Abstract
Aims: Integrins are multifunctional heterodimeric adhesion receptors that mediate the attachment between a cell and the extracellular matrix or other surrounding cells. In endothelial cells, integrins can modulate cell migration and motility. In particular, β3-integrin is expressed in angiogenic vessels. Signal transduction by β3-integrins requires the recruitment of intracellular signaling molecules. β3-endonexin is a highly spliced molecule that has been identified as a β3-integrin binding protein. β3-endonexin isoforms are expressed in endothelial cells and have been suggested to act as shuttle proteins between the membrane and the nucleus. However, their functional role in angiogenesis is unclear. In this study, we investigated whether β3-endonexin isoforms are involved in endothelial angiogenic processes under hypoxia. Results: The overexpression of β3-endonexin isoforms decreased endothelial proliferation and tube formation under hypoxia, while the depletion of β3-endonexin by RNAi promoted angiogenic responses in vitro and in vivo. In hypoxia, β3-endonexin accumulated in the nucleus, and prevention of this response by depletion of β3-endonexin increased hypoxic activation and induction of the hypoxia-inducible factor (HIF)-1 and its target genes VEGF and PAI-1. β3-endonexin diminished nuclear factor kappa B (NFκB) activation and decreased NFκB binding to the HIF-1α promoter under hypoxia, subsequently diminishing NFκB-dependent transcription of HIF-1α under hypoxia. Innovation: Our results indicate for the first time that the overexpression of β3-endonexin can decrease hypoxic induction and activation of HIF-1α and can prevent hypoxic endothelial proliferation and angiogenic responses. Conclusion: β3-endonexin can act as a novel anti-angiogenic factor specifically in the response to hypoxia due to its negative impact on the activation of HIF-1. Antioxid. Redox Signal. 20, 1964–1976.
Introduction
An adequate provision with oxygen is essential for eukaryotic cellular survival. Conditions of low oxygen availability, as, for example, found in the tumor environment, in ischemic heart disease or in stroke demand a rapid cellular program aiming at overcoming these critical conditions; for example, by adapting cellular metabolism and stimulating the growth of new vessels (angiogenesis) (3).
Innovation.
In this study, we demonstrated for the first time that the β3-integrin binding protein β3-endonexin acts as a novel anti-angiogenic molecule under hypoxic conditions. This anti-angiogenic function is based on negatively interfering with the hypoxia-inducible factor (HIF) pathway. Specifically, the overexpression of β3-endonexin prevented nuclear factor kappa B-dependent up-regulation of HIF-1α mRNA by hypoxia and subsequent HIF activation, thereby decreasing endothelial angiogenic processes. Thus, the promotion of β3-endonexin levels in hypoxic endothelial cells might be considered a novel anti-angiogenic option.
The family of hypoxia-inducible factors (HIFs) is essential in regulating these responses (26). In endothelial cells, HIFs are centrally involved in proliferative and angiogenic responses toward hypoxia (23). To date, three HIF family members have been identified. While HIF-1 is ubiquitously expressed and widely involved in the cellular process of hypoxic adaptation, HIF-2 appears to be more specifically involved in hypoxia signaling (22). HIF-3, on the other hand, might exert opposing effects (20). HIF transcription factors are α/β-heterodimers that consist of constitutively expressed HIF-1β (arylhydrocarbon receptor nuclear translocator [ARNT]) and a hypoxia-inducible α-protein which is unstable under normoxic conditions. Under hypoxic conditions, HIF-α proteins are stabilized and bind to ARNT. This complex initiates transcription by binding to hypoxia-response elements (HRE) in promoters and/or enhancers of hypoxia-inducible genes (28).
Subsequently, a variety of pro-angiogenic genes, including vascular endothelial growth factors (VEGFs) and plasminogen activator inhibitor-1 (PAI-1), are up-regulated, promoting the angiogenic response toward hypoxia (3, 17, 23).
In addition to these pro-angiogenic growth factors, important steps in the angiogenic process are regulated by integrins (11). Integrins are α/β-heterodimeric transmembrane receptors that bind extracellular matrix proteins or other adhesion receptors on neighboring cells (18, 25). However, despite the pivotal role that integrins play in the angiogenic process, their exact role in the response to hypoxia is still not completely clarified.
While many integrins are ubiquitously expressed, integrin αvβ3 has been specifically found to be expressed in activated pro-angiogenic endothelial cells and has been associated with vascular remodeling and tumor angiogenesis (8), although the specific mechanisms regulating β3-mediated signaling in the context of angiogenesis under normoxic and hypoxic conditions are not completely clear.
In recent years, β3-endonexin has been identified as a β3-integrin binding protein. This protein has been initially characterized as a β3-integrin interaction partner in a yeast 2-hybrid system (27), and it has been found to be expressed in various cells, including platelets, mononuclear leukocytes, and vascular cells (10, 27). Several splice variants of β3-endonexin have been described so far, including the two functionally important isoforms: β3-endonexin long (EN-L, 170 aa; 19.2 kDa) and β3-endonexin short (EN-S, 111 aa; 12.6 kDa). While only EN-S has been described to bind to β3-integrin (27), both forms can interact with cyclin A and inhibit cyclin A-Cdk2 kinase activity (21), suggesting that β3-endonexin isoforms differ in their physiological role and might exhibit functions in addition to integrin-linked signaling. In support, both β3-endonexin isoforms have been reported to decrease urokinase-type plasminogen activator receptor (uPAR) promoter activity in endothelial cells by interacting with the p50/p65 complex of nuclear factor kappa B (NFκB), thereby preventing its binding to the uPAR promoter (4, 5). Although uPAR has been associated with endothelial migration and angiogenesis (6), a role of β3-endonexin in these responses has not been shown to date.
In this study, we, thus, investigated whether β3-endonexin affects endothelial cell proliferation and tube formation under normoxia and hypoxia. We report that overexpression of β3-endonexin decreases proliferation and endothelial tube formation under hypoxia and concomitantly reduces hypoxic HIF activation. Hypoxia primarily promoted β3-endonexin accumulation in the nucleus. Detailed mechanistic analyses revealed that in response to intermediate and prolonged hypoxia, β3-endonexin decreased the transcription of HIF-1α by interfering with NFκB binding to the HIF-1α promoter. Thus, this novel pathway not only provides an explanation for the observation that HIF-1α mRNA levels decrease over prolonged periods of hypoxia but also identifies β3-endonexin as a novel anti-angiogenic molecule.
Results
β3-endonexin accumulates in the nucleus under hypoxia
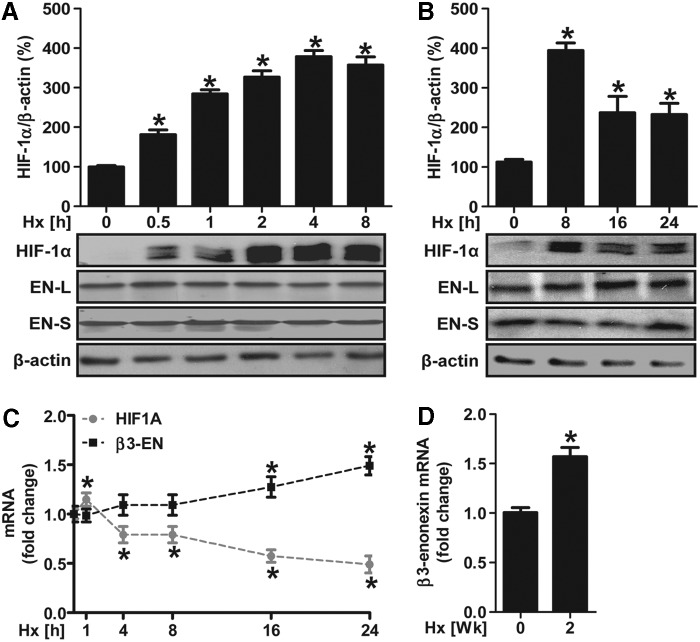
In order to evaluate the effects of hypoxia on the expression patterns of β3-endonexin in endothelial cells, human microvascular endothelial cells-1 (HMEC-1) were exposed to hypoxia (1% oxygen) for increasing time periods. Endogenous levels of β3-endonexin were subsequently identified by Western blot using a β3-endonexin antibody that detects the short (EN-S, 12.6 kDa) as well as the long (EN-L, 19.2 kDa) isoforms of β3-endonexin (4). Protein levels of neither of the β3-endonexin splice variants were significantly affected within 24 h of hypoxic exposure, although HIF-1α protein levels were up-regulated under hypoxia peaking at 4 to 8 h of hypoxia (Fig. 1A, B). Similarly, β3-endonexin mRNA levels were not affected by short- and intermediate-term hypoxia, although they increased with prolonged hypoxia extending to 16 h (Fig. 1C). Moreover, β3-endonexin transcripts were also identified in vivo in normoxic murine lung tissues and more pronounced in lung tissues derived from mice exposed to chronic hypoxia for 2 weeks (Fig. 1D). In contrast, hypoxia induced a rapid but transient increase in HIF-1α mRNA levels, which was followed by a decline to levels below the initial normoxic values (Fig. 1C).
FIG. 1.
Hypoxia differentially regulates β3-endonexin and HIF-1α expression. (A–C) Human microvascular endothelial cells-1 (HMEC-1) were exposed to hypoxia (1% oxygen, Hx) or normoxic conditions (0 h) for increasing time points. (A, B) Western blot analyses were performed with antibodies against HIF-1α and β3-endonexin isoforms. β-actin staining served as a loading control. Normoxic controls (0 h) were set equal to 100% (n=3, *p<0.05 vs. 0 h). (C) RT-qPCR was performed with primers amplifying human β3-endonexin and HIF-1α as well as 18S rRNA for normalization. Normoxic controls (0 h) were set equal to 1 (n=3, *p<0.05 vs. 0 h). (D) Total RNA was isolated from mice that were exposed to hypoxia (10% oxygen) for 2 weeks or from mice breathing room air (0 week). RT-qPCR was performed with primers amplifying murine β3-endonexin and 18S rRNA for normalization. Normoxic controls (0 week) were set equal to 1 (n=3, *p<0.05 vs. 0 week). HIF, hypoxia-inducible factor; PCR, polymerase chain reaction; RT-qPCR, real-time quantitative PCR.
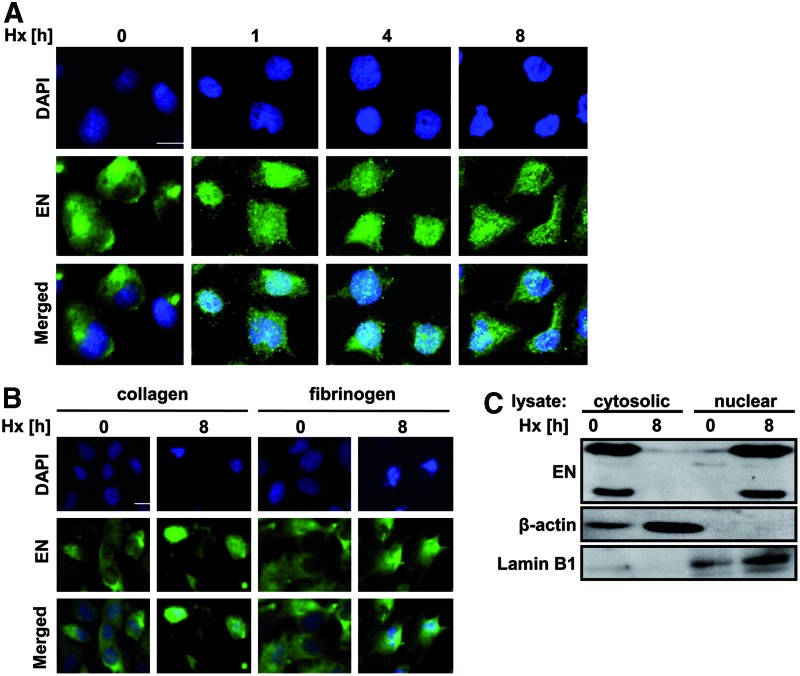
Immunofluorescence staining showed that β3-endonexin was localized in the cytoplasm and plasma membrane in normoxia, while under hypoxia, β3-endonexin was found accumulated in the nucleus (Fig. 2A). This response was independent of integrin signaling, as it remained robust even when cells were grown on collagen or fibronectin (Fig. 2B). Concomitantly, Western blot analysis showed that β3-endonexin was primarily present in the cytoplasmic fraction under normoxic conditions, while it accumulated in the nuclear fraction under hypoxia (Fig. 2C) indicating that β3-endonexin translocates to the nucleus on exposure to hypoxia.
FIG. 2.
β3-endonexin accumulates in the nucleus under hypoxia. (A/B) HMEC-1 cells were seeded on uncoated coverslips (A) or on coverslips coated with collagen or fibronectin (B) and exposed to hypoxia (Hx) or normoxia (0 h) for increasing time periods. Cells were fixed, and immunostaining was performed with an antibody against β3-endonexin and a secondary antibody conjugated with Alexa fluor 488. Nuclei were visualized by 4′, 6-diamidino-2-phenylindole (DAPI) staining. Scale bars represent 10 μm. (C) HMEC-1 cells were exposed to hypoxia (Hx) for 8 h or remained under normoxia (0 h). Western blot analyses were performed on nuclear and cytosolic fractions with antibodies against HIF-1α and β3-endonexin isoforms, β-actin and Lamin B1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
β3-endonexin modulates HIF activity and HIF-1α expression under hypoxia
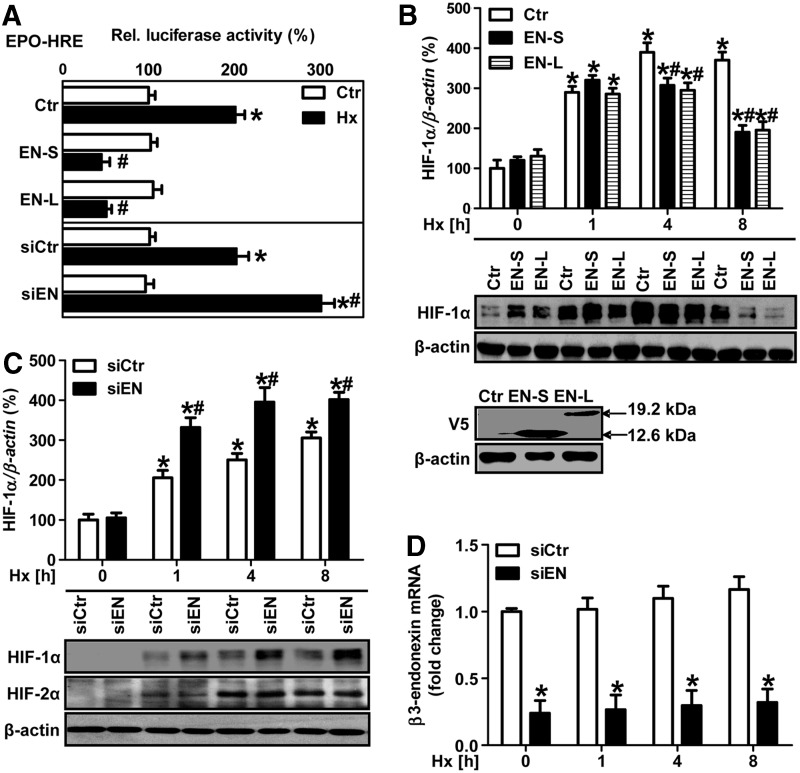
In the next step, we evaluated the possibility that β3-endonexin may interfere with the HIF system under hypoxia. First, we determined whether β3-endonexin affects HIF transactivation. To this end, HMEC-1 cells were co-transfected with a luciferase construct containing three HRE from the erythropoietin gene and expression vectors coding for either β3-endonexin short or long isoforms (Fig. 3A). Interestingly, hypoxia-induced HIF activity was completely abolished in cells overexpressing either of the β3-endonexin isoforms (Fig. 3A). In line, the overexpression of either of the β3-endonexin isoforms prevented hypoxic induction of HIF-1α protein (Fig. 3B). In contrast, the silencing of β3-endonexin by RNAi enhanced HIF activity (Fig. 3A) and HIF-1α protein levels under hypoxia (Fig. 3C), while it completely diminished β3-endonexin mRNA levels (Fig. 3D). This effect was specific to HIF-1α, as the induction of HIF-2α protein levels by hypoxia was not affected in β3-endonexin-depleted endothelial cells (Fig. 3C).
FIG. 3.
β3-endonexin decreases HIF activity and HIF-1α protein levels under hypoxia. (A) HMEC-1 cells were co-transfected with a luciferase construct driven by three hypoxia-response elements (HRE) from the erythropoietin gene (EPO-HRE) and expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) or with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr). HMEC-1 cells were exposed to hypoxia (1% oxygen, Hx) for 8 h, and luciferase assay was performed. Normoxic controls (Ctr) were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). (B) HMEC-1 cells were transfected with expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) and exposed to hypoxia for increasing time points or cultivated under normoxia (0 h). Western blot analyses were performed with antibodies against HIF-1α and β-actin. Normoxic controls (Ctr, 0 h) were set equal to 100% (n=3, *p<0.05 vs. Ctr 0 h; #p<0.05 vs. Ctr 1, 4 or 8 h). Expression of β3-endonexin short (EN-S) or long (EN-L) constructs was validated by Western blotting using a V5 antibody. (C, D) HMEC-1 cells were transfected with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr) and exposed to hypoxia for 1, 4, and 8 h or cultivated under normoxia (0 h). (C) Western blot analyses were performed with antibodies against HIF-1α and HIF-2α. β-actin levels served as a loading control. HIF-1α levels at normoxia (siCtr, 0 h) were set equal to 100% (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h). (D) RT-qPCR analyses were performed with primers amplifying human β3-endonexin and 18S rRNA for normalization. Control levels at normoxia (siCtr, 0 h) were set equal to 1 (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h).
Interestingly, the silencing of β3-endonexin also enhanced hypoxic HIF-1α mRNA levels (Fig. 4A). In addition, the silencing of β3-endonexin further enhanced hypoxic induction of the HIF target genes vascular endothelial growth factor A (VEGF-A) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fig. 4B, C). In addition, the overexpression of either of the β3-endonexin isoforms prevented the hypoxic induction of PAI-1 promoter activity (Fig. 4D). On the contrary, the silencing of β3-endonexin further enhanced hypoxic induction of the PAI-1 promoter; while this effect was abrogated when the HIF binding site in the PAI-1 promoter was mutated, indicating that β3-endonexin reduces hypoxic PAI-1 expression via an HIF-dependent mechanism.
FIG. 4.
β3-endonexin decreases mRNA levels of HIF-1α and its target genes under hypoxia. (A–C) HMEC-1 cells were transfected with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr) and exposed to hypoxia for 1, 4, and 8 h or cultivated under normoxia (0 h). RT-qPCR analyses were performed with primers amplifying human HIF-1α (A), VEGF (B), or GAPDH (C) as well as 18S rRNA for normalization. Control levels at normoxia (siCtr, 0 h) were set equal to 1 (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h). (D) HMEC-1 cells were co-transfected with a luciferase construct driven bý the wild-type PAI-1 promoter (PAI-796) or the PAI-1 promoter mutated at the HRE (PAI-796m) and expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) or with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr). Cells were exposed to hypoxia (Hx) for 8 h or remained under normoxia (Ctr), and luciferase assays were performed. Normoxic controls (Ctr) were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PAI-1, plasminogen activator inhibitor-1; siRNA, short interfering RNA; VEGF, vascular endothelial growth factor.
β3-endonexin diminishes NFκB-dependent induction of HIF-1α
In contrast to HIF-2α, HIF-1α has been previously shown to be regulated not only at the level of protein stability but also at the level of transcription as a direct target gene of NFκB (1, 7, 24). We, thus, hypothesized that β3-endonexin might be involved in the transcriptional regulation of HIF-1α by NFκB.
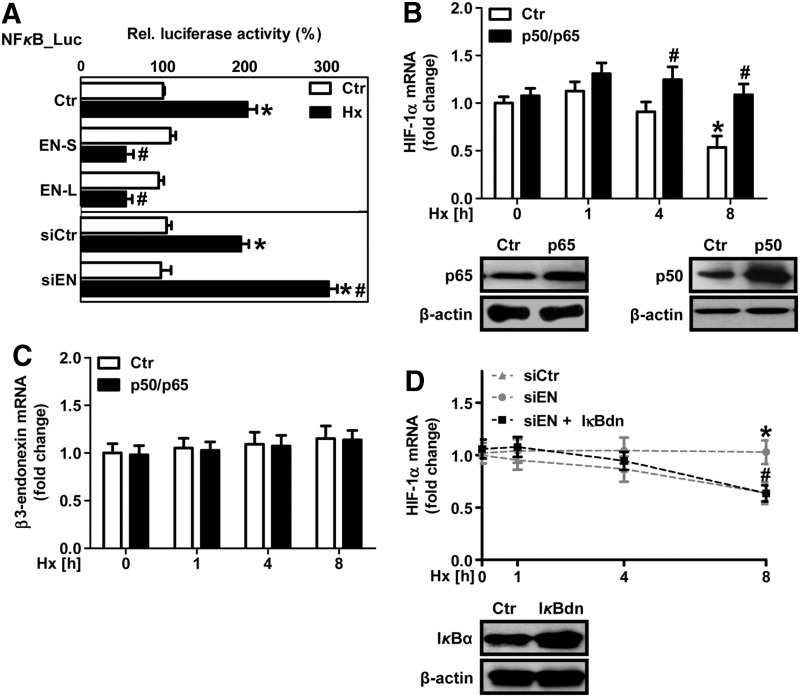
In fact, hypoxia increased NFκB-dependent luciferase activity in HMEC-1 cells, and this response was prevented by the overexpression of the β3-endonexin isoform (Fig. 5A). In contrast, the depletion of β3-endonexin further enhanced the hypoxic NFκB response (Fig. 5A), indicating that β3-endonexin diminishes hypoxic NFκB activation. Overexpression of the NFκB subunits p50 and p65 increased HIF-1α mRNA levels under hypoxia (Fig. 5B) similar to the situation with the depletion of β3-endonexin (Fig. 4A), while it did not affect β3-endonexin mRNA levels (Fig. 5C).
FIG. 5.
β3-endonexin modulates NFκB activity. (A) HMEC-1 cells were co-transfected with a luciferase construct driven by five NFκB binding-sites (NFκB-Luc) and expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) or with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr). Cells were exposed to hypoxia (1% oxygen, Hx) for 8 h or remained at normoxia (Ctr), and luciferase assays were performed. Normoxic controls were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). (B/C) HMEC-1 cells were transfected with expression vectors encoding for the NFκB subunits p50 and p65 or with control vector (Ctr) and exposed to hypoxia for 1, 4, and 8 h or cultivated under normoxia (0 h). Expression levels of p50 and p65 were validated by Western blotting. β-actin levels served as a loading control (B). RT-qPCR was performed for HIF-1α (B) and β3-endonexin (C) transcript levels. 18S rRNA levels were used for normalization. Control levels at normoxia (siCtr, 0 h) were set equal to 1 (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h). (D) HMEC-1 cells were co-transfected with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr) and with an expression vector coding for dominant-negative IκBα (IκBdn) and exposed to hypoxia for 1, 4, and 8 h or cultivated under normoxia (0 h). Expression of IκBα constructs was validated by Western blotting. β-actin served as a loading control. RT-qPCR was performed for HIF-1α transcript levels. 18S rRNA levels were used for normalization. Control levels at normoxia (siCtr, 0 h) were set equal to 1 (n=3, *p<0.05 vs. siCtr 0 h; #p<0.05 vs. siCtr 1, 4, or 8 h). NFκB, nuclear factor kappa B.
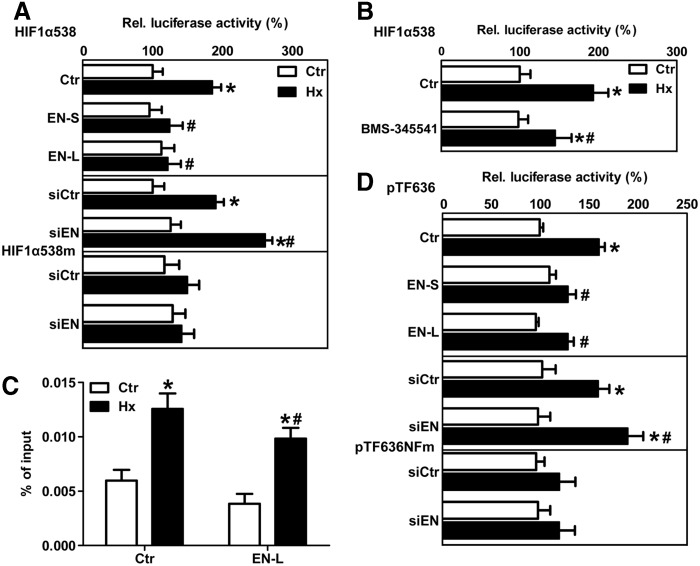
Moreover, the co-expression of dominant-negative IκBα (IκBdn) that inhibits NFκB activation prevented the up-regulation of HIF-1α mRNA in β3-endonexin-depleted cells under hypoxia (Fig. 5D), suggesting that β3-endonexin might act on HIF-1α mRNA expression via NFκB. In line, the depletion of β3-endonexin further enhanced the hypoxic induction of HIF-1α promoter activity (Fig. 6A), while the overexpression of β3-endonexin isoforms had opposite effects. Interestingly, the depletion of β3-endonexin had no effect on luciferase activity driven by the HIF-1α promoter mutated at the NFκB-binding site at −197/188 bp (7, 24), further indicating that β3-endonexin interferes with the regulation of HIF-1α by NFκB. In support, application of the IκB kinase inhibitor BMS-345541 (BMS, 100 μM) (9) decreased HIF-1α promoter activity (Fig. 6B).
FIG. 6.
β3-endonexin regulates hypoxic HIF-1α transcription via NFκB. (A) HMEC-1 cells were transfected with HIF-1α promoter luciferase constructs containing either the wild-type NFκB-binding site (HIF1α-538) or a mutated NFκB-binding site (HIF1α-538m) and with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr) or with expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr). Cells were exposed to hypoxia (1% oxygen, Hx) for 8 h or remained under normoxia (Ctr), and luciferase assays were performed. Normoxic controls were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). (B) HMEC-1 cells transfected with the HIF-1α promoter luciferase construct (HIF1α-538) were pretreated with the IκB kinase inhibitor BMS-345541 (BMS, 100 μM) and exposed to hypoxia (Hx) for 8 h or remained under normoxia (Ctr), and luciferase assays were performed. Normoxic untreated controls were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr; #p<0.05 vs. hypoxic Ctr). (C) HMEC-1 cells were transfected with expression vectors encoding β3-endonexin long form (EN-L) or control vector (Ctr). Cells were exposed to hypoxia (Hx) for 8 h or remained under normoxia (Ctr). Chromatin immunoprecipitation assay was performed using an antibody against p65 followed by RT-qPCR with primers amplifying the region of the human HIF-1α promoter containing the NFκB-binding site at −197/188 bp (n=3, *p<0.05 vs. normoxic Ctr; #p<0.05 vs. hypoxic Ctr). (D) HMEC-1 cells were co-transfected with a luciferase construct driven bý the wild-type tissue factor (TF) promoter (pTF636) or the TF promoter mutated at the NFκB site (pTF636NFm) and expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr) or with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr). Cells were exposed to hypoxia (Hx) for 8 h or remained under normoxia (Ctr), and luciferase assays were performed. Normoxic controls (Ctr) were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr).
To evaluate whether β3-endonexin would affect NFκB binding to the HIF-1α promoter, we performed chromatin immunoprecipitation assays in HMEC-1 cells overexpressing EN-L. While hypoxia increased the binding of p65 to the HIF-1α promoter in cells transfected with control vector, the overexpression of EN-L significantly decreased occupation of the HIF-1α promoter by p65 under hypoxic conditions (Fig. 6C), indicating that β3-endonexin interferes with NFκB binding to the HIF-1α promoter under hypoxic conditions and thereby inhibiting HIF-1α transcription under these conditions.
Interestingly, β3-endonexin was also able to modulate hypoxic induction of the NFκB target gene tissue factor (TF) (19). The overexpression of both β3-endonexin forms prevented the hypoxic induction of TF promoter activity (Fig. 6D). On the contrary, the silencing of β3-endonexin further enhanced hypoxic TF promoter activity. However, this response was blunted when the NFκB-binding site in the TF promoter was mutated, suggesting that β3-endonexin can act as a negative regulator of NFκB-dependent genes under hypoxia.
β3-endonexin diminishes proliferative and angiogenic responses under hypoxia
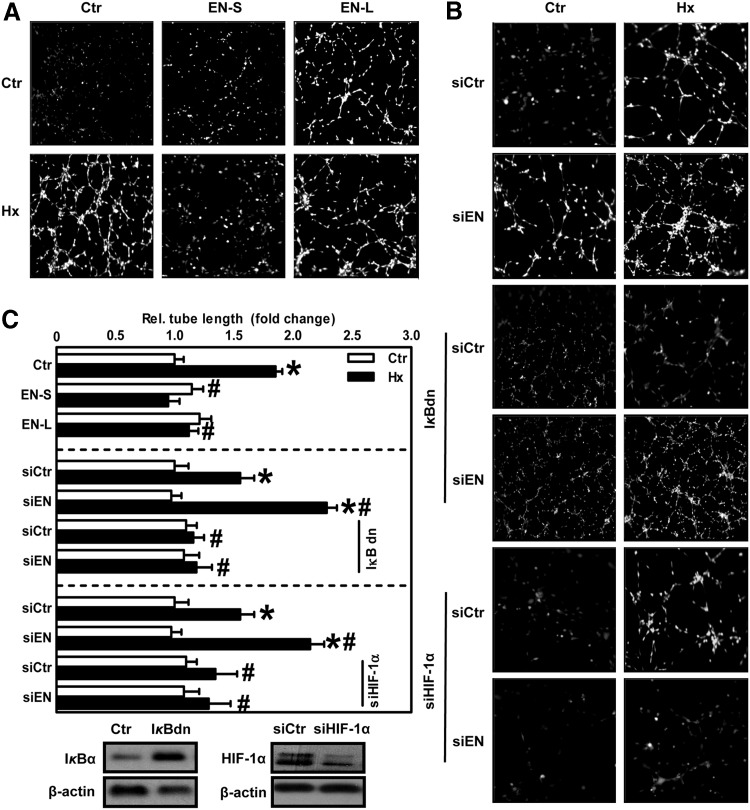
In the next step, we tested whether β3-endonexin is involved in endothelial proliferative and angiogenic responses under hypoxia. The expression of either of the β3-endonexin isoforms did not affect endothelial proliferation under normoxia as was determined by 5-bromo-2′deoxyuridine (BrdU) incorporation assay (Fig. 7) but prevented hypoxic induction of proliferation. In contrast, HMEC-1 silenced for β3-endonexin showed a further increase in proliferative activity under hypoxia (Fig. 7). However, on co-expression of an IκBdn that inhibits the activation of NFκB, the increased proliferative activity seen in hypoxic β3-endonexin depleted cells was diminished. Similarly, the depletion of HIF-1α prevented the hypoxic induction of proliferation in these cells (Fig. 7), indicating that HIF-1α and NFκB are involved in the inhibition of endothelial proliferation by β3-endonexin.
FIG. 7.

β3-endonexin diminishes endothelial proliferative responses under hypoxia. HMEC-1 cells were transfected with expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoform or with control vector (Ctr), or with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr). Alternatively, HMEC-1 cells were transfected with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr) and, in addition, transfected with an expression vector coding for IκBdn or with control vector (Ctr) or with siRNA against HIF-1α (siHIF-1α). Cells were exposed to hypoxia (1% oxygen, Hx) for 24 h or remained under normoxia (Ctr), and BrdU incorporation assay was performed. Normoxic controls were set equal to 100% (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). Western blot analyses were performed with antibodies against IκBα and HIF-1α. β-actin levels served as loading control. BrdU, 5-bromo-2′deoxyuridine.
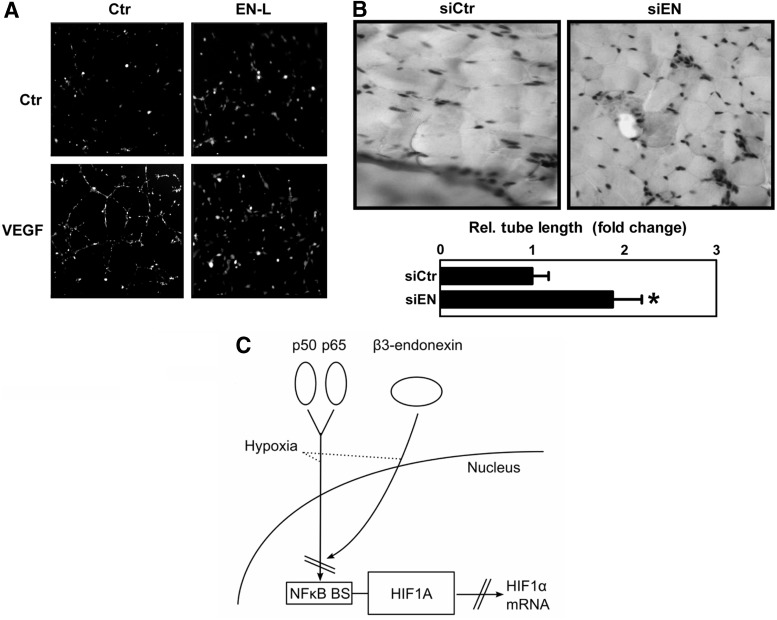
Next, we determined whether β3-endonexin affects tube formation in an in vitro matrigel assay as an indicator of angiogenesis. While under normoxic conditions, the modulation of β3-endonexin levels did not affect tube formation, the hypoxic induction of tube formation was diminished on the overexpression of β3-endonexin isoforms (Fig. 8A, C). In contrast, the silencing of β3-endonexin further enhanced hypoxic tube formation (Fig. 8B, C). However, in the presence of IκBdn or on depletion of HIF-1α, the effect of β3-endonexin silencing on hypoxic tube formation was abrogated. Similarly, the expression of EN-L was also able to decrease tube formation in response to the HIF target gene VEGF (Fig. 9A), indicating that β3-endonexin negatively affects tube formation by interfering with NFκB and HIF-1α.
FIG. 8.
β3-endonexin decreases hypoxic tube formation. (A) HMEC-1 cells were transfected with expression vectors coding for either β3-endonexin short (EN-S) or long (EN-L) isoforms or with control vector (Ctr). Cells were seeded on matrigel and exposed to hypoxia (1% oxygen, Hx) for 6 h or remained under normoxia (Ctr). (B) HMEC-1 cells were transfected with siRNA against β3-endonexin (siEN) or scrambled RNA (siCtr) and, in addition, transfected with an expression vector coding for IκBdn or with control vector or with siRNA against HIF-1α (siHIF-1α). Cells were seeded on matrigel and exposed to hypoxia as described earlier. (C) For quantification of tube formation assays, total tube lengths were evaluated. Normoxic controls were set equal to 1 (n=3, *p<0.05 vs. normoxic Ctr/siCtr; #p<0.05 vs. hypoxic Ctr/siCtr). IκBα and HIF-1α levels were determined in cells expressing IκBdn or siHIF-1α and the respective control cells using antibodies against IκBα and HIF-1α. Actin levels served as loading control. Representative figures are shown.
FIG. 9.
β3-endonexin deficiency promotes the angiogenic response in vivo. (A) HMEC-1 cells were transfected with an expression vector for β3-endonexin long (EN-L) or control vector (Ctr), seeded on matrigel, and exposed to VEGF (1 μg/μl) for 6 h or remained untreated (Ctr). A representative figure is shown (n=3). (B) HMEC-1 cells transfected with RNAi against β3-endonexin (siEN) or with scrambled RNA (siCtr) were mixed with matrigel. Plugs were injected subcutaneously in mice and excised after 7 days. Immunohistochemistry was performed with an antibody against CD31 to stain for human endothelial cells. Representative figures are shown (n=3, *p<0.05 vs. siCtr). (C) Under hypoxic conditions, NFκB subunits p50 and p65 bind to the HIF-1α promoter, thereby maintaining HIF-1α transcription. Subsequently, β3-endonexin translocates to the nucleus and prevents NFκB binding to the HIF-1α promoter at the NFκB-binding site (NFκB BS) by complexing p50 and p65, thus resulting in decreased HIF-1α mRNA levels.
To test the in vivo relevance of our findings, we performed in vivo matrigel plug assays. Similar to our in vitro model, the silencing of β3-endonexin significantly increased CD31 staining in the in vivo matrigel plug as an indicator of the angiogenic response (Fig. 9B), further supporting the concept that β3-endonexin is a negative regulator of endothelial proliferative and angiogenic processes.
Discussion
In this study, we demonstrated that β3-endonexin acts as a novel anti-angiogenic molecule under hypoxic conditions by interfering with the HIF pathway. Specifically, our studies provide evidence that β3-endonexin accumulates in the nucleus in response to hypoxia and that it contributes to the decline in HIF-1α mRNA levels observed after the onset of prolonged hypoxia. We propose a mechanism by which β3-endonexin interferes with NFκB activation of the HIF-1α promoter under hypoxia, thus preventing further induction of HIF-1α mRNA under these stress conditions.
αvβ3-integrins have been suggested to play an important role in the control of angiogenesis, although the precise mechanisms of action remain under dispute (8). It has been suggested that αvβ3-integrins may interact with multiple players in the angiogenic pathway and, depending on the interaction, have either pro-angiogenic or anti-angiogenic effects (16).
In this study, we found that β3-endonexin, which has been identified as a β3-integrin binding protein, is able to negatively control endothelial proliferation and angiogenic responses both in vitro and in vivo under hypoxic conditions.
β3-endonexin has been initially reported to exist in two alternatively spliced isoforms: the long (EN-L) and short (EN-S) (27). However, only EN-S has been identified as a binding protein to the cytoplasmic tail of β3-integrin (27), while both EN-S and EN-L have been shown to bind cyclin A (21). Similarly, in our study, EN-S as well as EN-L was able to decrease the angiogenic response toward hypoxia, indicating that β3-endonexin isoforms can act as anti-angiogenic molecules beyond their functions in regulating avidity and, thus, ligand-binding affinity of β3-integrin.
Our studies further showed that the anti-angiogenic function of β3-endonexin was linked to the family of HIFs, which have been described as promoting angiogenesis under hypoxia due to up-regulation of important proangiogenic factors such as VEGF and PAI-1 (23). In fact, VEGF and PAI-1 expression was enhanced in hypoxic β3-endonexin-deficient cells. Moreover, the depletion of HIF-1α prevented enhanced tube formation in β3-endonexin-deficient cells under hypoxia, while the overexpression of β3-endonexin diminished hypoxic as well as VEGF-induced tube formation, indicating a close link between the HIF pathway and β3-endonexin in the angiogenic response.
In fact, our studies indicated that β3-endonexin can act as a negative regulator of HIF activity under hypoxic conditions, as hypoxic HIF activity and HIF-1α protein levels were decreased in endothelial cells overexpressing either EN-L or EN-S. On the contrary, in β3-endonexin-deficient cells, the hypoxic induction of HIF activity and HIF-1α protein levels was further enhanced. Interestingly, this effect was specific to HIF-1α, as the induction of HIF-2α by hypoxia was not affected by the depletion of β3-endonexin. Moreover, in contrast to HIF-1α or HIF-2α, the protein as well as mRNA levels of β3-endonexin remained constant over a period of intermediate hypoxia, indicating that the inhibitory effect of β3-endonexin on the HIF pathway in this timeframe was not primarily mediated by increased expression of EN-L or EN-S under hypoxic conditions, although on onset of prolonged hypoxia or in an animal model of chronic hypoxia, β3-endonexin mRNA levels were elevated. While we cannot rule out the fact that on prolonged hypoxic stress, up-regulation of β3-endonexin expression might also be relevant for its response to hypoxia, our findings clearly show that the primary response of β3-endonexin to hypoxia is translocation to and accumulation in the nucleus. This mechanism was independent of integrin binding and might be facilitated by the presence of a nuclear localization sequence in the β3-endonexin protein (27).
While HIF-1α levels are predominantly regulated by hypoxia via a complex mechanism of protein stabilization, HIF-1α mRNA levels seem to be differentially affected by hypoxia in different cellular and organismal systems. We and others have reported in smooth muscle cells and other cells that hypoxia induces a rapid but transient increase in HIF-1α mRNA levels followed by a decline in prolonged hypoxia (1, 24). Interestingly, we could also observe a similar response to hypoxia in endothelial cells. However, when we depleted cells from β3-endonexin, the decline in HIF-1α mRNA levels on prolonged exposure to hypoxia was abrogated. These novel findings indicated that β3-endonexin might be involved in regulating HIF-1α mRNA levels under hypoxia.
We have previously shown that HIF-1α is a NFκB target gene (1, 7), an observation that has been confirmed in inflammatory conditions both in vitro and in vivo (24). We could also show that NFκB contributes to the transient induction of HIF-1α mRNA in response to hypoxia (1). In support, our data demonstrate that not only hypoxia increased NFκB transcriptional activity in endothelial cells but also β3-endonexin was able to prevent the activation of NFκB by hypoxia. Importantly, β3-endonexin was able to negatively affect NFκB-mediated HIF-1α transcription under hypoxia. This assumption was supported by our findings that forced expression of p50/p65 not only enhanced hypoxic HIF-1α mRNA levels but also prevented the decline on prolonged hypoxic exposure; while expression of an IκBdn that prevents activation of NFκB not only diminished the hypoxic up-regulation of HIF-1α mRNA levels in the absence of β3-endonexin, but also prevented endothelial proliferation and angiogenesis in β3-endonexin-deficient hypoxic cells. Moreover, the increase in hypoxic HIF-1α promoter activity on the depletion of β3-endonexin was completely blunted when the NFκB-binding site within the HIF-1α promoter that binds a p50/p65 complex (1, 24) was mutated. Importantly, hypoxic binding of the NFκB protein p65 to the HIF-1α promoter was decreased on the overexpression of EN-L, suggesting that NFκB binding to the HIF-1α promoter is prevented by β3-endonexin. In line, β3-endonexin has been reported to inhibit NFκB activity by binding and sterically blocking the active p50/p65 complex (4). These findings support a model by which under hypoxic conditions β3-endonexin translocates to the nucleus, binds to the p50/p65 complex, and, subsequently, prevents HIF-1α transcription and induction of HIF-1α mRNA (Fig. 9C). These findings also indicate that β3-endonexin is a novel regulator of the hypoxic response in endothelial cells by modulating the induction and activation of HIF-1α.
Although transcriptional regulation and de novo synthesis of HIF-1α take place under various conditions, in many cases regulated by NFκB (1, 24), this pathway may be less favourable under hypoxic conditions, where protein synthesis is limited. By activation of β3-endonexin as an endogenous inhibitor of HIF-1α transcription under hypoxic conditions, the stabilization of HIF-1α protein due to the inhibition of prolyl hydroxylases (PHD) activity will be favored, thus enabling a rapid and efficient provision of HIF-1α not affecting protein synthesis machinery. Since HIF-1α mRNA levels will not be replenished due to β3-endonexin blockade of NFκB as a major transcription factor regulating HIF-1α, HIF-1α mRNA levels will decline on prolonged exposure to hypoxia, a situation that might be overcome by enhanced levels of p50 and p65 exceeding the levels of β3-endonexin.
Our data further suggest that the inhibition of NFκB-dependent transcription under hypoxia by β3-endonexin might not only be limited to HIF-1α, as this protein is also able to negatively regulate hypoxic activation of the NFκB target gene TF. Along with our findings that under hypoxic conditions β3-endonexin accumulates in the nucleus, these data suggest that this protein might be a negative regulator of NFκB-dependent gene expression under hypoxia.
Taken together, our data indicate that β3-endonexin is a novel anti-angiogenic factor which is specifically important in the response to hypoxia due to the inhibition of NFκB-dependent up-regulation of HIF-1α and, subsequently, HIF1 activity and HIF1-dependent pro-angiogenic target gene expression. Since β3-endonexin expression was also evident in vivo in a model of chronic hypoxia characterized by structural alterations of the vessel wall and dysregulated vessel formation finally resulting in pulmonary hypertension, this molecule might represent an interesting novel therapeutic target in conditions in which HIF activation and angiogenesis need to be controlled.
Materials and Methods
Chemicals
All chemicals were from Sigma unless stated differently.
Cell culture
HMEC-1 were purchased from CDC Atlanta and grown according to the manufacturer's instructions. Experiments were performed with cells cultured in endothelial basal medium that was supplemented with 2% fetal calf serum (PAN). Cells were exposed to hypoxia (1% oxygen and 5% CO2) in a Ruskinn work station (IUL).
Transfections and luciferase assays
Expression vectors coding for the β3-endonexin short (EN-S) or long (EN-L) isoforms (pcDNA3.1EN-S-V5 and pcDNA3.1EN-L-V5) were cloned from endothelial cells through amplification by a polymerase chain reaction (PCR) using the previously published oligonucleotide sequences (14): 5′-GGG GCG ACG CGT ATG ATG CCT GTT AAA AGA TCA CTG AAG TTG GAT GGT CTG-3′ (fw)/5′-GGG GCG GCG GCC GCT TCA CAG AGG TTG TGA CAT CTG AGG CTG ACC TTT GTG-3′ (rev) (EN-L) or 5′-GGG GCG GCG GCC GCT TCA CTG TAT ACT ACT TAA ATT TTG CAT TAT CTC CAT-3′ (rev) (EN-S). The resulting PCR fragments were fused to the respective 3′ termini of a pcDNA3.1/V5 cloning cassette (Invitrogen). All constructs were identified by restriction digestion and verified by DNA sequencing. pcDNA3.1/V5 empty cassette served as a transfection control.
Expression vectors coding for IκBdn were from Becton Dickinson. Expression vectors coding for the NFκB subunits p50 and p65, HIF-1α promoter luciferase constructs HIF1α-538, HIF1α-538m, EPO-HRE-Luc, NFκB-Luc, and the PAI-1 promoter constructs PAI-796 and PAI-796m have been previously described (1, 7). The TF promoter constructs pTF636 and pTF636NFm have been previously described (2). For transfection, HMEC-1 cells were plated to a density of 50%–70%, cultured for 24 h, and transfected by FuGENE HD (Roche) reagent according to the manufacturer's protocol. Luciferase assays were performed as previously described (1). For gene silencing of β3-endonexin, HMEC-1 cells were transfected with short interfering RNA (siRNA) targeting human integrin-β3 binding protein ITGB3BP, 5′-CUGCCUCAGCCUUGGGAGUAGUUdGdG-3′ (sense) (ITGB3BP, NCBI Reference Sequence: NM_014288.4) or with control siRNA 5′-GACUACUGGUCGUUGAAGUdTdT-3′ (Eurogentec) using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. At 24 h after transfection, cells were placed in 2% fetal calf serum containing medium for 16 h before hypoxic stimulation. siRNA transfection efficiency was validated by real-time quantitative PCR (RT-qPCR), and was on average 80% to 90%.
Immunoblot analysis
Proteins were isolated from total cellular lysates or from nuclear and cytosolic fractions as previously described (7). Western blot analyses were performed as previously described (15) using antibodies against β3-endonexin (4), HIF-1α (Invitrogen), HIF-2α (Millipore), Lamin B1, p50, p65 (all Cell Signaling), IκBα, and β-actin (both Santa Cruz).
Gene expression analysis
Total RNA was isolated from HMEC-1 cells or from murine lung tissue using RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol. cDNA was synthesized from isolated RNA using high-capacity cDNA reverse transcription kit (Qiagen) according to the manufacturer's instructions in a 6000 Rotor Gene Real-Time PCR System (Qiagen). RT-qPCR was performed using human gene-specific primers: ITGB3BP (β3-endonexin consensus sequence) 5′-TCT CCA ACA ACT GGA ACT TGT C-3′(sense), 5′-TCC ATT TCT GTG CTT TTG CTC-3′ (anti-sense); HIF1A 5′-GAA GAC ATC GCG GGG AC-3′ (sense), 5′-TGG CTG CAT CTC GAG ACT TT-3′ (anti-sense); GAPDH 5′-GTG AAC ATG AGA AGT ATG ACA AC-3′ (sense), 5′-CAT GAG TCC TTC CAC GAT ACC-3′ (anti-sense); VEGFA 5′-AGG AGG AGG GCA GAA TCA TCA-3′ (sense), 5′-CTC GAT TGG ATG GCA AGT AGC T-3′ (anti-sense); 18S rRNA 5′-GTA ACC CGT TGA ACC CCA TT-3′ (sense), 5′-CCATCCAATCGGTAGTAGCG-3′ (anti-sense).
For gene analyses in the murine system, RT-qPCR was performed using murine gene-specific primers: Itgb3bp (β3-endonexin consensus sequence) 5′-GGA ACT TAT CAG TTG AGC CCA TT-3′ (sense), 5′-AGT TAC TCC GTT TCC TTG TTTCA-3′ (anti-sense); Hif1a 5′-GCA CTA GAC AAA GTT CAC CCT GAG A-3′ (sense), 5′-CGC TAT CCA CAT CAA AGC AA-3′ (anti-sense); 18S rRNA 5′-GCA ATT ATT CCC CAT GAA CG-3′ (sense), 5′-GGG ACT TAA TCA ACG CAA GC-3′ (anti-sense). For each independent experiment, samples were loaded in triplicate for each primer pair, and the value of each sample was normalized to 18S rRNA. All data were analyzed by the system software (Qiagen).
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation was performed as previously described (7). Specifically, HMEC-1 cells transfected with the expression vector encoding β3-endonexin long isoform (EN-L) or with control vector (Ctr) were exposed to hypoxia (1% oxygen) for 8 h or remained under normoxia (Ctr). Cells were fixed with formaldehyde, lysed, and sonicated to obtain 500 to 1000 bp DNA fragments. Chromatin was precipitated with an antibody against p65 (Cell Signaling) overnight at 4°C. Real-time PCR was performed to amplify a region of the human HIF-1α promoter containing the NFκB consensus sequence at −197/188 bp (forward, HIF1A 5′-GCT GAC CGC CTC CTG ATT G-3′, reverse 5′-TTC CTC GAG ATC CAA TGG CG-3′) using a Rotor-Gene 6000 Real-Time PCR System (Corbett Life Science). A gene-deficient region on chromosome 4 (Untr4) served as a negative control (forward, 5′-CTC CCT CCT GTG CTT CTC AG-3′ and reverse, 5′-AAT GAA CGT GTC TCC CAG AA-3′). As a background control, chromatin immunoprecipitation with isotype control antibody (IgG) was performed. Quantification was performed using a standard curve of the input. p65 binding to chromatin was revealed after background subtraction as a relative amount of the input used.
Indirect fluorescent labelling
β3-endonexin was detected using indirect immunofluorescence labeling. In brief, HMEC-1 cells were grown on uncoated cover slips or on cover slips coated with collagen or fibronectin (both Invitrogen) and fixed in 1:1 methanol-acetone solution, blocked with 2% bovine serum albumin solution for 25 min followed by incubation with the primary β3-endonexin antibody (1:100) for 1 h at room temperature in a humid chamber. For indirect fluorescent labeling, secondary mouse anti-rabbit antibody conjugated with Alexa fluor 488 (Invitrogen) was applied for 2 h at room temperature in a dark chamber. Cover slips were mounted with Fluorescent mounting medium (DAKO) on objective slides and analyzed on a Zeiss Axiophot microscope (Zeiss) with Axio Vision imaging software (Zeiss). Nuclei were visualized with 4′, 6-diamidino-2-phenylindole (DAPI, 1:10,000) staining (Invitrogen).
Proliferation assay
DNA synthesis in HMEC-1 cells was assessed by BrdU labeling (Roche) as previously described (13).
In vitro tube formation assay
HMEC-1 cells (5000 cells per well) were seeded on a micro-slide angiogenesis plate (Ibidi) that was mounted with growth factor-reduced Matrigel (BD Biosciences). Cells were subsequently incubated for 6 h either in hypoxia (1% oxygen) or under control conditions at 37°C, counterstained using Calcein AM (BD Biosciences). Alternatively, HMEC-1 cells were treated with VEGF (1 μg/μl; Invitrogen). The formation of capillary-like structures was assessed by fluorescence microscopy (Olympus) using the Openlab Modular Software for Scientific Imaging (Improvision) and was quantified for the total tube lengths using Image J software (Wright Cell Imaging Facility).
In vivo chronic hypoxia
C5BL/6 mice (8 weeks old, 20–25 g; Charles River) were exposed to chronic normobaric hypoxia (10% O2) in a ventilated chamber (Ing. Humbs) for 2 weeks. Age-matched control animals breathed room air. Mice were anesthetized with CO2 and sacrificed. Total mRNA was isolated from lung tissue, and RT-qPCR analysis was performed. All animal procedures were approved by the local legislation on the protection of animals (Government of Upper Bavaria).
In vivo matrigel plug assay
In vivo matrigel plug assay was performed as previously described (12). HMEC-1 cells were silenced for β3-endonexin (siEN) or transfected with scrambled RNA (siCtr). Cells (150,000 cells/plug) were mixed with 350 μl of growth factor reduced Matrigel (BD Biosciences) and subcutaneously injected into mice (C5BL6, 6 weeks old, male). At 7 days after the injection, matrigel plugs were excised, formalin fixed and paraffin embedded, sectioned, and prepared for immunohistochemical staining. The formation of capillary-like structures was assessed by staining with an antibody against CD31 (DAKO) in a 1:50 dilution. All animal procedures were approved by the local legislation on the protection of animals (Government of Upper Bavaria, Munich, Germany).
Statistical analysis
Values are presented as means±SD. Results were compared by analysis of variance for repeated measurements followed by Student–Newman–Keuls t-test. p<0.05 was considered statistically significant.
Abbreviations Used
- ARNT
arylhydrocarbon receptor nuclear translocator
- BrdU
5-bromo-2′deoxyuridine
- EN-L
β3-endonexin long isoform
- EN-S
β3-endonexin short isoform
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIF
hypoxia-inducible factor
- HMEC-1
human microvascular endothelial cells-1
- HRE
hypoxia-response elements
- IκBdn
dominant-negative IκBα
- Luc
luciferase
- NFκB
nuclear factor kappa B
- PAI-1
plasminogen activator inhibitor-1
- PCR
polymerase chain reaction
- PHD
prolyl hydroxylase
- RT-qPCR
real-time quantitative PCR
- siRNA
short interfering RNA
- TF
tissue factor
- uPAR
urokinase-type plasminogen activator receptor
- VEGF
vascular endothelial growth factor
Acknowledgments
The authors would like to thank Andreas Petry for help with editing this article; Zuwen Zhang, Anna Titova, and Frederick Vogel for help with animal experiments and sample processing. This work has been supported by grants from DFG GO709/4–5 and EU 7th framework program METOXIA.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Belaiba RS, Bonello S, Zahringer C, Schmidt S, Hess J, et al. . Hypoxia up-regulates hypoxia-inducible factor-1alpha transcription by involving phosphatidylinositol 3-kinase and nuclear factor kappaB in pulmonary artery smooth muscle cells. Mol Biol Cell 18: 4691–4697, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BelAiba RS, Djordjevic T, Bonello S, Artunc F, Lang F, et al. . The serum- and glucocorticoid-inducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res 98: 828–836, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bergers G. and Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer 3: 401–410, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Besta F, Massberg S, Brand K, Muller E, Page S, et al. . Role of beta(3)-endonexin in the regulation of NF-kappaB-dependent expression of urokinase-type plasminogen activator receptor. J Cell Sci 115: 3879–3888, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Besta F, Muller I, Lorenz M, Massberg S, Bultmann A, et al. . Reduced beta3-endonexin levels are associated with enhanced urokinase-type plasminogen activator receptor expression in ApoE−/− mice. Thromb Res 114: 283–292, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Binder BR, Mihaly J, and Prager GW. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemost 97: 336–342, 2007 [PubMed] [Google Scholar]
- 7.Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, et al. . Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol 27: 755–761, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, and Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest 96: 1815–1822, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, et al. . BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem 278: 1450–1456, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Byzova TV. and Plow EF. Activation of alphaVbeta3 on vascular cells controls recognition of prothrombin. J Cell Biol 143: 2081–2092, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Contois L, Akalu A, and Brooks PC. Integrins as “functional hubs” in the regulation of pathological angiogenesis. Semin Cancer Biol 19: 318–328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, et al. . Phosphodiesterase 2 mediates redox-sensitive endothelial cell proliferation and angiogenesis by thrombin via Rac1 and NADPH oxidase 2. Circ Res 104: 1169–1177, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Djordjevic T, Hess J, Herkert O, Gorlach A, and BelAiba RS. Rac regulates thrombin-induced tissue factor expression in pulmonary artery smooth muscle cells involving the nuclear factor-kappaB pathway. Antioxid Redox Signal 6: 713–720, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gawaz M, Besta F, Ylanne J, Knorr T, Dierks H, et al. . The NITY motif of the beta-chain cytoplasmic domain is involved in stimulated internalization of the beta3 integrin A isoform. J Cell Sci 114: 1101–1113, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Gorlach A, Diebold I, Schini-Kerth VB, Berchner-Pfannschmidt U, Roth U, et al. . Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ Res 89: 47–54, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Curr Opin Cell Biol 20: 514–519, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, and De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56: 549–580, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Mackman N, Brand K, and Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med 174: 1517–1526, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino Y, Cao R, Svensson K, Bertilsson G, Asman M, et al. . Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature 414: 550–554, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ohtoshi A. and Otoshi H. Analysis of beta3-endonexin mutants for their ability to interact with cyclin A. Mol Genet Genomics 266: 664–671, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Ratcliffe PJ. HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117: 862–865, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rey S. and Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res 86: 236–242, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, et al. . NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453: 807–811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sastry SK. and Horwitz AF. Integrin cytoplasmic domains: mediators of cytoskeletal linkages and extra- and intracellular initiated transmembrane signaling. Curr Opin Cell Biol 5: 819–831, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL. Hypoxia. Cross talk between oxygen sensing and the cell cycle machinery. Am J Physiol Cell Physiol 301: C550–C552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shattil SJ, O'Toole T, Eigenthaler M, Thon V, Williams M, et al. . Beta 3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin beta 3 subunit. J Cell Biol 131: 807–816, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16: 1151–1162, 2002 [DOI] [PubMed] [Google Scholar]