Abstract
Background: Low serum vitamin D levels have been associated with several autoimmune diseases, but their association with thyroid autoimmunity is unclear. We evaluated the association of serum vitamin D levels with the prevalence of autoimmune thyroid disease (AITD).
Methods: Our cross-sectional study included subjects who underwent routine health checkups, which included assays of serum 25-hydroxy vitamin D3 [25(OH)D3] and anti–thyroid peroxidase antibody (TPO-Ab), as well as thyroid ultrasonography (US) between 2008 and 2012 at the Asan Medical Center. We defined AITD according to the levels of TPO-Ab and US findings.
Results: A total of 6685 subjects (58% male; 42% female) were enrolled for this study. Overall prevalence of TPO-Ab positivity and both TPO-Ab/US positivity were 10.1% (6.3% male; 15.3% female) and 5.4% (2.3% male; 9.7% female) respectively. In female subjects, mean serum 25(OH)D3 levels were significantly lower in the TPO-Ab(+) (22.0 vs. 23.5 ng/mL, p=0.030) and TPO-Ab(+)/US(+) groups (21.6 vs. 23.4 ng/mL, p=0.027) compared with the control group, respectively. According to the levels of serum 25(OH)D3, the prevalence of TPO-Ab positivity (21.2%, 15.5%, and 12.6% in deficient, insufficient, and sufficient group, respectively; p=0.001) and both TPO-Ab and US positivity (14.7%, 9.9%, and 7.1% in deficient, insufficient, and sufficient group, respectively; p<0.001) decreased in female subjects. Interestingly, this pattern was significant only in pre-menopausal women (p=0.003 and p<0.001; respectively), but not in postmenopausal women. Multivariate analysis indicated that the adjusted odds ratios (OR) for AITD among those in the 25(OH)D3-deficient [TPO-Ab(+): OR 1.95, p=0.001; TPO-Ab(+)/US(+): OR 2.36, p<0.001] and -insufficient groups [TPO-Ab(+): OR 1.31, p=0.043; TPO-Ab(+)/US(+): OR 1.50, p=0.017] were significantly increased when compared with the sufficient group.
Conclusions: The levels of serum vitamin D were significantly lower in pre-menopausal women with AITD. Vitamin D deficiency and insufficiency were significantly associated with AITD in pre-menopausal women.
Introduction
The classical main function of vitamin D is a regulation of calcium homeostasis, which is primarily maintained via bone formation and resorption (1). In more recent years, the actions of vitamin D have been shown to go beyond calcium metabolism to include cell growth, differentiation, maturation and apoptosis, anticarcinogenic effects, and anti-autoimmune activities (2,3). In particular, the role of vitamin D as an immune modulator has been emphasized in recent reports (4–6).
Vitamin D deficiency has been found to correlate with an increased incidence of autoimmune diseases, including type 1 diabetes mellitus, rheumatoid arthritis, systemic lupus erythematosus, multiple sclerosis (MS), and Crohn's disease (2–7). In some animal experiments, vitamin D supplements prevented the development and progression of autoimmune disorders such as MS and type 1 diabetes mellitus (8–10). However, it is unclear whether low vitamin D levels closely correlate with the development of autoimmune thyroid disease (AITD). Small case-control studies have suggested lower serum vitamin D levels or a higher prevalence of vitamin D insufficiency in patients with AITD compared with healthy controls (11–16). However, another study reported no significant association between the serum vitamin D levels and thyroid autoimmunity (17). A recent study from the Amsterdam AITD cohort indicated that de novo development of anti–thyroid peroxidase antibody (TPO-Ab) was not associated with low vitamin D levels (18).
In our present study, we evaluated the serum 25-hydroxy vitamin D3 [25(OH)D3] levels according to the levels of serum TPO-Ab and thyroid ultrasonography (US) findings from a relatively large cohort. Importantly, we investigated the prevalence of TPO-Ab positivity and both TPO-Ab and US positivity according to the serum 25(OH)D3 status in men, as well as in both pre- and postmenopausal women. Our findings indicate that thyroid autoimmunity is significantly associated with lower vitamin D levels only in pre-menopausal women.
Materials and Methods
Subjects
We included subjects who underwent routine health checkups at the Asan Medical Center between 2008 and 2012. All subjects were interviewed and examined by physicians in the health promotion center. Information about menopausal status, history of previous disease and medication, and history of previous surgery for each subject was obtained via self-administered questionnaires. Subjects who were tested for serum levels of 25(OH)D3, calcium, and TPO-Ab, as well as simultaneously being examined using thyroid US, were enrolled in this study. However, subjects younger than 25 years of age or older than 80 years of age, pregnant women, and those with abnormal liver or kidney function, a history of any thyroid disease or thyroid surgery, or any medication history that involved taking calcium or vitamin D supplements were excluded. Seasons of health checkup date were categorized as spring (March to May), summer (June to August), autumn (September to November), and winter (February to December) (19). The study was approved by the institutional review board of the Asan Medical Center.
Laboratory measurement
Blood samples were collected in the morning from the subjects after an overnight fast. The serum 25(OH)D3 concentration was determined using the DIAsource 25OH-Vit.D3-Ria-CT Kit (DIAsource ImmunoAssays S.A., Louvain-La-Neuve, Belgium; Cobra II Auto-γ Counting System, Packard Instruments, Downers Grove, IL). We defined serum 25(OH)D3 levels as vitamin D deficient (<10 ng/mL, n=403), insufficient (10–30 ng/mL, n=4303), or sufficient (>30 ng/mL, n=1979) (20). The serum TPO-Ab concentration was determined using a BRAHMS anti-TPOn Radioimmunoassay (RAI) kit (Thermo Scientific, Limburg, Germany) with a functional sensitivity of 30 U/mL. A TPO-Ab level exceeding 60 U/mL was considered to constitute a positive TPO-Ab reading. Levels of serum thyrotropin (TSH) and free thyroxine (fT4) were measured using the TSH-CTK-3 immunoradiometric assay (IRMA) kit (DiaSorin S.p.A, Saluggia, Italy) and fT4 radioimmunoassay (RIA) kit (Beckman Coulter/Immunotech, Prague, Czech Republic), respectively. Standard liver function tests, kidney function tests, and serum calcium levels were measured using the TBA-200FR instrument (Toshiba Medical Systems, Tokyo, Japan) (21).
US examinations
All US examinations were performed using one of two US instruments: an iU22 unit (Philips Healthcare, Bothell, WA) or an HDI-5000 device (Philips Healthcare) equipped with a linear high-frequency probe (4–15 MHz). During US examination, the neck was extended in the supine position. The scanning protocol in all cases included both transverse and longitudinal real-time imaging of the thyroid. Examinations were performed by experienced radiologists with 2–16 years' US experience. Diffuse thyroid parenchymal disease was defined as having a heterogeneous parenchymal echogenicity on US and/or decreased vascularity on color Doppler sonogram.
Definition of AITD
We defined AITD in two different ways: serum TPO-Ab positivity [TPO-Ab(+) group, n=673] and both TPO-Ab and US positivity [TPO-Ab(+)/US(+) group, n=358].
Statistical analysis
Continuous variables were expressed as means (±standard deviation) and categorical variables are presented as numbers (percentage). Continuous variables were compared using the Student's t-test. Comparisons between each group according to categorical variables were done using a chi-square test (two-sided). The Cochran–Armitage trend test was used to compare the prevalence of AITD relative to vitamin D levels. We performed multivariate analysis using a binary logistic regression model. The R software package (v2.15.2; R Foundation for Statistical Computing, Vienna, Austria; www.R-project.org) was used for statistical analysis. All p-values were two-sided, and p<0.05 was considered to denote statistical significance.
Results
Clinical characteristics according to TPO-Ab levels
A total of 6685 subjects (58% male; 42% female) were enrolled for this study. The mean age was 54.1 years, and the mean body mass index (BMI) was 24.2 kg/m2. The overall prevalence of TPO-Ab positivity was 10.1% (6.3% male; 15.3% female). The characteristics of the study subjects in terms of the TPO-Ab positivity are summarized in Table 1.
Table 1.
Characteristics of Study Subjects in Terms of the Level of Serum Anti–Thyroid Peroxidase Antibody
| Total (n=6685) | TPO-Ab(−) (n=6012) | TPO-Ab(+) (n=673) | p-Value | |
|---|---|---|---|---|
| Age (years) | 54.1±8.9 | 54.1±8.9 | 54.4±8.3 | 0.414 |
| Female [n (%)] | 2793 (41.8) | 2366 (39.4) | 427 (63.4) | <0.001 |
| BMI (kg/m2) | 24.2±3.2 | 24.2±3.2 | 23.9±3.1 | 0.019 |
| Smoking [n (%)] | 3258 (48.7) | 3012 (50.1) | 246 (36.6) | <0.001 |
| Serum calcium (mg/dL) | 8.9±0.3 | 8.9±0.3 | 8.9±0.3 | 0.882 |
| TSHa (mU/L) | 2.0±1.0 | 2.0±1.0 | 2.1±1.0 | 0.02 |
| fT4 (ng/dL) | 1.3±0.2 | 1.3±0.2 | 1.3±0.4 | 0.153 |
| TPO-Aba (U/mL) | 13.4±10.1 | 10.5±10.1 | 522.7±10.2 | <0.001 |
| Health checkup seasons [n (%)] | 0.069 | |||
| Spring | 1914 (29) | 1741 (29) | 200 (30) | |
| Summer | 1912 (29) | 1712 (28) | 200 (30) | |
| Autumn | 1518 (23) | 1352 (22) | 166 (25) | |
| Winter | 1314 (20) | 1207 (20) | 107 (16) | |
| Health checkup months [n (%)] | 0.248 | |||
| Mar | 649 (10) | 590 (10) | 59 (9) | |
| Apr | 688 (10) | 607 (10) | 81 (12) | |
| May | 604 (9) | 544 (9) | 60 (9) | |
| Jun | 622 (9) | 555 (9) | 67 (10) | |
| Jul | 667 (10) | 594 (10) | 73 (11) | |
| Aug | 623 (9) | 563 (9) | 60 (9) | |
| Sep | 455 (7) | 406 (7) | 49 (7) | |
| Oct | 534 (8) | 469 (8) | 65 (10) | |
| Nov | 529 (8) | 477 (8) | 52 (8) | |
| Dec | 361 (5) | 337 (6) | 24 (4) | |
| Jan | 476 (7) | 432 (7) | 44 (7) | |
| Feb | 477 (7) | 438 (7) | 39 (6) | |
Geometric mean.
AITD, autoimmune thyroid disease; BMI, body mass index; fT4, free thyroxine; TPO-Ab, anti–thyroid peroxidase antibody; TSH, thyrotropin.
Serum 25(OH)D3 levels according to TPO-Ab positivity
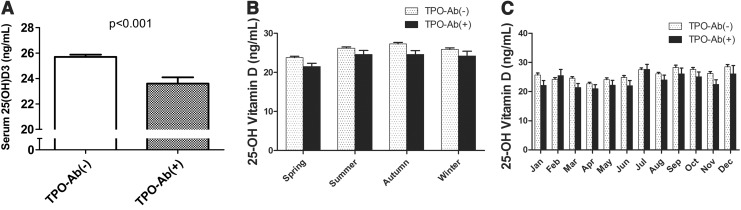
We compared the serum 25(OH)D3 levels between the TPO-Ab(−) group and TPO-Ab(+) group. The level of serum 25(OH)D3 was significantly lower in the TPO-Ab(+) group when compared with the TPO-Ab(−) group (23.6±0.5 vs. 25.7±0.2 ng/mL, p<0.001; Fig. 1A). The number of subjects who measured serum 25(OH)D3 levels in each season and months were not different between the two groups (Table 1). The difference in 25(OH)D3 levels between the two groups in each season are shown in Figure 1B. The 25(OH)D3 levels in the TPO-Ab(+) group tend to be lower than those in the TPO-Ab(−) group in each season, and there was a significant difference in spring and autumn. Serum 25(OH)D3 levels in each month between the two groups have a similar pattern except in February (Fig. 1C).
FIG. 1.
Serum levels of serum 25-hydroxy vitamin D3 [25(OH)D3] in the study subjects according to the anti–thyroid peroxidase antibody (TPO-Ab) positivity. (A) Levels of serum 25(OH)D3 in subjects from the TPO-Ab(−) group and TPO-Ab(+) group. (B) Serum levels of 25(OH)D3 according to each season. (C) Serum levels of 25(OH)D3 according to each month.
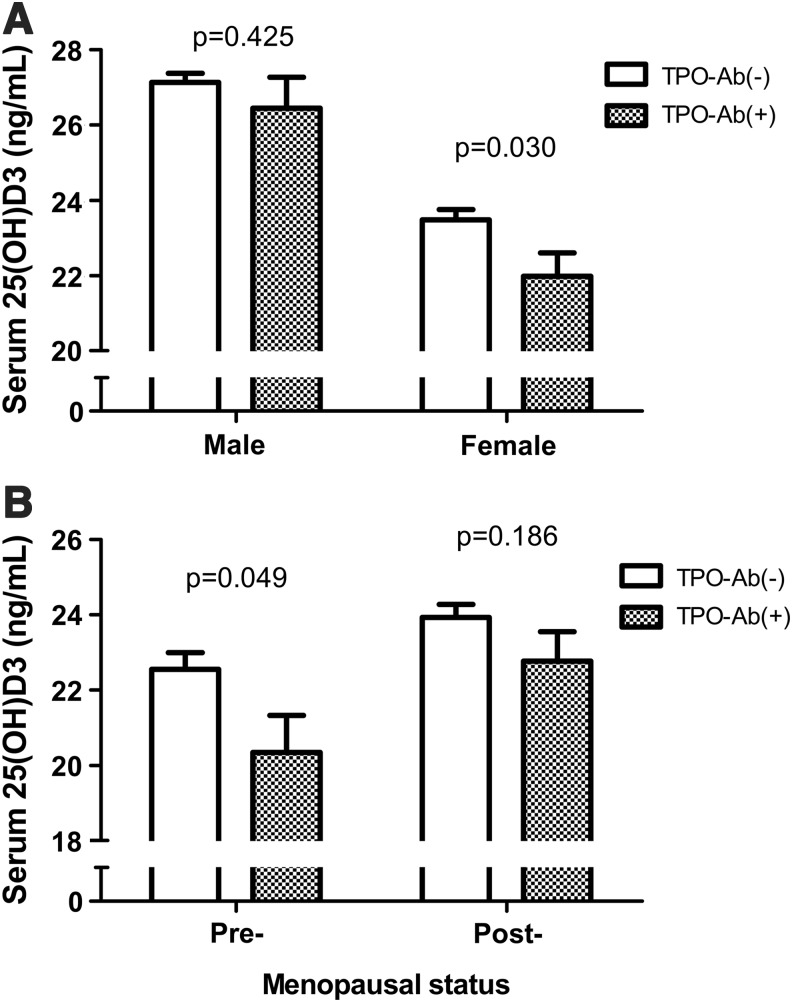
Next, we compared serum 25(OH)D3 levels between the TPO-Ab(+) and TPO-Ab(−) groups for male and female subjects (Fig. 2A). In male subjects, there was no difference in serum 25(OH)D3 levels between the two groups (26.5±0.8 vs. 27.1±0.2 ng/mL, p=0.425). In female subjects, the levels of serum 25(OH)D3 were significantly lower in the TPO-Ab(+) group when compared with the TPO-Ab(−) group (22.0±0.6 vs. 23.5±0.3 ng/mL, p=0.030). In subgroup analysis of pre- (n=908) and postmenopausal (n=1885) women, the serum 25(OH)D3 levels were only significantly different in pre-menopausal women (20.3±1.0 vs. 22.6±0.4 ng/mL, p=0.049; Fig. 2B).
FIG. 2.
Serum levels of 25(OH)D3 in the TPO-Ab(+) and TPO-Ab(−) group according to sex and menopause. (A) Serum 25(OH)D3 levels according to the level of TPO-Ab in male and female subjects. (B) Serum 25(OH)D3 levels according to the level of TPO-Ab in pre- and postmenopausal women.
Prevalence of TPO-Ab positivity according to serum 25(OH)D3 levels
We classified study subjects into three groups according to serum 25(OH)D3 levels: the vitamin D deficient (<10 ng/mL), insufficient (10–30 ng/mL), and sufficient (>30 ng/mL) groups. As shown in Figure 3A, the prevalence of TPO-Ab positivity in females was 21.2% in the deficient group, 15.5% in the insufficient group, and 12.6% in the sufficient group. A trend for the prevalence of TPO-Ab positivity to decrease as the levels of serum 25(OH)D3 increase was found to be significant in female subjects (p=0.001), although no significant differences in this regard were noted in male subjects. Next, we performed subgroup analysis in pre- and postmenopausal women (Fig. 3B). The same trend was significant for pre-menopausal women (25.0%, 15.6%, and 10.8% in deficient, insufficient, and sufficient groups, respectively; p=0.003) but not for postmenopausal women.
FIG. 3.
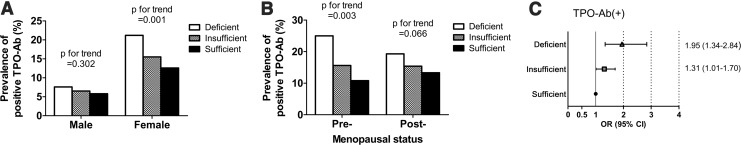
The prevalence of subjects with a positive TPO-Ab value according to serum 25(OH)D3 levels in men and women. Subjects were classified into three groups according to serum 25(OH)D3 levels: vitamin D deficient (<10 ng/mL), insufficient (10–30 ng/mL), and sufficient (>30 ng/mL). (A) The prevalence of subjects with a positive TPO-Ab in the vitamin D deficient group, insufficient group, and sufficient group. (B) The prevalence of TPO-Ab positivity between the three groups relative to the level of serum 25(OH)D3 in pre- and postmenopausal women. p-Values were calculated for trend analysis. (C) Adjusted odds ratios (ORs) for TPO-Ab positivity among members of the 25(OH)D3-deficient and -insufficient groups compared with the 25(OH)D3-sufficient group. A binary logistic regression model was used to calculate adjusted ORs after adjusting for age, body mass index, serum calcium levels, smoking, menopause, and season. Error bars represent the confidence intervals.
Given the findings of lower serum 25(OH)D3 levels in female subjects in the TPO-Ab(+) group, we evaluated the adjusted odds ratios (ORs) for women in the TPO-Ab(+) group. Adjusted ORs were calculated after adjusting for age, BMI, calcium levels, smoking history, menopausal history, and season using binary logistic regression model. As shown in Figure 3C, adjusted ORs for TPO-Ab positivity among subjects in the vitamin D deficient and insufficient groups were 1.95 ([confidence interval (CI) 1.34–2.84], p=0.001) and 1.31 ([CI 1.01–1.70], p=0.043), respectively when compared with the sufficient group. In this analysis, smoking history (including current and past) showed a significant association with TPO-Ab positivity (OR 1.51 [CI 1.02–2.21], p=0.036). However, other variables including age, BMI, calcium levels, menopausal history, and season had no significant association. When we further analyzed the adjusted ORs using month of vitamin D measurement instead of the season, the adjusted ORs for TPO-Ab positivity was 1.97 ([CI 1.34–2.88], p<0.001) in the vitamin D deficient group and 1.27 ([CI 0.98–1.67], p=0.071) in the vitamin D insufficient group, respectively.
Serum 25(OH)D3 levels according to both TPO-Ab and US positivity
The overall prevalence of both TPO-Ab and US positivity was 5.4% (2.3% male; 9.7% female). The characteristics of the study subjects in terms of the TPO-Ab/US positivity are summarized in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/thy). The serum 25(OH)D3 level was significantly lower in the TPO-Ab(+)/US(+) group when compared with the control group (23.3±0.7 vs. 25.6±0.2 ng/mL, p<0.001; Supplementary Fig. S1A). The serum 25(OH)D3 level was significantly lower in the TPO-Ab(+)/US(+) group only in females (21.6±0.8 vs. 23.4±0.3 ng/mL, p=0.027; Supplementary Fig. S1B), especially in pre-menopausal subjects (18.6±1.1 vs. 22.6±0.4 ng/mL, p=0.001; Supplementary Fig. S1C). The trend for prevalence of both TPO-Ab and US positivity to decrease as serum 25(OH)D3 levels increase was significant in female subjects (14.7%, 9.9%, and 7.1% in the deficient, insufficient, and sufficient groups, respectively; p<0.001), especially in pre-menopausal women (15.9%, 9.6%, and 3.4%, respectively; p<0.001; Supplementary Fig. S2A, B).
The adjusted OR for both TPO-Ab and US positivity among subjects in the vitamin D deficient and insufficient groups were 2.36 ([CI 1.50–3.73], p<0.001) and 1.50 ([CI 1.08–2.09], p=0.017) respectively when compared with the sufficient group (Supplementary Fig. S2C). Other factors had no significant correlation in this multivariate analysis. When we further analyzed the adjusted ORs using month of vitamin D measurement instead of the season, the adjusted ORs for TPO-Ab positivity were 2.33 ([CI 1.46–3.69], p<0.001) in the vitamin D deficient group and 1.45 ([CI 1.04–2.05], p=0.029) in the vitamin D insufficient group, respectively.
Discussion
Our present evaluation of changes in the levels of vitamin D according to the TPO-Ab positivity and US findings has revealed that serum vitamin D levels are significantly decreased in women with AITD relative to women without AITD. TPO-Ab positivity and both TPO-Ab and US positivity were also found to be more prevalent in vitamin D deficient and insufficient women compared with vitamin D sufficient women.
The discovery of the vitamin D receptor (VDR) in monocytes, dendritic cells, and activated T cells highlighted the potential involvement of vitamin D in the immune system and in the pathogenesis of autoimmune diseases (2). The significant association of VDR polymorphism with AITD has also indicated a potential role of vitamin D in the pathogenesis of AITD (22–24). Activated vitamin D modulates autoimmune reactions by regulating T lymphocytes to inhibit both the production and activity of cytokines (5). Vitamin D directly regulates T lymphocyte functions by inhibiting the proliferation of Th1 cells and increasing the number of Th2 cells (8). Vitamin D also suppresses the production of IL-2, IL-5, IFN-γ, and TNF-α, and increases the production of IL-4 and transforming growth factor in Th2 cells (12).
Our current results suggest a possible crosstalk between vitamin D and estrogen in the development of AITD. The association of lower vitamin D levels with the higher prevalence of AITD was found to be significant only in premenopausal women and not in men or postmenopausal women. Autoimmune thyroid diseases were more prevalent in women (25). Women have been reported to have higher absolute numbers of CD4+lymphocytes and higher rates of Th1 cytokine production than men (26). It is also well known that estrogen stimulates calcitriol accumulation in women (27–30). Investigation of experimental autoimmune encephalomyelitis (EAE) and MS has indicated that vitamin D also has more immune-modulatory effects in women than in men (31,32). These studies indicated that a possible crosstalk exists between 17-β estradiol (E2) and calcitriol during the pathogenesis of EAE and MS. Possible mechanisms have been proposed to explain this phenomenon (32). E2 can suppress CYP24A1 transcripts that encode the calcitriol-inactivating enzyme (24-hydroxylase) and also enhance VDR biosynthesis. E2 induces greater binding and internalization of vitamin D-binding protein (DBP) to self-reactive T cells and macrophages, which allows calcitriol to accumulate in immune cells. Although the mechanism underlying the immune-modulatory effects of calcitriol in the presence of estrogen remains unclear, the association of vitamin D deficiency with an increased prevalence of AITD in pre-menopausal women suggests that supplementation with vitamin D might offer a therapeutic approach to delay AITD in younger women. In this study, we included 29 pre-menopausal women and 128 postmenopausal women, who had a history of taking oral contraceptives or estrogen therapy. However, there was no difference in our results when we repeated the analysis after excluding these subjects.
In principle, AITD is a histological diagnosis. The pathology is characterized by lymphocytic infiltration of the thyroid parenchyma. But the diagnosis can be made by clinical evaluation, antibody measurement, and US findings. Most studies that have evaluated the association of vitamin D and thyroid autoimmunity have used serum TPO-Ab levels as a marker of thyroid autoimmunity (13,17,18). However, we have here defined AITD in two ways, in terms of the serum TPO-Ab levels and thyroid US findings suggestive of thyroiditis. In pre-menopausal women, a low serum vitamin D level was significantly associated with AITD using the two different definitions. Additionally, we subanalyzed the TPO-Ab(−) group according to US findings. In 6012 patients with TPO-Ab(−), 418 patients (7%) had evidence of thyroiditis on US findings. The vitamin D levels of these subgroup (22.1±0.6 ng/mL) were significantly lower than the ones of subjects with both negative TPO-Ab and US findings (25.9±0.2 ng/mL, p<0.001).
In our present study, we used 10–30 ng/mL of serum 25(OH)D3 as the cutoff value to classify the vitamin D status. The optimal level of vitamin D remains controversial. The definition of a clinically significant vitamin D deficiency may vary between different populations, and it is reported that serum vitamin D levels depend on age, sex, and BMI, and that they vary on a seasonal basis (13). Some studies have defined 20–30 ng/mL of serum 25(OH)D3 levels as constituting insufficient vitamin D to sustain levels of parathyroid hormone and bone health (33,34). However, another previous study defined a 10–30 ng/mL concentration of serum 25(OH)D3 as constituting vitamin D insufficiency (20). Recently, vitamin D deficiency became regarded as an important global health problem owing to its association with increased risk for metabolic bone diseases, as well as other chronic disease such as type 2 diabetes mellitus, cardiovascular disease, and cancer (35). According to the report of the Korea National Health and Nutrition Examination Survey (KNHANES), almost 80% of the Korean population had vitamin D levels between 10–30 ng/mL, with 10% of the population having vitamin D levels lower than 10 ng/mL (19).
Our current study has several limitations. First, we only evaluated cross-sectional data. A future follow-up of this cohort could thus provide more information about the possible role of vitamin D in thyroid autoimmunity. Second, there is a possibility of selection bias because our subjects were not selected from a community-based cohort. Third, seasonal variation could still be a confounding factor in this study. In Korea, it is known that there are seasonal variations in vitamin D levels (19). In our study, the measurements of the vitamin D levels were not done in a single season. However, there was no significant difference in the distribution of subjects between the AITD and the control group according to months or seasons. Fourth, we have used a definition of AITD without consideration of thyroid function tests in this study. A further longitudinal study is needed to evaluate the effect of vitamin D levels on thyroid dysfunction in our study subjects.
In summary, we found in our present analyses that low serum vitamin D levels are significantly associated with AITD, especially in pre-menopausal women. These findings suggest a possible role of vitamin D in the development of AITD and a potential crosstalk mechanism between vitamin D and estrogen in the pathogenesis of AITD. A future longitudinal cohort study and prospective interventional trials based on the community population may further clarify the role of vitamin D in AITD.
Supplementary Material
Acknowledgments
This study was supported by grants (No. 2013-374 and No. 2013-582) from the Asan Institute for Life Sciences, Seoul, Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arnson Y, Amital H, Shoenfeld Y.2007Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis 66:1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeke F, Takiishi T, Korf H, Gysemans C, Mathieu C.2010Vitamin D: modulator of the immune system. Curr Opin Pharmacol 10:482–496 [DOI] [PubMed] [Google Scholar]
- 3.Deluca HF, Cantorna MT.2001Vitamin D: its role and uses in immunology. FASEB J 15:2579–2585 [DOI] [PubMed] [Google Scholar]
- 4.Cantorna MT, Mahon BD.2004Mounting evidence for vitamin D as an environmental factor affecting autoimmune disease prevalence. Exp Biol Med (Maywood) 229:1136–1142 [DOI] [PubMed] [Google Scholar]
- 5.Marques CD, Dantas AT, Fragoso TS, Duarte AL.2010The importance of vitamin D levels in autoimmune diseases. Rev Bras Reumatol 50:67–80 [PubMed] [Google Scholar]
- 6.Szodoray P, Nakken B, Gaal J, Jonsson R, Szegedi A, Zold E, Szegedi G, Brun JG, Gesztelyi R, Zeher M, Bodolay E.2008The complex role of vitamin D in autoimmune diseases. Scand J Immunol 68:261–269 [DOI] [PubMed] [Google Scholar]
- 7.Munger KL, Levin LI, Massa J, Horst R, Orban T, Ascherio A.2013Preclinical serum 25-hydroxyvitamin D levels and risk of type 1 diabetes in a cohort of US military personnel. Am J Epidemiol 177:411–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antico A, Tampoia M, Tozzoli R, Bizzaro N.2012Can supplementation with vitamin D reduce the risk or modify the course of autoimmune diseases? A systematic review of the literature. Autoimmun Rev 12:127–136 [DOI] [PubMed] [Google Scholar]
- 9.Badenhoop K, Kahles H, Penna-Martinez M.2012Vitamin D, immune tolerance, and prevention of type 1 diabetes. Curr Diab Rep 12:635–642 [DOI] [PubMed] [Google Scholar]
- 10.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L.2002A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes 51:1367–1374 [DOI] [PubMed] [Google Scholar]
- 11.Bozkurt NC, Karbek B, Ucan B, Sahin M, Cakal E, Ozbek M, Delibasi T.2013The association between severity of vitamin D deficiency and Hashimoto's thyroiditis. Endocr Pract 19:479–484 [DOI] [PubMed] [Google Scholar]
- 12.Camurdan OM, Doger E, Bideci A, Celik N, Cinaz P.2012Vitamin D status in children with Hashimoto thyroiditis. J Pediatr Endocrinol Metab 25:467–470 [PubMed] [Google Scholar]
- 13.Kivity S, Agmon-Levin N, Zisappl M, Shapira Y, Nagy EV, Danko K, Szekanecz Z, Langevitz P, Shoenfeld Y.2011Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol 8:243–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rotondi M, Chiovato L.2013Vitamin D deficiency in patients with Graves' disease: probably something more than a casual association. Endocrine 43:3–5 [DOI] [PubMed] [Google Scholar]
- 15.Tamer G, Arik S, Tamer I, Coksert D.2011Relative vitamin D insufficiency in Hashimoto's thyroiditis. Thyroid 21:891–896 [DOI] [PubMed] [Google Scholar]
- 16.Yasuda T, Okamoto Y, Hamada N, Miyashita K, Takahara M, Sakamoto F, Miyatsuka T, Kitamura T, Katakami N, Kawamori D, Otsuki M, Matsuoka TA, Kaneto H, Shimomura I.2012Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves' disease. Endocrine 42:739–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goswami R, Marwaha RK, Gupta N, Tandon N, Sreenivas V, Tomar N, Ray D, Kanwar R, Agarwal R.2009Prevalence of vitamin D deficiency and its relationship with thyroid autoimmunity in Asian Indians: a community-based survey. Br J Nutr 102:382–386 [DOI] [PubMed] [Google Scholar]
- 18.Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM.2012Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol 167:43–48 [DOI] [PubMed] [Google Scholar]
- 19.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, Kim KM, Kim KJ, Rhee Y, Lim SK.2011Vitamin D insufficiency in Korea—a greater threat to younger generation: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J Clin Endocrinol Metab 96:643–651 [DOI] [PubMed] [Google Scholar]
- 20.Rosen CJ.2011Clinical practice. Vitamin D insufficiency. N Engl J Med 364:248–254 [DOI] [PubMed] [Google Scholar]
- 21.Kim BJ, Lee SH, Bae SJ, Kim HK, Choe JW, Kim HY, Koh JM, Kim GS.2010The association between serum thyrotropin (TSH) levels and bone mineral density in healthy euthyroid men. Clin Endocrinol (Oxf) 73:396–403 [DOI] [PubMed] [Google Scholar]
- 22.Feng M, Li H, Chen SF, Li WF, Zhang FB.2013Polymorphisms in the vitamin D receptor gene and risk of autoimmune thyroid diseases: a meta-analysis. Endocrine 43:318–326 [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Lopez E, Kurylowicz A, Bednarczuk T, Paunkovic J, Seidl C, Badenhoop K.2005Vitamin D receptor polymorphisms are associated with Graves' disease in German and Polish but not in Serbian patients. Thyroid 15:1125–1130 [DOI] [PubMed] [Google Scholar]
- 24.Stefanic M, Papic S, Suver M, Glavas-Obrovac L, Karner I.2008Association of vitamin D receptor gene 3′-variants with Hashimoto's thyroiditis in the Croatian population. Int J Immunogenet 35:125–131 [DOI] [PubMed] [Google Scholar]
- 25.Pearce EN, Farwell AP, Braverman LE.2003Thyroiditis. N Engl J Med 348:2646–2655 [DOI] [PubMed] [Google Scholar]
- 26.Whitacre CC.2001Sex differences in autoimmune disease. Nat Immunol 2:777–780 [DOI] [PubMed] [Google Scholar]
- 27.Elenkov IJ, Wilder RL, Bakalov VK, Link AA, Dimitrov MA, Fisher S, Crane M, Kanik KS, Chrousos GP.2001IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. J Clin Endocrinol Metab 86:4933–4938 [DOI] [PubMed] [Google Scholar]
- 28.Gray TK, McAdoo T, Hatley L, Lester GE, Thierry M.1982Fluctuation of serum concentration of 1,25-dihydroxyvitamin D3 during the menstrual cycle. Am J Obstet Gynecol 144:880–884 [DOI] [PubMed] [Google Scholar]
- 29.Spach KM, Hayes CE.2005Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol 175:4119–4126 [DOI] [PubMed] [Google Scholar]
- 30.van Hoof HJ, van der Mooren MJ, Swinkels LM, Sweep CG, Merkus JM, Benraad TJ.1999Female sex hormone replacement therapy increases serum free 1,25-dihydroxyvitamin D3: a 1-year prospective study. Clin Endocrinol (Oxf) 50:511–516 [DOI] [PubMed] [Google Scholar]
- 31.Nashold FE, Spach KM, Spanier JA, Hayes CE.2009Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol 183:3672–3681 [DOI] [PubMed] [Google Scholar]
- 32.Correale J, Ysrraelit MC, Gaitan MI.2010Gender differences in 1,25 dihydroxyvitamin D3 immunomodulatory effects in multiple sclerosis patients and healthy subjects. J Immunol 185:4948–4958 [DOI] [PubMed] [Google Scholar]
- 33.Holick MF.2007Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 34.Adams JS, Hewison M.2010Update in vitamin D. J Clin Endocrinol Metab 95:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holick MF.2012The D-lightful vitamin D for child health. JPEN J Parenter Enteral Nutr 36:9S–19S [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.