Abstract
The survival of transplanted cells and their resulting efficacy in cell-based therapies is markedly impaired due to serum deprivation and hypoxia (SD/H) resulting from poor vascularization within tissue defects. Lysophosphatidic acid (LPA) is a platelet-derived growth factor with pleiotropic effects on many cell types. Mesenchymal stromal cells (MSC) exhibit unique secretory and stimulatory characteristics depending on their differentiation state. In light of the potential of MSC in cell-based therapies, we examined the ability of LPA to abrogate SD/H-induced apoptosis in human MSC at increasing stages of osteogenic differentiation in vitro and assessed MSC survival in vivo. Undifferentiated MSC were rescued from SD/H-induced apoptosis by treatment with both 25 and 100 μM LPA. However, MSC conditioned with osteogenic supplements responded to 25 μM LPA, and cells conditioned with dexamethasone-containing osteogenic media required 100 μM LPA. This rescue was mediated through LPA1 in all cases. The addition of 25 μM LPA enhanced vascular endothelial growth factor (VEGF) secretion by MSC in all conditions, but VEGF availability was not responsible for protection against apoptosis. We also showed that codelivery of 25 μM LPA with MSC in alginate hydrogels significantly improved the persistence of undifferentiated MSC in vivo over 4 weeks as measured by bioluminescence imaging. Osteogenic differentiation alone was protective of SD/H-induced apoptosis in vitro, and the synergistic delivery of LPA did not enhance persistence of osteogenically induced MSC in vivo. These data demonstrate that the capacity of LPA to inhibit SD/H-induced apoptosis in MSC is dependent on both the differentiation state and dosage. This information will be valuable for optimizing osteogenic conditioning regimens for MSC before in vivo implementation.
Introduction
Mesenchymal stromal cells (MSC) are popular candidates for regenerative medicine due to their ready availability, nonimmunogenicity, and ability to differentiate into multiple tissue types.1,2 In particular, MSC can be differentiated toward an osteoblastic lineage using chemical cues and supplements3,4 and hold the potential for treating millions of critical-sized bone defects and nonunion fractures that occur annually. Current approaches under investigation seek to direct differentiation of MSC in conjunction with biocompatible scaffolds and other delivery systems,5,6 but successful translation to in vivo environment is initially hampered by the harsh environment, including serum deprivation and reductions in local oxygen tension (hypoxia) (SD/H) at the defect or fracture site. Indeed, 99% of MSC do not survive culture under ischemia after 3 days7 and 99% of MSC implanted into ischemic heart tissue die within 96 h,8 severely limiting the therapeutic potential of such treatments. Without overcoming such poor conditions, extensive apoptosis can significantly impede or prevent tissue formation, regardless of the cell transplantation method.9–11
Although growth factors such as angiopoietin-1 have been shown to protect MSC against ischemia-induced apoptosis,12 the high cost of producing and purifying recombinant proteins and the difficulty of accurate delivery render large-scale implementation impractical. Lysophosphatidic acid (LPA) is a glycerophospholipid signaling molecule that binds to cognate G-protein-coupled receptors and has a wide range of effects on many different cell types.13–16 Naturally present in serum at low micromolar concentrations,15 LPA plays regulatory roles in the adhesion, migration, and proliferation of endothelial cells as well as neurons.14,17,18 Additionally, LPA affects actin polymerization in fibroblasts, osteoblasts, and other cell types to modulate cytoskeletal tension and contractile forces.13,15
Of particular interest for tissue engineering applications is the capacity for LPA to reduce apoptosis in MSC. Previous studies have demonstrated that LPA rescues rat MSC from SD/H-induced apoptosis in vitro9 and in vivo19 through ERK-dependent mediation of endoplasmic reticulum stress and signaling pathways.10 The ability of LPA to attenuate apoptosis,9,19 combined with its relative low cost (currently, ∼$10/mg versus ∼$12,000/mg for recombinant human angiopoietin20), makes LPA an attractive target for continued investigation as a means to enhance the effectiveness of MSC-based tissue engineering strategies by improving cell viability in ischemia.
Despite the promising, but limited, antiapoptotic effects of LPA in rat cells, further assessment of its potential for clinical application requires thorough characterization of its effects on human MSC. Undifferentiated MSC can be used to support angiogenesis in defect sites or modulate immune responses,21 while treatment strategies that target direct bone repair may benefit from inducing MSC toward the osteoblastic lineage before implantation to promote rapid bone formation and calcium deposition.22,23 Since MSC exhibit unique secretory and stimulatory characteristics depending on their differentiation state,4,24 it is important to account for variations in cellular responses to LPA at different degrees of differentiation.
We hypothesized that LPA would rescue human MSC from SD/H-induced apoptosis and that the capacity for LPA to attenuate apoptosis is a function of the degree of osteogenic differentiation. We investigated this hypothesis by conditioning MSC with three media types of increasing osteogenic capacity and exposing them to SD/H in the presence of varying concentrations of LPA. We assessed apoptosis in cells maintained in each media type and also probed the expression of LPA receptors to elucidate the ones involved in promoting cellular survival. Additionally, we quantified the effect of LPA on the production of vascular endothelial growth factor (VEGF) and examined possible mechanisms for LPA-mediated apoptotic attenuation. Finally, we used an alginate hydrogel delivery vehicle and noninvasive bioluminescence imaging (BLI) to track cell survival in vivo over 4 weeks.
Materials and Methods
Cell culture
For in vitro studies, human bone marrow-derived MSC (Lonza, Walkersville, MD) were expanded without further characterization in a growth medium (GM) consisting of the minimum essential alpha medium (α-MEM; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS; JR Scientific, Woodland, CA) and 1% penicillin–streptomycin (P/S; Mediatech, Manassas, VA). MSC were transduced to express firefly luciferase (MSC-Luc) for in vivo studies as previously described.25,26 Cells were cultured under standard conditions in a humidified incubator and utilized at passages 5–6. To induce osteogenic differentiation, cells were cultured in either osteogenic media (OM: GM supplemented with 10 mM β-glycerophosphate and 50 μg/mL ascorbate-2-phosphate; Sigma-Aldrich, St. Louis, MO) or in OM supplemented with dexamethasone (OM+: OM with 10 nM dexamethasone, Sigma-Aldrich).4 All media were replaced every 3 days.
For all experiments examining the effects of SD/H, MSC were preconditioned in GM, OM, or OM+ for 7 days in T-225 tissue culture flasks and subsequently seeded on six-well tissue culture plates at 30,000 cells/cm2. After attaching overnight, cells were washed 3×with PBS to eliminate all traces of serum. To induce apoptosis, media were replaced with serum-free GM, OM, or OM+ supplemented with 0.1% (w/v) fatty acid-free BSA, and cells were incubated in hypoxia for 24 h (n=6). In studies examining caspase activity and VEGF secretion in SD/H, cells were incubated in airtight chambers (Billups-Rothenberg, Del Mar, CA) at an oxygen tension of ≤1%, as previously described.27 In studies for LPA receptor expression, cells were incubated for in Heracell 150i tri-gas incubators (Thermo Scientific, Waltham, MA) at 1% oxygen. Negative controls for apoptosis were cultured for the same duration in 21% O2 in GM, OM, or OM+ with full serum.
Biochemical characterization of osteogenic differentiation
MSC were plated at 30,000 cells/cm2 on 12-well tissue culture plates in GM, OM, or OM+ and maintained for 7 days (n=4). The intracellular alkaline phosphatase (ALP) activity was quantified for cells in each medium at each time point and normalized to the DNA content as previously described.28,29
qPCR analysis of osteogenic markers and LPA receptor expression
Total RNA was collected from MSC exposed to SD/H after preconditioning in GM, OM, or OM+ (n=4) using an RNeasy Mini kit (Qiagen, Valencia, CA). About 270 ng of total RNA was reverse transcribed with the QuantiTect Reverse Transcription kit (Qiagen). qPCR was performed using a QuantiFast Probe PCR kit (Qiagen) on a Mastercycler realplex2 (Eppendorf, Westbury, NY). Primers and probes for RPL13 (HS00204173_m1), RUNX2 (Hs00231692_m1), LPAR1 (Hs00173500_m1), LPAR2 (Hs01113287_m1), LPAR3 (Hs00173857_m1), LPAR4 (Hs00271072_s1), and LPAR5 (Hs00252675_s1) were purchased from Applied Biosystems (Foster City, CA). Amplification conditions were 95°C for 3 min, followed by 40 cycles at 95°C for 3 s and 60°C for 30 s. Quantitative PCR results were normalized to RPL13 transcript levels to yield ΔCt, and fold change in expression relative to the housekeeping gene was calculated using 2−ΔCt.30
Visual and quantitative assessment of MSC exposed to SD/H
MSC conditioned in GM were exposed to SD/H as described above, and the morphological characteristics of MSC in each condition were observed and recorded at 100×magnification. DNA from MSC in each condition (n=3) was quantified using a Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen).
Induction and rescue of SD/H-induced apoptosis
MSC were preconditioned in GM, OM, or OM+ and exposed to SD/H for 24 h. To explore the antiapoptotic potential of LPA, MSC were cultured in serum-free GM, OM, or OM+ containing 0.1% fatty acid-free BSA and supplemented with LPA (Enzo Life Sciences, Plymouth Meeting, PA) to a final concentration of 1, 10, 25, and 100 μM. A subset of cells received the LPA1/3 inhibitor Ki16425 (10 μM; Cayman Chemical, Ann Arbor, MI) to abrogate LPA binding10 in the presence of media containing 25 μM LPA. This LPA concentration was selected as an optimum dose due to its effectiveness in multiple media types. For experiments testing the effects of VEGF in SD/H, cells in GM were exposed to SD/H with 25 μM LPA and the saturating addition of 10 μg/mL VEGF165/121 antibody (AB-293-NA; R&D Systems, Minneapolis, MN)4 or 5 ng/mL recombinant VEGF (Lonza) with 25 μM LPA and 10 μM Ki16425 (n=6).
Total protein was collected in 50 μL of lysis buffer containing 0.1% Triton X-100 (Sigma-Aldrich), 1 mM Tris, pH 8.0, 1 mM EDTA (Sigma-Aldrich), and 1% Protease Inhibitor Cocktail Set I (EMD Chemicals, Darmstadt, Germany). Protein concentrations were determined using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's instructions. Apoptosis was quantitatively assessed by analyzing 10 μg of protein per sample using a Caspase-Glo 3/7 assay (Promega, Madison, WI). Luminescence was detected on a microplate reader (BIO-TEK Synergy HTTR, Wisnooski, VT) and normalized to protein collected from control cells grown in 21% O2 supplemented with 10% FBS.
Quantification of MSC VEGF production
VEGF secretion into media by MSC in response to SD/H, 25 μM LPA, and 10 μM Ki16425 was measured using a human VEGF ELISA kit according to the manufacturer's instructions (R&D Systems). Data were normalized to the quantity of total protein collected from the cells in each well (n=4).
Alginate gel synthesis
Alginate gels were fabricated largely as previously described.31,32 Briefly, hydrogels were formed from a 2% (w/v) solution of irradiated alginate polymer coupled to oligopeptides containing the Arg-Gly-Asp (RGD) cell adhesion sequence. 6.66×106 undifferentiated or OM+ preconditioned MSC-Luc were suspended in each mL of the 2% solution, which was then mixed thoroughly with a supersaturated calcium sulfate slurry using two syringes and a three-way stopcock (40 μL CaSO4 per mL alginate). When used, LPA was added to the 2% solution to yield a final concentration of 25 μM in the polymerized gels.
Murine subcutaneous injection model and noninvasive imaging of implanted cells
Treatment of experimental animals was in accordance with the UC Davis animal care guidelines and the all National Institutes of Health animal-handling procedures. Eight-week-old nonobese diabetic/severe combined immunodeficient gamma (NSG, NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) mice (Jackson Laboratories–West, Sacramento, CA) were anesthetized and maintained under a 2% isoflurane/O2 mixture delivered through a mask. Every animal received four subcutaneous injections of 150 μL each, containing MSC-Luc conditioned in GM or OM + and with or without 25 μM LPA (n=12). One injection was made over each shoulder and hip.
Cell persistence was measured using in vivo BLI at 1, 3, 7, 14, 21, and 28 days on an IVIS Spectrum (Perkin Elmer, Waltham, MA) as previously described.25,26 Briefly, mice were injected with D-Luciferin, Firefly (Caliper, Hopkinton, MA; 10 μL/g body weight), and luminescence was measured using Living Image software (Perkin Elmer). Total photons per second per centimeter were recorded from each bioluminescent region of interest. Data are normalized to luminescence from gels containing undifferentiated cells within each animal at each time point.
Animals were euthanized 7 and 28 days postsurgery (n=6) and each gel was excised and fixed in 10% formalin for 72 h. Fixed gels were processed for histology using standard techniques. The presence of surviving MSC was assessed by immunohistochemistry for human CD90 (ab133350, 1:100; Abcam, Cambridge, MA) and a rabbit-specific HRP/DAB detection kit (ab64261; Abcam).
Statistical analysis
Data are presented as mean±standard error unless otherwise stated. Statistical analysis was performed using paired Student's t-tests and one-way ANOVA with the Tukey's multiple comparison post-test, where applicable. p-values less than 0.05 were considered statistically significant.
Results
Media composition differentially drives osteogenic differentiation
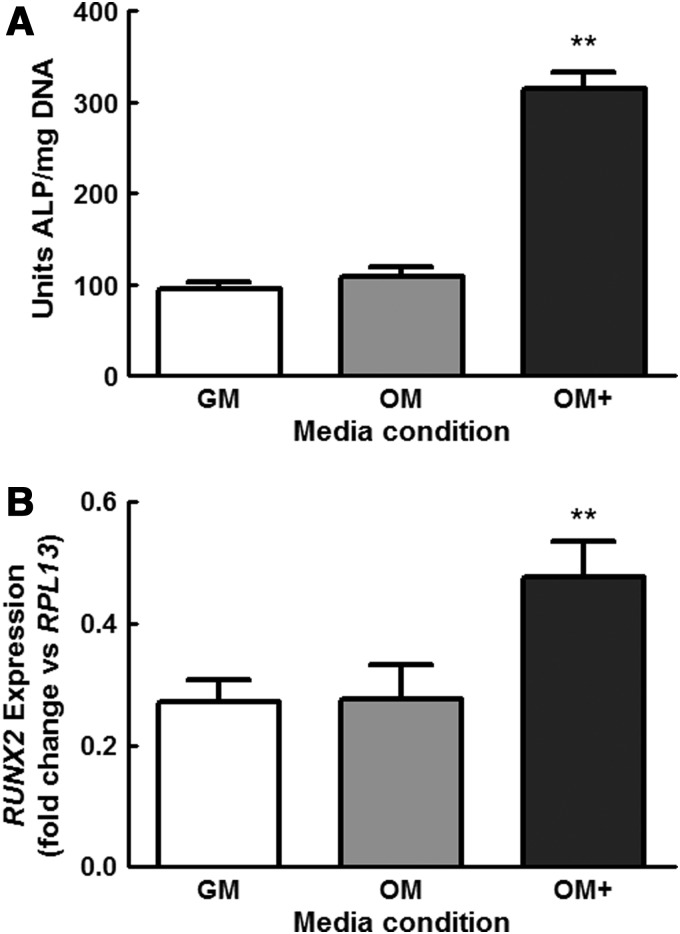
MSC cultured in OM+ exhibited significantly higher levels of ALP activity at 7 days compared with cells maintained in GM and OM (Fig. 1A). MSC in OM exhibited modestly a greater ALP activity compared with GM, although we did not observe statistically significant increases. Similarly, cells conditioned in OM+ expressed significantly higher levels of the osteogenic transcription factor RUNX2 compared with cells cultured in GM or OM (Fig. 1B). Based on these and previous data,6 we selected 7 days as the consistent duration for osteogenic preconditioning for the remainder of these studies.
FIG. 1.
Osteogenic differentiation of mesenchymal stromal cells (MSC) grown in growth medium (GM), osteogenic media (OM), and OM+ for 7 days as determined by (A) intracellular alkaline phosphatase (ALP) activity and (B) RUNX2 expression. **p<0.001 versus GM and OM, (n=4).
LPA receptor expression is dependent upon differentiation state
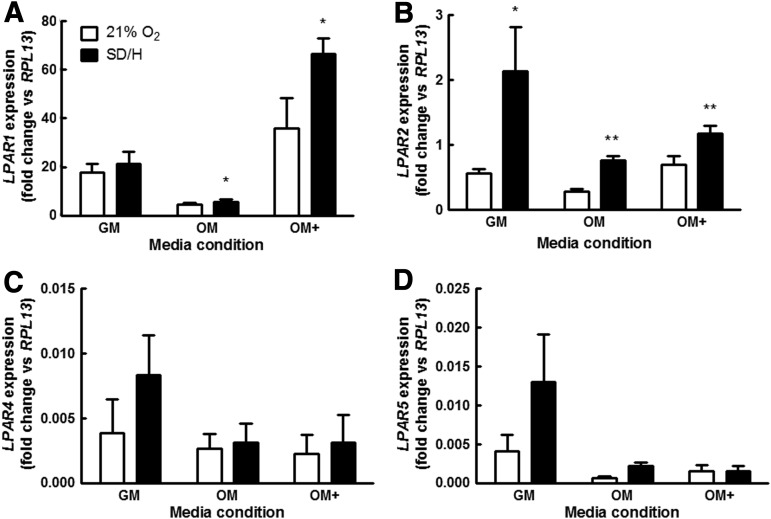
MSC exposed to SD/H had expressed greater transcript for LPAR1, LPAR2, LPAR4, and LPAR5, regardless of osteogenic preconditioning, compared with samples cultured at 21% O2 in full serum. LPAR1 expression was significantly increased by SD/H in MSC cultured in OM and OM+ (Fig. 2A), while LPAR2 expression was higher in SD/H in all media (Fig. 2B). LPAR4 and LPAR5 similarly showed trends for increased expression in SD/H compared with 21% O2, but did not achieve statistical significance (Fig. 2C, D). Notably, MSC preconditioned in OM expressed significantly lower levels of LPAR1 and LPAR2 compared with cells cultured in both GM and OM+. LPAR3 was not found at detectable levels under the tested culture conditions (data not shown), consistent with other reports indicating that MSC do not express LPAR3.
FIG. 2.
Lysophosphatidic acid (LPA) receptor expression is significantly affected by the MSC differentiation state. (A) LPAR1 expression is significantly increased by serum deprivation and hypoxia (SD/H) in OM and OM+, while (B) LPAR2 expression is higher in SD/H for all media conditions. SD/H also increases expression of (C) LPAR4 and (D) LPAR5, but not to statistically significant levels. LPAR1 and LPAR2 expression was significantly higher in GM and OM+ than OM. LPAR3 was not detected MSC in any media type. *p<0.05 versus control, **p<0.01 versus control.
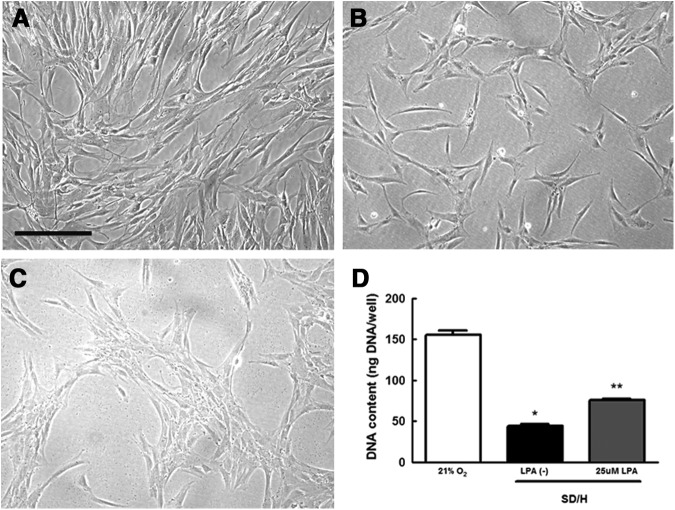
SD/H causes loss of MSC in culture
Compared with MSC maintained in 21% O2 and full serum (Fig. 3A), we observed a significant loss of MSC exposed to SD/H (Fig. 3B). The number of MSC in culture was visually reduced, and the addition of 25 μM LPA mitigated this loss (Fig. 3C, D). These observations were quantitatively confirmed by measuring the DNA content in the culture (Fig. 3D).
FIG. 3.
SD/H induces apoptosis in MSC in GM after 24 h. (A) Representative images of MSC in 21% O2 with 10% fetal bovine serum (FBS). (B) MSC exposure to SD/H results in significant loss of cells from the culture dish that is partially rescued by the addition of (C) 25 μM LPA. Images taken at 100×; scale bar represents 100 μm. (D) Quantification of cell number by DNA content confirms visual assessment. *p<0.001 versus control, **p<0.001 versus SD/H (n=3).
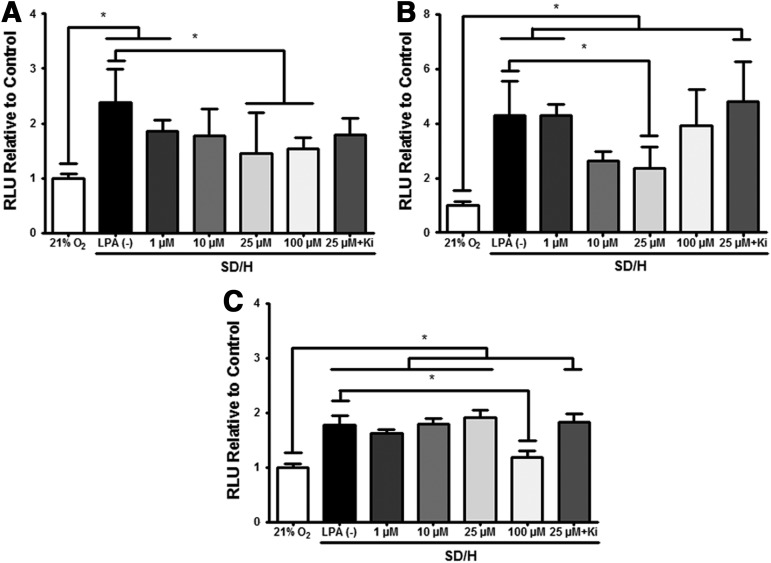
LPA-mediated rescue of SD/H-induced apoptosis is dependent upon differentiation state
MSC in SD/H conditions exhibited increased caspase 3/7 activity compared with control cells maintained in 21% O2 and 10% FBS, regardless of the differentiation state (Fig. 4A–C). The addition of LPA to the culture medium resulted in a dose-dependent effect on the caspase activity that was also related to the differentiation state. For MSC in GM and SD/H, the addition of 25 and 100 μM LPA significantly reduced caspase 3/7 activity, a widely accepted quantitative measure of apoptosis,9,33 compared with no LPA (Fig. 4A). MSC preconditioned in OM exhibited significant reduction in caspase activity to control levels when stimulated with 25 μM LPA, and the addition of the LPA1/3 receptor inhibitor Ki16425 negated this effect (Fig. 4B).
FIG. 4.
LPA rescues MSC from SD/H-induced apoptosis in a dose-dependent manner that varies by differentiation state as induced by the following: (A) GM, (B) OM, and (C) OM+. Data are normalized to RLU from cells in 21% O2 and media containing 10% FBS. *p<0.01 (n=6).
MSC preconditioned in OM+ displayed significantly greater cell death after 24 h in SD/H compared with OM+ conditioned control cells in 21% O2 with full serum. Significant attenuation of caspase activity occurred in the presence of 100 μM LPA and was abrogated by Ki16425. Unlike MSC conditioned in OM or GM, 25 μM LPA was insufficient to protect cells against apoptosis, as measured by caspase 3/7 activity (Fig. 4C).
LPA increases VEGF production in MSC
VEGF production by MSC decreased as a function of increased osteogenic differentiation, as directed by the addition of dexamethasone to the cocktail of osteogenic supplements (Fig. 5A). MSC exposed to SD/H in GM increased VEGF secretion compared with control cells exposed to 21% oxygen with 10% FBS, but we did not observe increased VEGF secretion under SD/H for MSC cultured in other media. Compared with MSC in GM or OM, VEGF secretion was impaired in MSC preconditioned in OM+ and significantly reduced further in SD/H. For MSC in GM, the addition of 25 μM LPA in SD/H increased VEGF secretion beyond concentrations seen for cells in 21% O2 or SD/H, while Ki16425 abrogated this increase in VEGF in response to exogenous LPA. Compared with cells without LPA, we observed a trend for increased VEGF production in MSC cultured in OM upon the addition of 25 μM LPA in SD/H, although this did not achieve statistical significance. Similar to MSC in GM, the addition of LPA to MSC in OM+ in SD/H significantly increased VEGF production and Ki16425 negated this response.
FIG. 5.
(A) LPA significantly increases production of vascular endothelial growth factor (VEGF) by MSC in GM and OM+, which is mitigated by the addition of Ki16425 (n=4). However, (B) the addition of VEGF does not affect MSC survival under SD/H (n=6). *p<0.0001 versus control, **p<0.01 versus SD/H, #p<0.001 versus SD/H+25 μM LPA, and ***p<0.01 versus LPA(−).
To determine if LPA-mediated VEGF protects MSC against apoptosis, cells in GM were exposed to SD/H with 25 μM LPA and saturating addition of 10 μg/mL VEGF165/121 antibody; no further decrease in the caspase activity was detected (Fig. 5B). Similarly, 5 ng/mL VEGF did not improve cell survival when added to GM in conjunction with 25 μM LPA and 10 μM Ki16425.
LPA and osteogenic differentiation promote MSC survival in vivo
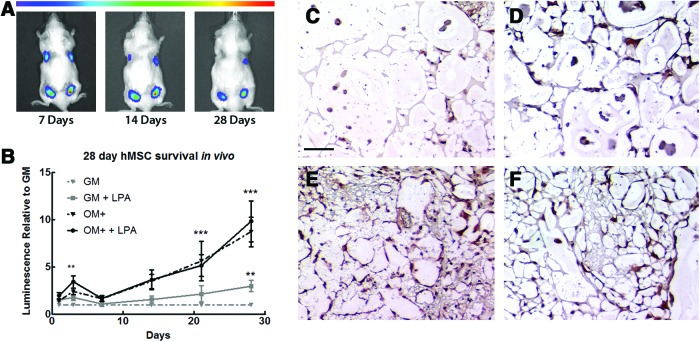
To test whether the protective effects of LPA translate to in vivo applications, we entrapped MSC-Luc in alginate gels containing 25 μM LPA and injected them subcutaneously in NSG mice. We compared undifferentiated cells versus MSC preconditioned in OM+ because these groups had the widest range of LPA receptor expression in SD/H compared with 21% O2 and exhibited less sensitivity to SD/H than cells in OM. Noninvasive BLI revealed that alginate-entrapped, GM conditioned MSC-Luc undergo significant cell death over 4 weeks in vivo (Fig. 5A, B). By the final time point, virtually no luminescence was detected from this group of gels. Codelivery of 25 μM LPA significantly improved survival of undifferentiated cells at 28 days and maintained sustained, readily observable luminescence.
In agreement with our in vitro caspase 3/7 data, MSC-Luc preconditioned in OM+ were dramatically more resistant to apoptosis. Similarly, the addition of 25 μM LPA did not further inhibit cell loss. CD90 staining of MSC at 28 days (Fig. 5C–F) confirms an almost total absence of undifferentiated cells that was improved with LPA treatment, as well as the continued presence of OM+conditioned cells.
Discussion
Widespread cell death following implantation into sites of ischemic injury remains a major hurdle to successful implementation of MSC-based solutions for tissue repair. Although recombinant proteins can promote cell survival, they are costly to produce in large quantities and carry distinct challenges for long-term stabilization and delivery. In this study, we examined the ability of LPA to inhibit SD/H-induced apoptosis in human MSC. Previous groups have shown that LPA, an inexpensive glycerophospholipid, inhibits apoptosis in undifferentiated rat MSC in vitro,9 but these results lack translational relevance because of the inherent differences in behavior between human and rat MSC. Human MSC signaling and phenotype are affected by their differentiation state,4 and many strategies for tissue repair utilize different preconditioning regimens1,2,6 for increased efficacy. For example, osteogenically inducing MSC before implantation enhances in vivo bone regeneration,6 whereas undifferentiated MSC secrete higher levels of growth factors.34 Therefore, we specifically assessed the protective effects of LPA on cells exposed to three increasing degrees of osteogenic conditioning.
After preconditioning MSC in varying media (GM, OM, and OM+) for 1 week, the cellular response to LPA in SD/H was significantly different at both the transcriptional and functional levels. qPCR analysis revealed that cells in 21% O2 with 10% FBS expressed LPAR1 and LPAR2 at the highest levels, while LPAR4 and LPAR5 expression was much lower (Fig. 2). LPAR3 was undetected in this population. This is in agreement with previous studies characterizing LPA receptor expression in MSC expressing recombinant human telomerase.35 Following exposure to SD/H, LPA receptor expression increased in cells in all media types, although to varying degrees of statistical significance. Notably, the expression levels of LPAR1 and LPAR2 were statistically different in each media condition, and MSC preconditioned in OM showed lower levels of expression for all receptors compared with cells in GM and OM+.
We selected a luciferase-based caspase 3/7 activity assay to rapidly quantify protein markers directly involved in late-stage apoptosis. A number of techniques are available for apoptotic assessment, including flow cytometry and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). However, these techniques do not reliably discriminate between apoptotic and necrotic cells, and TUNEL may yield false positives from cells undergoing DNA repair and transcription.36 Caspase 3/7 activation has long been established as a late-stage marker of apoptosis that is conserved across many species.37 Increases in the caspase activity among a cell population are widely accepted as being reflective of increases in apoptosis.9,33 The caspase 3/7 activity revealed that LPA rescues SD/H-induced apoptosis in human MSC at all three differentiation states, but cells conditioned in each media type require different effective concentrations (Fig. 4). 25 and 100 μM of LPA inhibited SD/H-mediated increases in caspase activity for cells conditioned in GM, while only 25 μM LPA had significant effects in OM. 100 μM of LPA was required to detect reductions in apoptosis for cells in OM+. Furthermore, we found that caspase 3/7 activity in MSC conditioned in OM and exposed to SD/H were nearly twice as high as those in cells cultured in GM and OM+. Additionally, SD/H induced the lowest apoptotic response in MSC preconditioned in OM+. Because dexamethasone protects MSC from confluence-induced apoptosis,38 we examined the potentially protective role of dexamethasone (10 nM) when added to cells in GM and exposed to SD/H for 24 h. No antiapoptotic effects were found (data not shown), indicating that differences in caspase activity between OM and OM+ were not dexamethasone dependent.
The addition of the LPA1/3 inhibitor Ki16425 abrogated LPA-mediated rescue of apoptosis in all media conditions, although significance was achieved only in OM and OM+. Combined with the absence of detectable LPAR3, this indicates that apoptotic inhibition is mediated through LPA1, which is further supported by greater levels of transcript expression. Additional comparisons between apoptotic responses and LPA receptor expression levels show a correlation between relatively low levels of LPA1 in OM-conditioned MSC and higher caspase levels in SD/H, suggesting that cells with fewer receptors are more sensitive to ischemic conditions. Similarly, MSC under hypoxia exhibit increased expression of receptors for erythropoietin and hepatocyte growth factor,8 as well as receptors CX3CR1 (interleukin 8 alpha) and CXCR4.39 Ongoing work is examining the role of cAMP/PKA and Ca2+ in the ability of LPA to rescue cells from SD/H.
In addition to possessing osteogenic potential, MSC can play a critical role in tissue repair by serving as support cells that secrete trophic factors to accelerate angiogenesis and wound healing.2,26,40 One such potent angiogenic factor, VEGF, is antiapoptotic for a number of cells, including endothelial cells,41 cardiomyocytes,42 and neurons.43 Although MSC do not express VEGF receptors,44 they produce increased amounts of VEGF in response to ischemia.45–47 Indeed, MSC conditioned in GM secreted significantly higher levels of VEGF in SD/H, which was further augmented by the addition of 25 μM LPA (Fig. 5A). Ki16425 decreased VEGF production when added with LPA, again implicating LPA1-mediated signaling. The addition of VEGF-neutralizing antibody or exogenous VEGF had no effect on caspase activity, confirming that LPA rescues apoptosis independently of VEGF (Fig. 5B).
As human MSC undergo osteogenic differentiation, they lose their proangiogenic potential,4 which is in accordance with our observed decreases in VEGF production by OM- and OM+treated cells. However, the addition of LPA to OM+ still resulted in significantly increased VEGF secretion, which was abrogated by Ki16425. The secretion of VEGF by MSC in OM in response to LPA exhibited a trend that mirrored OM+, but lacked statistical significance, possibly as a result of the lower receptor expression levels described previously.
We applied our findings in vivo by injecting alginate-entrapped MSC-Luc with LPA subcutaneously in NSG mice. Using BLI, we noninvasively observed persistence over 4 weeks and found that undifferentiated cells indeed suffered from widespread cell death upon implantation (Fig. 6). The addition of 25 μM of LPA significantly improved survival, confirming that LPA can be effectively implemented in a hydrogel delivery system. Of particular note, GM MSC entrapped in LPA-containing gels experienced sustained increases in survival from localized delivery of LPA at every time point over the course of the study. This prolonged response contradicts the burst release profile that frequently hampers the effectiveness of recombinant proteins.48 Additionally, our ability to incorporate LPA and cells directly into gels during synthesis allows for straightforward clinical applications, especially compared with previously established methods that require rat MSC to be preconditioned with LPA in culture before injection in vivo.19
FIG. 6.
LPA promotes cell survival of MSC over 4 weeks in vivo. (A) BLI reveals that more GM-preconditioned MSC-Luc survive over 28 days when delivered with 25 μM LPA (top right) compared with untreated cells (top left). OM+ preconditioned MSC-Luc (bottom left) have higher persistence over time, but also exhibit improved survival in LPA-containing gels (bottom right). (B) Quantification of luminescence over time shows that LPA significantly improves survival of GM MSC-Luc. OM+ conditioning alone had a protective effect over 28 days, regardless of LPA treatment. Data are normalized to luminescence from gels containing undifferentiated cells within each animal at each time point. (C) Representative CD90 staining from gels containing GM-conditioned MSC confirms poor cell survival at 28 days that is (D) improved with the addition of LPA. (E, F) MSC conditioned in OM+ persisted at 28 days both in the absence (E) or presence (F) of LPA. Magnification is 100×; scale bar represents 100 μm. **p<0.01 versus GM, ***p<0.001 versus GM (n=6). Color images available online at www.liebertpub.com/tea
Consistent with our in vitro data, osteogenically induced MSC-Luc were inherently more resistant to apoptosis, with LPA providing no significant additional protection at 25 μM. Temporal quantification of luminescence revealed a universal decrease in intensity over time in all conditions, indicating that differences between groups are not driven by proliferation (data not shown). These data have significant implications for cell-based therapies for tissue repair, with potential applications for two unique treatment strategies. In one case, LPA, which has mitogenic effects on endothelial cells,49 could be used to promote survival and VEGF secretion in undifferentiated MSC to further enhance angiogenesis for a natural wound healing response. This approach may markedly enhance the efficacy of MSC when implanted to drive neovascularization for use in advanced vascular disease, slow healing wounds, or promoting collateralization during bone repair. Conversely, osteogenically induced MSC treated with LPA would exhibit increased resistance to SD/H-induced apoptosis in a defect site, potentially enabling these cells to more effectively and directly contribute to forming new bone. Either of these approaches will significantly improve the efficacy of MSC-based tissue engineering treatments in a cost-effective, readily applicable manner
Our results demonstrate that LPA-mediated rescue of human MSC viability in SD/H is indeed dependent on osteogenic differentiation, with cells conditioned in GM requiring the smallest effective dose and cells in OM exhibiting the most adverse reaction to ischemia. Additionally, LPA treatment significantly increased VEGF production in GM and OM+. All of these responses appear to be dependent on LPA1, as determined by treatment with an LPA1/3 inhibitor, coupled with the lack of LPAR3 expression. We also successfully codelivered LPA with MSC in vivo to promote survival of undifferentiated cells and showed that differentiated MSC are more resistant to ischemic cell death. This study provides valuable insight into the considerations necessary to optimize in vivo cell survival in differentiation-mediated tissue engineering applications.
Acknowledgments
The authors would like to thank Allison Hoch for assay development assistance and technical discussion. This project was supported by grants from the National Institutes of Health 1R21AG036963 and the AO Craniomaxillofacial Foundation (C10-39L) to JKL, and the National Institutes of Health R03 AR057547 to DCG. This project was also supported by Award Number T32EB003827 from the National Institute of Biomedical Imaging and Bioengineering (BYB) and the California Institute for Regenerative Medicine UC Davis Stem Cell Training Program (CIRM T1-00006, CIRM TG2-01163).
Disclosure Statement
No competing financial interests exist.
References
- 1.Caplan A.I.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213,341, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Caplan A.I.New era of cell-based orthopedic therapies. Tissue Eng Part B Rev 15,195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peter S.J., Liang C.R., Kim D.J., Widmer M.S., and Mikos A.G.Osteoblastic phenotype of rat marrow stromal cells cultured in the presence of dexamethasone, beta-glycerolphosphate, and L-ascorbic acid. J Cell Biochem 71,55, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Hoch A.I., Binder B.Y., Genetos D.C., and Leach J.K.Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PloS One 7,e35579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu H.H., Zhao L., and Weir M.D.Stem cell-calcium phosphate constructs for bone engineering. J Dental Res 89,1482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang S.W., Lee J.S., Park M.S., Park J.H., and Kim B.S.Enhancement of in vivo bone regeneration efficacy of human mesenchymal stem cells. J Microbiol Biotechnol 18,975, 2008 [PubMed] [Google Scholar]
- 7.Potier E., Ferreira E., Meunier A., Sedel L., Logeart-Avramoglou D., and Petite H.Prolonged hypoxia concomitant with serum deprivation induces massive human mesenchymal stem cell death. Tissue Eng 13,1325, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Das R., Jahr H., van Osch G.J., and Farrell E.The role of hypoxia in bone marrow-derived mesenchymal stem cells: considerations for regenerative medicine approaches. Tissue Eng Part B Rev 16,159, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Baydoun A.R., Xu R., Deng L., Liu X., Zhu W., et al. . Lysophosphatidic acid protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis. Stem Cells 26,135, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Li Z., Wei H., Liu X., Hu S., Cong X., and Chen X.LPA rescues ER stress-associated apoptosis in hypoxia and serum deprivation-stimulated mesenchymal stem cells. J Cell Biochem 111,811, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Sun Y., Nam J.S., Han D.H., Kim N.H., Choi H.K., Lee J.K., et al. . Lysophosphatidic acid induces upregulation of Mcl-1 and protects apoptosis in a PTX-dependent manner in H19-7 cells. Cell Signal 22,484, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Liu X.B., Jiang J., Gui C., Hu X.Y., Xiang M.X., and Wang J.A.Angiopoietin-1 protects mesenchymal stem cells against serum deprivation and hypoxia-induced apoptosis through the PI3K/Akt pathway. Acta Pharmacol Sin 29,815, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Moolenaar W.H.Lysophosphatidic acid, a multifunctional phospholipid messenger. J Biol Chem 270,12949, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Noguchi K., Herr D., Mutoh T., and Chun J.Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol 9,15, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Tigyi G.Aiming drug discovery at lysophosphatidic acid targets. Br J Pharmacol 161,241, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yanagida K., and Ishii S.Non-Edg family LPA receptors: the cutting edge of LPA research. J Biochem 150,223, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kleger A., Liebau S., Lin Q., von Wichert G., and Seufferlein T.The impact of bioactive lipids on cardiovascular development. Stem Cells Int 2011,916180, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teo S.T., Yung Y.C., Herr D.R., and Chun J.Lysophosphatidic acid in vascular development and disease. IUBMB Life 61,791, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu X., Hou J., Shi L., Chen J., Sang J., Hu S., et al. . Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev 18,947, 2009 [DOI] [PubMed] [Google Scholar]
- 20.R&D Systems. Annual Catalog. 2012. www.rndsystems.com/products.aspx
- 21.Le Blanc K., Rasmusson I., Sundberg B., Gotherstrom C., Hassan M., Uzunel M., et al. . Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363,1439, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Viateau V., Guillemin G., Bousson V., Oudina K., Hannouche D., Sedel L., et al. . Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res 25,741, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Schmitt A., van Griensven M., Imhoff A.B., and Buchmann S.Application of stem cells in orthopedics. Stem Cells Int 2012,394962, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenberg A.R., Ouyang L., and Elisseeff J.H.Mesenchymal stem cell stimulation of tissue growth depends on differentiation state. Stem Cells Dev 20,405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarantal A.F., Lee C.C., Batchelder C.A., Christensen J.E., Prater D., and Cherry S.R.Radiolabeling and in vivo imaging of transplanted renal lineages differentiated from human embryonic stem cells in fetal rhesus monkeys. Mol Imaging Biol 14,197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J., Decaris M.L., and Leach J.K.Bioceramic-mediated trophic factor secretion by mesenchymal stem cells enhances in vitro endothelial cell persistence and in vivo angiogenesis. Tissue Eng Part A 18,1520, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decaris M.L., Lee C.I., Yoder M.C., Tarantal A.F., and Leach J.K.Influence of the oxygen microenvironment on the proangiogenic potential of human endothelial colony forming cells. Angiogenesis 12,303, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis H.E., Rao R.R., He J., and Leach J.K.Biomimetic scaffolds fabricated from apatite-coated polymer microspheres. J Biomed Mater Res Part A 90,1021, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Decaris M.L., and Leach J.K.Design of experiments approach to engineer cell-secreted matrices for directing osteogenic differentiation. Ann Biomed Eng 39,1174, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak K.J., and Schmittgen T.D.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25,402, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Sun Q., Silva E.A., Wang A., Fritton J.C., Mooney D.J., Schaffler M.B., et al. . Sustained release of multiple growth factors from injectable polymeric system as a novel therapeutic approach towards angiogenesis. Pharm Res 27,264, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva E.A., Kim E.S., Kong H.J., and Mooney D.J.Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci U S A 105,14347, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis H.E., Binder B.Y., Schaecher P., Yakoobinsky D.D., Bhat A., and Leach J.K.Enhancing osteoconductivity of fibrin gels with apatite-coated polymer microspheres. Tissue Eng Part A 19,1773, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan A.I., and Dennis J.E.Mesenchymal stem cells as trophic mediators. J Cell Biochem 98,1076, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y.B., Kharode Y., Bodine P.V., Yaworsky P.J., Robinson J.A., and Billiard J.LPA induces osteoblast differentiation through interplay of two receptors: LPA1 and LPA4. J Cell Biochem 109,794, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Elmore S.Apoptosis: a review of programmed cell death. Toxicol Pathol 35,495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicholson D.W., and Thornberry N.A.Caspases: killer proteases. Trends Biochem Sci 22,299, 1997 [DOI] [PubMed] [Google Scholar]
- 38.Song I.H., Caplan A.I., and Dennis J.E.Dexamethasone inhibition of confluence-induced apoptosis in human mesenchymal stem cells. J Orthop Res 27,216, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Hung S.C., Pochampally R.R., Hsu S.C., Sanchez C., Chen S.C., Spees J., et al. . Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PloS One 2,e416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burlacu A., Grigorescu G., Rosca A.M., Preda M.B., and Simionescu M.Factors secreted by mesenchymal stem cells and endothelial progenitor cells have complementary effects on angiogenesis in vitro. Stem Cells Dev 22,643, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deveza L., Choi J., Imanbayev G., and Yang F.Paracrine release from nonviral engineered adipose-derived stem cells promotes endothelial cell survival and migration in vitro. Stem Cells Dev 22,483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai Y., Xu M., Wang Y., Pasha Z., Li T., and Ashraf M.HIF-1alpha induced-VEGF overexpression in bone marrow stem cells protects cardiomyocytes against ischemia. J Mol Cell Cardiol 42,1036, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y., Liu W., Wang Y., Chao X., Qu Y., Wang K., et al. . VEGF protects rat cortical neurons from mechanical trauma injury induced apoptosis via the MEK/ERK pathway. Brain Res Bull 86,441, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Fierro F.A., Kalomoiris S., Sondergaard C.S., and Nolta J.A.Effects on proliferation and differentiation of multipotent bone marrow stromal cells engineered to express growth factors for combined cell and gene therapy. Stem Cells 29,1727, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.He J., Genetos D.C., Yellowley C.E., and Leach J.K.Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J Cell Biochem 110,87, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Liu L., Yu Q., Lin J., Lai X., Cao W., Du K., et al. . Hypoxia-inducible factor-1alpha is essential for hypoxia-induced mesenchymal stem cell mobilization into the peripheral blood. Stem Cells Dev 20,1961, 2011 [DOI] [PubMed] [Google Scholar]
- 47.An S.S., Jin H.L., Kim K.N., Kim D.S., Cho J., Liu M.L., et al. . Neuroprotective effect of combined hypoxia-induced VEGF and bone marrow-derived mesenchymal stem cell treatment. Child's Nerv Syst 26,323, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Davis H.E., and Leach J.K.Designing bioactive delivery systems for tissue regeneration. Ann Biomed Eng 39,1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ren B., Hale J., Srikanthan S., and Silverstein R.L.Lysophosphatidic acid suppresses endothelial cell CD36 expression and promotes angiogenesis via a PKD-1-dependent signaling pathway. Blood 117,6036, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]