Abstract
Modular tissue engineering is a method of building vascularized tissue-engineered constructs. Submillimeter-sized collagen pieces (modules) coated with a layer of endothelial cells (EC; vascular component), and with embedded functional cells, are self-assembled into a larger, three-dimensional tissue. In this study, we examined the use of developmental endothelial locus-1 (Del-1), an extracellular matrix protein with proangiogenic properties, as a means of tipping the angiogenic balance in human umbilical vein endothelial cells incorporated in modular tissue-engineered constructs. The motivation was to enhance the vascularization of these constructs upon transplantation in vivo, in this case, without the use of exogenous mesenchymal stromal cells. EC were transduced using a lentiviral construct to overexpress Del-1. The Del-1 EC formed more sprouts in a fibrin gel sprouting assay in vitro compared with eGFP (control) transduced EC, as expected. Del-1 EC had a distinct profile of gene expression (upregulation of matrix metalloproteinase-9 [MMP-9], urokinase-type plasminogen activator [uPA/PLAU], vascular endothelial growth factor [VEGF-A], and intercellular adhesion molecule-1 [ICAM-1]; downregulation of angiopoietin-2 [Ang2]), also supporting the notion of “tipping the angiogenic balance”. On the other hand, contrary to our expectations, when Del-1 EC-coated modules were implanted subcutaneously in a severe combined immunodeficient/beige animal model, the proangiogenic effect of Del-1 was less remarkable. There was only a small increase in the number of blood vessels formed in Del-1 implants compared with the eGFP implants, and only few blood vessels formed at the implant site in both cases. This was presumed due to limited EC survival after transplantation. We speculate that if we could improve EC survival in our study (for example, by adding other prosurvival factors or supporting cells), we would see a greater Del-1-induced angiogenic benefit in vivo as a consequence of increased Del-1 secretion by a higher number of surviving cells.
Introduction
The lack of rapid vascularization of tissue-engineered constructs upon transplantation remains a critical issue in tissue engineering. A vascular supply is required for circulation of nutrients, signaling molecules, gas exchange, and waste removal. In the absence of an internal vascular network that can rapidly connect to the host vasculature, the majority of the transplanted therapeutic cells do not remain viable. In this study, we report a potential means of enhancing the vascularization of tissue-engineered constructs. Our hypothesis was that we could use transduction with developmental endothelial locus-1 (Del-1), an extracellular matrix (ECM) protein shown to have angiogenic properties in vivo, as a means of tipping the angiogenic balance from a quiescent to a proangiogenic phenotype in endothelial cells (EC) incorporated in tissue-engineered constructs.
Del-1 is a matricellular protein containing three EGF-like repeats and two discoidin I-like domains.1 Matricellular proteins are ECM proteins whose primary role is not to provide structural support to the tissue, but to modulate cell behavior by interacting with and coordinating the functions of several biological factors, including cells, cytokines, growth factors, proteases, and other ECM components.2,3 Del-1 was shown to initiate angiogenesis in vivo in the absence of exogenous growth factors in the hindlimb, cerebral, and cardiac ischemia animal models.4–7 In vitro, Del-1 supports the attachment and migration of EC and triggers the expression of the transcription factor HoxD3, which then induces the expression of a number of factors involved in angiogenesis, such as integrin αVβ3 and urokinase-type plasminogen activator (uPA).4 Del-1 also protects EC from apoptosis8 and supports the adhesion, migration, and proliferation of smooth muscle cells.9 Del-1 was also shown to interfere with lymphocyte function-associated molecule-1 (LFA-1)-dependent leukocyte-EC adhesion,10 and thus act as an endogenous inhibitor of inflammation.10–13
To fabricate the tissue-engineered constructs in our study, we used a modular approach. Modular tissue engineering was first introduced by the Sefton group as a method to build larger tissue-engineered constructs with an intrinsically built-in vascular component.14 Submillimeter-sized tissue units (modules) are typically made of collagen type I and EC are seeded on the outer surface of the modules. Vascular supporting cells or therapeutic cells of interest can be embedded inside the modules. Larger tissue structures are formed by mixing together several modules.15 Using this modular approach, our group previously showed that when empty collagen modules coated with human umbilical vein endothelial cells (HUVEC) were implanted subcutaneously in severe combined immunodeficient/beige (SCID/Bg) mice, HUVEC assembled into vascular tube structures in the area between the modules, although the extent of vascularization was limited, due to limited EC survival.16 Adding adipose-derived mesenchymal stromal cells (adMSC) to the modules lead to extensive tissue vascularization beginning at day 7 in SCID/Bg mice with subcutaneous implants using human microvascular EC to coat the modules; at day 21, the vessels were perfuseable by microCT analysis.17 In a Sprague-Dawley rat, drug-immunosuppressed, omental pouch model, allogeneic rat aortic EC-coated modules were able to give rise to a rich vascular network.18 The maturation and functionality of these blood vessels was improved through the addition of bone marrow-derived rat MSC (bmMSC).19
The goal was to explore using Del-1 transduction as an alternative to MSC incorporation. Specifically, this study aimed to (i) produce HUVEC that overexpress Del-1; (ii) characterize in vitro the behavior of the modified HUVEC; and (iii) characterize in vivo the remodeling and the vascular network formed upon implantation of collagen modules coated with Del-1 HUVEC in a SCID/Bg subcutaneous implant model, in the absence of any vascular supporting cells. The effects of Del-1 and such support cells is described in a separate article.20 HUVEC vessel formation has not hitherto been characterized in the same detail as that reported here.
Materials and Methods
Cells
Primary HUVEC (Lonza) were maintained in the EGM-2 cell culture medium (Lonza) at 37°C in a 5% CO2 humidified air atmosphere, with the cell culture medium changed every 2–3 days. HUVEC were stably transduced with HIV-1-based recombinant lentivirus encoding for either eGFP alone (eGFP HUVEC) or Del-1-IRES-eGFP (Del-1 HUVEC), with mouse Del-1 major cDNA, a kind gift from Dr. T. Quertermous, Stanford University. After transduction (multiplicy of infection [MOI] of 5, 24-h incubation with the lentivirus in the EGM-2 supplemented with 8 μg/mL protamine sulfate [Sigma]), transduced HUVEC were maintained in culture and used for experiments up to passage 6. The transduction efficiency was measured by flow cytometry for both eGFP HUVEC and Del-1 HUVEC, and ∼100% of cells were consistently eGFP+ throughout the cell culture period (Beckman Coulter Epics XL cytometer with Expo32 ADC version 1.1C software).
The lentivirus constructs were designed and prepared by Dr. J. Medin's laboratory, University Health Network, Toronto. Briefly, the transfer vector (pDY.Del-1.IRES-eGFP or pDY.eGFP.WS; see Fig. 1), the packaging plasmid (pCMVdeltaR8.91), and the envelope plasmid (pMDG), were first mixed together with polyethylenimine (Sigma-Aldrich). Next, 293T cells (cultured in DMEM [Sigma] supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin [Sigma]) were incubated with the plasmid mixture for 16–18 h at 37°C and 5% CO2 atmosphere. At the end of the incubation period, the medium was replaced with a fresh cell culture medium. The supernatant was collected 24 and 48 h after the medium change, filtered (0.45-μm filter), concentrated by ultracentrifugation, resuspended in the DMEM, and stored at −80°C. The viral titer was determined by transduction of 293T cells.
FIG. 1.
Maps of transfer vectors included in lentiviral constructs: (A) vector containing Del1-IRES-eGFP sequence; (B) vector containing eGFP-alone sequence, used as control for experiments. Color images available online at www.liebertpub.com/tea
Sprouting assay
As adapted from,21,22 Cytodex3™ beads (gelatin-coated microcarriers; GE Healthcare) were coated with EC (0.6×106 HUVEC/103 beads in EGM-2 complete medium) by gentle shaking every 20 min for 4 h at 37°C, followed by overnight incubation. The beads were resuspended in a fibrin gel the next day. To prepare the fibrin gel, a fibrinogen solution (Sigma; 2 mg/mL dissolved in saline solution) was first mixed with aprotinin (Sigma; 0.15 U/mL). Next, the beads coated with HUVEC were resuspended in the fibrinogen solution (∼400 beads/mL fibrinogen solution). The beads and fibrinogen were then mixed with thrombin (Sigma; 0.625 U/mL) in the wells of a 24-well plate and allowed to form a clot at 37°C for 20 min. The EC culture medium was added to the wells after the clot was formed and changed periodically, as per standard cell culture protocols. Sprout formation was monitored over 1 week (day 1, 4, and 7). Pictures of the beads were taken using a Zeiss Axiovert light microscope with a 5× objective lens and equipped with a CCD camera (N=3; 10 beads/condition at each time point). The sprouts were counted and measured manually using ImageJ software (ImageJ 1.45; NIH).
Quantitative reverse transcription polymerase chain reaction
HUVEC (Del-1 or eGFP) were plated on thin collagen gels (type-I bovine dermal collagen solution; PureCol, Inamed Biomaterials; 3.1 mg/mL) or on tissue culture-treated polystyrene (TCPS) plates (5×103 cells/cm2). The collagen gels were prepared by adding a thin layer of neutralized endotoxin-free collagen solution to a nontissue culture-treated cell culture dish (30 μL/cm2) and allowing the collagen to gel for 1 h at 37°C before seeding the cells. After 7 days in culture, the cells were lysed and the RNA was isolated using the Qiagen RNeasy® Fibrous Tissue Mini Kit (Qiagen) according to the manufacturer's instructions. The concentration and purity of the RNA were measured using a NanoDrop Spectrophotometer (ND1000; Thermo Scientific). The 260/280 ratio was greater than 2.0 for all samples. cDNA synthesis from RNA was performed using the SuperScript™ III First-Strand Synthesis kit (Invitrogen) according to the manufacturer's instructions, with both random and oligodT primers. The Applied Biosystems 7900HT Real-Time PCR system with the SDS 2.3 software (Applied Biosystems) was used for fluorescence detection during polymerase chain reaction (PCR) (SYBR® Green chemistry). Gene-specific primers (Table 1) were designed using either Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast) or PrimerBank (http://pga.mgh.harvard.edu/primerbank/) and synthesized by Sigma Genosys. The data analysis was performed as described elsewhere.23 To perform the analysis, the individual threshold cycle values (Ct) and the mean amplicon efficiencies (E) were estimated using the SDS 2.3 software and the LinRegPCR software (LinRegPCR version 12; Heart Failure Research Center), respectively. GAPDH was used as the reference gene for relative quantification. The normalized relative quantity (NRQ) of each gene of interest relative to the endogenous reference gene (GAPDH) was first calculated for each sample using the following formula:
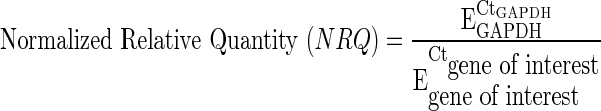 |
Table 1.
Primer Sequences for qRT-PCR
| Probe | Forward (F) and reverse (R) primers | GenBank accession |
|---|---|---|
| GAPDH | F: AAATTGAGCCCGCAGCCTCCC | NM_002046.3 |
| R: TGACCAGGCGCCCAATACGAC | ||
| Edil3 | F: GGTTTACTGCTGTCCTGCAGGCG | AF031524.1 |
| R: CAACCAAAAGCCAGGCTGCTACC | ||
| Ang2 | F: CAAACTCAGCTAAGGACCCCACTGT | NM_001147.2 |
| R: CGCTGCCATCCTCACGTCGC | ||
| FGF2 | F: ATCAAAGGAGTGTGTGCTAACC | NM_002006 |
| R: ACTGCCCAGTTCGTTTCAGTG | ||
| CXCL12 | F: ATGCCCATGCCGATTCTTCG | NM_000609 |
| R: GCCGGGCTACAATCTGAAGG | ||
| PDGF-B | F: TCTCTGCTGCTACCTGCGT | NM_002608.2 |
| R: CAAAGGAGCGGATCGAGTGG | ||
| VEGF-A | F: CAACATCACCATGCAGATTATGC | NM_003376 |
| R: GCTTTCGTTTTTGCCCCTTTC | ||
| HoxD3 | F: AGAGTCTCGACAGAACTCCAAG | NM_006898 |
| R: GCGTTCCGTGAGATTCAGC | ||
| ITGB3 | F: ATACCTGGCCCTGTGCCTTGGT | NM_000212.2 |
| R: AGGCCACACGTGCTGATACAACTG | ||
| ICAM-1 | F: TTGAACCCCACAGTCACCTAT | NM_000201 |
| R: CCTCTGGCTTCGTCAGAATCA | ||
| PLAU | F: AGCGACTCCAAAGGCAGCAATGAA | NM_002658.3 |
| R: TTCTTTGGGCAGTTGCACCAGTGA | ||
| MMP2 | F: GGAAAGCCAGGATCCATTTT | NM_004530 |
| R: ATGCCGCCTTTAACTGGAG | ||
| MMP9 | F: AGACGGGTATCCCTTCGACG | NM_004994 |
| R: AAACCGAGTTGGAACCACGAC | ||
| MMP14 | F: GAAGCCTGGCTACAGCAATATG | NM_004995 |
| R: TGCAAGCCGTAAAACTTCTGC |
The relative expression ratio for each gene of interest was then calculated as the ratio between the mean NRQ for Del-1 HUVEC samples and the mean NRQ for eGFP HUVEC samples for that gene of interest:
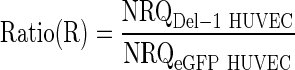 |
Results were presented as the relative expression ratio±standard error of the mean, N=3, for each gene of interest. A t-test (paired, one tailed) statistical analysis was performed on the log transformed NRQ to determine if there are any statistical differences between paired Del-1 HUVEC and eGFP HUVEC samples. Differences were considered significant when 0.67≤R≥1.5 and p<0.05. In some cases, as noted in the text p<0.10.
Implants
Submillimeter-sized collagen cylinders (modules, initial size ∼2 mm long and 0.6 mm in diameter) were prepared by neutralizing and gelling a type-I bovine dermal collagen solution (PureCol, 3.1 mg/mL; Inamed Biomaterials) inside polyethylene tubing (Intramedic™ PE60; Becton Dickson), followed by cutting of the tubing into small pieces with an automatic tube cutter (FCS Technology, Inc.), and vortexing of the cut tubing to separate the gelled collagen modules from the polyethylene tubing, as described elsewhere.15 All the collagen modules obtained after gelling of ∼1.5 mL collagen solution inside 3 m of PE60 polyethylene tubing completely filled with the collagen solution were collected in a 15-mL tube and seeded with either Del-1 HUVEC or eGFP HUVEC overnight (∼4×106 HUVEC [one confluent T75 tissue culture flask]). The next day, ∼0.1 mL of HUVEC-contracted, settled modules (i.e., all modules available from one pack of 3 m tubing; size of individual contracted modules: ∼0.4 mm long and 0.3 mm in diameter ellipsoids), and 0.5 mL of PBS were injected subcutaneously, through an 18-gauge needle, in the dorsum of SCID/Bg mice (6–7 weeks of age, male, N=4; Charles River Laboratories), similar to.17 After surgery, mice were individually housed in sterile cages and provided free access to sterilized food and water under the approval of the University of Toronto animal care committee.
Immunohistochemistry
In vivo samples
The modules were explanted 7, 14, and 21 days after the implant, fixed in 10% neutral buffered formalin, and processed for immunohistochemistry. Paraffin-embedded, 4-μm-thick sections were analyzed using the following stains and antibodies: hematoxylin and eosin (H&E), Masson Trichrome, anti-GFP (Abcam; #AB6556, 1:1000, rabbit polyclonal antibody, detects (e)GFP expressing cells), Biotinylated Ulex Europaeus Agglutinin I (UEA I; Vector Laboratories; #B1065, 1:400, binds to human EC), anti-CD31 (Santa Cruz Biotechnology, Inc.; #SC-1506-R, 1:2000, rabbit polyclonal antibody, detects both mouse and human CD31), and anti-α smooth muscle actin (SMA) (Sigma; #A5228, 1:200, mouse monoclonal antibody, detects both mouse and human αSMA).
In vivo blood vessel counts: The number of donor-derived (GFP+) blood vessels (with defined lumen), as well as the total number of blood vessels (donor+host, CD31+) present at the implant site were counted manually using digitized histology slides (ScanScope XT brightfield scanner, 20× objective lens; Aperio Technologies) and the Aperio ImageScope viewing software (Aperio Technologies; version 11). The number of vessels in the implant area was normalized to the area occupied by the implant on the whole histological section to obtain the vessel density at the implant site (N=4). The diameter of the GFP+ and CD31+ vessels was manually measured using the Aperio ImageScope viewing software. Vessels were binned based on their size, with capillaries <9 μm, small arterioles or venules 9–15 μm, large arterioles or venules 15–75 μm, and others (abnormal) ≥75 μm, similarly to.19 Digitized SMA-stained histology slides were analyzed using the Positive Pixel Count Algorithm available with the Aperio ImageScope software to determine the SMA density at the implant site (N=4).
In vitro samples
Some modules were collected immediately after coating with HUVEC (N=4) or were cultured in vitro for an additional 7 days under standard cell culture conditions (N=4). Samples were collected and fixed in 4% paraformaldehyde (Electron Microscopy Sciences), embedded in a 2% agarose block (Roche Diagnostics), processed for histology (paraffin sections), and stained with an anti-Ki67 antibody (Novus Biologicals; #NB110-90592, 1:1000, rabbit polyclonal antibody) to detect proliferating cells. Some modules were also kept and cultured in vitro for 7 days in a complete medium, and then cultured for another 7 days in a serum-free medium to induce apoptosis (N=4). Modules were then collected and fixed in 4% paraformaldehyde, embedded in a 2% agarose block, processed for histology (paraffin sections), and stained with an anti-cleaved caspase 3 antibody (Cell Signaling Technology; #9661, 1:600, rabbit polyclonal antibody) to detect apoptotic cells.
Cell counts for in vitro samples: Proliferating cells (Ki67+) and apoptotic cells (cleaved caspase 3+) were counted on histology slides prepared from in vitro samples. An Olympus BX61 light microscope with a 20× objective lens and equipped with an Olympus DP70 camera was used to take up to five pictures (hot spots) per sample (N=4, n≤5). ImageJ software (ImageJ 1.45; NIH) was used to analyze the images (manual counting) and the average of the counts/sample was used for statistical analysis.
Statistical analysis
A one-way analysis of variance with LSD post hoc was used to compare means between multiple groups, unless otherwise noted. Differences between means were considered statistically significant at p<0.05. Statistical analysis was performed with the SPSS Statistics software (IBM Corp.; version 20).
Results
Delivery of Del-1 through lentiviral transduction
The maps of the transfer vectors included in the lentiviral constructs are shown in Figure 1. The eGFP sequence was included in both Del-1 and control eGFP vectors to enable tracking of implanted HUVEC in vivo.
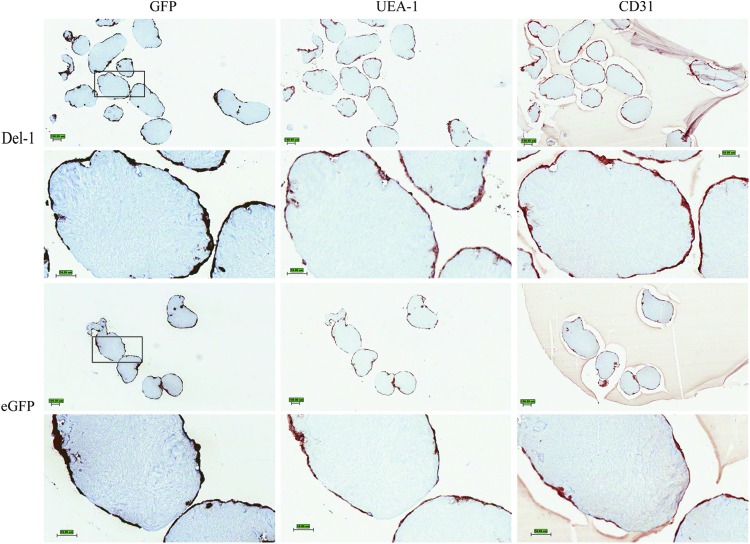
Modules coated with transduced HUVEC were analyzed by immunohistochemistry immediately after fabrication to confirm eGFP expression by both Del-1 HUVEC and eGFP HUVEC and also to confirm good coverage of the modules with the HUVEC before transplantation (Fig. 2). Qualitatively, the histology images indicated that all the EC (UEA-1+, CD31+) also expressed eGFP (GFP+). Additionally, good cell coverage was observed for both Del-1 HUVEC and eGFP HUVEC; thus, an equally high number of cells were transplanted in both cases.
FIG. 2.
Representative histology images of modules in vitro immediately after fabrication. The samples were serially cut; the same modules are seen with the different histology stains. All the EC (UEA-1+, CD31+) appeared to express eGFP (GFP+). Good EC coverage of the modules was observed for both Del-1 and eGFP samples. Scale bar is 100 μm for lower magnification images and 50 μm for higher magnification (black squares indicate areas that are shown in higher magnification images). Each oval structure in the high-magnification images is one module. Del-1, developmental endothelial locus-1; EC, endothelial cells. Color images available online at www.liebertpub.com/tea
Sprouting assay
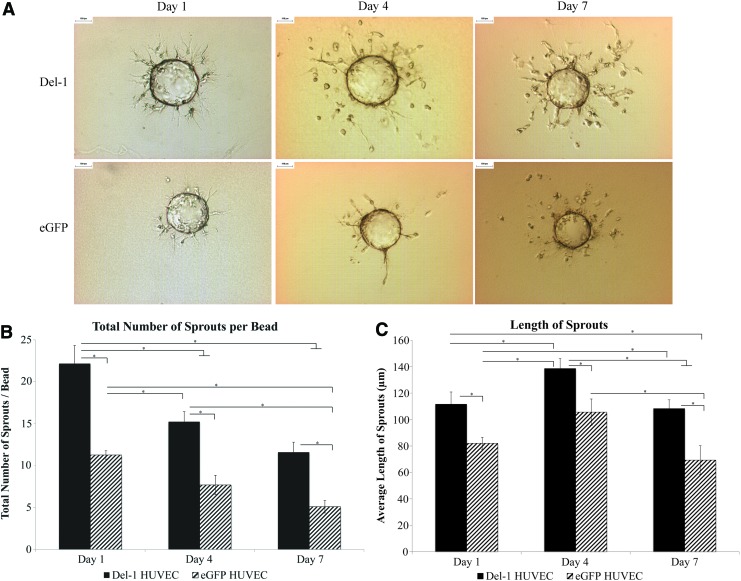
Del-1 HUVEC formed significantly more sprouts (p<0.05) compared with eGFP HUVEC (Fig. 3B) at all time points. The sprouts formed by the Del-1 HUVEC were also significantly (p<0.05) longer than eGFP HUVEC for all time points (Fig. 3C). The initial elongation from day 1 to 4 was followed by regression of the sprouts between day 4 and 7 (decrease in both the number and length of sprouts) for both Del-1 HUVEC and eGFP HUVEC. This is expected for EC, especially in the absence of supporting cells that delay sprout disintegration in vitro.21,24
FIG. 3.
Cytodex3™ bead fibrin gel sprouting assay. (A) Representative images of sprouts. Scale bar=100 μm. (B) Del-1 HUVEC formed more sprouts compared with eGFP HUVEC as early as day 1, and more sprouts were apparent for Del-1 HUVEC for at least 7 days in culture. (C) The length of the sprouts was also greater for Del-1 HUVEC compared with eGFP HUVEC for all time points. Sprouts were measured for 10 beads per condition. Graphs show average±SEM; N=3 (n=10); ANOVA with LSD post hoc and p<0.05 (*) considered significant. ANOVA, analysis of variance; HUVEC, human umbilical vein endothelial cells; SEM, standard error of the mean. Color images available online at www.liebertpub.com/tea
qRT-PCR
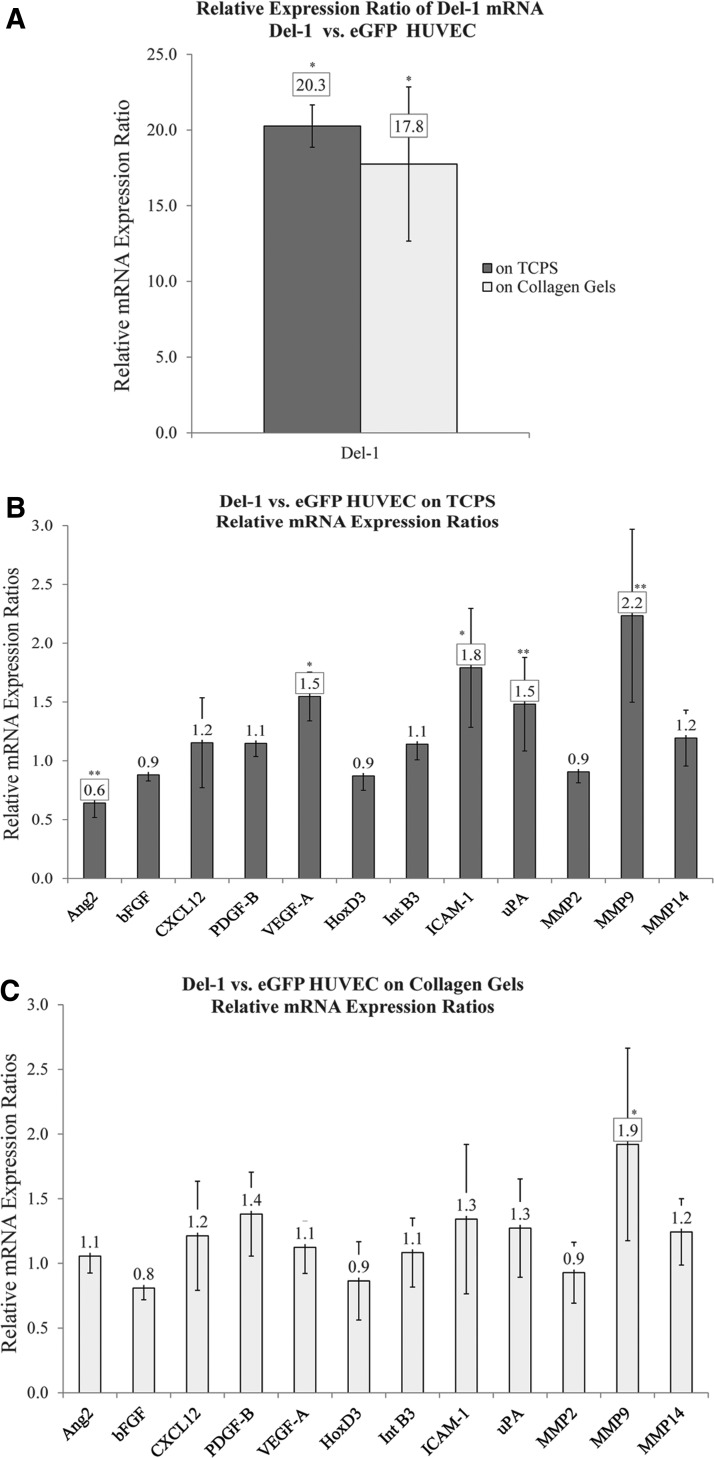
qRT-PCR data confirmed (Edil3) expression by cells transduced with the integrating Del-1 lentivirus. Del-1 HUVEC expressed 20 or 18 times more Del-1 on average than eGFP HUVEC (p<0.05) when cultured on TCPS or on collagen gels, respectively (Fig. 4A).
FIG. 4.
Expression of genes involved in angiogenesis in Del-1 HUVEC versus eGFP HUVEC. (A) Del-1 HUVEC expressed much higher levels of Del-1 mRNA compared with eGFP HUVEC. (B) When cultured on TCPS for 7 days, Del-1 HUVEC differentially expressed Ang2, VEGF-A, ICAM-1, PLAU, and MMP-9 compared with eGFP HUVEC. Differences were small, but statistically significant (at least at p<0.1). (C) When cultured on collagen, MMP-9 was upregulated in Del-1 HUVEC compared with eGFP HUVEC. Data are presented as ratios of relative gene expression (Del-1 HUVEC vs. eGFP HUVEC, relative to GAPDH). Graphs show average of ratios±SEM; N=3; paired t-test one tailed with p<0.05 (*) or p<0.10 (**) and 0.67≤Ratio ≥1.5 highlighted. Ang2, angiopoietin-2; ICAM-1, intercellular adhesion molecule-1; MMP-9, matrix metalloproteinase-9; TCPS, tissue culture-treated polystyrene; uPA, urokinase-type plasminogen activator; VEGF-A, vascular endothelial growth factor.
qRT-PCR data also showed that Del-1 HUVEC had a distinct expression profile of genes involved in angiogenesis compared with eGFP HUVEC, particularly when cells were cultured on TCPS. Differences in gene expression were small, but statistically significant (at least at p<0.1). Specifically, when cultured on TCPS for 7 days, Del-1 HUVEC differentially expressed angiopoietin-2 (Ang2), vascular endothelial growth factor (VEGF-A), intercellular adhesion molecule-1 (ICAM-1), PLAU, and matrix metalloproteinase-9 (MMP-9) compared with eGFP HUVEC (Fig. 4B). When cultured on collagen, MMP-9 was upregulated in Del-1 HUVEC compared with eGFP HUVEC (Fig. 4C). For the rest of the genes analyzed, including HOXD3 and integrin β3 (INTB3), there was no significant difference.
In vitro proliferation and apoptosis
As expected from the literature,8 we did not observe any statistically significant difference in cell proliferation (Ki67+ cells) for Del-1 HUVEC versus eGFP HUVEC, both initially after seeding the cells onto the collagen modules (day 0), as well as after culturing the EC-coated modules for 7 days in vitro (day 7). We observed a higher number of proliferating cells at day 0 (about 20–25 per field) compared with day 7 (<5 per field) for both Del-1 HUVEC and eGFP HUVEC.
We also did not observe any statistically significant difference in the number of apoptotic cells between Del-1 HUVEC and eGFP HUVEC, when HUVEC were cultured under serum-free conditions on collagen modules, although there were slightly more (1.2×) apoptotic cells for the Del-1 samples (about 40 caspase 3-positive cells per field in the absence of serum, relative to about 20 with serum for Del-1).
In vivo vascularization and remodeling
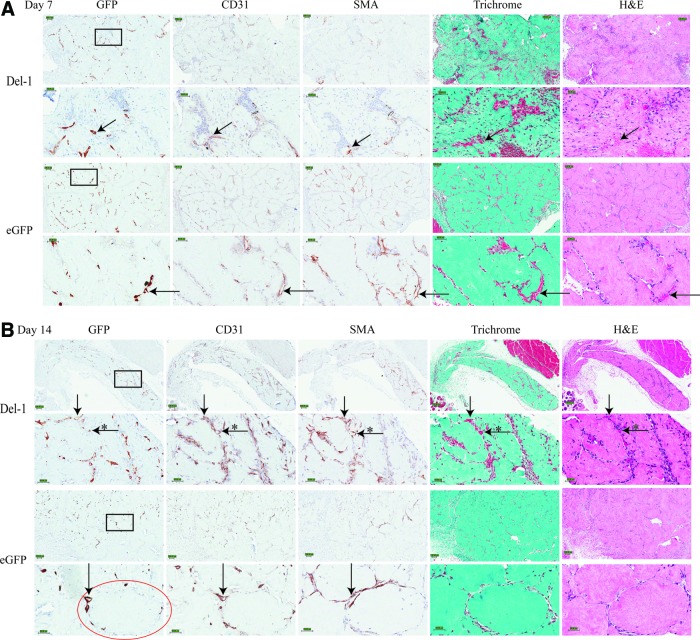
Representative images of the visual appearance of the tissues at explant are shown in Figure 5, with some blood vessels visible (red color) inside the implant area. Figure 6 shows various histological stains of the implants at day 7, 14, and 21. The GFP+ cells are the implanted HUVEC (either Del-1 HUVEC or eGFP HUVEC). Both Del-1 HUVEC and eGFP HUVEC can be seen in the area surrounding the modules, forming EC-lined tubular structures (donor-derived blood vessels) as early as day 7 and persisting for at least 21 days. The CD31 images identify all blood vessels, both donor derived and host derived. By visually comparing the serially cut GFP images to the CD31 images, we found that while the majority of blood vessels at the implant site were donor derived (both CD31+ and GFP+), there were also a few host-derived blood vessels (CD31+, but GFP−). The host-derived blood vessels were found in most cases in the area close to the edge of the implant, at the border with the host tissue.
FIG. 5.
Representative photographs of tissues at explant. Some blood vessels are visible within the implant area. Arrows indicate the location of implants. Insets focus on the implants. Color images available online at www.liebertpub.com/tea
FIG. 6.
Representative histology images of serially cut tissue samples at (A) Day 7; (B) Day 14; (C) Day 21 after implantation. Implanted HUVEC migrated off the surface of the modules and formed blood vessels in the area between the modules (GFP+ for donor-derived vessels; CD31+ for donor- OR host-derived EC-lined blood vessels). Examples of donor- or host-derived blood vessels are indicated with arrows; examples of host-derived vessels are indicated with (*). Examples of smooth muscle cell invested vessels (SMA+) are indicated with arrows; erythrocyte-containing vessels are also indicated with arrows. Insets show details of inside space of vessels. Scale bar is 100 μm for lower magnification images and 50 μm for higher magnification. Black squares indicate areas that are shown in higher magnification images. Circle highlights one individual module. SMA, smooth muscle actin. Color images available online at www.liebertpub.com/tea
We also investigated the recruitment of host pericytes and smooth muscle-positive (SMA+) cells, some of which were likely myofibroblasts, around the newly formed endothelial-lined tubes. We found blood vessels invested with an SMA layer as early as day 7 and up to day 21; nevertheless, the overall SMA staining at the implant site was rather limited, as the number of blood vessels present was also relatively low. Trichrome and H&E histology images show the presence of red blood cells in some of these blood vessels as early as day 7, suggestive of a connection to the host vasculature and perfusion (Fig. 6).
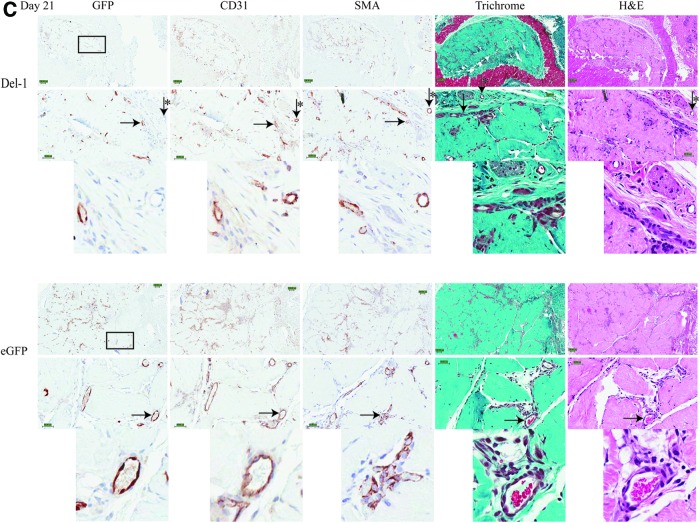
The average density of both donor-derived (GFP+) and total number of blood vessels (CD31+) was slightly higher for Del-1 implants compared with eGFP implants at both day 7 and 14 (Fig. 7A, B), although these differences were small. Both the number of donor-derived, as well as the total number of blood vessels remained relatively constant over time (Fig. 7A, B). SMA staining was also quantified (Fig. 7C). The average size of the blood vessels (host or donor) at the implant site did not change over time (all around 10–12 μm in diameter for donor-derived vessels, and all around 12–14 μm for all vessels [donor and host]) and was similar between Del-1 implants and eGFP implants. Most of the vessels were categorized as capillaries or small arterioles or venules.
FIG. 7.
Density of blood vessels and SMA staining at the implant site. Transplantation of Del-1 HUVEC (black bars) led to a slightly higher number of donor-derived blood vessels (GFP+; A), as well as total number of blood vessels (donor+host, CD31+; B) and SMA staining (C) compared with eGFP HUVEC (patterned bars). The number of blood vessels remained relatively constant over time; the differences were small. All vessels (with defined lumen) were counted and normalized to the area occupied by the implant on the whole histological section. The Aperio ImageScope Positive Pixel Count Algorithm was used for SMA staining. Graphs show average±SEM; N=4; ANOVA with LSD post hoc and p<0.05 (*) considered significant.
Discussion
Increased sprouting and differential angiogenic gene expression for Del-1 HUVEC
Del-1 was clearly expressed more after transduction at the mRNA level. Our data here and in the follow-on article20 demonstrate that some (but not all) of the expected biological effects were observed. However, we were unable to find an antibody for Del-1 so that we were unable to analyze for the protein directly. Del-1 protein is apparently sticky and hard to purify (T. Quatermous, personal communication). We were also unsuccessful in removing the EC from the culture dish, while preserving the ECM and so conducting a functional assay on the EC-secreted matrix. Hence, we cannot exclude the possibility that the effects that we observed were in fact due to intracellular Del-1-mediated proangiogenic signaling (if Del-1 was indeed not secreted by the EC), rather than due to signaling triggered by integrin binding to Del-1 after Del-1 deposition in the ECM, as established in all previous literature reports. However, to our knowledge, there are no reports in the literature indicating any biological effects of intracellular Del-1.
Some in vitro results (sprouting, mRNA) were consistent with the expectation that Del-1 can enhance the angiogenic state of transduced HUVEC. We speculate that the increased sprouting for Del-1 HUVEC may be, at least in part, a consequence of increased protease activity (MMPs, uPA, etc.). One of the steps involved in the sprouting of EC from the surface of the beads is the degradation of the surrounding ECM by the cells to form vascular guidance channels in the matrix, and several literature reports showed the involvement of both MMPs and uPA in sprout formation in fibrin gels.24–26 Our qRT-PCR showed that Del-1 HUVEC expressed more MMP-9 and PLAU compared with eGFP HUVEC, although measured on collagen gels and TCPS, not on fibrin, and with the differences significant only at p<0.1 in some cases. The upregulation of these proteases in Del-1 HUVEC is consistent with a more invasive and proangiogenic state for Del-1 HUVEC compared with eGFP HUVEC. The role of these proteases in angiogenesis is reviewed elsewhere.27–29
Other than the increased expression of MMP-9 and PLAU mRNA, we observed a distinct pattern of gene expression for Del-1 HUVEC versus eGFP HUVEC for other factors involved in angiogenesis as well. For example, VEGF-A was upregulated (and Ang2 was downregulated slightly) in Del-1 HUVEC compared with eGFP HUVEC when cells were cultured on TCPS. VEGF is a potent mitogen and prosurvival factor for EC, it stimulates sprout formation in in vitro models, and it stimulates angiogenesis in vivo.30–32 Ang2 is upregulated by hypoxia, at sites of vascular remodeling, or under pathological conditions (for example, tumor growth), and it is involved in both vascularization and vessel regression, depending on the context (as reviewed by Thomas and Augustin33).
ICAM-1 was also upregulated in Del-1 HUVEC compared with eGFP HUVEC (on TCPS). Physiologically, ICAM-1 is expressed at low basal levels on the surface of EC and it is upregulated on the activated endothelium, such as during the inflammation process associated with angiogenesis. The ICAM-1 upregulation may therefore confirm the enhanced angiogenic state of Del-1 HUVEC relative to eGFP HUVEC. The upregulation of ICAM-1 perhaps also suggests a compensatory mechanism triggered in Del-1 transduced cells, as Del-1 was shown to inhibit leukocyte-EC binding through its antagonizing interaction with the LFA-1,10 the main leukocyte ligand of ICAM-1.34
In contrast with the above data in support of “tilting the balance,” there were other data that did not support this notion. In our hands, Del-1 transduced HUVEC did not show upregulation of the proangiogenic transcription factor HoxD3 or the integrin αVβ3 compared with eGFP HUVEC, contrary to literature reports.4,35 We speculate that the differences with the literature might be due to the intrinsic experimental differences between the means of exposure of cells to Del-1, specifically cell transduction in our case versus an acute mode of exposure in the literature reports with high amounts of Del-1. RT-PCR literature reports4 were based on characterizing EC plated on basement membrane ECM and with the Del-1 protein added to the culture medium (10 μg/mL Del-1 protein) for 24 h. In our experimental model, the cells continuously produced the Del-1 during 7 days in culture and the amount of Del-1 was also likely much less than 10 μg/mL in our case.
No difference in proliferation or antiapoptotic effect in vitro
Our in vitro proliferation results are consistent with literature reports showing that Del-1 did not affect proliferation of EC.8 The decrease in proliferation over time was also expected, as HUVEC on collagen modules form a confluent, quiescent cell layer on the surface of the collagen modules after a few days of culture in vitro.14,36,37 The apoptosis results, however, contradicted a literature report where HUVEC plated on Del-1-coated plates were protected from apoptosis relative to bovine serum albumin (BSA)-coated plates, an effect mediated by integrin binding and adhesion to Del-1.8 We suspect that in contrast to Del-1, the BSA surfaces did not provide strong attachment of HUVEC, and thereby BSA surfaces facilitated apoptosis (or anoikis). In this study, HUVEC were seeded onto collagen modules, thus having integrin binding in both cases (although perhaps through a different combination of integrins).
In vivo vascularization
Some blood vessels formed in the implant area, and the presence of red blood cells inside the lumen of some of these blood vessels suggests they were perfused, although these blood vessels also had limited SMA+ coverage, and it is likely that they failed to fully mature. Although the in vitro results showed a clear Del-1-induced effect on sprouting (Fig. 3), the in vivo angiogenic benefits of Del-1 in our system were less dramatic; we observed only a small increase in the number of blood vessels formed in Del-1 implants compared with the eGFP implants. We suspect that a limited number of HUVEC survived after transplantation, and as a consequence, a limited amount of Del-1 was also produced at the implant site by the remaining surviving HUVEC.
HUVEC are susceptible to undergo apoptosis upon transplantation even in immunocompromised animal models such as SCID/Bg mice,16,38 presumably triggered by the hostile inflammatory response, as well as by the hypoxic, nutrient-poor environment. Others have improved the outcome of transplantation by using genetic manipulation with antiapoptotic genes (transduction of EC with Bcl-2 in38,39), by changing the composition of the ECM to improve HUVEC survival (added fibronectin16), or by cotransplantation with supporting cells (bmMSC or adMSC17,19). In this first in vivo study using Del-1 transduced EC, we did not use any supporting cells or any additional prosurvival factors other than Del-1, testing whether the literature reported properties of Del-1 are sufficient to compensate for the absence of MSC.
We speculated that if we could improve HUVEC survival in our study (for example, by adding other prosurvival factors or supporting cells, such as adMSC17), we would see a greater Del-1-induced angiogenic benefit in vivo as a consequence of increased Del-1 secretion by a higher number of surviving cells. Indeed, in a follow-up article, we describe the substantive vascularization benefit of using Del-1 transduced HUVEC.20
In vivo remodeling
EC-coated module implants remodel upon implantation to form the observed vasculature. This process depends on a variety of factors, including the host animal and the nature of the transplanted EC. In this study, transduction with Del-1 had a limited effect on remodeling (unlike the subsequent study20), yet other aspects of this process are worthy of comment.
In addition to the HUVEC-lined (donor-derived) blood vessels, we also found host-derived vessels, mostly present at the periphery of the implant site and presumably supplying the donor-derived vasculature. Indirect evidence of connection of the donor-derived vasculature to the host vasculature is provided by the presence of red blood cells inside the lumen of HUVEC-lined vessels as early as day 7. Others (using other EC systems38–43) have also seen the presence of host-derived blood vessels in the implant area, mostly restricted to the edge of the implant.
Platform for future manipulations
The modular approach, along with lentiviral transduction of EC with proangiogenic molecules, can serve as a versatile platform for future studies. First of all, transduction of EC with various molecules of interest is a highly adaptable in vivo delivery method, where cells act not only as drivers of in vivo tissue reconstruction, but also as delivery vehicles. Second, the modular approach is intrinsically versatile, as one can envision that modules coated with EC transduced to overexpress various molecules of interest can be mixed together in controlled ratios to elicit a desired outcome in vivo. Alternatively, the same strategy can be used as a screening tool to better understand in vivo responses to various combinations of stimuli and better inform the design of tissue-engineered constructs.
Both preclinical and clinical studies with lentiviral systems have shown that their use as gene delivery systems was safe and did not lead to tumorigenesis following viral insertion into the host genome.44–46 In fact, several mutations are believed to be necessary to eventually lead to oncogenesis, which suggests that the risk of occurrence of disease due to insertional mutagenesis in several wrong spots exists, but is low.47 Moreover, the lentiviral delivery system could be further manipulated to generate transient instead of permanent expression of certain molecules by using nonintegrating instead of integrating lentiviruses.
We propose that our system can be further improved in vivo by using a combination of proangiogenic and prosurvival signals rather than using Del-1 alone and these signals may be a combination of ECM factors and growth factors. The cross talk and synergy between integrins and growth factor receptors in angiogenesis is well recognized and is the subject of several reviews.48–50 For example, cross activation between the β3 subunit of integrin αVβ3 and vascular endothelial growth factor receptor 2 (VEGFR2) in EC has been described.51 Careful selection of combinations of ECM molecules (to bind specific integrins) and growth factors (to bind specific growth factor receptors) may lead to increased vascularization of tissue-engineered constructs by comparison to delivery of growth factors alone or ECM molecules alone.
Conclusion
In this study, we used transduction with the proangiogenic ECM protein Del-1 as a means of tipping the angiogenic balance in EC incorporated in modular tissue-engineered constructs. We showed that HUVEC transduced to overexpress Del-1 have increased sprouting activity and a distinct angiogenic gene expression profile compared with eGFP HUVEC in vitro, although the proangiogenic effect of Del-1 in vivo was less remarkable.
Acknowledgments
We thank C. Lo for performing the animal surgeries. We also thank O. Lopez-Perez, B. Au and Dr. J. Medin at the University Health Network, Toronto for generating the lentiviruses used in this study. We are also grateful to Dr. T. Quertermous, Stanford University, for supplying the Del-1 cDNA. The Toronto General Hospital Pathology Research Program performed all the immunohistochemistry staining. E. Ciucurel was a recipient of the Fonds Québécois de la Recherche sur la Nature et les Technologies doctoral scholarship. This research was funded by the Canadian Institutes of Health Team Grant 111624.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hidai C., Zupancic T., Penta K., Mikhail A., Kawana M., Quertermous E.E., et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alpha vbeta 3 integrin receptor. Genes Dev 12,21, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein P., and Sage E.H.Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14,608, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Bornstein P.Matricellular proteins: an overview. J Cell Commun Signal 3,163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhong J.Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein del-1. J Clin Invest 112,30, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho H.K., Jang J.J., Kaji S., Spektor G., Fong A., Yang P., et al. Developmental endothelial locus-1 (del-1), a novel angiogenic protein: its role in ischemia. Circulation 109,1314, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Fan Y., Zhu W., Yang M., Zhu Y., Shen F., Hao Q., et al. Del-1 gene transfer induces cerebral angiogenesis in mice. Brain Res 1219,1, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kown M.H., Suzuki T., Koransky M.L., Penta K., Sakamoto G., Jahncke C.L., et al. Comparison of developmental endothelial locus-1 angiogenic factor with vascular endothelial growth factor in a porcine model of cardiac ischemia. Ann Thorac Surg 76,1246, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Kundu R.K., Longaker M.T., Quertermous T., and Yang G.P.The angiogenic factor del1 prevents apoptosis of endothelial cells through integrin binding. Surgery 151,296, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Rezaee M., Penta K., and Quertermous T.Del1 mediates vsmc adhesion, migration, and proliferation through interaction with integrin alpha(v)beta(3). Am J Physiol Heart Circ Physiol 282,H1924, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Choi E.Y., Chavakis E., Czabanka M.A., Langer H.F., Fraemohs L., Economopoulou M., et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science 322,1101, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eskan M.A., Jotwani R., Abe T., Chmelar J., Lim J.H., Liang S., et al. The leukocyte integrin antagonist del-1 inhibits il-17-mediated inflammatory bone loss. Nat Immunol 13,465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavakis E., Choi E.Y., and Chavakis T.Novel aspects in the regulation of the leukocyte adhesion cascade. Thromb Haemost 102,191, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi E.Y.Inhibition of leukocyte adhesion by developmental endothelial locus-1 (del-1). Immune Netw 9,153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuigan A.P., and Sefton M.V.Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A 103,11461, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuigan A.P., Leung B., and Sefton M.V.Fabrication of cell-containing gel modules to assemble modular tissue-engineered constructs [corrected]. Nat Protoc 1,2963, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Cooper T.P., and Sefton M.V.Fibronectin coating of collagen modules increases in vivo huvec survival and vessel formation in scid mice. Acta Biomater 7,1072, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler M.J., and Sefton M.V.Cotransplantation of adipose-derived mesenchymal stromal cells and endothelial cells in a modular construct drives vascularization in scid/bg mice. Tissue Eng Part A 18,1628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain M.D., Gupta R., and Sefton M.V.Chimeric vessel tissue engineering driven by endothelialized modules in immunosuppressed sprague-dawley rats. Tissue Eng Part A 17,151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chamberlain M.D., Gupta R., and Sefton M.V.Bone marrow-derived mesenchymal stromal cells enhance chimeric vessel development driven by endothelial cell-coated microtissues. Tissue Eng Part A 18,285, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciucurel E.C., and Sefton M.V.Del-1 overexpression in endothelial cells increases vascular density in tissue engineered implants containing endothelial cells and adipose-derived mesenchymal stromal cells. Tissue Eng Part A; [DOI: 10.1089/ten.tea.2013.0242 Epub ahead of print]; PMID: , 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich F., and Lelkes P.I.Fine-tuning of a three-dimensional microcarrier-based angiogenesis assay for the analysis of endothelial-mesenchymal cell co-cultures in fibrin and collagen gels. Angiogenesis 9,111, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Nehls V., and Drenckhahn D.A novel, microcarrier-based in vitro assay for rapid and reliable quantification of three-dimensional cell migration and angiogenesis. Microvasc Res 50,311, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Rieu I., and Powers S.J.Real-time quantitative rt-pcr: design, calculations, and statistics. Plant Cell 21,1031, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kachgal S., and Putnam A.J.Mesenchymal stem cells from adipose and bone marrow promote angiogenesis via distinct cytokine and protease expression mechanisms. Angiogenesis 14,47, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghajar C.M., Kachgal S., Kniazeva E., Mori H., Costes S.V., George S.C., et al. Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316,813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroon M.E., Koolwijk P., van Goor H., Weidle U.H., Collen A., van der Pluijm G., et al. Role and localization of urokinase receptor in the formation of new microvascular structures in fibrin matrices. Am J Pathol 154,1731, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binder B.R., Mihaly J., and Prager G.W.Upar—upa—pai-1 interactions and signaling: a vascular biologist's view. Thromb Haemost 97,336, 2007 [PubMed] [Google Scholar]
- 28.van Hinsbergh V.W., and Koolwijk P.Endothelial sprouting and angiogenesis: matrix metalloproteinases in the lead. Cardiovasc Res 78,203, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Ghajar C.M., George S.C., and Putnam A.J.Matrix metalloproteinase control of capillary morphogenesis. Crit Rev Eukaryot Gene Expr 18,251, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N.Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25,581, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Ferrara N.Vegf-a: a critical regulator of blood vessel growth. Eur Cytokine Netw 20,158, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Ho Q.T., and Kuo C.J.Vascular endothelial growth factor: biology and therapeutic applications. Int J Biochem Cell Biol 39,1349, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas M., and Augustin H.G.The role of the angiopoietins in vascular morphogenesis. Angiogenesis 12,125, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Lawson C., and Wolf S.Icam-1 signaling in endothelial cells. Pharmacol Rep 61,22, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Boudreau N., Andrews C., Srebrow A., Ravanpay A., and Cheresh D.A.Induction of the angiogenic phenotype by hox d3. J Cell Biol 139,257, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuigan A.P., and Sefton M.V.The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials 28,2547, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.She M., McGuigan A.P., and Sefton M.V.Tissue factor and thrombomodulin expression on endothelial cell-seeded collagen modules for tissue engineering. J Biomed Mater Res A 80,497, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Schechner J.S., Nath A.K., Zheng L., Kluger M.S., Hughes C.C., Sierra-Honigmann M.R., et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci U S A 97,9191, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enis D.R., Shepherd B.R., Wang Y., Qasim A., Shanahan C.M., Weissberg P.L., et al. Induction, differentiation, and remodeling of blood vessels after transplantation of bcl-2-transduced endothelial cells. Proc Natl Acad Sci U S A 102,425, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng G., Liao S., Kit Wong H., Lacorre D.A., di Tomaso E., Au P., et al. Engineered blood vessel networks connect to host vasculature via wrapping-and-tapping anastomosis. Blood 118,4740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jay S.M., Shepherd B.R., Andrejecsk J.W., Kyriakides T.R., Pober J.S., and Saltzman W.M.Dual delivery of vegf and mcp-1 to support endothelial cell transplantation for therapeutic vascularization. Biomaterials 31,3054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jay S.M., Shepherd B.R., Bertram J.P., Pober J.S., and Saltzman W.M.Engineering of multifunctional gels integrating highly efficient growth factor delivery with endothelial cell transplantation. FASEB J 22,2949, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Critser P.J., Kreger S.T., Voytik-Harbin S.L., and Yoder M.C.Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res 80,23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papayannakos C., and Daniel R.Understanding lentiviral vector chromatin targeting: working to reduce insertional mutagenic potential for gene therapy. Gene Ther 20,581, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Persons D.A.Lentiviral vector gene therapy: effective and safe? Mol Ther 18,861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montini E., Cesana D., Schmidt M., Sanvito F., Ponzoni M., Bartholomae C., et al. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat Biotechnol 24,687, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Hahn W.C., and Weinberg R.A.Rules for making human tumor cells. N Engl J Med 347,1593, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Somanath P.R., Ciocea A., and Byzova T.V.Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys 53,53, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smyth S.S., and Patterson C.Tiny dancers: the integrin-growth factor nexus in angiogenic signaling. J Cell Biol 158,17, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eliceiri B.P.Integrin and growth factor receptor crosstalk. Circ Res 89,1104, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Mahabeleshwar G.H., Feng W., Reddy K., Plow E.F., and Byzova T.V.Mechanisms of integrin-vascular endothelial growth factor receptor cross-activation in angiogenesis. Circ Res 101,570, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]