Abstract
Multipotent human adipose-derived stromal/stem cells (hADSCs) hold a great promise for cell-based therapy for many devastating human diseases, such as spinal cord injury and stroke. If exogenous hADSCs can be cultured in a three-dimensional (3D) scaffold with effective proliferation and differentiation capacity, it will better mimic the in vivo environment, which will have profound impact on the therapeutic application of hADSCs. In this study, a group of elastic-dominant, porous bioscaffolds from photocurable chitosan and gelatin were fabricated and proven to be biocompatible with both hADSCs and hADSC-derived neuron-like cells (hADSC-NLCs) in vitro. The identity of harvested hADSCs was confirmed by their positive immunostaining of mesenchymal stem cell surface markers, CD29, CD44, and CD105, and also positive expression of stem markers, Sox-2, Oct-4, c-Myc, Nanog, and Klf4. Their multipotency was further confirmed by trilineage differentiation of hADSCs toward adipocyte, osteoblast, and chondrocyte. It was found that hADSCs could be conditioned to differentiate into neurons in vitro as determined by immunostaining the markers of Tuj1, MAP2, NeuN, and Synapsin. The hADSCs and hADSC-NLCs were proven to be biocompatible with 3D scaffold, which actually facilitated the proliferation and differentiation of hADSCs in vitro, by MTT assay and their neuronal gene expression profiling. Moreover, hADSC-NLCs, which were mixed with 3D scaffold and transplanted into traumatic brain injury mouse model, survived in vivo and led to the better repair of the damaged brain area. The immunohistochemical studies revealed that 3D scaffold indeed improved the viability of transplanted cells, their ability to incorporate into the in vivo neural circuit, and their capacity for tissue repair. This study indicates that hADSCs would have great therapeutic application potential as seeding cells for in vivo transplantation to treat various neurological diseases when co-applied with porous chitosan/gelatin bioscaffolds.
Introduction
Human adipose-derived stromal/stem cells (hADSCs) has been one of the ideal seeding cells for cell-replacement therapy, due to their merits such as easy availability, merely noninvasion to patients, adequate amount in the adipose tissue, easy in vitro expansion or passage, reliable biosafety, and free of immunogenicity.1–3 Zuk et al. first harvested hADSCs from suction-assisted lipectomy and found differentiation capability of hADSCs into adipogenic, osteogenic, chondrogenic, and myogenic cells.1 Besides, Gronthos et al. showed that hADSCs express CD surface markers similar to those of human bone marrow stromal/stem cells (hBMSCs),2 implying that the hADSCs can potentially be used as an alternative cell source in replacement of hBMSCs.4 Therefore, multilineage differentiation of hADSCs to adipocytes, osteoblast, and chondrocytes provides a new solution for various human diseases.5,6 In the past 5 years, the differentiation of hADSCs into neuron cells has attracted much attention from scientists and clinicians of translational medicine because of their potential clinical application in neurodegenerative diseases, such as Parkinson and Alzheimer diseases.7–19 Previous studies are typically involved in the evaluation and mechanism of in vitro neuronal differentiation of hADSCs or hBMSCs rather than their clinical translation. For hADSC clinical application to treat neural degeneration or related diseases, it is essential to create an optimal niche to ensure the viability of transplanted cells and to promote them in vivo proliferation and differentiation of hADSCs.
Three-dimensional (3D) bioscaffolds are attractive candidates as temporary supports for cell accommodation and growth, due to their tissue-like elasticity and high water content, mimicking an interstitial tissue environment.20,21 Chitosan, a naturally abundant degradable polysaccharide, has been used to prepare porous scaffolds for stem cell-based tissue regeneration. For example, Schwartz et al. reported that chitosan is quite suitable for the induction of the chondrogenic differentiation of the human bone marrow mesenchymal stem cells.22 Haipeng et al. reported that neurons cultured on the chitosan membrane grew well and that chitosan tube promote repair of the peripheral nervous system.23 Yuan et al. found that chitosan fibers supported the adhesion, migration, and proliferation of Schwann cells, which provide a similar guide for regenerating axons to Büngner bands in the nervous system.24 However, to the best of our knowledge, no report has been appeared yet on chitosan-based scaffolds for the differentiation of hADSC into neuron-like cells (hADSC-NLCs).
The purposes of this study were to fabricate photocurable scaffolds that contain chemically modified chitosan and gelatin and to investigate their potential role for facilitating hADSC differentiation into hADSC-NLCs. To this end, we chemically modified hydroxyl groups of chitosan with benzoic groups and methacrylate groups. Upon UV light exposure, the chitosan derivatives were crosslinked via a polymerization of methacrylate residues to form 3D scaffold. Next, gelatin was incorporated to form chitosan–gelatin hybrid scaffolds. Since gelatin is derived from denatured collagen and has both negatively and positively charged patches,25 it carries specific peptide sequences to facilitate cell attachment and proliferation.26 It is assumed that the hybrid scaffolds contain interconnected pores for easy loading of hADSCs as well as handy incorporation of growth factors and nutrients, thereby allowing hADSC attachment, proliferation, and differentiation into hADSC-NLCs both in vitro and in vivo.27–29
Materials and Methods
Synthesis of light-curable chitosan and 3D scaffold fabrication
Light-curable chitosan was synthesized according to the method we described below. Chitosan (2 g, purchased from Sigma-Aldrich) was dissolved in 45 g methane sulfonic acid (purchased from Aladdin) while constantly stirring until the chitosan was completely dissolved. Followed by dropwise adding the mixture of 2.2 g benzoyl chloride (purchased from Aladdin) and 2.45 g methacryloyl chloride (purchased from Aladdin). The solution was kept at room temperature (RT) under stirring for 3–4 h. Precipitate was obtained in cold water followed by neutralization with ammonium hydroxide (purchased from Aladdin). After repeat filtering and rinsing, the product was dried in vacuum over P2O5 for 2 days and stored at 4°C until further use.
Light-curable chitosan of 1 g was dissolved in 20 g of DMSO containing 1 g gelation under stirring to obtain 5% gelatin-5% photocurable chitosan-DMSO mixture. Then, 10 mg Iragure 2959 (purchased from Ciba Specialty Chemicals) was added into the solution. To obtain porous scaffolds, salt crystal (NaCl, 250–380 μm) was used as porgen. First, the mold (8 mm in diameter and 8 mm in height) was filled with salt crystals. Then, the solution was added dropwise on the top of the salt and filled the interspaces between the salt crystals, followed by exposing to the UV light until the solution was completely cured. The discs were immersed in DI water and washed several times to remove the remaining solvent and salt. To improve the mechanical strength of the photocured discs, one set of the discs were further crosslinked by immersing into 1:4 water–acetone (v/v) containing 1% EDC, which crosslinked gelatin and gelatin with chitosan, followed by washing with DI water.
Characterizations of light-curable chitosan and 3D scaffolds
FITR-ATR spectra of scaffolds were recorded directly on an infrared spectrometer (Bruker Tensor 27).
1H NMR spectra of samples were recorded on a Varian Inova spectrometer (Bruker AV 400 MHZ) by using d-DMSO solution (20 mg samples dissolved in 0.6 mL of d-DMSO).
The morphology (shape and size), elemental composition of scaffolds, and cells surfaces were examined by scanning electron microscopy (SEM) (Hitachi S-4800) coupled with an energy dispersive X-ray analyzer (EDS). Samples were incubated for 1 h. with glutaraldehyde 1.5% (v/v) in 0.05 M phosphate buffer, pH 7.4. Then, samples were washed three times with the same buffer for 5 min. Samples were dehydrated successively with EtOH 50%, 75%, 90%, and 100%. After solvent evaporation, samples were coated with gold in a sputter coater model 3 (Pelco; Ted Pella, Inc.).
The compressive properties of the scaffolds were measured using the Dynamic Mechanical Analyzer Q800 (DMA 800). And the compression test was carried out with a constant strain rate at 1 mm/min and the trigger force is 18 N. The initial elastic modulus was calculated based on the slope of the stress–strain curve at low strains (<3%). For the cyclic compression test, under a constant strain rate of 1 mm/min, the scaffolds were first compressed to 60% strain and then recovered to 30% strain by retreating the force. After that, the scaffolds were repeatedly compressed and recovered between 30% and 60% strain at 1 mm/min strain rate for four more cycles. Each measurement was performed three times and averaged.
hADSCs isolation
Isolation of hADSCs was carried out according to the previously published references.1,2 Fresh liposuction adipose liquid from the clinical surgery was washed extensively with Lactated Ringer's solution to remove contaminating debris and red blood cells. Then, the tissue was cut and minced into small pieces by sterile surgical scissors, then digested with 0.075% collagenase (Roche Diagnostics) for 40 min at 37°C with gentle agitation. Neutralization of the homogenate was carried out with the culture medium supplemented with 10% fetal bovine serum (FBS) after digestion and filtered through a 100-μm nylon mesh to remove cellular debris and were collected into new tubes. After centrifuging at 400 g for 5 min at RT, cells were suspended in the culture medium and left onto plates in the humidified incubator at 37°C with 5% CO2. After 24 h incubation, the plates were washed extensively with phosphate-buffered saline (PBS) to remove residual nonadherent red blood cells. For hADSC expansion, cells were seeded at 6×104 cells/cm2 in T-75 flasks (Falcon; Becton Dickinson) in the basal medium Dulbecco's modified Eagle's medium (DMEM)/F-12 medium (Invitrogen) containing 20% FBS (JRH Bioscience), 100 IU penicillin, and 100 mg/mL streptomycin (Invitrogen), and incubated at 37°C with 5% CO2 and humidified incubator.
RNA extraction and qRT-PCR
Total RNA was isolated from ∼106 cells of hADSCs, embryonic stem cells (ESCs), and differentiated hADSCs (hADSC-NLCs) with Qiagen RNeasy Mini Kit (#74104) by following the standard operation manual. The extracted RNA was used only when the OD260/280 value was above 1.8. Reverse transcription from the above prepared total RNA was carried out using the Thermo Scientific RevertAid First Strand cDNA Synthesis kit (#K1621). The RT-PCR program including incubating for 60 min at 42°C followed by terminating the reaction by heating at 70°C for 5 min. The primers used for these five stem transcription factors were designed with primer design software, and the sequences are listed in Table 1. Semiquantitative RT-PCR using the above prepared first-strand cDNA as the template, following the protocol: template 500–1000 ng, primer 1 μL, 2× Pfu PCR Master Mix (KP201; Tiangen) 6.25 μL, and DNase-RNase free water to a final total volume of 12.5 μL. The PCR reaction was performed on Bio-Rad (S1000™ Thermal Cycler) for semiquantitative RT-PCR and on AB Applied Biosystem 7500 for quantitative RT-PCR. The PCR products were analyzed by running electrophoresis on 2.0% agarose gel. The data were processed by the ABI 7500 software.
Table 1.
Primer Sequence for the Related Genes
| Gene symbol | Primer sequence | Tm (°C) | Product size (bp) |
|---|---|---|---|
| Sox2 | For 5′-ACCTCTTCCTCCCACTCC-3′ Rev 5′-CTCCCATTTCCCTCGTTT-3′ |
58 | 249 |
| Oct4 | For 5′-AGCGAACCAGTATCGAGAAC-3′ Rev 5′-CCCTGAGAAAGGAGACCC-3′ |
60 | 224 |
| c-Myc | For 5′-AGAAGCGACAGAATCAAA-3′ Rev 5′-GGACCTGGTATGTGGAGA-3′ |
55 | 210 |
| Nanog | For 5′-AGGCAACTCACTTTATCC-3′ Rev 5′-CCACAAATCACAGGCATA-3′ |
52 | 235 |
| KLF4 | For 5′-ACCAGGCACTACCGTAAACA-3′ Rev 5′-CCCTCATCGGGAAGACAG-3′ |
60 | 192 |
| Tuj1 | For 5′-GCCAAGAACATGATGGCC-3′ Rev 5′-TGTTCTTGCTCTGGATGGC-3′ |
56 | 127 |
| MAP2 | For 5′-CCTGTGTTAAGCGGAAAACC-3′ Rev 5′-AGAGACTTTGTCCTTTGCCTGT-3′ |
60 | 86 |
| NFL | For 5′-TCCTACTACACCAGCCATGT-3′ Rev 5′-TCCCCAGCACCTTCAACTTT-3′ |
60 | 284 |
| NFM | For 5′-TGGGAAATGGCTCGTCATTT-3′ Rev 5′-CTTACTGGAAGCGGCCAATT-3′ |
58 | 333 |
| GFAP | For 5′-GCAGAGATGATGGAGCTCAATGACC-3′ Rev 5′-GTTTCATCCTGGAGCTTCTGCCTCA-3′ |
66 | 266 |
| MaxiK | For 5′-ACAACATCTCCCCCAACC-3′ Rev 5′-TCATCACCTTCTTTCCAATTC-3′ |
56 | 310 |
| Kv4.2 | For 5′-ACCGTGACCCAGACATCTTC-3′ Rev 5′-CACTGTTTCCACCACATTCG-3′ |
62 | 365 |
| NE-Na | For 5′-GCTCCGAGTCTTCAAGTTGG-3′ Rev 5′-GGTTGTTTGCATCAGGGTCT-3′ |
62 | 446 |
| CACNA1G | For 5′-AGGGCAGGACTGTCTTCTGA-3′ Rev 5′-TCTGAGTCAGGCATTTCACG-3′ |
58 | 416 |
| Beta-actin | For 5′-TCACCCACACTGTCCCATCTACGA-3′ Rev 5′-TGAGGTAGTCAGTCAGGTCCCG-3′ |
66 | 200 |
| GAPDH | For 5′-CAAGATCATCAGCAATGCCTCCTG-3′ Rev 5′-GCCTGCTTCACCACCTTCTTGA-3′ |
66 | 363 |
Immunocytochemistry and lineage-specific staining
The isolated hADSCs were characterized by detecting series of specific CD surface markers of CD44 (47-0441-80; eBioscience), CD29 (11-0299-41; eBioscience), CD105 (12-1057-41; eBioscience), and CD45 (11-9459-41; eBioscience) as reported previously by Zuk et al. and Gronthos et al.1,2 The immunocytochemistry staining was performed as follows: the hADSCs were fixed with 4% paraformaldehyde (PFA) in PBS for 15–20 min at RT, the cells were permeabilized with 0.25% Triton X-100 in PBS for 10 min at RT; blocked in block solution with 3% BSA or donkey serum for 30 min, and incubated with the primary antibodies (diluted block solution) for 1 h at RT or overnight at 4°C; at the same time, the IgG isotype controls corresponding to each primary antibody were set to exclude the false-positive detection; incubated in fluorochrome-conjugated secondary antibodies for 1 h at RT and DAPI solution for another 10 min to stain the nuclear; ultimately mounted on a slide using antifade mounting solution. Fluorescence signals can be detected using the confocal microscope (Leica SP5) under the proper exciting wavelength. For detection of multi-potential lineage commitment toward the mesodermal layers, such as adipocyte, osteoblast, and chondrocyte, Oil Red O (O0625-25G; Sigma), Alizarin (3381-60; HGB), and Toluidine blue (TB0962-1G; Bio Basic, Inc.) were, respectively, used to perform staining. The detailed protocols followed the previously published reference.1
Differentiation and characterization of hADSC-NLCs
hADSCs have been proved to be able to differentiated into hADSC-NLCs by growing in a specific neuron induction cocktail medium.7,15–18 The induction protocol used in this study mainly referred to the previously published7 with minor modification by preincubating hADSCs with DKK1 (SRP3258, 10 ng/mL; Sigma) for 24 h before grown in the neuron differentiation cocktail medium. In another set of experiment, to promote the maturation of the hADSC-NLCs, we added BDNF (10 ng/mL), NT3 (10 ng/mL), and NGF (10 ng/mL) to the cocktail medium 3 days after treatment with the cocktail medium. Immunocytochemistry and semiquantitative RT-PCR were used to determine the identity of hADSC-NLCs.
Seeding of hADSCs on the 3D scaffolds and their neuronal differentiation
According to the previous evidence on the capability of hADSC differentiation into hADSC-NLCs, it should be very meaningful to mimic an in vivo supportive and transplantable scaffold environment for these cells better survival after transplantation into injured sites. To achieve this goal, hADSCs were first expanded at passage 3, collected, and adjusted with the growth medium to a final concentration of 106/mL. Then, 10 μL of the hADSC solution were loaded onto the scaffolds cylinder with diameter of 2 mm and height of 2 mm through a syringe. After incubation for 4–6 h, these scaffolds loaded with hADSCs were dipped into the growth medium for 24 h incubation. To determine their supportive effect on hADSC proliferation, MTT assay with a time course of 96 h was first performed paralleled with their counterparts cultured on plastic culture dishes. MTT assays were performed according to previously published protocols.30 For their neuronal differentiation, the growth medium was first replaced with serum-free DMEM/F12 medium supplemented with DKK1 (10 ng/mL) and incubated for another 24 h. After that, the neuron differentiation cocktail medium was used to replace the above medium. At the same time, hADSCs growing and neuron differentiation on the tissue culture plastic dishes were carried out in parallel as controls due to the impossibility of performing immunocytochemistry to characterize the hADSC-NLCs. After 3 days of differentiation with the cocktail medium, Tuj1, MAP2, and Synapsin markers were determined by immunocytochemistry. After another 3 days of incubation with the cocktail medium supplemented with neurotrophic factors of BDNF, NGF, and NT3, hADSC-NLCs cultured on the plastic dishes were performed immunocytochemistry with NeuN, Synapsin, and vGAT markers. At the same time, hADSCs and hADSC-NLCs from the plastic dishes and the 3D scaffolds were extracted total RNA to perform later semiquantitative RT-PCR to analyze the neuronal marker expression variance during this induction. The primers for these neural markers used in this study are listed in Table 1.
Transplanting hADSC-NLCs/chitosan/gelatin 3D scaffold into traumatic brain injury model
Traumatic brain injury (TBI) mouse model was made according to the following the protocols published by Guo et al.31 hADSCs at P3–P5 were first infected with enhanced green fluorescent protein (EGFP)-expressing lentivirus, then collected and loaded into the afore-prepared light-curable chitosan/gelatin 3D scaffold. After 24 h incubation with the hADSC growth medium, this construct was washed with PBS and incubated with the neuron cocktail induction medium for another 24 h. Then, the mixture of EGFP expressing hADSC-NLCs/chitosan/gelatin scaffold was obtained. To transplant this mixture into the TBI (n=6) mouse brain injury site, we minced this scaffold into cylinder-like pieces. Meanwhile, TBI mice, which only received PBS placebo treatment, served as the control group (n=6). To trace the fate of hADSCs and 3D scaffold in the brain of the TBI mice, the mice were subjected to anesthesia and intracardial perfusion before sacrifice at day 2 and 7 after transplantation. Brain dissection and immunohistochemical studies were performed following the previously published protocols.32
Results
Spectrum characterization of light-curable chitosan
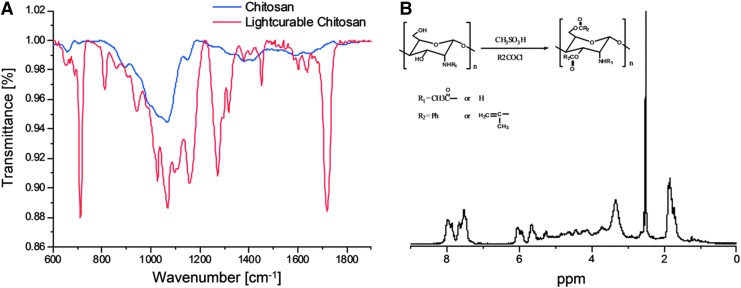
From the IR spectra of each sample (Fig. 1A), characteristic peaks at around 1720 cm−1 show the existence of ester carbonyl groups, indicating that the acyl chloride groups have reacted with the hydroxyl groups in the chitosan. The presence of amino group peaks at around 1600 cm−1, with no peaks at 1670 cm−1 (amide I) and 1536 cm−1 (amide II), indicating that the amino groups were protected during the reaction due to the protonation of the amino groups when reacting with methane sulfonic acid. Moreover, the peak at 710 cm−1 indicates the existence of the aromatic ring belonging to the benzoyl group. In 1H NMR (Fig. 1B), the protons in the pyranose ring appear at δ=2.8–5.2 ppm. The signals for the protons on the benzoyl group appear at δ=7.5–8.2 ppm, and for the protons on the methylene in the olefin, the signal appears at δ=5.7–6.3 ppm.
FIG. 1.
FTIR-ATR (A) and 1H NMR (B) characterization of light-curable chitosan.
The mechanical properties of 3D scaffolds
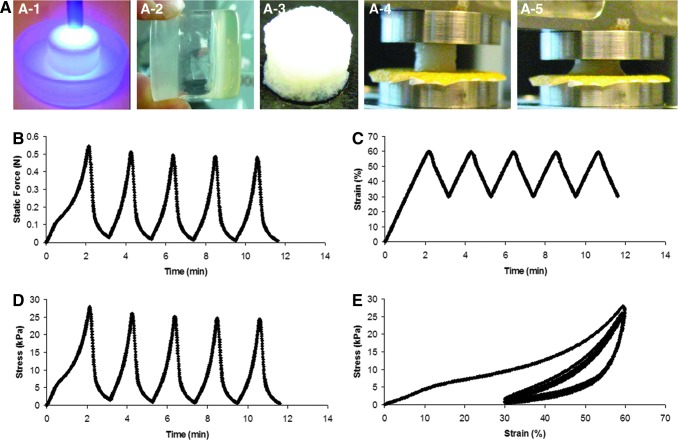
We further evaluated the mechanical properties of the chitosan–gelatin hybrid scaffolds. Cyclic compression test was performed on the Gtn-Cht scaffolds to assess the dynamic load-bearing capacity of the scaffolds at a constant strain rate of 1 mm/min and at strains ranging from 30% to 60% (Fig. 2). At a medium-range strain rate of 1 mm/min, the repeated static force-time or stress-time cycles indicate full recovery of the scaffold in gross dimensions after each cycle within the range of the strain (30%–60%) (Fig. 2A, C). The stress–strain curve (Fig. 2D) indicates that the scaffold recovers to 30% strain instead of the initial no strain condition after the first cycle and reproducibly deforms and recovers along the same stress–strain loop in the following cycles, suggesting a temporal retardation during the recovery process possibly due to the creeping behavior of the scaffold.
FIG. 2.
(A) Three-dimensional (3D) scaffolds fabrication scheme (A-1, A-2, and A-3: UV exposure; A-4 and A-5: compression test) and cyclic compression test on the chitosan–gelatin hybrid scaffolds (5%–5%) at a constant strain rate of 1 mm/min and a strain range of 30%–60%. (B) Static force versus time; (C) strain versus time; (D) stress versus time; and (E) stress–strain curve.
Morphology and surface elements of 3D scaffolds and cells
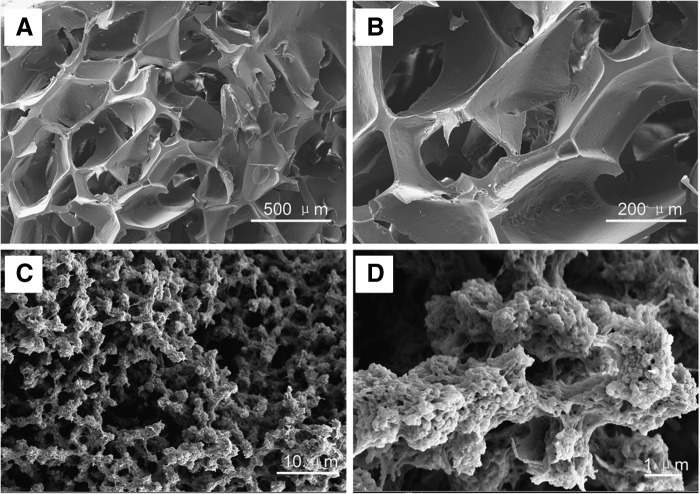
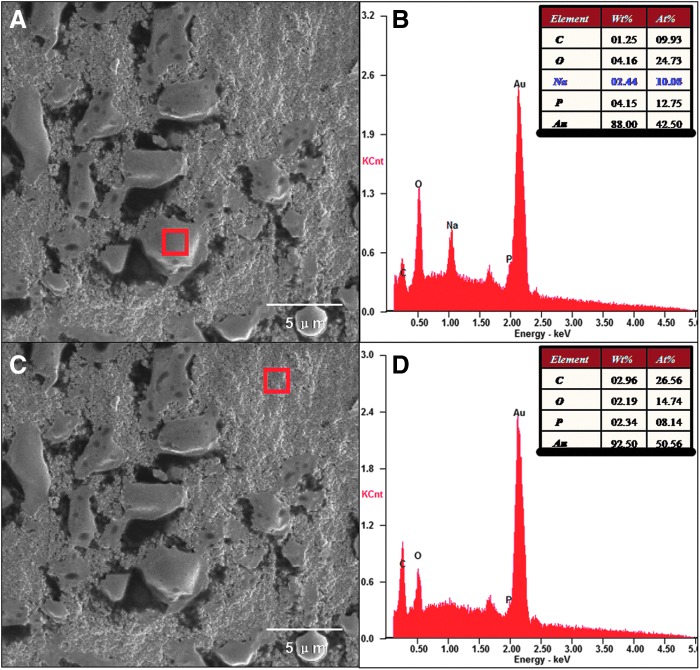
SEM coupled with an EDS were used to study the morphology (shape and size) (Fig. 3) and elemental composition of scaffolds and cells surfaces (Fig. 4). The surfaces and innerstructures of the hybrid chitosan–gelatin scaffolds at higher magnifications revealed the presence of nanoscale structures (e.g., pores, beads) (Fig. 3C, D) on the inside and outside surface of scaffold skeleton. The region of cells and scaffolds (Fig. 4A, C) were simultaneously analyzed by EDS; this spectrum shows energetic lines (Ka) for carbon, oxygen, sodium, and phosphorus. The relative semiquantitative weight % (Ka) for sodium was 2.44% in cells surfaces (Fig. 4B), whereas it was not observed even at a low concentration on the scaffolds surfaces (Fig. 4D).
FIG. 3.
Scanning electron microscopy (SEM) morphology of the 3D scaffolds (5% gelatin-5% light-curable chitosan) made with NaCl (250–380 μm) as porgen: (A, B) top view; (C, D) cross section with nanostructures, such as gelatin beads.
FIG. 4.
SEM images of the 3D scaffolds coupled with day 6 human adipose-derived stromal/stem cell (hADSC)-neuron-like cells (NLCs) (A, C). The corresponding energy dispersive X-ray analyzer (EDS) spectrum on cell surface (A) and scaffold surface (C) showed energetic lines for carbon, oxygen, Na+ and phosphrous (B, D).
Characterization of hADSCs by cell morphology and CD surface markers
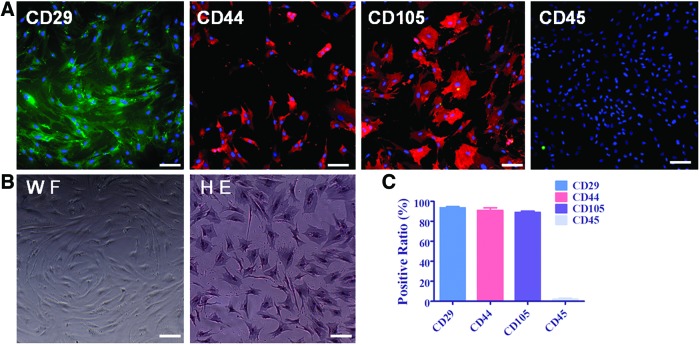
The processed liposuction tissue cells were seeded at 6×107 cells/cm2 in T-75 flasks in the basal medium DMEM/F-12 medium containing 20% FBS. The medium was changed twice every week. After 15 days of culture, hADSCs grew adherent, swirling and displaying typical spindle-like morphology. Hematoxylin and eosin (H&E) staining demonstrated hADSCs as mononuclear cells, as shown in Figure 5B. To identify hADSCs, immunocytochemistry staining with CD surface markers were performed with the data shown in Figure 5A and the quantification of CD marker positive ratio is shown in Figure 5C. These results demonstrated that more than 85% of hADSCs positively expressed mesenchymal stem cell markers of CD29, CD44, and CD105 but negatively expressed hematopoietic stem cell marker of CD45 (<5%). These results were consistent with the previous report by Gronthos et al.2
FIG. 5.
Characterization of hADSCs by cell morphology and CD surface markers. hADSCs grow swirling with spindle-like morphology. (A) Shows more than 85% of hADSCs at P3 positively express CD29, CD44, and CD105 and less than 5% of hADSCs express CD45. P3 of hADSCs plated on plastic culture dish in Dulbecco's modified Eagle's medium (DMEM)/F12 medium supplemented with 10% fetal bovine serum (FBS) taken under white field (WF) and hematoxylin and eosin (H&E) staining to observe hADSC morphology and nuclear was shown on (B). (C) Shows the quantification of CD-positive ratio of cells by taking average of at least five microscope fields. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Multipotential properties of hADSCs determined by expression of stem cell-specific transcriptional factors and trilineage differentiation capability
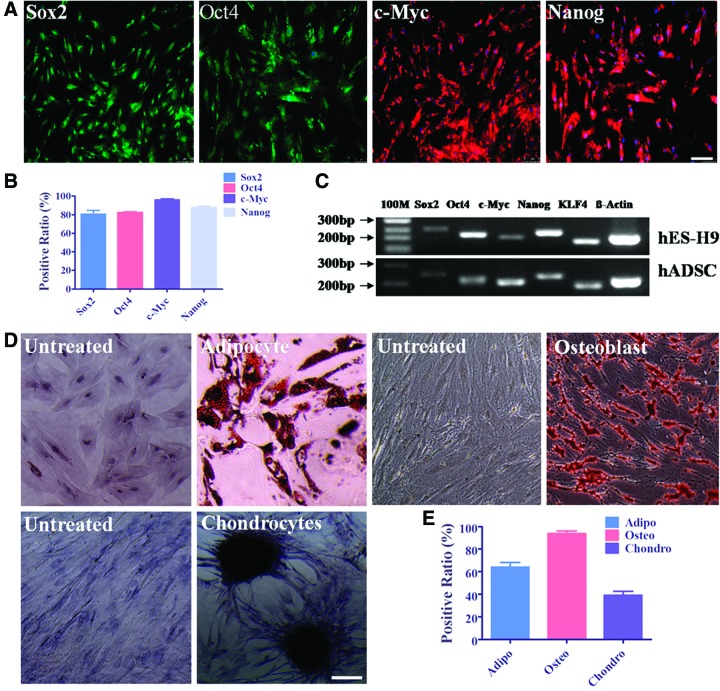
Classical stem cell transcriptional markers, such as Sox2, Oct4, c-Myc, Nanog, and KLF4, have been widely used to characterize induced pluripotent stem cells.31 To characterize the stemness of hADSCs, this study performed immunocytochemistry staining and designed the transcriptional factor marker primers and performed semiquantitative RT-PCR to detect these factors expression comparing with ESCs. Data showed that hADSCs expressed Sox2, Oct4, c-Myc, and Nanog at protein level by immunocytochemistry staining (Fig. 6A) and expressed Sox2, Oct4, c-Myc, Nanog, and KLF4 with human ES cell line H9 as the positive control by semiquantitative RT-PCR (Fig. 6C), whereas Oct4, Nanog, and KLF4 were expressed notably higher in hESCs. Data on the quantification of positively expressed stem cell transcriptional markers demonstrated that more than 80% of hADSCs at P3 positively expressed these four markers (Fig. 6B), which was consistent with the RT-PCR result (Fig. 6C). Furthermore, hADSCs demonstrated their multidifferentiation potential through in vitro trilineage differentiation assay induced by the lineage-specific differentiation medium. The isolated hADSCs can be induced into adipocytes stained with Oil Red O, osteoblast cells stained with Alizarin, and chondrocytes stained with Toluidine blue (Fig. 6D). The percentage of the positively stained cells among all the hADSCs were quantified by taking the average among five microscope fields. The data are shown in Fig. 6E. These data demonstrated that the isolated hADSCs possessed the multipotency as previously reported.1–4,7
FIG. 6.
Stem cell transcriptional factor expression determination and trilineage differentiation of hADSCs. Immunocytochemistry staining with Sox2, Oct4, c-Myc, and Nanog showed that more than 80% of hADSCs positively express these markers, which is consistent with their mRNA level expression comparing with human ES cell line of H9 (A–C). hADSCs can be specifically induced into adipocytes stained with Oil Red O, osteoblast cells stained with Alizarin, and chondrocytes stained with Toluidine blue by culturing in certain and specific differentiating medium for 14 days (D). The untreated means hADSCs were cultured in normal medium and performed later staining in parallel with those cultured in the differentiating medium. The medium was changed every 3 days. The differentiation positive ratio of trilineages was collected by taking average of at least five microscope view fields (E). Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Neuronal differentiation of hADSCs into hADSC-NLCs on plastic culture dishes
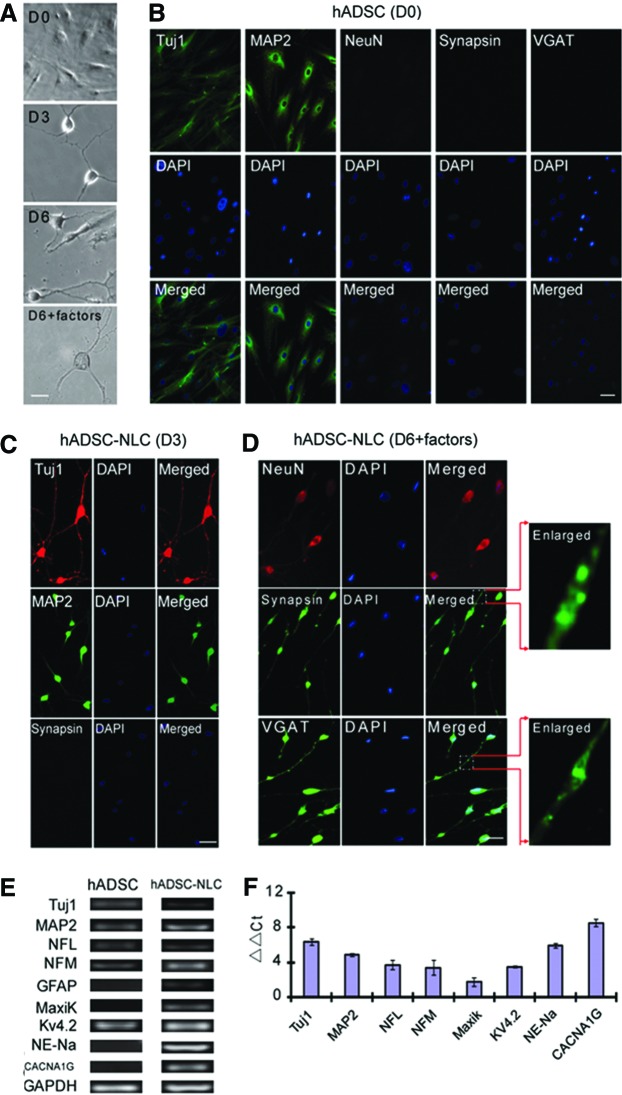
According to the published data on DKK1, which can promote the ESCs differentiation toward neurons by inhibiting Wnt pathway.32–34 This neuronal induction protocol started with incubating the hADSCs with DKK1 for 24 h, then followed by 3 days of cocktail medium induction as reported by Safford et al.7 During this induction period, hADSCs morphology changed dramatically with cytoplasm retracting toward nucleus, and cell body becoming spherical and refractile as shown in Figure 7A. To confirm that the neuronal marker expression is really induced by cocktail induction, hADSCs without any treatment were first immunostained with Tuj1, MAP2, NeuN, Synapsin1/2, and vGAT. Data in Figure 7B showed that hADSCs have some low-level expression of Tuj1 and MAP2 with diffusive pattern without any detectible expression of NeuN, Synapsin1/2, and vGAT. These results imply that hADSCs may preferentially commit toward neuronal lineage differentiation due to their basic expression of some neuronal markers such as Tuj1 and MAP2, but spontaneous neuronal differentiation is barely detectible without induction. After 3 days of induction, the hADSCs started to become apparently polarized with extensive neurite-like structures, and IHC studies with neuronal markers of Tuj1, MAP2, and Synapsin1/2 showed that the expression of Tuj1 and MAP2 increased significantly while Synapsin1/2 staining remains negative as demonstrated in Figure 7C. To further promote the maturation of these immature NLCs, neurotrophic factors such as BDNF, NGF, and NT3 was further supplemented into the cocktail medium for another 3 days. Immunostaining with mature neuronal markers of NeuN, Synapsin1/2, and vGAT showed that after neurotrophic factor incubation, hADSC-NLCs began to express these mature and functional neuron markers, as shown in Figure 7D. The spiny synaptic structures can be observed after neurotrophic factor treatment (Fig. 7D, enlarged). To further evaluate the molecular nature of neuronal differentiation of hADSCs, qRT-PCR was applied to detect the expression level of genes, which specify the neuronal function and related ion channels on day 6 of induction in comparison with undifferentiated hADSCs. The hADSC-NLCs started to express the genes of major ion channels, such as sodium, potassium, and calcium channels, which explicitly demonstrated their neuronal merits and functional maturation. The primers are listed in Table 1, and the PCR product electrophoresis and expression fold variance are shown in Figure 7E and F.
FIG. 7.
hADSCs were induced into (A) NLCs with morphology changing continuously as time lapse from day 0 (D0) to day 6 (D6). (B) Without any treatment, hADSCs positively express Tuj1 and MAP2 and negatively express NeuN, Synapsin1/2, and vGAT. After 3-day incubation with cocktail medium, most hADSCs-NLCs positively expressed Tuj1, MAP2 but not Synapsin1/2 (C). After additional 3-day incubation with the cocktail medium supplemented with neurotrophic factors, most cells began to express neuron function-related markers of NeuN, Synapsin, and vGAT (D). qRT-PCR data demonstrated that neuron markers such as NFL, NFM, as well as function- and ion channel-related genes such as Kv4.2, NE-Na, and CACNA1G were all upregulated (E, F). Scale bar=25 μm for (A), scale bar=50 μm for (B–D). Color images available online at www.liebertpub.com/tea
3D scaffolds are biocompatible with hADSCs and facilitate their neuronal differentiation into hADSC-NLCs
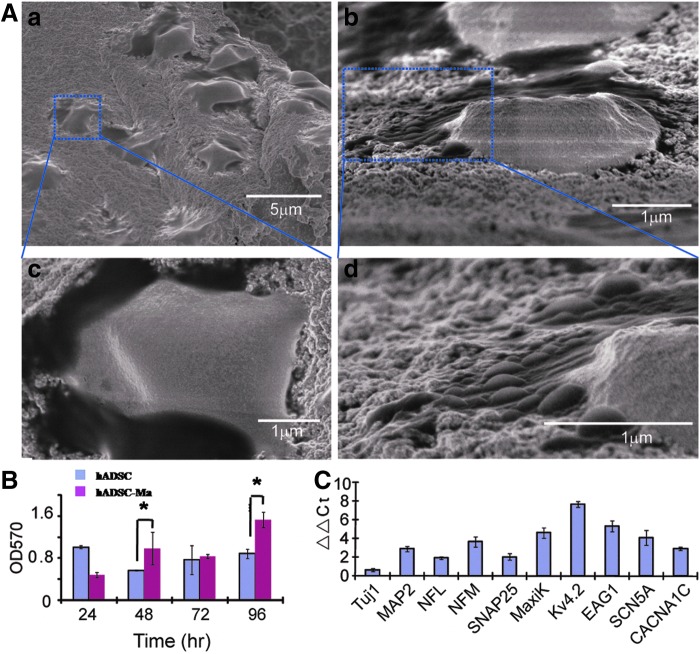
Since the previous evidence has demonstrated the capability of hADSC differentiation into hADSC-NLCs and hADSC can be cultured on 3D scaffolds, we further tested that this transplantable scaffold can support the neuronal differentiation of hADSCs. We observed that after seeding hADSCs onto the hybrid scaffolds in the culture medium, hADSCs rapidly attached, spread, and infiltrated into the bulk of the scaffolds through the interconnected pores (Fig. 8A). The cells exhibited high viability and could actively proliferate on the scaffolds as analyzed by MTT assay (Fig. 8B). These cells also expressed various neuronal markers as demonstrated by qRT-PCR (Fig. 8C). Collectively, these results suggested a high biocompatibility of the gelatin–chitosan hybrid scaffolds that are fabricated by governing the transient phase separation in a polysaccharide-protein-organic solvent system, and the potential of the hybrid scaffolds for tissue engineering applications.
FIG. 8.
hADSCs cultured on the 3D scaffold were subjected to the neuron cocktail medium with similar operation on the plastic dishes. After 6 day differentiation, hADSC-NLCs were detected the morphology by SEM (A). To monitor hADSC proliferation capability on this 3D scaffold material, MTT assay were carried out within 96 h at a time course of 24 h (B). Both hADSC-NLCs and hADSCs on 3D scaffold at D6 were subjected to trizol digestion for total RNA extraction. qRT-PCR were carried out to detect the neuron-related gene expression variance (C). ADSC-Ma means hADSCs cultured on the 3D scaffold material to compare with the hADSCs cultured on the plastic dishes. Two-way ANOVA with Bonferroni post-tests were performed for the data in (B) using GraphPad Prism version 5.00 for Windows, GraphPad Software (San Diego, CA), www.graphpad.com. *p<0.05.
hADSC-NLC/chitosan/gelatin construct can ensure the survival of hADSCs and their in vivo neuronal differentiation and lead to the repair of damaged brain tissue in TBI mouse model
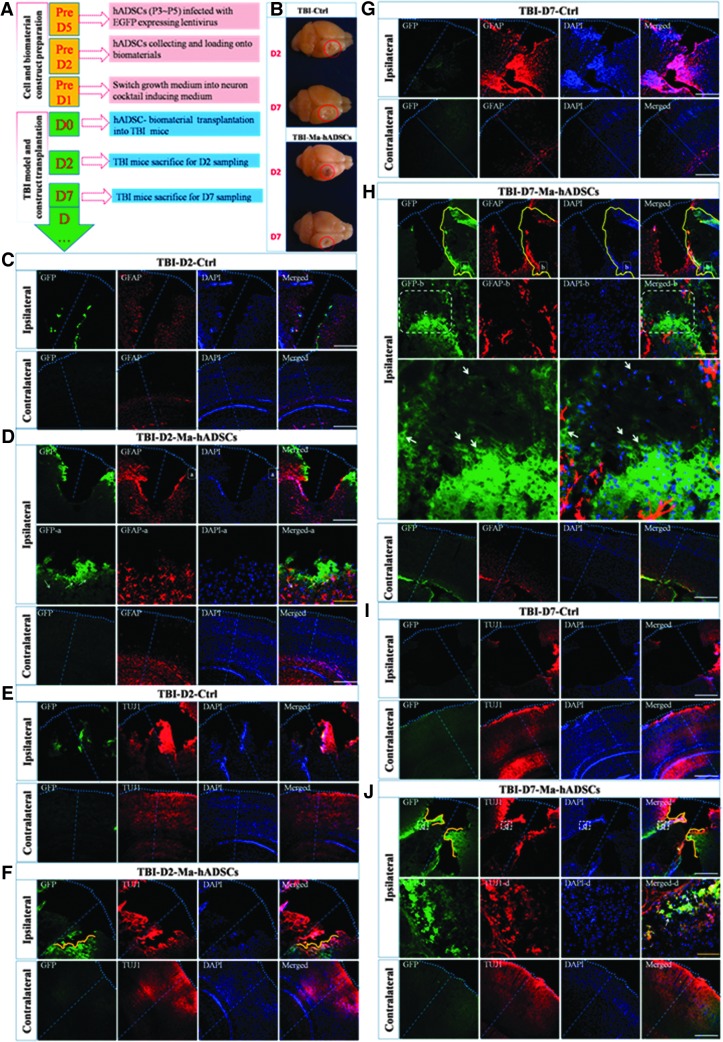
To further test the biocompatibility of this 3D scaffold with hADSCs in vivo, we applied the mixture of hADSC-NLCs/chitosan/geletin construct to the injuried site of the mouse brain in TBI model, which carries high mortality and severe disability to human beings. The experimental paradigm transplantation is shown in Figure 9A. Smaller injury sizes were found in the group transplanted with hADSC-NLCs/chitosan/gelatin scaffold (labeled as TBI-Ma-hADSCs) compared with the PBS-injected control group (labeled as TBI-Ctrl) at each time point of D2 and D7 (Fig. 9B). Comprehensive immunohistochemical studies on TBI brain slices with astrocytic marker GFAP and neuronal marker TUJ1 are presented in Figure 9(C–J). In comparision with the PBS control, co-transplantation of hADSCs with 3D scaffold significantly facilitated the migration of endogenous cells into injured sites and integrated with transplanted cells as shown by the fully intermingled GFAP-positive and GFP-positive cells, as shown in Figure 9D and H. However, interestingly, co-localization of GFP and GFAP was rarely observed in Figure 9D and H, which suggest that hADSC-NLCs did not change their predestinated fate and were not committed into astrocytic differentiation in vivo. On the contrary, co-localization of GFP and TUJ1 were observed around the rim of the injury sites in Figure 9F and J, which demonstrated the viability and neuron lineage fate of transplanted hADSC-NLCs when mixed with 3D scaffold. These results gave us very strong hints that our fabricated light-curable chitosan 3D scaffold can well support hADSC-NLCs survival and guide their neuron lineage transmission in vivo as what has been proved in our in vitro experiments (Fig. 8B).
FIG. 9.
Traumatic brain injury (TBI) mouse model for tracing the hADSC-NLC/chitosan/gelatin scaffold meanwhile using phosphate-buffered saline (PBS) as the control group (n=6). (A) Shows the protocols to perform this experiment; (B) shows the representative TBI brain morphology at different time points (D2 and D7) in each group (n=6). Brain dissection and histoimmunostaining with GFAP and TUJ1 were performed at time course of 2 days (D2) and 7 days (D7) after TBI surgery and cell material construct transplantation. In figures (C, E, G, I), TBI-D2-Ctrl and TBI-D7-Ctrl means TBI mice without treatment but PBS placebo injection as the control group sacrificed on D2 or D7 after TBI surgery. In figures (D, F, H, J), TBI-D2-Ma-hADSCs and TBI-D7-Ma-hADSCs means TBI mice transplanted with hADSC-NLC/chitosan/gelatin scaffold sacrificed on D2 or D7 after TBI surgery. Scale bar (yellow line=100 μm, white line=500 μm).
Discussion
hADSCs have been widely accepted as one of the most promising cell sources for cell replacement therapy in translational medicine while their therapeutic effects remain to be improved and the underlying mechanism needs to be unraveled. Since biocompatible materials may allow the cells to grow three-dimensionally and facilitate the reconstitution of vascular structure, when applied together with hADSCs, it may improve the efficacy and efficiency of cell replacement therapy significantly. Based on this rational analysis, this study focused on the feasibility of co-culturing hADSCs with photocurable high elastic 3D scaffolds, which can be potentially applied for in vivo transplantation.
Raw chitosan has very poor solubility in organic solvents because of its semicrystalline structure, and it can only be dissolved in some acids, such as acetic acid. After modification, light-curable chitosan has very good solubility in many organic solvents (up to 50%), including dimethylformamide (DMF), DMSO, dimethylacetamide (DMAC), acetone, methane chloride, and so forth, which facilitates the fabrication of scaffolds with desired structures. To determine the practical ratio of grafted benzoyl and methacryloyl groups, the peak area of benzoyl and methacryloyl groups was integrated and the ratio was calculated according to the practical ratio of benzoyl and methacryloyl groups after integration of the peak area. The results show that the reactivity of benzoyl chloride is much higher than methacryloyl chloride as the practical ratio of benzoyl groups is always higher than its feeding ratio. So the content of methacryloyl groups is influenced not only by the feeding ratio but also by the reactivity of these two acyl chlorides.
Natural tissue in the body resides in a complicated biomechanical environment that is constantly subjected to cyclic mechanical loadings. As candidate scaffolds for tissue engineering applications, the biomechanical functions in response to cyclic mechanical loadings are very important. The recovery stress–strain curves indicates an energy loss during the cyclic compression process: the compressive loading energy absorbed by the scaffold during the compression process was stored in the material and was only partially released during the recovery potentially due to the internalization/dissipation of energy by the material. The area under the stress–strain curve is usually defined as toughness, an index of the material's ability to absorb energy during deformation. The cyclic compression tests demonstrate that the scaffolds are primarily elastic in nature and possess the appropriate strength and toughness to withstand cyclic compressive loadings that simulate physiological conditions.
Compared with other organs in the body, the central nervous system is a very unique tissue, due to its very unusual ECM structures, as well as many other unique characteristics, including its soft physical properties. Natural brain tissue, which has an elastic modulus of around 500 Pa, is very soft compared to other tissues, such as muscle, which possesses an elastic modulus of around 104 Pa.35,36 Hydrogel mechanical properties obviously play a major role in regulating various cell behaviors,37 it is not surprising that hydrogels with biomechanical properties that are not similar to local environmental conditions have a negative effect on cell behavior. It is well known that hydrogels that possess biomechanical properties, which closely mimic those of the natural ECM, provide the best environment for the differentiation of progenitor cells into the mature phenotypes adherent to that particular tissue.38 The initial elastic modulus of the hydrogel scaffolds range from 330 to 400 Pa. It is more similar to natural brain tissue and will have a positive effect on hADSCs neurogenesis.
After using the differential velocity adherent method to isolate hADSCs from the liposuction or surgery, we characterized the isolated hADSCs with CD surface markers of CD44, CD29, CD105, and CD45, stem cell markers of Sox2, Oct4, c-Myc, Nanog, and KLF4. The results showed that hADSCs in this study were relatively pure (>80%) and possess the mesenchymal stem cell identity. These results were consistent with the previously published studies.1,2,4,7,21,22 To further determine their multipotential differentiation capability, trilineage differentiation toward adipocytes, osteoblast, and chondrocytes was induced with specific induction medium. hADSCs show their stem cell properties and can also be specifically induced into NLCs by a cocktail induction protocol, which is consistent with the previous reports.6–8 To promote these NLCs to become more mature, neurotrophic factors such as BDNF, NGF, and NT3 were supplemented after 3 days of preinduction incubation. Improved expression of NeuN, Synapsin1/2, and vGAT demonstrated that these factors were functional in promoting neuronal maturation. Previous published data on DKK1 showed that DKK1 can promote the ESCs differentiation toward neurons by inhibiting Wnt pathway.32–34 This neuronal induction protocol started with incubating the hADSCs with DKK1 for 24 h, then followed by 3 days of cocktail medium induction as reported by Safford et al.7 Safford et al. used EGF/FGF to preincubate the HuADSC or MuDSCs for 7 days before using neuronal cocktail to induce. They did not use neurotrophic factors to promote neuron maturation. In terms of induction results, our NLCs became more mature as demonstrated by their expression of puncta-like Synapsin1/2 and vGAT.
Since the surfaces of the scaffold skeleton are the interfaces between the two phases (biopolymer-rich phase and solvent-rich phase) during the coacervation process, these SEM images again suggest biphasic distribution of gelatin and the ability of gelatin to form nanoscale structures at the interfaces during the transient phase separation. Cell culture experiments with hADSCs using these scaffolds indicated that nanoscale structures at the surfaces were critical to mediate cell attachment, differentiation, and biomineralization potentially through selectively enhancing the adsorption of specific types of proteins that are favorable for cell–cell interactions, matrix productions, cell–matrix interactions, and bioactivity. In a similar manner, the nanoscale architectures that we have created on the skeleton of the hybrid chitosan–gelatin scaffolds may serve as adhesive domains to promote cell attachment, spreading, ECM production, and functioning.
SEM–EDS was used to study the morphology and elemental composition of material surfaces. In recent years, SEM–EDS also have been increasingly used in life science field, which has helped to demonstrate the formation of the interface between the tissue and the implant. In this work, we used SEM–EDS to obtain additional information related to elemental composition variations in the scaffolds and cells surfaces. The semiquantitative weight (%) for phosphorus increases from 2.34% in the scaffolds to 4.15% in the cells membrane, for sodium is 2.44% in the cells surface, while it was not observed even at a low concentration on the scaffolds surface. Oxygen in the cell membranes is 4.16%; it is almost two times higher than in the scaffolds surface (2.19%). Carbon in the cells surface is 1.25%; it is more two times lower than in the scaffolds surface (2.96%). EDS data provide a good evidence for the difference of scaffolds and bioactive cells on the surface. The difference of elemental composition, especially for sodium, maybe caused by sodium cationic channel activation in cell membrane or cell membrane-specific adsorption of protein during the culture process.39–41
To understand whether the in vivo situation is consistent with the in vitro results, TBI mouse model was peformed to trace the chitosan/hADSC-NLCs construct. To avoid the disturbance of the self-emitted fluorescence from the biomaterials, we remove the biomaterial out of the injury site. Then, we focused on the rim of the injury site to observe the migration of the hADSC-NLCs loaded in the biomaterial before transplantation. As what we expected, we found lots of GFP+/TUJ1+/DAPI+ cells, which demonstrated that these cells could migrate to the rim of the injury site and maintain their neuron lineage characteristics. Our results suggest that the 3D porous scaffolds can promote the hADSC proliferation and subsequently its neuronal differentiation both in vitro and in vivo as shown in Figures 8 and 9. Especially, the highly porous structure of this 3D scaffold had facilitated the migration of both endogenous and exogenous cells to be fully integrated as shown in Figure 9D. It is conceivable that this 3D scaffold may also promote the reconstitute the vascular structure in vivo at injured site and help to repair the damaged brain more efficiently (Fig. 9B). In summary, we have reported a novel approach in creating 3D porous scaffolds of polysaccharide and protein with tunable microstructures, superior mechanical properties, and excellent cytocompatibility. Meanwhile, hADSCs can be induced to differentiate into NLCs labeled by specific neuron markers on the plastic culture dish and the 3D scaffolds as well as the in vivo brain of TBI mouse model, implying the feasibility of combining these hADSCs with 3D scaffolds for cell replacement therapy to treat some neurodegenerative diseases and other neurological diseases such as TBI.
Acknowledgments
This study is supported by the following grants: the Ministry of Science and Technology of China (2010CB945200, 2012CB966300, 2011CB966200); the National Natural Science Foundation of China (81271369, 81070910); National program for support of Top-notch young professionals; New Century Excellent Talents in University (NCET-10-0606); Shanghai Rising-Star program (11QA1407100); Projects of International Cooperation and Exchanges NSFC (81261130318); and the Fundamental Research Funds for the Central Universities.
Disclosure Statement
No competing financial interests exist.
References
- 1.Zuk P.A, Zuh M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7,211, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Gronthos S., Franklin D.M., Leddy H.A., Robey P.G., Storms R.W., and Gimble J.M.Surface protein characterization of human adipose tissue-derived stromal cells. J Cell Physiol 189,54, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13,4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu Y., Liu T., Song K., Fan X., Ma X., and Cui Z.Adipose-derived stem cell: a better stem cell than BMSC. Cell Biochem Funct 26,664, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Denker A.E., Nicoll S.B., and Tuan R.S.Formation of cartilage-like spheroids by micromass cultures of murine C3H10T1/2 cells upon treatment with transforming growth factor beta-1. Differentiation 59,25, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A., et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164,247, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Safford K.M., Hicok K.C., Safford S.D., Halvorsen Y.D., Wilkison W.O., Gimble J.M., et al. Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem Biophys Res Commun 294,371, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Safford K.M., Safford S.D., Gimble J.M., Shetty A.K., and Rice H.E.Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp Neurol 187,319, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Case J., Horvath T.L., Howell J.C., Yoder M.C., March K.L., and Srour E.F.Clonal multilineage differentiation of murine common pluripotent stem cells isolated from skeletal muscle and adipose stromal cells. Ann NY Acad Sci 1044,183, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Woodbury D., Reynolds K., and Black I.B.Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J Neurosci Res 69,908, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Woodbury D., Schwarz E.J., Prockop D.J., and Black I.B.Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61,364, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Yaghoobi M.M., Mowla S.J., and Tiraihi T.Nucleostemin, a coordinator of self-renewal, is expressed in rat marrow stromal cells and turns off after induction of neural differentiation. Neurosci Lett 390,81, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Guilak F., Lott K.E., Awad H.A., Cao Q., Hicok K.C., Fermor B., et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 206,229, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Deng W., Obrocka M., Fischer I., and Prockop D.J.In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun 282,148, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Ashjian P.H., Elbarbary A.S., Edmonds B., DeUgarte D., Zhu M., Zuk P.A., et al. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plast Reconstr Surg 111,1922, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Suon S., Jin H., Donaldson A.E., Caterson E.J., Tuan R.S., Deschennes G., et al. Transient differentiation of adult human bone marrow cells into neuron-like cells in culture: development of morphological and biochemical traits is mediated by different molecular mechanisms. Stem Cells Dev 13,625, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimura J., Ogawa R., Mizuno H., Fukunaga Y., and Suzuki H.Neural differentiation of adipose-derived stem cells isolated from GFP transgenic mice. Biochem Biophys Res Commun 333,116, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Yang L.Q., Geng J., Cao J.Q., Gu R., Chen F., Kong J., et al. Directed differentiation of motor neuron cell-like cells from human adipose-derived stem cells in vitro. Neuroreport 22,370, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Gronthos S., Zannettino A.C., Hay S.J., Shi S., Graves S.E., Kortesidis A., et al. Molecular and cellular characterization of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116,1827, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Schmitt C., Sanchez C., Desobry-Banon S., and Hardy J.Structure and technofunctional properties of protein-polysaccharide complexes: a review. Crit Rev Food Sci Nutr 38,689, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Zeman L., and Patterson D.Effects of the solvent on polymer incompatibility in solution. Macromolecules 5,513, 1972 [Google Scholar]
- 22.Schwartz Z., Griffon D.J., Fredericks L.P., Lee H.B., and Weng H.Y.Hyaluronic acid and chondrogenesis of murine bone marrow mesenchymal stem cells in chitosan sponges. Am J Vet Res 72,42, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Haipeng G., Yinghui Z., Jianchun L., Yandao G., Nanming Z., and Xiufang Z.Studies on nerve cell affinity of chitosan-derived materials. J Biomed Mater Res, 52,285, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Yuan Y., Zhang P., Yang Y., Wang X., and Gu X.The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials 25,4273, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Gupta A.N., Bohidar H.B., and Aswal V.K.Surface patch binding induced intermolecular complexation and phase separation in aqueous solutions of similarly charged gelatin-chitosan molecules. J Phys Chem B 111,10137, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Perng C.K., Kao C.L., Yang Y.P., Lin H.T., Lin W.B., Chu Y.R., et al. Culturing adult human bone marrow stem cells on gelatin scaffold with pNIPAAm as transplanted grafts for skin regeneration. J Biomed Mater Res A, 84A, 3,622, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto M., Takahashi Y., and Tabata Y.Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials 24,4375, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Rosso F., Marino G., Giordano A., Barbarisi M., Parmeggiani D., and Barbarisi A.Smart materials as scaffolds for tissue engineering. J Cell Physiol 203,465, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Stevens M.M., and George J.H.Exploring and engineering the cell surface interface. Science 310,1135, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Gao S., Lai C.K.M., and Cheung P.C.K.Non-digestible carbohydrates isolated from medicinal mushroom sclerotias as novel prebiotics. Int J Med Mushr 11,1, 2009 [Google Scholar]
- 31.Guo D., Zeng L., Brody D.L., and Wong M.Rapamycin attenuates the development of posttraumatic epilepsy in a mouse model of traumatic brain injury. PLoS One 8,e64078, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takeuchi S., Wada K., Nawashiro H., Uozumi Y., Otani N., Nagatani K., Kobayashi H., and Shima K.Decrease in plasma adiponectin level and increase in adiponectin immunoreactivity in cortex and hippocampus after traumatic brain injury in rats. Turk Neurosurg 23,349, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Welstead G.G., Brambrink T., and Jaenisch R.Generating iPS cells from MEFS through forced expression of Sox-2, Oct-4, c-Myc, and Klf4. J Vis Exp 14,734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu C.Y., Whye D., Mason R.W., and Wang W.Embryonic stem cell neurogenesis and neural specification. J Cell Biochem 111,535, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Leipzig N.D., Shoichet M.S.The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials 30,6867, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Lu Y.B., Franze K., Seifer G., Steinhäuser C., Kirchhoff F., Wolburg H., Guck J., Janmey P., Wei E.Q., Käs J., Reichenbach A.Viscoelastic properties of individual glial cells and neurons in the CNS. Proc Natl Acad Sci U S A 103,17759, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Discher D.E., Janmey P., Wang Y.Tissue cells feel and respond to the stiffness of their substrate. Science 310,1139, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Engler A.J., Sen S., Sweeney H.L., Discher D.E.Matrix elasticity directs stem cell lineage specification. Cell 126,677, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Ribeiro D., Ellwanger K., Glagow D., Theofilopoulos S., Corsini N.S., Martin-Villalba A., et al. Dkk1 regulates ventral midbrain dopaminergic differentiation and morphogenesis. PLoS One 6,e15786, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardozo A.J., Gómez D.E., and Argibay P.F.Transcriptional characterization of Wnt and Notch signaling pathway in neuronal differentiation of human adipose tissue derived stem cells. J Mol Neurosci 44,186, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Maria P.F., and Julio S.C.SEM–EDS probing of morphological and physiological changes produced by a porphyrin photosensitizer in Psammobatis extenta electrocytes. Micron 38,668, 2007 [DOI] [PubMed] [Google Scholar]