtained 1 h after LPS stimulation, and the levels of TNF-α and IL-6 were measured by enzyme-linked immunosorbant assay (ELISA). Data are expressed as the means±standard deviation (SD; n=9–12). *P<.05, **P<.01, ***P<.005 compared with the control group.
Effect of PYE and its fractions on macrophage cytotoxicity in vitro
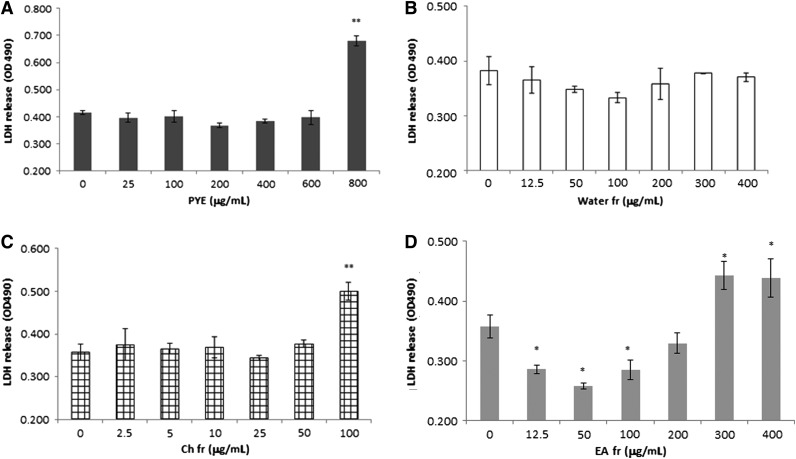
Because macrophages are the main cell sources of TNF-α and IL-6 secretion in response to LPS, peritoneal macrophages were isolated and used for in vitro assays. First, we measured extracellular LDH in order to investigate cytotoxicity. PYE was cytotoxic at high concentrations (800 μg/mL; Fig. 3A). Further, fractional cytotoxicity was measured using the same method. The concentration range of the water, ethyl acetate, and chloroform fractions was determined based on the ratio of yield. In case of the chloroform fraction, we extended its maximum concentration up to 100 μg/mL, much more than required. At high concentrations, the ethyl acetate fraction (400 μg/mL) and the chloroform fraction (100 μg/mL) were cytotoxic to cells (Fig. 3B–D).
FIG. 3.
Cytotoxicity of PYE and its solvent partitioned fractions in vitro. Peritoneal macrophages were isolated from thioglycollate-injected mice and cultured in the presence of PYE, water, ethyl acetate, or chloroform for 24 h. Extracellular lactate dehydrogenase (LDH) release was presented as optical density. Each bar represents means±SD of four independent experiments. (A) PYE, (B) water fraction, (C) chloroform fraction, and (D) ethyl acetate fraction. *P<.05, **P<.01 compared with untreated cells.
Effect of PYE and its fractions on LPS-induced TNF-α and IL-6 gene expression in vitro
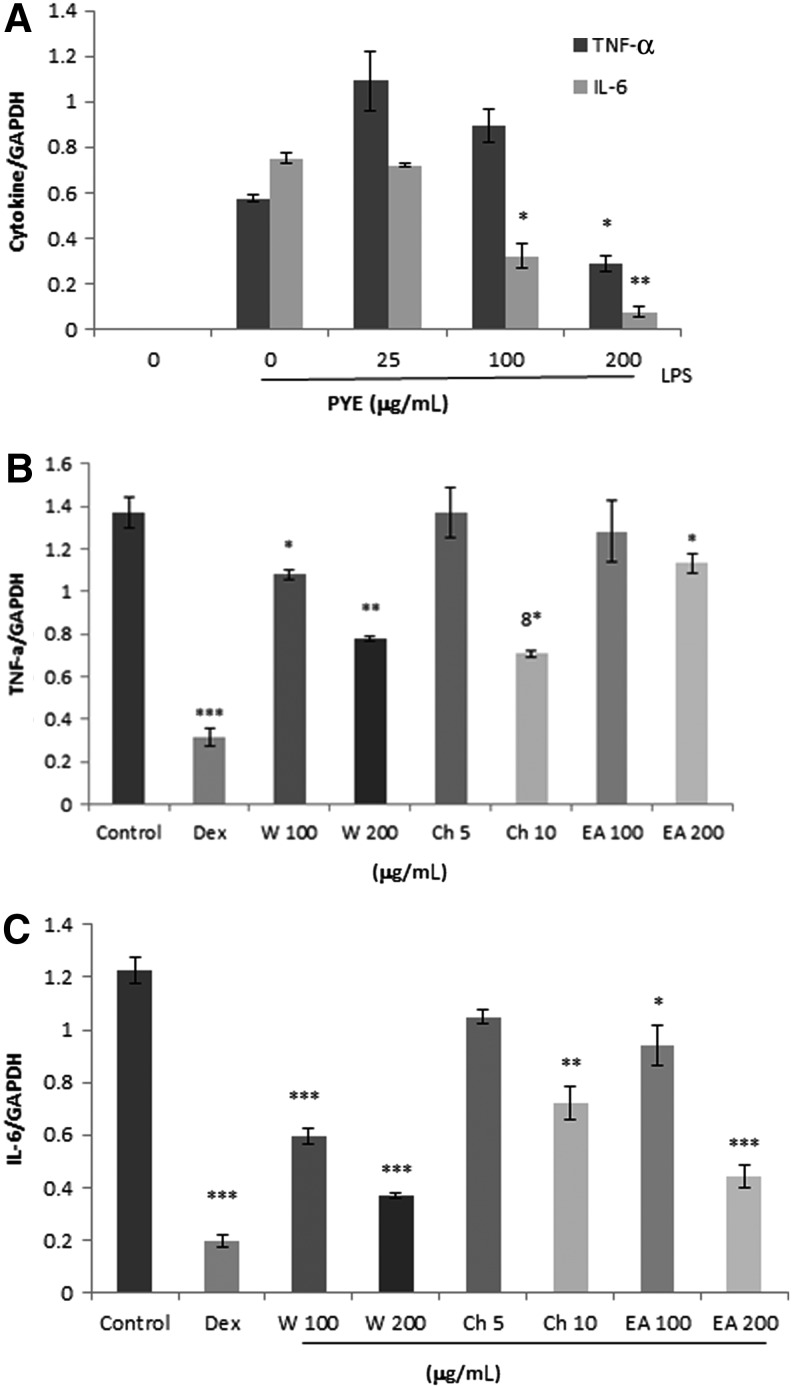
We investigated whether PYE could affect TNF-α and IL-6 at the transcriptional level. Macrophages were pretreated for 1 h with 25, 100, and 250 μg/mL of PYE followed by LPS stimulation for 4 h. Using real-time PCR, the expression of TNF-α and IL-6 genes were analyzed. IC50 values for TNF-α and IL-6 mRNA expression were 200 and 100 μg/mL respectively (Fig. 4A). Consistent with the in vivo results, IL-6 was more sensitive than TNF-α to PYE treatment in vitro. Subsequently, we measured the ability of the water, ethyl acetate, and chloroform fractions to inhibit these cytokine genes. Likewise, 100 and 200 μg/mL of the water and ethyl acetate fractions and 5 and 10 μg/mL of the chloroform fraction were chosen according to the percentage of each fraction. The concentration of dexamethasone (1 μM) was described as previously.10 TNF-α and IL-6 expression decreased with increasing concentration of the water fraction (Fig. 4B). The ethyl acetate and chloroform fractions reduced the expression of IL-6 gene in a dose-dependent manner, and decreased TNF-α gene expression was observed at high concentrations (Fig. 4B).
FIG. 4.
Effect of PYE and its fractions on LPS-induced TNF-α and IL-6 gene expression. Peritoneal macrophages were pretreated 1 h with PYE (A) or its fractions (B) and (C), and then stimulated for 4 h with LPS (100 ng/mL); 1 μM dexamethasone was used as a reference drug. Total RNA was extracted and real-time polymerase chain reaction (PCR) was performed. GAPDH was used as an internal control. Data are expressed as means±SD of three independent experiments. *P<.05, **P<.005, ***P<.001 compared with cells treated with LPS only (control).
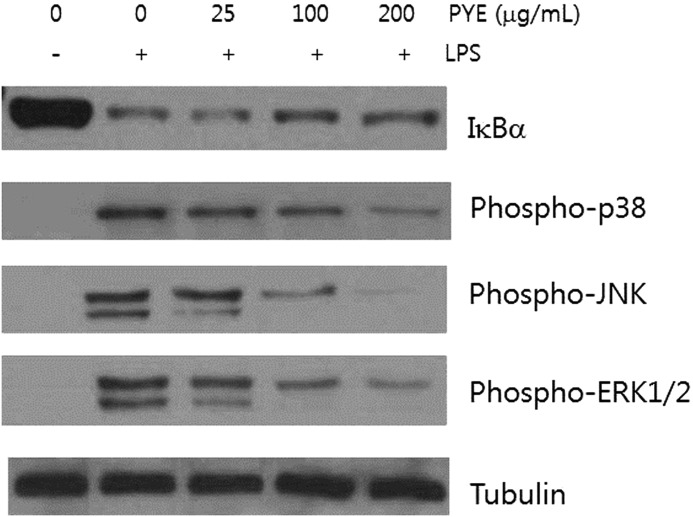
Effect of PYE and its fractions on IκBα and MAPK signaling molecules
NF-κB and MAPK play an important role in relaying external inflammatory signals to the nucleus and promoting the expression of inflammatory proteins. Under normal conditions, NF-κB exists as a complex with IκB proteins in the cytosol. Various stimuli, including LPS, can induce a series of membranous and cytosolic adaptor proteins to phosphorylate IκB kinase (IKK), which can then phosphorylate IκB proteins. This leads to ubiquitination and subsequent degradation of IκB by the proteasome, resulting in translocation of free NF-κB to the nucleus. We obtained whole protein lysates after exposure to LPS for 30 min in the presence of PYE. Degradation of IκBα, a major member of the IκB family, was decreased in cells treated with 100 and 200 μg/mL of PYE (Fig. 5A). These results suggested that PYE may affect NFκB signaling by interfering with pathways upstream of IκBα degradation.
FIG. 5.
Effect of PYE on LPS-induced IκBα degradation and MAPK activation. Peritoneal macrophages were pretreated 1 h with PYE and then stimulated for 30 min with LPS. Whole protein was extracted and examined by Western blot analysis. Tubulin was used as an internal control. One of the three experiments is shown.
p38, JNK, and ERK1/2, which are the major mammalian MAPKs, are activated by upstream protein kinases in response to inflammatory stimuli and phosphorylate nuclear proteins to enhance the expression of TNF-α and IL-6 genes, and stabilize cytokine mRNA in the cytosol.6 PYE at 100 and 200 μg/mL decreased the activity of p38, JNK, and ERK1/2 (Fig. 5).
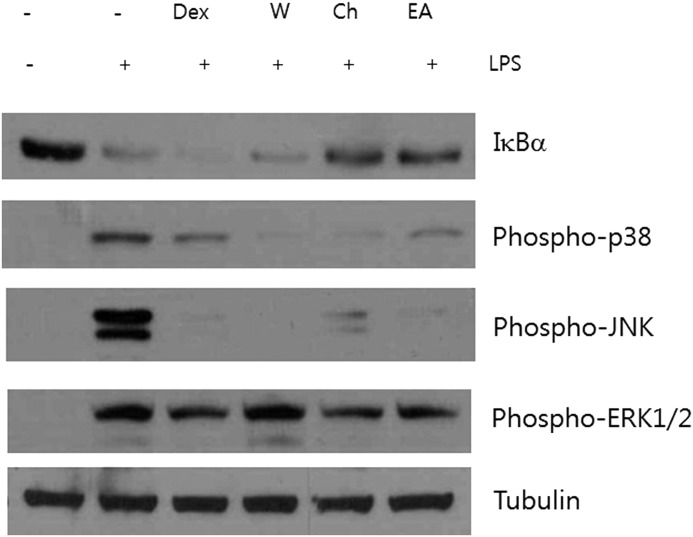
We next examined the ability of each fraction to inhibit IκBα degradation and MAPK phosphorylation. The chloroform and ethyl acetate fractions inhibited the degradation of IκBα, but dexamethasone did not, as previously described11 (Fig. 6). The suppressive effects on phosphorylated p38 and JNK were observed in all these fractions, and the inhibitory effects on ERK1/2 activation were in the chloroform and ethyl acetate fractions.
FIG. 6.
Effect of water, chloroform, and ethyl acetate fractions from PYE on LPS-induced IκBα degradation and MAPK activation. Peritoneal macrophages were pretreated 1 h with 1 μM dexamethasone (Dex), 200 μg/mL water fraction (W), 10 μg/mL chloroform fraction (Ch), and 200 μg/mL ethyl acetate fraction (EA), and then stimulated for 4 h with LPS. Whole protein was extracted and examined by Western blot analysis. Tubulin was used as an internal control. One of the three experiments is shown.
Discussion
The results of the present study show that oral treatment with PYE decreased serum levels of TNF-α and IL-6 using the LPS-stimulated inflammation model. At the cellular level, PYE affected LPS-induced NF-κB and MAPK signaling molecules. Among the fractions, the chloroform fraction showed anti-inflammatory activities at much lower concentrations than the water and ethyl acetate fractions.
Oral application of PYE in vivo was more effective for decreasing IL-6 release than for TNF-α, which was consistent with our in vitro results. Although TNF-α and IL-6 overlap with respect to their biological functions, induction of TNF-α occurs more rapidly than that of IL-6, and the pathways involved in the production of these cytokines are not identical.12,13 IL-6 is synthesized not only in response to LPS but also to TNF-α, although the latter occurs at later time points.14 Thus, the decreased levels of IL-6 by PYE were likely not due to a secondary effect exerted by decreased levels of TNF-α. Different fractions of PYE appeared to act on each of these pathways, although it appeared that PYE contained components that are more inhibitory toward IL-6 than TNF-α. Specifically, our fractionation studies showed that the IC50 value of the chloroform fraction for TNF-α was 10 μg/mL, a concentration 20 times lower than that of the water fraction, whereas all of the fractions inhibited IL-6, albeit with varying potencies. Since the concentration range of the chloroform fraction assayed was based on the relative ratio of yield, its maximum concentration was very low. But, when assayed at a high concentration (100 μg/mL) that would be contained in 2000 μg/mL of PYE, the chloroform fraction completely inhibited the secretion of TNF-α and IL-6 (data not shown).
According to our HPLC analysis, the chloroform fraction contained genistein, prunetin, naringenin, sakuranetin, and amygdalin. Genistein, a phytoestrogen, showed in vivo anti-inflammatory effects in both a delayed type hypersensitivity model in response to oxazolone and collagen-induced arthritis.15 Genistein reduced the expression of nitric oxide, TNF-α, and IL-6 by modulating NF-κB in various macrophage cell lines.16,17 Prunetin, a methylated genistein prodrug, is itself biologically active. Recently, intraperitoneal application of prunetin decreased the serum levels of TNF-α and IL-6 in LPS-injected mice, and lowered the mRNA expression of TNF-α but not IL-6.18 The same study also demonstrated an inhibitory role of prunetin in IκBα degradation and activation of p38 and JNK. Naringenin was reported to decrease LPS-stimulated TNF-α production in macrophages.19 Sakuranetin showed anti-inflammatoy activity in an experimental asthma model in vivo via control of NF-κB activation.20 Amygdalin, a cynogenic compound, occurs in several species of the Prunus genus. The presence of amygdalin may account for the cytotoxicity observed in PYE. Amygdalin was reported to exert anti-inflammatory activity by inhibiting LPS-induced TNF-α production in mouse RAW264.7 macrophage cells.21 The inflammatory activity displayed by the ethyl acetate and water fractions may be partly attributable to amygdalin.
Taken together, our results indicate that PYE was able to inhibit LPS-induced expression of TNF-α and IL-6, the latter of which was more prominent. The effects of PYE on inflammatory cytokine synthesis may involve modulation of NF-κB and MAPK activation.
Acknowledgment
This study was supported by a grant of the Traditional Korean Medicine R&D Project, Ministry of Health and Welfare, Republic of Korea (B110081).
Author Disclosure Statement
The authors declare that they have no competing financial interests.
References
- 1.Matsuoka N, Ikeda T, El-Aasr M,Manabe H, Murakami Y, Deguchi H, Nohara T: Study of the chemical constituents of Pruni Cortex and its related parts. J Nat Med 2011;65:166–171 [DOI] [PubMed] [Google Scholar]
- 2.Jung HA, Kim AR, Chung HY, Choi JS: In vitro antioxidant activity of some selected Prunus species in Korea. Arch Pharm Res 2002;25:865–872 [DOI] [PubMed] [Google Scholar]
- 3.Jo K, Lee SE, Lee SW, Hwang JK: Prunus yedoensis Matsum. stimulates glucose uptake in L6 rat skeletal muscle cells by activating AMP-activated protein kinase and phosphatidylinositol 3-kinase/Akt pathways. Nat Prod Res 2012;26:1610–1615 [DOI] [PubMed] [Google Scholar]
- 4.Kang GJ, Lee HJ, Yoon WJ, Yang EJ, Park SS, Kang HK, Park MH, Yoo ES: Prunus yedoensis inhibits the inflammatory chemokines, MDC and TARC, by regulating the STAT1-signaling pathway in IFN-g-stimulated HaCaT human keratinocytes. Biomol Ther 2008;16:394–402 [Google Scholar]
- 5.Semenzato G: Tumour necrosis factor: a cytokine with multiple biological activities. Br J Cancer 1990;61:354–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaestel M, Kotlyarov A, Kracht M: Targeting innate immunity protein kinase signalling in inflammation. Nat Rev 2009;8:480–499 [DOI] [PubMed] [Google Scholar]
- 7.Hayden MS, Ghosh S: Shared principles in NF-kappaB signaling. Cell 2008;132:344–362 [DOI] [PubMed] [Google Scholar]
- 8.Aoki K, Ishida Y, Kikuta N,Kawai H, Kuroiwa M, Sato H: Role of CXC chemokines in the enhancement of LPS-induced neutrophil accumulation in the lung of mice by dexamethasone. Biochem Biophys Res Commun 2002;294:1101–1108 [DOI] [PubMed] [Google Scholar]
- 9.Purswani MU, Eckert SJ, Arora HK, Noel GJ: Effect of ciprofloxacin on lethal and sublethal challenge with endotoxin and on early cytokine responses in a murine in vivo model. J Antimicrob Chemother 2002;50:51–58 [DOI] [PubMed] [Google Scholar]
- 10.Bhattacharyya S, Ratajczak CK, Vogt SK,Kelley C, Colonna M, Schreiber RD, Muglia L: TAK1 targeting by glucocorticoids determines JNK and IκB regulation in Toll-like receptor-stimulated macrophages. Blood 2010; 115:1921–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M: Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995;270:286–290 [DOI] [PubMed] [Google Scholar]
- 12.Wang SW, Pawlowski J, Wathen ST,Kinney SD, Lichenstein HS, Manthey CL: Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm Res 1999;48:533–538 [DOI] [PubMed] [Google Scholar]
- 13.Kim K, Duramad O, Qin XF, Su B: MEKK3 is essential for lipopolysaccharide-induced interleukin-6 and granulocyte-macrophage colony-stimulating factor production in macrophages. Immunology 2007;120:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghezzi P, Sacco S, Agnello D,Marullo A, Caselli G, Bertini R: LPS induces IL-6 in the brain and in serum largely through TNF production. Cytokine 2000;12:1205–1210 [DOI] [PubMed] [Google Scholar]
- 15.Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A: Genistein as an anti-inflammatory agent. Inflamm Res 2003;52:341–346 [DOI] [PubMed] [Google Scholar]
- 16.Ji G, Zhang Y, Yang Q,Cheng S, Hao J, Zhao X, Jiang Z: Genistein suppresses LPS-induced inflammatory response through inhibiting NF-kappaB following AMP kinase activation in RAW 264.7 macrophages. PLoS One 2012;7:e53101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Mazza G: Effects of anthocyanins and other phenolic compounds on the production of tumor necrosis factor alpha in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem 2002;50:4183–4189 [DOI] [PubMed] [Google Scholar]
- 18.Yang G, Ham I, Choi HY: Anti-inflammatory effect of prunetin via the suppression of NF-kappaB pathway. Food Chem Toxicol 2013;58C:124–132 [DOI] [PubMed] [Google Scholar]
- 19.Lyu SY, Park WB: Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch Pharm Res 2005;28:573–581 [DOI] [PubMed] [Google Scholar]
- 20.Toledo AC, Sakoda CP, Perini A,Pinheiro NM, Magalhaes RM, Grecco S, Tiberio IF, Camara NO, Martins MA, Lago JH, Prado CM: Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br J Pharmacol 2013;168:1736–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin KM, Kim IT, Hong SP, Hong JP, Lee KT: In vitro antiinflammatory activity of amygdalin in murine macrphages RAW 264.7 cells. Kor J Pharmacogn 2003;34:223–227 [Google Scholar]