Abstract
The cellular microenvironment plays a crucial role in directing proliferation and differentiation of stem cells. Cells interact with their microenvironment via integrins that recognize certain peptide sequences of extracellular matrix proteins. This receptor-ligand binding has profound impact on cell fate. Interactions of human bone marrow mesenchymal stem cells (hMSCs) with the triple helical collagen mimetic, GPC(GPP)5-GFOGER-(GPP)5GPC-NH2, and the fibronectin adhesion peptide, RGD, were studied in degradable or nondegradable polyethylene glycol (PEG) gels formed by Michael-type addition chemistry. Proliferation, cytoskeletal morphology, and chondrogenic differentiation of encapsulated hMSCs were evaluated. The hMSCs adopted a highly spread morphology within the GFOGER-modified gels, whereas RGD induced a star-like spreading of the cells. hMSCs within GFOGER-modified degradable gels had a high proliferation rate compared with cells in peptide-free gels (p=0.017). Gene expression of type II collagen was highest in GFOGER-modified degradable gels after 21 days. Peptide incorporation increased GAG production in degradable gels after 7 and 21 days and GFOGER-modified degradable hydrogels had on average the highest GAG content, a finding that was confirmed by Alcian blue staining. In conclusion, the GFOGER peptide enhances proliferation in degradable PEG gels and provides a better chondrogenic microenvironment compared with the RGD peptide.
Introduction
The limited ability of cartilage tissue to self-repair after injury or disease has driven intensive efforts to engineer replacement tissue.1,2 Cell-based cartilage tissue engineering holds strong promise to provide solutions for cartilage repair.3–5 Chondrogenic differentiation of stem cells has been widely studied using pellet cultures,6–8 micromass cultures,9 and hydrogels.10 Both natural10–14 and synthetic15–17 materials have been employed to induce chondrogenesis of stem cells in 3D cultures. Synthetic gels such as polyethylene glycol (PEG) are attractive for 3D culturing of cells,18–21 as they can be functionalized with specific adhesion sequences,16 growth factor binding sites,22,23 and protease-sensitive motifs.24 A PEG hydrogel modified with a matrix metalloproteinase (MMP)-sensitive peptide was shown to increase chondrocyte proliferation and upregulate mRNA levels of type II collagen and aggrecan in bovine chondrocytes.25
The extracellular microenvironment is believed to play a key role in the biological response of stem cells.26,27 Modification of hydrogels with small functional groups such as amines and phosphates induced differentiation of stem cells into the osteogenic or adipogenic pathways.28 Further, the introduction of adhesion sequences such as RGD into materials has shown its role in adhesion,24 viability,29 proliferation,30 and differentiation of stem cells,31,32 however, an inhibition of chondrogenesis was also reported in RGD-modified alginate hydrogels.33 Chondrocytes form primary interactions with type II collagen and hyaluronic acid in the pericellular matrix.34 The GFOGER sequence was identified by Knight et al.35 as the recognition site in type I and IV collagen. Aggregation of chondrocytes in suspension was also shown to be mediated by α10β1 integrin binding to the GFOGER peptide.36 Moreover, type II collagen-induced GAG deposition in chondrocytes was inhibited by GFOGER integrin blocking peptides.37
The GFOGER sequence has been used to modify surfaces38–40 and hydrogels37,41 to control adhesion and gene expression of chondrocytes and differentiation of stem cells. When bone marrow stromal cells were encapsulated in an agarose gel with covalently coupled GFOGER peptides, type II collagen, and aggrecan mRNA stimulation and GAG deposition by transforming growth factor beta (TGF-β) and dexamethasone was inhibited.41 However, human bone marrow mesenchymal stem cells (hMSC) encapsulated in a nondegradable PEG hydrogel modified with the GFOGER peptide showed enhanced chondrogenesis compared with the unmodified and the stiffer modified hydrogels.42
We hypothesized that GFOGER functionalized PEG hydrogels provide a better chondrogenic environment than RGD and that providing MMP-sensitive motifs would further enhance this response. To test this hypothesis, hMSC were encapsulated in degradable (MMP-sensitive) or nondegradable PEG hydrogels containing RGD or GFOGER sequences and cell morphology, proliferation, gene expression, and synthesis of chondrogenic markers were analyzed.
Materials and Methods
Materials
Phosphate buffer saline (PBS), fetal bovine serum (FBS), cell culture media (DMEM-Glutamax), antibiotic-antimycotic, Trypsin/EDTA, 4′,6-diamidino-2-phenylindole dilactate (DAPI), and Alexa 488 goat anti-mouse-conjugated IgG IgM (H+L) were from Invitrogen AG. Formaldehyde, L-proline, dexamethasone, tris (2-carboxyethyl) phosphine hydrochloride solution (TCEP), acetic acid, Spectra-Por Float-A-Lyzer G2 black 3.5–5 kDa, sodium chloride (NaCl), immunoglobin G (IgG), pronase E from Streptomyces griseus, chondroitinase ABC (C2905), bovine serum albumin (BSA), Triton×100, phalloidin TRITC-labeled mixed isomers (phalloidin-rhodamine), ethylenediaminetetraacetic acid (EDTA), sodium phosphate, 1,9-dimethyl-methylene blue (DMMB), sodium formate, formic acid, ethanol, papain from papaya latex, L-cysteine, and chondroitin 4-sulfate sodium salt from bovine trachea were purchased from Sigma Aldrich Chemie GmbH. ITS+ Premix and nontissue culture treated 24-well plates were from Becton Dickson AG Allschwill. L-Ascorbic acid phosphate magnesium salt was obtained from Wako (IG instrumenten-Gesellschaft AG). Type II collagen antibody II-II6B3 was from Developmental Studies Hybridoma Bank, University of Iowa. MMP-Degradable, without RGD-peptide (Ref. 1004), MMP-NonDegradable without RGD-peptide (Ref. 1010), disc casters, and RGD peptide were obtained from QGel. Human fibroblast growth factor-2 (FGF-2) and human TGF-β3 were from Peprotech. The GFOGER peptide with the sequence GPC(GPP)5-GFOGER-(GPP)5GPC-NH2 was obtained from Richard Farndale (University of Cambridge, Cambridge, UK) and preparation of the peptide for hydrogel incorporation was followed according to an established protocol.35
Peptide preparation and cell culture
2 mg GFOGER peptide was reduced in 10 mM acetic acid 2 mM TCEP then heated for 2 min to 70°C and allowed to form triple helices at 4°C for 24 h. The triple helical peptides (11.1 kDa) were dialyzed against 10 mM acetic acid with a 3.5–5 kDa cutoff dialysis column to remove unfolded peptides and the TCEP. Passage 2 human mesenchymal stem cells from four independent donors (hMSC) were seeded and expanded until passage 6 in DMEM supplemented with 10% FBS, 1% antibiotic-antimycotic and 1 ng/mL FGF-2, which has been shown to maintain the multilineage and chondrogenic potential of stem cells.43 Cells were released with trypsin/EDTA and encapsulated at 1×107 cells/mL in degradable or nondegradable PEG gels modified with 100 μM RGD or 100 μM GFOGER peptide or nonmodified according to the manufacturer's protocol (QGel). The cell/PEG solution was cast in discs of 30 μL volume each. After 45 min incubation at 37°C, discs were removed from the caster and cultured in a nontissue culture 24-well plate prefilled with 1 mL chondrogenic media/well (DMEM 31966, 1% antibiotic-antimycotic, 50 μg/mL L-ascorbic acid, 1% ITS+ premix, 40 μg/mL L-proline, 100 ng/mL dexamethasone, and 10 ng/mL TGF-β3). Nonmodified nondegradable gels cultured in chondrogenic media were designated as controls. Cell pellets were also prepared by centrifuging a 250,000 cell suspension for 5 min at 300 g in 15 mL falcon tubes then culturing the pellets in 500 μL chondrogenic or control media as differentiation controls. The media was changed every 3 days.
Mechanical properties of hydrogels
Degradable and nondegradable hydrogels modified with 100 μM RGD, 100 μM GFOGER, or nonmodified were cultured in cell culture medium in the incubator for 24 h after production. Compressive moduli of the hydrogels were measured using a texture analyzer (TA.XTPlus; Stable Microsystems). Samples were compressed without preload at a speed of 0.01 mm/s and the force was measured at 0.1 mm compression, where the gels showed linear behavior. Young's moduli E were calculated according to the formula:
 |
where stress σ is the measured force per hydrogel area and ɛ is the strain.
Cell morphology
Cell nuclei and the f-actin were visualized using double labeling by DAPI and rhodamine-labeled phalloidin respectively. Cells were fixed with 5% formalin for 30 min at 4°C, washed 3× with PBS, permeabilized with 0.4% Triton X-100 for 20 min, and washed again. F-actin fibers were stained for 1 h total with 0.13 μg/mL phalloidin. After 30 min, 2 μg/mL of DAPI was added at 1:1 (v/v) to the phalloidin solution for the remaining 30 min. All staining was done at 4°C and in complete darkness. After staining, samples were washed and stored in PBS. Samples were imaged with a two photon microscope and 150 μm z-stacks were acquired starting approximately 200 microns from the disc surface (SP8 Leica Microsystems).
Proliferation within hydrogels
Cell proliferation was assessed with a bromodeoxyuridine assay (Calbiochem® BrdU Cell Proliferation Assay) using a slight modification of the manufacturer's protocol. Briefly, gels were transferred to a 96-well plate and incubated in media containing 1:200 concentration of BrdU label for 24 h. The media was removed and gels were dissolved by incubation with 200 μL Trypsin/EDTA for 20 min with 500 rpm shaking. The plate was centrifuged for 10 min at 1000 rpm, the liquid in the wells was carefully removed and wells were dried with a gentle stream of nitrogen. Samples were then incubated with a fixative/denaturing solution for 30 min, the anti-BrdU antibody for 1 h, and the HRP-conjugate for 30 min. Substrate was added for color development followed by addition of the stop solution. Absorbance was measured at dual wavelengths of 450–540 nm with a spectrophotometric plate reader (Synergy; BioTek Instruments, Inc.).
Gene expression
After 7 or 21 days in culture, hydrogels were washed once in PBS and 350 μL RLT Plus Lysis Buffer (Qiagen) was added. Samples were processed using a homogenizer (TH 220 tissue homogenizer, Omni International, LabForce). A volume of 540 μL of RNA-free water and 10 μL Protease K solution (Qiagen) were then added to each sample and samples were incubated for 10 min at 55°C with continuous shaking at 1000 rpm. RNA was isolated using the RNeasy Mini Kit (Qiagen) following the fibrous tissue isolation protocol given by the manufacturer. Total RNA was quantified using a spectrophotometer (Nanodrop ND-1000) and the measured 260/280 ratio was consistently 2.0±0.1 for all samples. Total RNA was reverse transcribed and gene expression of type II collagen (Col2A7), type I collagen (Col1), and aggrecan were determined using quantitative real-time PCR (StepOnePlus; Applied Biosystems). Pellet cultures were used as chondrogenesis controls, donors showing no response to chondrogenic media in pellets were thus excluded from the study (see Supplementary Figs. S1 and S2; Supplementary Data are available online at www.liebertpub.com/tea). Ribosomal protein L13 (RPL13) was used as a housekeeping gene as it has been recently demonstrated to be more stable than GAPDH.44 The Livak method was used for analysis of quantitative real-time PCR data.45 The primers used in this study are listed in Table 1.
Table 1.
Genes Used in Quantitative Reverse Transcription-Polymerase Chain Reaction Analysis
| Gene | Accession number | Primer sequence (5′-3′) | Product size (BP) |
|---|---|---|---|
| RPL13 | NM_012423 | F–AAGTACCAGGCAGTGACAG | 100 |
| R–CCTGTTTCCGTAGCCTCATG | |||
| Col2A7 | NM_001844 | F–GGAATTCGGTGTGGACATAGG | 92 |
| R–ACTTGGGTCCTTTGGGTTTG | |||
| Col1A7 | NM_000088 | F–CAGCCGCTTCACCTACAGC | 83 |
| R–TTTTGTATTCAATCACTGTCGCC | |||
| Aggrecan | NM_001135 | F–GAATGGGAACCAGCCTATACC | 98 |
| R–TCTGTACTTTCCTCTGTTGCTG |
DNA quantification in gels
Gels, collected at days 7 and 21 for assessment of DNA content, were dissolved in 100 μL of papain lysis buffer (10 mM EDTA, 100 mM sodium phosphate, 10 mM L-cysteine, and 125 μg/mL papain type III at pH 6.3) overnight at 60°C with shaking at 1000 rpm. DNA quantification was carried out with Quant-iTTM PicoGreen dsDNA Kit (Invitrogen). All samples were diluted 1:10 in TE buffer supplied by the kit. DNA standard was diluted in TE buffer to 2 μg/mL, 200 ng/mL, 20 ng/mL, 2 ng/mL, and 0.2 ng/mL concentrations. Lysis buffer was diluted 1:10 and PicoGreen reagent was diluted 1:200 in TE buffer. A volume of 100 μL of each sample and DNA standard was transferred to a 96-well plate in triplicates and an equal volume of PicoGreen solution was added in each well. Measurements were performed using an excitation wavelength of 480 nm and emission wavelength of 520 nm with a plate reader (Synergy; BioTek Instruments, Inc.).
DMMB assay
DMMB dye was prepared according to Estes et al.46 Briefly, 21 mg of DMMB was dissolved in 5 mL ethanol with 2 mg sodium formate and mixed into 800 mL distilled water. The pH was adjusted to 3.0 with formic acid followed by bringing up the solution to 1 L. Gels were collected at days 7 and 21 for assessment of GAG content and dissolved in 100 μL of papain lysis buffer (10 mM EDTA, 100 mM sodium phosphate, 10 mM L-cysteine, and 125 μg/mL papain type III at pH 6.3) overnight at 60°C with shaking at 1000 rpm. Chondroitin 4- sulfate (Sigma) was diluted in papain buffer dilutions from 0 to 35 μg/mL, which were used to create the standard curve. A volume of 40 μL of each sample or standard was pipetted into a 96-well plate. A volume of 125 μL of the DMMB dye was added to each well and optical density was measured at 595 nm with a plate reader. All GAG quantification was normalized to DNA content for each gel.
Histology and immunostaining
After 21 days in culture, disks were washed once in PBS for 3 min, fixed for 30 min with 4% paraformaldehyde, 0.2% Triton-X100, washed in PBS for 3 min, and stored in PBS. Disks were embedded in Optimum Cutting Temperature (O.C.T) compound and frozen for 5 min on a dry ice block. Six micrometers thick sections (CryoStar NX70; ThermoScientific) were fixed in ethanol and retrieval of type II collagen antigens was carried out using 1 mg/mL pronase at 37°C for 15 min. Sections were incubated with 5% BSA in PBS for 1 h at room temperature to block unspecific binding. Sections were incubated with the type II collagen primary antibody at 1:10 dilutions in 1% BSA in PBS overnight at 4°C. Sections were then washed 3× with PBS followed by incubation for 1 h at room temperature with 1:400 Alexa-488 goat anti-mouse secondary antibody. Controls were prepared following the same procedure but omitting the primary antibody. Images of the samples were taken using a confocal microscope (Carl Zeiss AG/LSM 510, equipped with a 40×0.6 NA objective). GAG deposition of cells encapsulated within hydrogels in the various conditions was analyzed using Alcian blue. Briefly, frozen sections of the hydrogels were defrosted at room temperature, washed in PBS for 5 min and fixed with 4% formaldehyde in PBS for 7 min. Samples were washed 3×5 min each in PBS, hydrated with ultra-pure water, and then stained in Alcian blue solution (pH 2.5) for 20 min. Slides were washed in running tap water until unspecific staining was washed away, samples were rinsed in ultra-pure water and dehydrated using ascending alcohol solutions (95% to absolute ethanol) for 3 min each. Sections were cleaned in xylene, air-dried, and mounted with resinous mounting medium (Mounting Medium Ref. 4112; Richard-Allan Scientific). A cover slip was added on the top of the section and sealed with nail polish. Images were acquired using a Leica DFC420 C digital microscope camera equipped with a 40×objective.
Statistical analysis
Quantitative data were obtained using a minimum of three independent donors and expressed in all figures and text as the mean±standard error. Analysis of variance (ANOVA) and post-hoc Tukey's tests where used for statistical evaluation where p values of less than 0.05 were considered significant. OriginPro version 8.1 was used to carry out the statistical analysis.
Results
Hydrogel modification did not affect mechanical properties
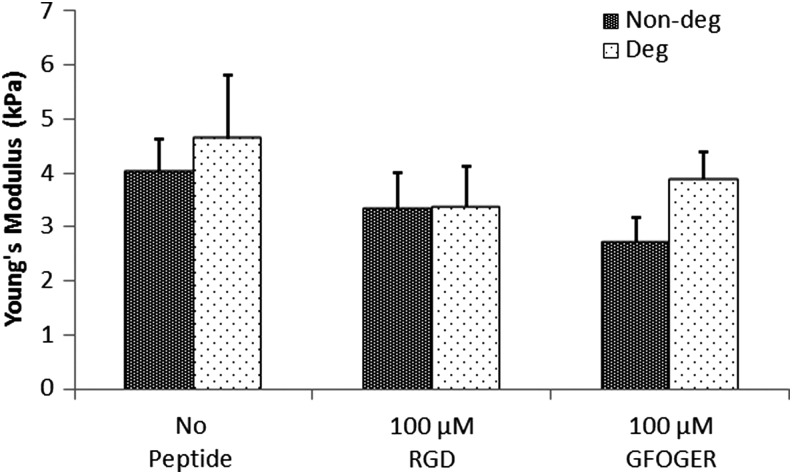
To test whether the mechanical properties of the gels could be affected by peptide incorporation, the compressive moduli of the hydrogels were measured. No significant difference was found among the different tested conditions (Fig. 1). A two-way ANOVA test revealed that there was no significant influence in mechanical properties, neither due to degradability of the gels (p=0.35) nor from the addition of the adhesion peptide (p=0.32). The interaction term between degradability and peptide also showed no significance (p=0.75). We can therefore assume that cell behavior was not affected by differences in the initial mechanical properties of the gels. The average compressive modulus of the hydrogels (3.7±0.3 kPa) was similar to that reported by the manufacturer.
FIG. 1.
Compression test of hydrogels containing 100 μM RGD, 100 μM GFOGER or without adhesion peptides. Young's moduli are shown for the nondegradable gels (Nondeg) and for the degradable gels (Deg).
Cell morphology
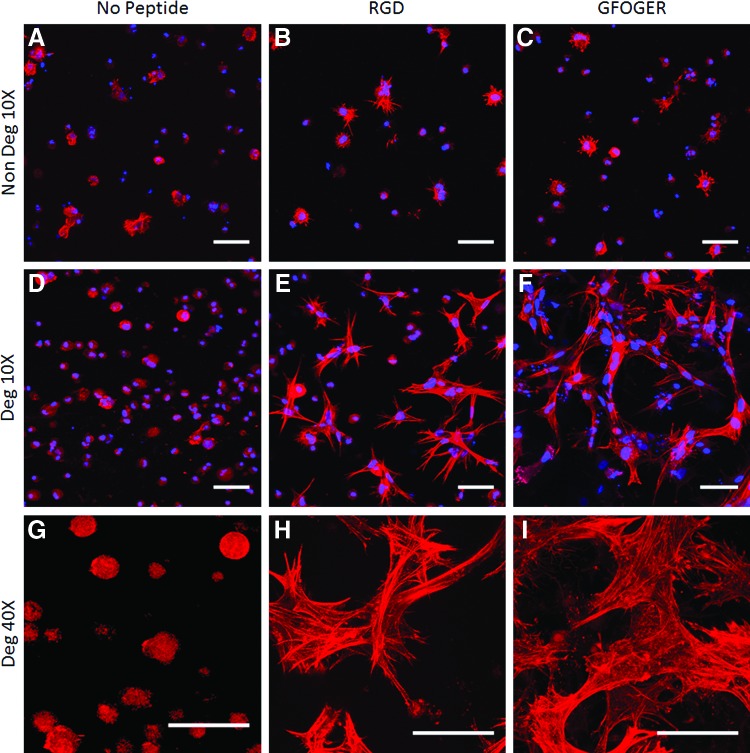
MSCs in PEG hydrogels exhibited very different actin cytoskeletal structure depending on the specific integrin-binding domain incorporated into the PEG matrix. If no adhesion peptide was used, cells displayed a round morphology in the maximum intensity projections of the z-stacks (Fig. 2A, D, G). In RGD and GFOGER containing PEG gels, cells spread and developed stress fibers (Fig. 2B, C, E, F, H, I). This effect was much more pronounced in the degradable gels that permitted the cells to remodel their matrix and physically free space in the 3D network for this elongated morphology (Fig. 2E, F, H, I). Cell protrusions were observed in nondegradable peptide-containing gels, though spreading was clearly restricted. The shape of spread cells strikingly varied in RGD-PEG versus GFOGER-PEG gels. In RGD-PEG gels, the cells tended to be smaller and contained thin star-like projections of cytoskeleton ending at a matrix attachment site in a sharp point (Fig. 2B, E, H). In contrast, in GFOGER-PEG gels, cells interacted with the gel using more uniform, evenly distributed matrix attachment sites (Fig. 2C, F, I). This effect was also observed in chondrocytes encapsulated within RGD and GFOGER-modified degradable hydrogels (See Supplementary Fig. S3).
FIG. 2.
Human bone marrow mesenchymal stem cells cultured for 21 days in chondrogenic media in modified PEG gels after fixation and staining for nuclei (DAPI, blue) and actin filaments (phalloidin, red). PEG modifications included an MMP-cleavable peptide (degradable gels, D–I) or MMP-insensitive peptides (nondegradable, A–C) and incorporation of the adhesion peptides RGD (B, E, H), GFOGER (C, F, I), or no adhesion peptide (A, D, G). Gel degradability improved cell spreading in hydrogels modified with adhesion peptides. Cells in RGD-containing gels displayed star-like morphologies with thick, individually-discernible actin fibers. GFOGER-modified gels induced a more homogenous spreading of cells with thinner and more dispersed actin filaments (I). Depicted are maximum intensity projections through 150 μm thick Z-stack images, scale=50 μm. Dapi was excluded from high magnification figures to allow a clearer visualization of the cytoskeletal organization. PEG, polyethylene glycol; MMP, matrix metalloproteinase. Color images available online at www.liebertpub.com/tea
Cell proliferation was highest in GFOGER degradable hydrogels
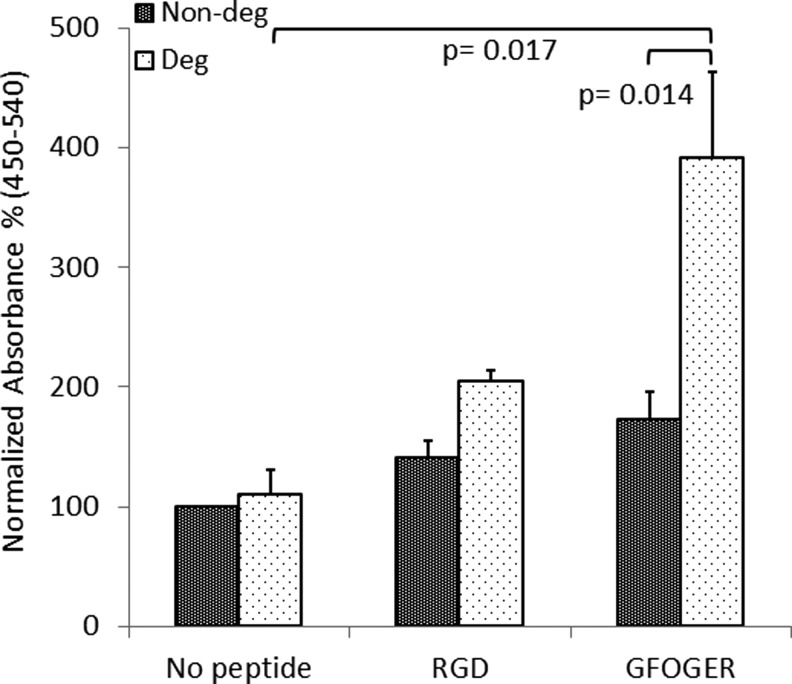
Peptide incorporation in the gels yielded an increase in cell proliferation for both degradable and nondegradable gels (Fig. 3). GFOGER-modified degradable gels had the best proliferation rate, which was higher than both no adhesion peptide gels (p=0.017) and RGD-modified gels (p=0.069). Degradable GFOGER-modified gels were shown to cause a significant increase (p=0.014) in cell proliferation compared with GFOGER nondegradable gels, whereas such effect was not seen between degradable and nondegradable versions of RGD- and no adhesion peptide gels.
FIG. 3.
Cell proliferation in gels was assessed after 7 days with a bromodeoxyuridine (BrdU) assay. Absorbance values (450–540 nm) for all hydrogel conditions were normalized to nondegradable gels with no adhesion peptide incorporation and relative quantitation is shown, (n=3).
Gene expression
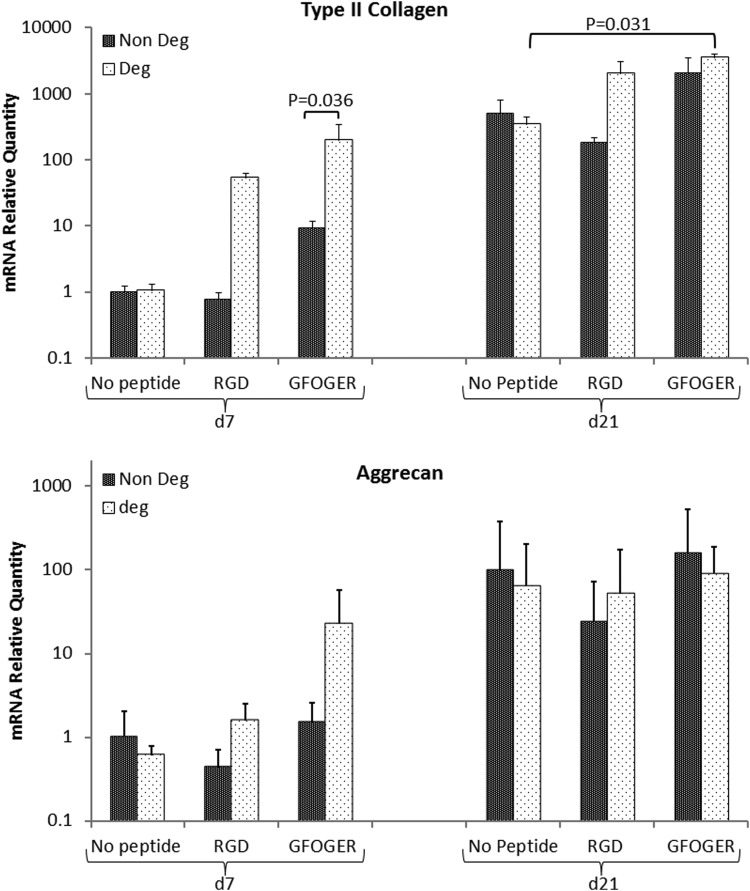
Gene expression of type II collagen was highest in GFOGER-modified degradable gels when compared with peptide-free degradable controls after 21 days in culture (Fig. 4). Gene expression of type II collagen and aggrecan changed significantly from day 7 to 21 (p=8.7E-5) and (p=1.4E-3) respectively. The upregulation in type II collagen between days 7 and 21 in RGD-modified degradable, GFOGER-modified nondegradable, and GFOGER-modified degradable exceeded 100-fold on average (p=0.001, p=0.002 and p=0.006 respectively) indicating a stronger chondrogenic induction in these samples. Aggrecan upregulation with time exceeded 100-fold only for GFOGER nondegradable gels p=0.007. Gel degradability was a significant factor in type II collagen induction at day 7, only for GFOGER samples (p=0.036), however, degradability showed no significant effects in any sample at day 21. The effect of peptide incorporation was significant only in GFOGER samples compared with no peptide (p=0.017) and the GFOGER degradable gels expressed significantly higher type II collagen at day 21 compared with peptide-free degradable gels (p=0.031). Expression of type I collagen was not significantly different among all studied conditions after 7 and 21 days of culture (see Supplementary Fig. S4).
FIG. 4.
Gene expression of type II collagen and aggrecan was determined using quantitative real-time polymerase chain reaction (PCR) (StepOnePlus; Applied Biosystems). Nondegradable hydrogels without modification (Non Deg No peptide) were used as controls and RPL13 was used as a reference gene, (n=3).
GAG production in peptide-modified gels
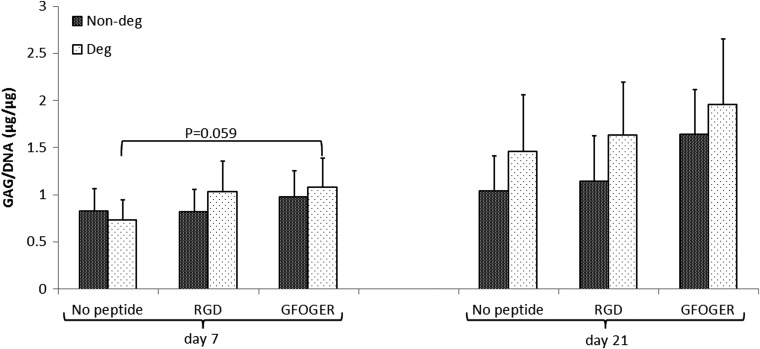
Although there was no significant effect on GAG production as a response to time in culture, adhesion peptide incorporation or degradability, the GFOGER-modified degradable hydrogels tended to have the highest GAG content among the tested conditions (Fig. 5). After 7 days GFOGER-modified degradable gels had an almost significant increase in GAG/DNA (p=0.059).
FIG. 5.
GAG/DNA ratio where DNA was quantified using PicoGreen and GAG was quantified using the DMMB assay, (n=3).
Histology and immunostaining
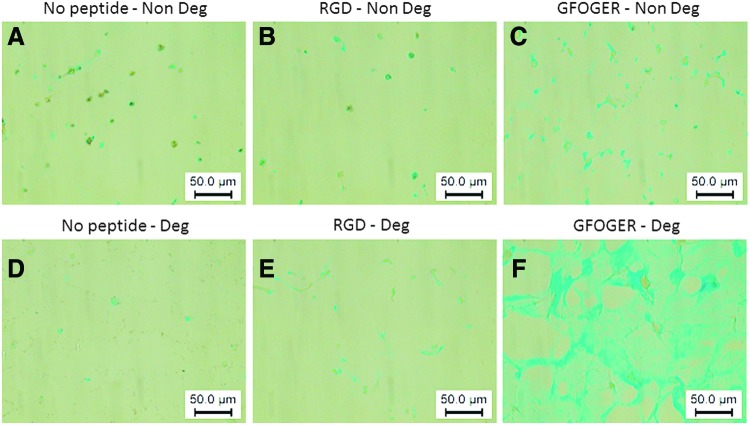
GAG synthesis was confirmed qualitatively using Alcian blue staining. GFOGER degradable hydrogels showed the most prominent staining compared to all other hydrogel conditions (Fig. 6). In hydrogels with no adhesion peptide modification (Fig. 6A, D) and in RGD-modified gels (Fig. 6B, E), staining was limited to the immediate vicinity of the cells. In GFOGER-modified gels, staining in the nondegradable hydrogels was very strong around the cells only with little GAGs in the matrix (Fig. 6C). In GFOGER degradable gels, the staining was strongly distributed throughout the whole sample (Fig. 6F). Although the overall Alcian blue staining intensity varied from donor to donor, the staining was consistently highest in GFOGER degradable gels. Alcian blue staining in most hydrogels except GFOGER degradable hydrogels was very limited indicating that no background staining occurs due to the hydrogel or the incorporated peptide.
FIG. 6.
Alcian blue staining of hMSC cultured in PEG gels of different compositions. hMSC cultured in nondegradable PEG gels without adhesion peptides (No peptide–Non Deg, A), nondegradable PEG gels with RGD peptides (RGD–Non Deg, B), nondegradable PEG gels with GFOGER peptides (GFOGER–Non Deg, C), degradable PEG gels without adhesion peptides (No peptide–Deg, D), degradable with RGD peptides (RGD–Deg, E), and degradable with GFOGER peptides (GFOGER–Deg, F). All hydrogels were cultured for 21 days in chondrogenic medium. Images were acquired with a 40× objective. hMSC, human bone marrow mesenchymal stem cells. Color images available online at www.liebertpub.com/tea
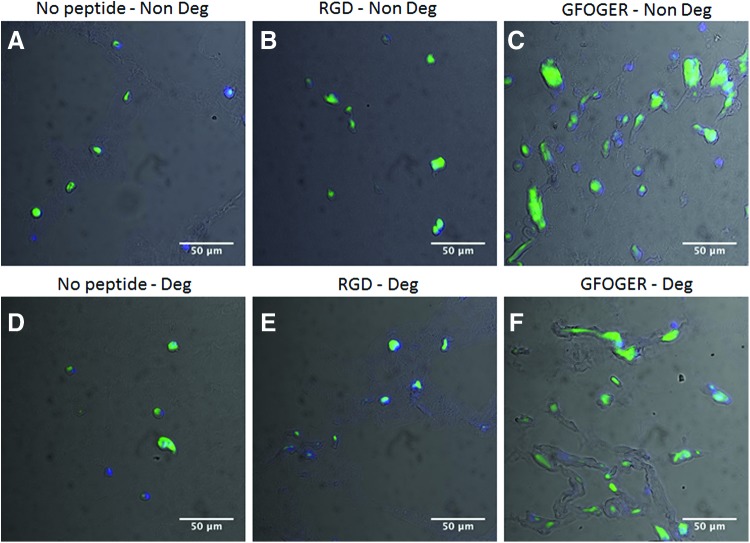
Type II collagen immunostaining considerably varied among samples and was strongest in the GFOGER-modified hydrogels (Fig. 7C, F) compared with all other conditions (Fig. 7A, B, D, E).
FIG. 7.
Type II collagen immunostaining of hMSC cultured in PEG gels of different compositions. hMSC cultured in nondegradable PEG gels without adhesion peptides (No peptide–Non Deg, A), nondegradable PEG gels with RGD peptides (RGD–Non Deg, B), nondegradable PEG gels with GFOGER peptides (GFOGER–Non Deg, C), degradable PEG gels without peptides (No peptide–Deg, D), degradable with RGD peptides (RGD–Deg, E), and degradable with GFOGER peptides (GFOGER–Deg, F). All hydrogels were cultured for 21 days in chondrogenic medium. Images were acquired with a 40× objective. Color images available online at www.liebertpub.com/tea
Discussion
In the current study we encapsulated hMSCs in degradable and nondegradable PEG hydrogels modified with the peptides RGD and GFOGER, which are key adhesion sequences of cartilage extracellular matrix proteins fibronectin and collagens respectively. Cell proliferation was highest in GFOGER-modified degradable hydrogels. Proliferation was four-fold higher in GFOGER-modified degradable hydrogels compared with degradable hydrogels without any adhesion sequences (p=0.017) and was two-fold higher than in RGD-modified degradable gels (p=0.069). GAG/DNA synthesis measured by a DMMB assay and qualitatively confirmed with Alcian blue staining was greatest in the GFOGER-modified degradable gels. Although Alcian blue staining was most prominent in GFOGER-Deg cultures, the GAG/DNA level was not significantly higher in GFOGER-Deg gels compared with the other conditions. This is due to the high proliferation rate exhibited in GFOGER-Deg cultures reflecting an overall reduced GAG synthesis per cell. Moreover, gene expression and synthesis of type II collagen was highest in GFOGER-modified degradable hydrogels. Cell morphology strikingly varied between RGD and GFOGER-modified gels. Cells in GFOGER-modified hydrogels had a more uniformly spread morphology with relatively weak stress fibers compared with RGD-modified hydrogels, which were star-like in shape with strong actin fibers as shown in Figure 2. Viability was higher in degradable hydrogels modified with RGD or GFOGER sequences when compared with the other conditions. The viability was also higher in the RGD and GFOGER-modified nondegradable hydrogels compared with nonmodified nondegradable hydrogels (see Supplementary Fig. S5).
Several studies have employed RGD sequences for improving cell adhesion,24 viability29 and chondrogenesis16,31,32 of stem cells in 3D PEG gels. However, very few studies investigated the incorporation of the GFOGER peptide in 3D hydrogels and their influence on chondrogenesis.41,42 In particular, the effect of hydrogel degradability on the response of stem cells to the GFOGER peptide has not been reported. Moreover, a study comparing the effect of presenting equal molar concentrations of GFOGER and RGD peptides on chondrogenesis is presented here for the first time. As expected, the presence of MMP motifs in the PEG hydrogels greatly influenced the cell response to GFOGER and RGD peptides in terms of cell spreading and proliferation. Further, degradability was essential to induce chondrogenesis in hydrogels modified with adhesion peptides.
A major requirement for an engineered 3D biomaterial to be used for tissue engineering applications is to maintain cell viability for a long culturing period. Additionally, increased proliferation may be desirable for tissue formation.47 Although the viability of fibroblasts24 or chondrocytes25 is not significantly affected by the materials degradability, stem cells have been reported to have a higher viability in degradable hydrogels.47 Further, incorporation of adhesion sequences like RGD,48 IKVAV,47 and GFOGER42 were shown to improve stem cell viability in PEG gels. We show here that the presence of MMP motifs alone did not have a significant effect on cell proliferation or cell viability (see Supplementary Fig. S2). However, the incorporation of peptides in both degradable and nondegradable hydrogels improved cell viability and cell proliferation, which is consistent with the literature.42,47,48 In general, incorporation of adhesion peptide sequences improved proliferation, however, only GFOGER degradable hydrogels had a significantly higher proliferation (p=0.017) compared with the degradable hydrogels without any adhesion peptides. Degradability was also only a significant factor in the presence of GFOGER sequences (p=0.014).
Morphology is a key characteristic of cells in mature cartilage tissue where a round cell shape is typical of chondrocytes.49,50 Cell morphology is also implicated in chondrogenic differentiation where the precartilage condensation is accompanied by alterations from a spread to a round cell shape.51,52 Chondrogenesis of bone marrow stem cells has been shown to be inhibited in RGD-modified alginate.33 Moreover, chondrogenesis in agarose was inhibited in the presence of RGD sequences and this inhibition was blocked by disruption of the f-actin cytoskeleton using cytochalasin D.53 These results are in contradiction with the findings of the current study where chondrogenic differentiation was strongest in RGD and GFOGER-modified degradable hydrogels in which cells had a clearly more spread morphology than the other conditions. This might be due to different properties of PEG compared with agarose and alginate but also due to the fact that the latter hydrogels are not degradable by cellular proteases. The current results are in agreement with a study by Hwang et al.16 where human embryonic stem cells exhibited increased gene expression and matrix production of cartilage markers in RGD-modified PEG-diacrylate compared with RGD-free controls. In their work, RGD-containing hydrogels promoted a larger cell diameter compared with RGD-free controls.16 In the current study, cells encapsulated in RGD-modified degradable hydrogels had a star-like morphology with strong actin fibers, whereas cells in GFOGER had more diffuse and less intense actin fibers (Fig. 2H, I). The contradictory results presented in the literature, and the results of the current study suggest that cell shape alone does not control chondrogenesis. Cell–matrix interactions have been shown to be critical in directing stem cell fate.54 Therefore, GFOGER sequences might result in favorable chondrogenic cell signaling compared with RGD. Further, the differential cell morphologies and cytoskeletal organizations induced by GFOGER and RGD sequences may induce varying intracellular tensile and compressive forces. These forces may activate specific biochemical pathways according to the tensegrity model.55,56
In general, the trend observed in the current study was that the increase in cell spreading and proliferation was associated with an increase in type II collagen expression. Stem cell fate in various culture conditions is always in flux between either self-renewal or lineage specification. Elucidation of the factors that contribute to this decision making is critical in directing their fate. The results of the current study indicate that cell spreading and proliferation do not necessarily inhibit chondrogenesis as might be expected in 2D and that the 3D microenvironment, cell–matrix interactions and growth factors may be stronger players in the initial phase of chondrogenesis. It is possible that a culturing period exceeding 6 weeks would allow the cells in GFOGER degradable hydrogels to finally adopt a round morphology and commit to a chondrogenic phenotype.
GFOGER-modified hydrogels represent a new biomimetic option for cell growth in a 3D hydrogel. The GFOGER peptide induces higher proliferation of stem cells and maintains on average a higher expression of the chondrogenic markers type II collagen and aggrecan and a higher GAG production when compared with the commonly used RGD peptide. Degradability and the presence of adhesion peptides in 3D cultures are shown here to be necessary to maintain a high viability and initiate chondrogenesis of MSCs. Taken together, the results of the current study suggest that GFOGER peptides presented in a degradable hydrogel provide a better microenvironment for proliferation and chondrogenesis of hMSCs compared with RGD. The combination of proliferation while maintaining the expression and synthesis of type II collagen and aggrecan might be used for MSC-based treatments of cartilage lesions.
Supplementary Material
Acknowledgments
This work was funded by the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement n° NMP4-SL-2009-229292 (Find&Bind). The type II collagen antibody II-II6B3 developed by T.F. Linsenmayer was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We also thank Janos Vörös (Laboratory of Biosensors and Bioelectronics, ETH Zurich, Switzerland) for his valuable scientific input, Nadiia Kondratiuk for help with immunostaining, Richard Farndale for providing the GFOGER peptide and for his advice on cross-linking and Celine Gandar (QGel SA) for her assistance with the PEG gels.
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Becerra J., Andrades J.A., Guerado E., Zamora-Navas P., López-Puertas J.M., and Reddi A.H.Articular cartilage: structure and regeneration. Tissue Eng Part B Rev 16,617, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Grande D.A., Southerland S.S., Manji R., Pate D.W., Schwartz R.E., and Lucas P.A.Repair of articular cartilage defects using mesenchymal stem cells. Tissue Eng 1,345, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Chiang H., and Jiang C.C.Repair of articular cartilage defects: review and perspectives. J Formos Med Assoc 108,87, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., and Peterson L.Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 331,889, 1994 [DOI] [PubMed] [Google Scholar]
- 5.Strauss E.J., Fonseca L.E., Shah M.R., and Yorum T.Management of focal cartilage defects in the knee-Is ACI the answer? Bull NYU Hosp Jt Dis 69,63, 2011 [PubMed] [Google Scholar]
- 6.Johnstone B., Hering T.M., Caplan A.I., Goldberg V.M., and Yoo J.U.In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238,265, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi Y., Sekiya I., Yagishita K., and Muneta T.Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52,2521, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mackay A.M., Beck S.C., Murphy J.M., Barry F.P., Chichester C.O., and Pittenger M.F.Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng 4,415, 1998 [DOI] [PubMed] [Google Scholar]
- 9.De Bari C., Dell'Accio F., Tylzanowski P., and Luyten F.P.Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44,1928, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Erickson G.R., Gimble J.M., Franklin D.M., Rice H.E., Awad H., and Guilak F.Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells in Vitro and in Vivo. Biochem Biophys Res Commun 290,763, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Awad H.A., Quinn Wickham M., Leddy H.A., Gimble J.M., and Guilak F.Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 25,3211, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ma H.-L., Hung S.-C., Lin S.-Y., Chen Y.-L., and Lo W.-H.Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res Part A 64A,273, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Mauck R.L., Yuan X., and Tuan R.S.Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthritis Cartilage 14,179, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Wang Y., Kim U.-J., Blasioli D.J., Kim H.-J., and Kaplan D.L.In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 26,7082, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Cho J.H., Kim S.-H., Park K.D., Jung M.C., Yang W.I., Han S.W., et al. . Chondrogenic differentiation of human mesenchymal stem cells using a thermosensitive poly(N-isopropylacrylamide) and water-soluble chitosan copolymer. Biomaterials 25,5743, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Hwang N.S., Varghese S., Zhang Z., and Elisseeff J.Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng 12,2695, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Zhou G., Liu W., Cui L., Wang X., Liu T., and Cao Y.Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng 12,3209, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lutolf M.P., and Hubbell J.A.Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotech 23,47, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., and Elisseeff J.Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27,12, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Williams C.G., Kim T.K., Taboas A., Malik A., Manson P., and Elisseeff J.In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng 9,679, 2003 [DOI] [PubMed] [Google Scholar]
- 21.McCall J.D., Luoma J.E., and Anseth K.S.Covalently tethered transforming growth factor beta in PEG hydrogels promotes chondrogenic differentiation of encapsulated human mesenchymal stem cells. Drug Deliv Transl Res 2,305, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C.-C., and Anseth K.S.Controlling affinity binding with peptide-functionalized poly(ethylene glycol) hydrogels. Adv Funct Mater 19,2325, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J.Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 31,4639, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B., et al. . Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A 100,5413, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y., Lutolf M.P., Hubbell J.A., Hunziker E.B., and Wong M.Bovine primary chondrocyte culture in synthetic matrix metalloproteinase-sensitive poly(ethylene glycol)-based hydrogels as a scaffold for cartilage repair. Tissue Eng 10,515, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Hwang N.S., Varghese S., and Elisseeff J.Controlled differentiation of stem cells. Adv Drug Deliv Rev 60,199, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson A., and Trumpp A.Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6,93, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Benoit D.S.W., Schwartz M.P., Durney A.R., and Anseth K.S.Small functional groups for controlled differentiation of hydrogel-encapsulated human mesenchymal stem cells. Nat Mater 7,816, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salinas C.N., and Anseth K.S.The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med 2,296, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y.-C., Brayfield C.A., Gerlach J.C., Peter Rubin J., and Marra K.G.Peptide modification of polyethersulfone surfaces to improve adipose-derived stem cell adhesion. Acta Biomater 5,1416, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Liu S.Q., Tian Q., Wang L., Hedrick J.L., Hui J.H.P., Yang Y.Y., et al. . Injectable biodegradable poly(ethylene glycol)/RGD peptide hybrid hydrogels for in vitro chondrogenesis of human mesenchymal stem cells. Macromol Rapid Commun 31,1148, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Salinas C.N., and Anseth K.S.The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29,2370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Connelly J.T., García A.J., and Levenston M.E.Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials 28,1071, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Loeser R.F.Integrins and cell signaling in chondrocytes. Biorheology 39,119, 2002 [PubMed] [Google Scholar]
- 35.Knight C.G., Morton L.F., Peachey A.R., Tuckwell D.S., Farndale R.W., and Barnes M.J.The Collagen-binding A-domains of Integrins α1β1 and α2β1recognize the same specific amino acid sequence, GFOGER, in native (Triple-helical) collagens. J Biol Chem 275,35, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Gigout A., Jolicoeur M., Nelea M., Raynal N., Farndale R., and Buschmann M.D.Chondrocyte aggregation in suspension culture is GFOGER-GPP- and β1 integrin-dependent. J Biol Chem 283,31522, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Chiu L.-H., Chen S.-C., Wu K.-C., Yang C.-B., Fang C.-L., Lai W.-F.T., et al Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes. J Cell Physiol 226,1981, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Wojtowicz A.M., Shekaran A., Oest M.E., Dupont K.M., Templeman K.L., Hutmacher D.W., et al. . Coating of biomaterial scaffolds with the collagen-mimetic peptide GFOGER for bone defect repair. Biomaterials 31,2574, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raynor J.E., Petrie T.A., García A.J., and Collard D.M.Controlling cell adhesion to titanium: functionalization of poly[oligo(ethylene glycol)methacrylate] brushes with cell-adhesive peptides. Adv Mater 19,1724, 2007 [Google Scholar]
- 40.Reyes C.D., Petrie T.A., Burns K.L., Schwartz Z., and García A.J.Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 28,3228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connelly J., Petrie T., García A., and Levenston M.Fibronectin-and collagen-mimetic ligands regulate bone marrow stromal cell chondrogenesis in three-dimensional hydrogels. Eur Cell Mater 22,168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu S.Q., Tian Q., Hedrick J.L., Po Hui J.H., Rachel Ee P.L., and Yang Y.Y.Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 31,7298, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Tsutsumi S., Shimazu A., Miyazaki K., Pan H., Koike C., Yoshida E., et al. . Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288,413, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Studer D., Lischer S., Jochum W., Ehrbar M., Zenobi-Wong M., and Maniura-Weber K.Ribosomal protein L13a as a reference gene for human bone marrow-derived mesenchymal stromal cells during expansion, Adipo-, Chondro-, and Osteogenesis. Tissue Eng Part C Methods 18,761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Estes B.T., Diekman B.O., Gimble J.M., and Guilak F.Isolation of adipose-derived stem cells and their induction to a chondrogenic phenotype. Nat Protocols 5,1294, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jongpaiboonkit L., King W.J., and Murphy W.L.Screening for 3D environments that support human mesenchymal stem cell viability using hydrogel arrays. Tissue Eng Part A 15,343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nuttelman C.R., Tripodi M.C., and Anseth K.S.Synthetic hydrogel niches that promote hMSC viability. Matrix Biol 24,208, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Von Der Mark K., Gauss V., Von Der Mark H., and Muller P.Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267,531, 1977 [DOI] [PubMed] [Google Scholar]
- 50.Benya P.D., and Shaffer J.D.Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 30,215, 1982 [DOI] [PubMed] [Google Scholar]
- 51.DeLise A.M., Fischer L., and Tuan R.S.Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage 8,309, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Gao L., McBeath R., and Chen C.S.Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-Cadherin. Stem Cells 28,564, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connelly J.T., García A.J., and Levenston M.E.Interactions between integrin ligand density and cytoskeletal integrity regulate BMSC chondrogenesis. J Cell Physiol 217,145, 2008 [DOI] [PubMed] [Google Scholar]
- 54.Guilak F., Cohen D.M., Estes B.T., Gimble J.M., Liedtke W., and Chen C.S.Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 5,17, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ingber D.E.Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci 116,1157, 2003 [DOI] [PubMed] [Google Scholar]
- 56.Ingber D.E.Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci 116,1397, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.