Abstract
Human mesenchymal stem cells (hMSCs) have the ability to differentiate into mesenchymal lineages. In this study, we hypothesized that treatment of embryoid bodies (EBs) composed of either human embryonic stem cells (hESCs) or human induced pluripotent stem cells (hiPSCs) with a hMSC-conditioned medium (CM) can stimulate the induction of the mesodermal lineage and subsequent differentiation toward the osteogenic and chondrogenic lineage. Quantitative real-time reverse transcription–polymerase chain reaction (qRT-PCR) analysis indicated that the hMSC-CM treatment increased gene expression related to the mesodermal lineage and decreased gene expression related to the endodermal and ectodermal lineage in EBs. Fourteen days after culturing the mesodermal lineage-induced EBs in the osteogenic or chondrogenic differentiation medium, we observed enhanced osteogenic and chondrogenic differentiation compared with untreated EBs, as evaluated using qRT-PCR, cytochemistry, immunocytochemistry, and flow cytometry. This method may be useful for enhancing the osteogenic or chondrogenic differentiation of hESCs or hiPSCs.
Introduction
The promotion of human embryonic stem cell (hESC) or human induced pluripotent stem cell (hiPSC) differentiation toward a specific lineage is necessary for the clinical application of the cells. Embryoid bodies (EBs) create a suitable environment for hESC or hiPSC differentiation into cells of the three germ layers.1 After EB formation, EBs are plated onto tissue culture dishes for differentiation into various lineages.2 However, hESCs form teratomas, which are tumor-like formations containing three-germ-layer tissues, upon injection into immune-deficient mice.3 This phenomenon is a major obstacle for the clinical application of hESCs.4 The promotion of hESC or hiPSC differentiation toward a specific lineage in vitro may help to suppress teratoma formation and to obtain a transplantable dosage of a homogenous population of a desired cell type. Therefore, methods for the promotion of hESC differentiation toward a specific lineage need to be developed for clinical use.5
hESCs and hiPSCs have a potential use in cell therapy for the regeneration of various tissues, such as bone and cartilage.6–9 To obtain osteogenically and chondrogenically differentiated cells, ESCs or iPSCs can be induced to form EBs that can initiate spontaneous differentiation into cells representing the three germ layers, followed by culture on tissue culture dishes with osteogenic and chondrogenic induction media. However, the proportions of osteoblasts and chondrocytes produced by this method are very low.10 Previously, a stepwise differentiation protocol has been reported to enhance the efficiency of hESC differentiation to chondrocytes.11 The protocol is based on stepwise differentiation to primitive streak mesendoderm, followed by mesoderm, and finally chondrocytes. Each step of differentiation is induced by specific types of growth factors and cytokines. For example, mesodermal differentiation is induced by fibroblast growth factor (FGF2), bone morphogenic protein 4 (BMP-4), follistatin, and neurotrophin-4. A study has also reported that activin-A and transforming growth factor β1 (TGF-β1) induced mesodermal differentiation of hESCs.12
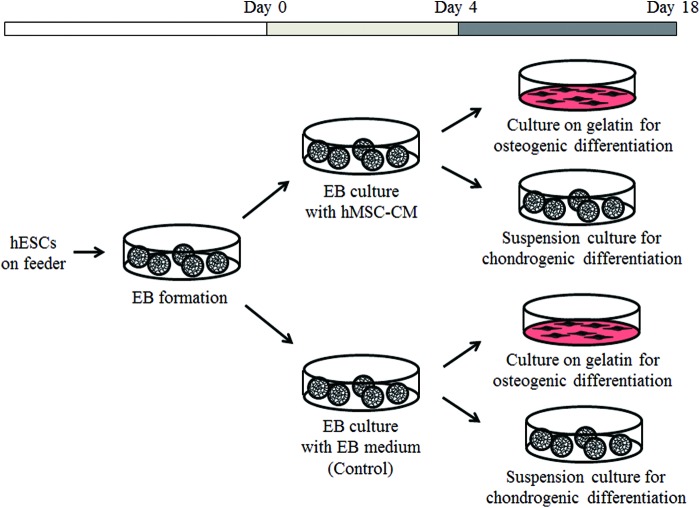
The conditioned medium (CM), which contains growth factors and differentiation regulation factors that are released from the cultured cells,13 could be used to promote ESC or iPSC differentiation into specific lineages. Previously, it was reported that treatment of murine ESCs (mESCs) that were cultured in monolayers before EB formation with CM from HepG2 cells, a human hepatocarcinoma cell line, enhanced mesoderm induction and the subsequent osteogenic differentiation of mESCs.5 It has been reported that human mesenchymal stem cells (hMSCs) secrete growth factors, including FGF2, BMP4, and TGF-β1,14–16 which have been shown to induce the mesodermal differentiation of hESCs. In this study, we hypothesized that the hMSC-CM can be used to promote hESC and hiPSC differentiation toward mesodermal lineage and subsequent osteogenic and chondrogenic differentiation. We used the hMSC-CM to culture hESCs and hiPSCs at the EB stage, rather than at the post-EB stage (Fig. 1), to promote mesodermal lineage differentiation and suppress endodermal and ectodermal lineage differentiation. To induce osteogenic and chondrogenic differentiation, the mesodermal lineage-induced EBs were cultured on tissue culture dishes with osteogenic and chondrogenic induction media (Fig. 1). This method may be an effective way to enhance the differentiation of hESCs and hiPSCs toward the osteogenic and chondrogenic lineage, which can be used to regenerate bone and cartilage tissues in cell-based therapies.
FIG. 1.
A schematic diagram of the protocol for mesodermal lineage induction of EBs derived from hESCs and subsequent differentiation to the osteogenic and chondrogenic lineages. To induce mesodermal induction of EBs, EBs composed of hESCs were cultured in the hMSC-CM. EB culture with EB media served as a control. Subsequently, the EBs were induced to undergo osteogenic or chondrogenic differentiation. CM, conditioned medium; EB, embryoid body; hESC, human embryonic stem cell; hMSC, human mesenchymal stem cell. Color images available online at www.liebertpub.com/tea
Materials and Methods
Culture of hESCs and hiPSCs
SNUhES31 (Institute of Reproductive Medicine and Population, Medical Research Center, Seoul National University, Seoul, Korea), an hESC line, and previously established hiPSCs17 (Kor-WT-iPSC 1, Yonsei University College of Medicine, Seoul, Korea) were maintained as an undifferentiated state by culture on feeder layers of mitomycin-C (Sigma-Aldrich, St. Louis, MO)-treated STO cells (a mouse embryonic fibroblast cell line, American Type Culture Collection; ATCC, Manassas, VA) in the Dulbecco's Modified Eagle's Medium (DMEM)/F12 (Gibco BRL, Gaithersburg, MD) supplemented with 20% (v/v) knockout serum replacement (Invitrogen, Carlsbad, CA), 4 ng/mL FGF2 (R&D Systems, Minneapolis, MN), 1% nonessential amino acid (Invitrogen), 0.1 mM β-mercaptoethanol (Sigma), and 0.2% primocin (InvivoGen, San Diego, CA). The culture medium was changed daily, and the hESCs were passaged onto fresh STO cells every week. The hESC or hiPSC colonies were fragmented into uniform sizes using the STEMPRO® EZPassage system (Invitrogen). The culture medium for STO cell expansion consisted of the DMEM (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin (Invitrogen).
Preparation of hMSC-CM
To prepare the hMSC-CM, hMSCs (Lonza, Walkersvile, MD) were cultured in a growth medium consisting of the DMEM (Gibco) supplemented with 10% (v/v) FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. hMSCs were plated 3×103 cells/cm2 (total 5×105 cells) on a 150-mm culture dish and the volume of the culture medium was 20 mL. After 7 days, when the cells were confluent, the supernatant was collected, filter sterilized through a 0.22-μm filter (Corning, Inc., Corning, NY), and stored at −20°C for short-term storage up to 1 week and in a −80°C freezer for long-term storage.
Formation of EBs and mesodermal induction
To form EBs, hESC and hiPSC colonies were detached from the feeders using collagenase type IV (2 mg/mL; Worthington, Lakewood, NJ). The detached hESC and hiPSC colonies were subsequently transferred to a low-attachment culture dish. The EBs were formed by incubation in the FGF2-depleted ESC culture medium, which was defined as the EB medium. To induce mesodermal induction, the hMSC-CM was mixed with the EB medium at a 1:1 ratio (v/v) and supplied to the EBs for 4 days (hMSC-CM group) (Fig. 1). The medium was changed every other day. The EBs without hMSC-CM treatment served as controls.
Quantitative real-time reverse transcription–polymerase chain reaction
After 4 days, the EBs were harvested from four independent cultures for each group and analyzed using quantitative real-time reverse transcription–polymerase chain reaction (qRT-PCR). Total RNA was extracted from the hMSC-CM EBs and untreated EBs. The RNA was reverse-transcribed into cDNA. The expression levels of ZIC, SOX1, FOXQ1, GSC, CER, BRACHYURY (T), WNT3, and MIXL1 mRNA were determined by qRT-PCR. qRT-PCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA) with FAST SYBR Green PCR master mix for 45 cycles (Applied Biosystems). Each cycle consisted of the following times and temperatures: 94°C for 3 s and 60°C for 30 s. The primers are shown in Table 1.
Table 1.
Human-Specific Primers for Each Gene
| Gene | Primer |
|---|---|
| GAPDH | Sense 5′-GTC GGA GTC AAC GGA TTT GG-3′ |
| Antisense 5′-GGG TGG AAT CAA TTG GAA CAT-3′ | |
| ZIC1 | Sense 5′-GCG CTC CGA GAA TTT AAA GA-3′ |
| Antisense 5′-CGT GGA CCT TCA TGT GTT TG-3′ | |
| SOX1 | Sense 5′-TAC AGC CCC ATC TCC AAC TC-3′ |
| Antisense 5′-GCT CCGACT TCA CCA GAG AG-3′ | |
| FOXQ1 | Sense 5′-GCG CGG ACT TGC ACT TT-3′ |
| Antisense 5′-GCA CGT TTG ATG GAG ATT TTA AAA-3′ | |
| GSC | Sense 5′-GAG GAG AAA GTG GAG GTC TGG TT-3′ |
| Antisense 5′-CTC TGA TGA GGA CCG CTT CTG-3′ | |
| CER | Sense 5′-ACA GTG CCC TTC AGC CAG ACT-3′ |
| Antisense 5′-ACA ACT ACT TTT TCA CAG CCT TCG T-3′ | |
| T | Sense 5′-ATC ACA AAG AGA TGA TGG AGG AA-3′ |
| Antisense 5′-GGT GAG TTG TCA GAA TAG GTT GG-3′ | |
| WNT3 | Sense 5′-CTG CCA GGA GTG TAT TCG CAT C-3′ |
| Antisense 5′-GAG AGC CTC CCC GTC CAC AG-3′ | |
| MIXL1 | Sense 5′-GGT ACC CCG ACA TCC ACT T-3′ |
| Antisense 5′-TGG AAG GAT TTC CCA CTC TG-3′ | |
| Alkaline phosphatase | Sense 5′-CCC TTG ACC CCC ACA ATG T-3′ |
| Antisense 5′-GTT GTT CCT GTT CAG CTC GTA-3′ | |
| Collagen type I | Sense 5′-CAG CCG CTT CAC CTA CAG C-3′ |
| Antisense 5′-TTT TGT ATT CAA TCA CTG TCT T-3′ | |
| Osteopontin | Sense 5′-GCC GAC CAA GGA AAA CTC ACT-3′ |
| Antisense 5′-CAG AAC TTC CAG AAT CAG CCT-3′ | |
| Osteonectin | Sense 5′-TCC ACA GTA CCG GAT TCT CTC T-3′ |
| Antisense 5′-TCT ATG TTA GCA CCT TGT CTC CAG-3′ | |
| Osteocalcin | Sense 5′-CCT CAC ACT CCT CGC CCT ATT-3′ |
| Antisense 5′-CCC TCC TGC TTG GAC ACA AA-3′ | |
| Collagen type II | Sense 5′-ATA AGG ATG TGT GGA AGC CG-3′ |
| Antisense 5′-TTT CTG TCC CTT TGG TCC TG-3′ | |
| Aggrecan | Sense 5′-ACC CTG GAA GTC GTG GTG AAA-3′ |
| Antisense 5′-CGT GGC AAT GAT GGC ACT G-3′ |
Osteogenic and chondrogenic differentiation of hESCs and hiPSCs
For osteogenic differentiation, EBs and hMSC-CM EBs were cultured on gelatin-coated (0.1 μg/cm2) dishes with an osteogenic induction medium for 14 days (Fig. 1). The osteogenic induction medium was the DMEM (Gibco) supplemented with 10% FBS (Gibco), 0.1 μM dexamethasone (Sigma), 50 μM ascorbate-2-phosphate (Sigma), and 10 mM β-glycerophosphate (Sigma). The medium was changed every 2 days. For chondrogenic differentiation, EBs and hMSC-CM EBs were cultured in pellets with a chondrogenic induction medium consisting of the DMEM supplemented with 1% (v/v) Insulin-Transferrin-Selenium-A (Gibco), 0.1 μM dexamethasone (Sigma), 50 μg/mL ascorbate-2-phosphate (Sigma), and 10 ng/mL TGF-β3 (R&D Systems) for 14 days. The medium was changed every 2 days. Osteogenic and chondrogenic differentiation was analyzed using qRT-PCR, cytochemistry, and immunocytochemistry.
qRT-PCR of osteogenically and chondrogenically differentiated hESCs and hiPSCs
After 14 days of osteogenic or chondrogenic induction, the cells were harvested from four independent cultures for each group and analyzed for osteogenic and chondrogenic marker gene expression using qRT-PCR. For the osteogenic differentiation analysis, the expressions of the alkaline phosphatase (ALP), collagen type I (COL I), osteopontin (OP), osteonectin (ON), and osteocalcin (OC) genes were analyzed. For chondrogenic differentiation analysis, the expressions of the collagen type II (COL II) and aggrecan (AGG) genes were analyzed. qRT-PCR was performed using the StepOnePlus real-time PCR system (Applied Biosystems) with FAST SYBR Green PCR master mix for 45 cycles (Applied Biosystems). Each cycle consisted of the following times and temperatures: 94°C for 3 s, 60°C for 30 s. The primers are shown in Table 1.
Chemical staining and immunocytochemistry of osteogenically and chondrogenically differentiated hESCs
After 14 days of osteogenic or chondrogenic induction, the cells were fixed with 4% (w/v) paraformaldehyde in phosphate-buffered saline (PBS) for 10 min at room temperature. Osteogenically differentiated cells were stained with Alizarin Red S staining and von Kossa staining. The chondrogenic differentiated cell pellets were embedded in Optimal Cutting Temperature compound (Tissue-Tek; Sakura Finetk, Torrance, CA) and frozen at −20°C. Sections (10 μm in thickness) were made using Cryostat Cryocut Microtome (CM3050S; Leica, Nussloch, Germany) and then fixed using 4% (w/v) paraformaldehyde in PBS for 10 min at room temperature. The sections were stained by Alcian Blue staining and Safranin O staining. The images were examined using a light microscope (Model IX71; Olympus, Tokyo, Japan). For immunocytochemistry, osteogenically differentiated cells were stained with an anti-human OC antibody (Abcam, Cambridge, United Kingdom), and the chondrogenically differentiated cell pellets were stained with an anti-human COL II antibody (Abcam). The OC staining results were visualized using rhodamine-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). The COL II staining results were visualized using fluorescein-isothiocyanate-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). The cells were counterstained with 4,6 diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) and examined using a fluorescence microscope (Model IX71; Olympus).
Flow cytometric analysis
Osteogenically differentiated cells were treated with trypsin (0.25% [w/v] in PBS) and dissociated by gentle pipetting. The dissociated cells were washed with PBS. The osteogenically differentiated cells were labeled with a rhodamine-conjugated OC antibody (R&D Systems) and then analyzed by FACS Aria I (BD Bioscience, San Jose, CA) using CellQuest software (BD Bioscience).
Statistical analysis
All quantitative data are expressed as the mean±standard deviation. The statistical analysis was performed using the Student's t-test (SAS software; SAS Institute, Cary, NC). A p-value<0.05 was considered statistically significant.
Results
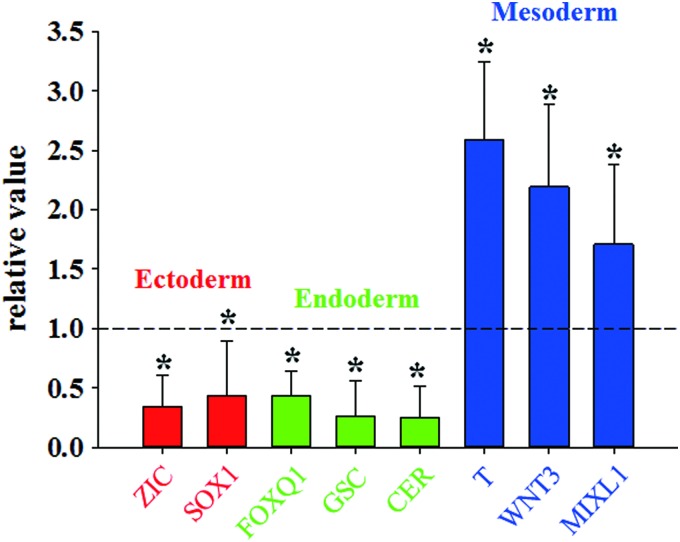
To determine whether the hMSC-CM treatment promotes mesodermal differentiation of EBs derived from hESCs, the expressions of the three-germ-layer lineage-specific genes were evaluated by qRT-PCR. The expressions of mesodermal genes (T, WNT3, and MIXL1) in the hMSC-CM EBs were increased compared with the untreated EBs (Fig. 2). In contrast, the expressions of ectodermal genes (ZIC and SOX1) and endodermal genes (FOXQ1, GSC, and CER) were decreased in the hMSC-CM EBs. The hMSC-CM-treated EBs derived from hiPSCs also showed enhanced mesodermal gene (WNT3 and MIXL1) and decreased ectodermal gene (ZIC and SOX1) expressions compared with untreated EBs derived from hiPSCs (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).
FIG. 2.
Enhanced mesodermal lineage induction of hESC EBs through the treatment of EBs with the hMSC-CM for 4 days. The gene expressions of mesodermal (T, WNT3, and MIXL1), endodermal (FOXQ1, GSC, and CER), and ectodermal (ZIC1 and SOX1) of hMSC-CM-treated EBs (hMSC-CM EBs) were determined by qRT-PCR and normalized to the levels of the untreated EB group (n=4, *p<0.05 vs. untreated EB). qRT-PCR, quantitative real-time reverse transcription–polymerase chain reaction. Color images available online at www.liebertpub.com/tea
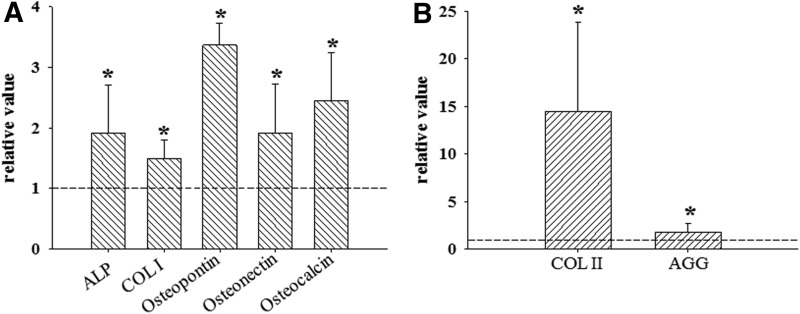
After 4 days of EB culture with or without hMSC-CM treatment, hMSC-CM EBs and untreated EBs were cultured for 14 days with the osteogenic or chondrogenic medium to investigate whether the hMSC-CM treatment enhanced the osteogenic and chondrogenic differentiation of hESCs. qRT-PCR analysis showed that the expressions of osteogenic marker genes (ALP, COL I, OP, ON, and OC) were significantly enhanced in the hMSC-CM treatment group compared with the untreated control group (Fig. 3A). For chondrogenic differentiation, COL II and AGG expressions were significantly enhanced in the hMSC-CM treatment group compared with the untreated control group (Fig. 3B). In hiPSC-derived EBs, hMSC-CM treatment also enhanced osteogenic marker genes (COL I, ON, and OC) and chondrogenic marker genes (COL II, and AGG) (Supplementary Fig. S2A, B).
FIG. 3.
Enhanced osteogenic and chondrogenic differentiation of mesodermal lineage-induced hESCs at day 14. The mRNA expressions of (A) osteogenic differentiation markers in osteogenic differentiation-induced hMSC-CM hESCs and (B) chondrogenic differentiation markers in chondrogenic differentiation-induced hMSC-CM hESCs were determined using qRT-PCR and normalized to the levels of the osteogenic or chondrogenic differentiation-induced, untreated hESCs. *p<0.05 versus untreated hESCs.
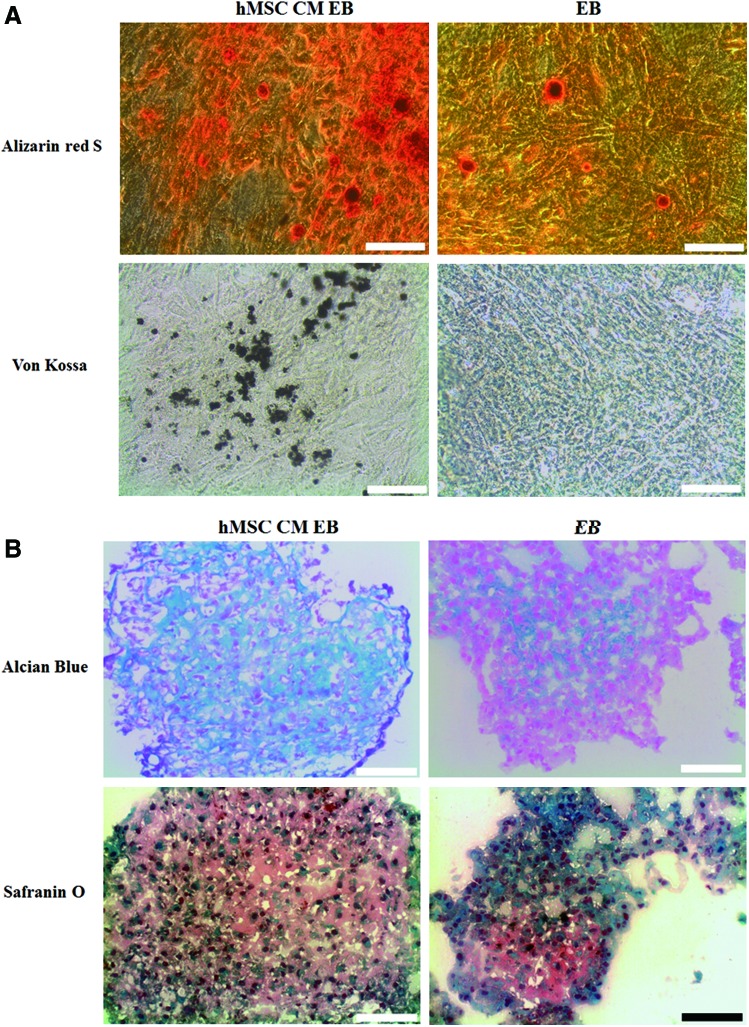
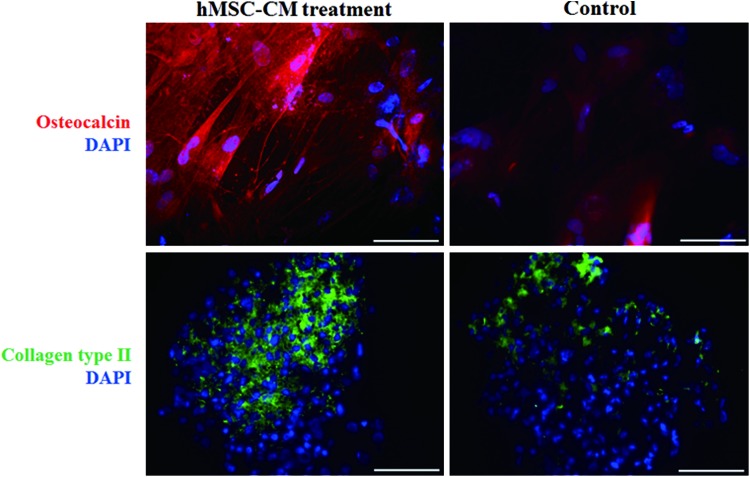
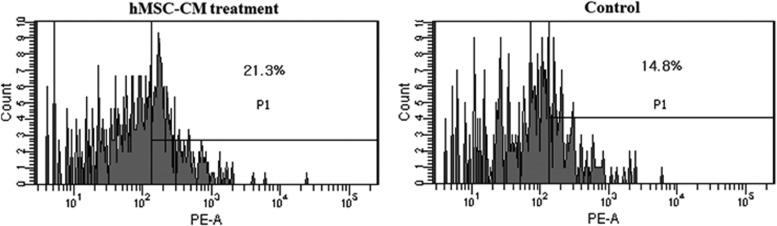
The calcium deposition of osteogenically differentiated hMSC-CM hESCs and untreated hESCs was evaluated by Alizarin Red S and von Kossa staining (Fig. 4A). The calcium deposition was enhanced in hMSC-CM hESCs compared with untreated hESCs, as hMSC-CM EBs were stained more positively for Alizarin Red S (red color) and von Kossa (black color) staining. hMSC-CM hESCs were more positively stained with Alcian Blue and Safranin O staining for cartilage-specific sulfated proteoglycans and sulfated glycosaminoglycans compared with untreated hESCs (Fig. 4B), indicating a larger extent of chondrogenic differentiation in the hMSC-CM treatment group. The immunocytochemistry analysis showed a similar tendency. hMSC-CM-treated hESCs showed more positive signals for OC (red) and COL II (green) compared with the untreated hESCs (Fig. 5). Flow cytometric analysis showed that 21.3% of the hMSC-CM hESCs cultured in the osteogenic medium were OC positive, whereas only 14.8% of the untreated hESCs were positive (Fig. 6).
FIG. 4.
Enhanced osteogenic and chondrogenic differentiation of mesodermal lineage-induced hESCs. Light microscopic images of (A) Alizarin Red S and von Kossa staining and (B) Alcian Blue and Safranin O staining of hMSC-CM-treated hESCs and untreated hESCs following osteogenic or chondrogenic differentiation induction for 14 days. The scale bars indicate 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 5.
Enhanced osteogenic and chondrogenic differentiation of mesodermal lineage-induced hESCs. Immunocytochemistry for human osteocalcin (red) and human collagen type II (green) in hMSC-CM-treated hESCs and untreated hESCs following osteogenic or chondrogenic differentiation induction for 14 days. The nuclei were stained with DAPI (blue). The scale bars indicate 100 μm. DAPI, 4,6 diamidino-2-phenylindole. Color images available online at www.liebertpub.com/tea
FIG. 6.
Flow cytometric analysis of hMSC-CM-treated hESCs and untreated hESCs using an osteocalcin antibody following osteogenic differentiation induction for 14 days.
Discussion
A previous study reported that mESCs cultured in the HepG2 cell-CM enhanced mesoderm formation and osteogenic cell derivation from mESCs.5 In this study, we directly cultured hMSC-CM-treated EBs to enhance mesodermal differentiation. qRT-PCR results showed that hMSC-CM treatment promoted mesodermal induction compared with untreated hESCs (Fig. 2). Subsequently, mesodermal lineage-induced EBs exhibited an enhancement of osteogenic and chondrogenic differentiation compared with untreated EBs.
hMSC-CM treatment enhanced the mesodermal lineage differentiation of hESCs and hiPSCs in the present study. During in vitro culture of MSCs, various growth factors and cytokines, such as BMP-4 and TGF-β1 that are secreted from MSCs, accumulate in the culture medium.18,19 BMP-4 and TGF-β1 have been previously reported to stimulate a signaling pathway that induces mesodermal differentiation.15,16 BMP signaling is required for the separation of the ectodermal and mesodermal fate and the induction of mesoderm formation in vivo.20 TGF-β1 is known to inhibit endodermal and ectodermal differentiation, but allow the mesodermal differentiation of EBs.12 BMP-4 and TGF-β1 present in the hMSC-CM may accelerate the mesodermal differentiation of hESCs and hiPSCs. Our data showed that the expressions of mesodermal markers, such as BRACHYURY, WNT3, and MIXL1,21 were significantly upregulated in EBs cultured with the hMSC-CM (Fig. 2). WNT3 and MIXL1 expression is involved in the BMP-4 and TGF-β signaling pathway, which is important for mesoderm induction.21 WNT3a signaling induces paraxial mesodermal formation.22 The hMSC-CM is known to contain WNT3.23 As shown in Figure 2, the WNT3 level in the hMSC-CM-treated EBs was 2.3-fold higher compared with controls. Furthermore, WNT3 may have a synergic effect on the mesodermal differentiation of hESCs and hiPSCs when combined with FGF2 contained in the hMSC-CM.14,24,25
hMSC-CM treatment seems to be more effective for mesodermal differentiation of EBs than BMP-4 and FGF2 treatment. Previously, we have reported that BMP-4 and FGF2 treatment of EBs enhanced the mesodermal lineage differentiation of hESCs.26 However, coadministration of BMP-4 and FGF2 to EBs upregulated mesodermal mRNA expression of WNT3 by only 30%. Compared with the previous results, WNT3 expression was enhanced by 230% by hMSC-CM treatment in the present study (Fig. 2). This result suggests that other cytokines, with the exception of BMP-4 and FGF2, are present in the hMSC-CM, and these cytokines also stimulate the mesodermal differentiation of EBs.
Through the induction of the mesodermal lineage in hESCs and hiPSCs at the EB stage, chondrogenic and osteogenic differentiation of hESCs was promoted. Among the three germ layers, the mesoderm lineage provides the progenitor cells for skeletal tissue.5 Chondrocytes and osteoblasts, two major cell types of the skeleton, have a mesenchymal origin.27 Thus, enhanced mesodermal differentiation of hESCs and hiPSCs may promote osteogenic and chondrogenic differentiation. EBs cultured with the hMSC-CM exhibited an enhanced differentiation potential toward cartilage and bone cells.
In summary, hMSC-CM treatment of EBs derived from hESCs promoted differentiation to a mesodermal lineage. The mesodermal lineage-induced EBs exhibited enhanced osteogenic and chondrogenic differentiation when cultured in the osteogenic and chondrogenic medium compared with untreated EBs. This study represents another step toward achieving fully directed cell differentiation. Further studies are necessary to define the specific growth factors that are need for mesodermal lineage differentiation. The effects of the hMSC-CM on other types of differentiation and further in vivo studies are also necessary.
Supplementary Material
Acknowledgment
This study was supported by grants (2010-0020352 and 2012M3A9C6049724) from the National Research Foundation of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Itskovitz-Eldor J., Schuldiner M., Karsenti D., Eden A., Yanuka O., Amit M., Soreq H., and Benvenisty N.Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6,88, 2000 [PMC free article] [PubMed] [Google Scholar]
- 2.Höpfl G., Gassmann M., and Desbaillets I.Differentiating embryonic stem cells into embryoid bodies. Methods Mol Biol 254,79, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Prokhorova T.A., Harkness L.M., Frandsen U., Ditzel N., Schrøder H.D., Burns J.S., and Kassem M.Teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of Matrigel. Stem Cells Dev 18,47, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Przyborski S.A.Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells 23,1242, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Hwang Y.S., Randle W.L., Bielby R.C., Polak J.M., and Mantalaris A.Enhanced derivation of osteogenic cells from murine embryonic stem cells after treatment with HepG2-conditioned medium and modulation of the embryoid body formation period: application to skeletal tissue engineering. Tissue Eng 12,1381, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Cohen S., Leshansky L., Zussman E., Burman M., Srouji S., Livne E., Abramov N., and Itskovitz-Eldor J.Repair of full-thickness tendon injury using connective tissue progenitors efficiently derived from human embryonic stem cells and fetal tissues. Tissue Eng Part A 16,3119, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Domev H., Amit M., Laevsky I., Dar A., and Itskovitz-Eldor J.Efficient engineering of vascularized ectopic bone from human embryonic stem cell-derived mesenchymal stem cells. Tissue Eng Part A 18,2290, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bigdeli N., Karlsson C., Strehl R., Concaro S., Hyllner J., and Lindahl A.Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem Cells 27,1812, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Kitagawa M., and Era T.Differentiation of mesodermal cells from pluripotent stem cells. Int J Hematol 91,373, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Kawaguchi J., Mee P.J., and Smith A.G.Osteogenic and chondrogenic differentiation of embryonic stem cells in response to specific growth factors. Bone 36,758, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Oldershaw R.A., Baxter M.A., Lowe E.T., Bates N., Grady L.M., Soncin F., Brison D.R., Hardingham T.E., and Kimber SJ.Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol 28,1187, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Schuldiner M., Yanuka O., Itskovitz-Eldor J., Melton D.A., and Benvenisty N.Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 97,11307, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baer P.C., Bereiter-Hahn J., Missler C., Brzoska M., Schubert R., Gauer S., and Geiger H.Conditioned medium from renal tubular epithelial cells initiates differentiation of human mesenchymal stem cells. Cell Prolif 42,29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnaird T., Stabile E., Burnett M.S., Shou M., Lee C.W., Barr S., Fuchs S., and Epstein S.E.Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation 109,1543, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Wislet-Gendebien S., Bruyère F., Hans G., Leprince P., Moonen G., and Rogister B.Nestin-positive mesenchymal stem cells favour the astroglial lineage in neural progenitors and stem cells by releasing active BMP4. BMC Neurosci 5,33, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C.H., and Hwang S.M.Cytokine interactions in mesenchymal stem cells from cord blood. Cytokine 32,270, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Jang J., Yoo J.E., Lee J.A., Lee D.R., Kim J.Y., Huh Y.J., Kim D.S., Park C.Y., Hwang D.Y., Kim H.S., Kang H.C., and Kim D.W.Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med 44,202, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L., Tredget E.E., Wu P.Y., and Wu Y.Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3,e1886, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osugi M., Katagiri W., Yoshimi R., Inukai T., Hibi H., and Ueda M.Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A 18,1479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loebel D.A., Watson C.M., De Young R.A., and Tam P.P.Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev Biol 264,1, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Zhang P., Li J., Tan Z., Wang C., Liu T., Chen L., Yong J., Jiang W., Sun X., Du L., Ding M., and Deng H.Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111,1933, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa Y., Fujimori T., McMahon A.P., and Takada S.Evidence that absence of Wnt-3a signaling promotes neuralization instead of paraxial mesoderm development in the mouse. Dev Biol 183,234, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Etheridge S.L., Spencer G.J., Heath D.J., and Genever P.G.Expression profiling and functional analysis of wnt signaling mechanisms in mesenchymal stem cells. Stem Cells 22,849, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Ciruna B., and Rossant J.FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell 1,37, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Chapman D.L., and Papaioannou V.E.Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391,695, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Lee T.J., Jang J., Kang S., Jin M., Shin H., Kim D.W., and Kim B.S.Enhancement of osteogenic and chondrogenic differentiation of human embryonic stem cells by mesodermal lineage induction with BMP-4 and FGF2 treatment. Biochem Biophys Res Commun 430,793, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Colnot C.Cellular and molecular interactions regulating skeletogenesis. J Cell Biochem 95,688, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.