Abstract
Background: Low serum albumin concentration is a predictor of failure of source control for intra-abdominal infection. However, data on dynamics of albumin synthesis in these patients and to what extent these changes contribute to hypoalbuminemia are relatively scarce. We investigated in a group of patients with gastrointestinal fistula the dynamic response of liver albumin synthesis to intra-abdominal abscess and how these related to hypoalbuminemia and circulating endocrine hormone profiles.
Methods: Eight gastrointestinal fistula patients scheduled to undergo percutaneous abscess sump drainage were enrolled prospectively to measure albumin synthesis rates at different stages of the inflammatory response (immediately after diagnosis and 7 d following sump drainage when clinical signs of intra-abdominal sepsis had been eradicated). Eight age-, sex-, and body mass index–matched intestinal fistula patients were studied as control patients. Consecutive arterial blood samples were drawn during a primed-constant infusion (priming dose: 4 micromol·kg−1, infusion rate: 6 micromol·kg−1·min−1) to determine the incorporation rate of L-[ring-2H5]-phenylalanine directly into plasma albumin using gas chromatography/mass spectrometry analysis.
Results: Patients suffering from intra-abdominal infection had reduced plasma albumin and total plasma protein concentrations, compared with control patients. Albumin fractional synthesis rates in patients with intra-abdominal abscess were decreased, compared with those in the control group. When the source of infection was removed, albumin synthesis rates returned to control values, whereas albumin concentrations did not differ significantly from the corresponding concentrations in control subjects and patients with intra-abdominal abscess.
Conclusion: Despite nutritional intervention, albumin synthesis rate is decreased in intestinal fistula patients with intra-abdominal abscess; albumin synthesis returns to control values during convalescence.
Despite vigorous nutritional support, low serum albumin concentrations still prevail among patients with complicated intra-abdominal infections (cIAI). Moreover, a low albumin concentration is a predictor of failure of source control for intra-abdominal infection according to guidelines by the Surgical Infection Society and the Infectious Diseases Society of America [1]. Besides, the results of a 2010 meta-analysis suggest that hypoalbuminemia is a significant independent predictor of the development of acute kidney injury and subsequent clinical outcomes in intensive care unit patients [2]. Thus, gaining insight into the mechanisms responsible for a decreased serum albumin concentration complicating critical illness is important and might lead ultimately to novel therapeutic approaches for these patients.
Serum albumin depletion may be as a result of a disparity between the rates of protein synthesis and degradation, or distribution between the vascular and extravascular compartments. In the past several years, there was broad agreement that both the rate of albumin degradation and the loss of albumin to the tissue spaces increases in patients with sepsis [3,4], and attention has turned to measurement of albumin synthesis. However, in vivo studies have yielded conflicting results regarding the effects of infection on albumin synthesis (decreased [5], increased [6], or unchanged [7]). Meanwhile, measurements of albumin synthesis in human beings have been conducted at only one time point, mostly during the acute phase, and several studies have addressed the possibility of albumin synthesis responding differently depending on the stage of the inflammatory process and various stimuli. In fact, a biphasic response (no change, then a decrease) of albumin synthesis has been confirmed in a rat model of sepsis [8]. The pathophysiologic process of intra-abdominal abscess following gastrointestinal fistula differs from those with an acute-phase response and possibly elicits different kinetics of albumin synthesis. However, data on dynamics of albumin synthesis in these patients are relatively scarce. Thus, changes in liver albumin synthesis rates in patients with gastrointestinal fistula in conjunction with inflammation need to be characterized.
The present study was designed to evaluate in a group of patients with gastrointestinal fistula the response of liver albumin synthesis to intra-abdominal abscess (IAA). In this study, we determined in vivo the albumin fractional synthesis rate (FSR) in duplicate—at the time of diagnosis of intra-abdominal abscess and one week after sump drainage when clinical signs of intra-abdominal abscess have subsided—and how these relate to albumin levels and circulating endocrine hormone profiles.
Materials and Methods
Materials
L-[ring-2H5]-phenylalanine (99 atom percent excess, Cambridge Isotopes, Andover, MA) was dissolved in sterile 0.9% NaCl to a final concentration of 6 g/L. Then, the solution was passed through a 0.22 micron filter and sterilized before being dispensed in ampoules by the pharmacy department at the Jinling Hospital, Nanjing, China. Tests were performed to ensure the correct identity and concentration, as well as a sterile and pyrogen-free product.
Subjects
Eight patients with intra-abdominal abscess admitted to the Research Institute of General Surgery at Jinling Hospital, Nanjing, China were included prospectively in this investigation. In parallel, eight patients with controlled enterocutaneous fistula matched for age, gender, and body mass index served as controls. Patients with IAA were eligible for inclusion if they were aged 18–60 years, showed clinical evidence of IAA, and were scheduled to undergo percutaneous abscess sump drainage using a 12-mm trocar [9] within 1 d of study entry. The diagnosis of IAA was made primarily on the basis of computed tomography findings and supplemented with clinical parameters [1], including one or more of the following: Fever or hypothermia, leukocytosis, high C-reactive protein (CRP) concentration, abdominal wall involuntary guarding, and abdominal tenderness or pain. The diagnosis was confirmed by aspiration and culture of pus retrieved from the abscess. All patients received a worldwide-accepted protocol designed for the treatment of patients with enterocutaneous fistulas [10]. All subjects were screened prior to study and were excluded for hepatic, renal, or endocrine disease, having diabetes mellitus or other metabolic abnormalities, showing clinical evidence of infections at other body sites, or having received somatostatin therapy. Subjects with rapidly progressing disease or immediately life-threatening illness also were excluded.
The study protocol was reviewed and approved by the ethics review board of Jinling Hospital, Nanjing, China. Written informed consent for participation in the study was obtained from all participants after the purposes and potential risks of the experimental procedures had been explained in detail.
Experimental protocol
Measurement of albumin synthesis in patients with IAA was performed in duplicate, immediately before (Period 1) and one week following sump drainage when clinical signs of intra-abdominal sepsis had been eradicated (Period 2; Fig. 1). In control patients, albumin synthesis rates were determined at one time point only (Period 0). Albumin fractional synthesis rate was measured from the rate of incorporation of L-[ring-2H5]-phenylalanine into plasma albumin and all studies were conducted in the absorptive state following an overnight fast of at least 8 h [11]. On the morning of study, subjects' height was measured in triplicate with a stadiometer to the nearest 0.1 cm and weight with an electronic scale to the nearest 0.1 kg. An intravenous catheter was then placed in an antecubital vein for the infusion of stable isotopically labeled amino acid and a second catheter in the radial artery of the contralateral arm for blood sampling. The hand was kept warm with a heating blanket to relieve discomfort. After baseline blood samples were taken for determination of natural isotopic enrichments, liver enzymes, amino acids and hormone concentrations, a primed, constant infusion of L-[ring-2H5]-phenylalanine (priming dose: 4 micromol·kg−1, infusion rate: 6 micromol·kg−1·min−1) was started at 0700 and maintained during the whole course of the protocol (6 h). Additional 3-mL arterial blood samples were drawn at hourly intervals throughout the infusion into pre-chilled tubes containing ethylenediaminetetraacetic acid (EDTA) and centrifuged immediately at 4°C; the plasma was stored at −80°C until further analysis. Physiologic parameters (i.e., body temperature, heart rate) were monitored and recorded hourly during the study.
FIG. 1.

Schematic representation of the isotope infusion protocol. Patients with intra-abdominal abscess (IAA) were studied at the day when IAA was diagnosed (Period 1) and one week after sump drainage (Period 2). For the control patients, only one measurement was taken (Period 0). After obtaining blood samples for background enrichment at 0 min, a primed continuous infusion of L-[ring-2H5]-phenylalanine was administrated intravenously for six hours. Arrows indicate blood sampling times.
Analytical procedures
Precursor pool enrichment
For measurement of plasma free phenylalanine enrichment, initially plasma samples were deproteinized by adding 10 microliters of 5-sulphosalicylic acid solution (45%) to 100 microliters of sample [12]. After vortex mixing, the samples were centrifuged at 1,000 rmp for 10 min at 4°C. The supernatant was acidified to pH 3 and loaded to a solid phase extraction (SPE) cartridge [13]. Amino acids trapped on the cartridge were washed out by 2 mL of 3 mol/L ammonia water, and effluents were collected and dried in vacuo.
Albumin pool enrichment
Albumin in plasma samples was isolated by differential solubility in absolute ethanol from trichloroacetic acid (TCA) precipitated proteins, as described by Debro and Korner [14]. In short, the plasma was precipitated with cold 10% TCA and centrifuged. The acid was poured off and cold absolute ethanol was then added to dissolve the protein pellet. The resulting supernatant contained exclusively albumin and was dried under vacuum. The purity and identity of the extracted albumin has been demonstrated previously [15, 16]. Further to remove traces of free amino acids, the residue was suspended in 0.3 mol/L sodium hydroxide at 37°C for 1 h and further reprecipitated in 2 mol/L perchloric acid (HClO4). Following washing with 0.2 mol/L HClO4, albumin was hydrolyzed with 6 mol/L hydrogen chloride in an evacuated sealed tube at 110°C for 24 h. The hydrolysates were further purified by passing through a 0.22 micron filter and were evaporated to dryness for measurement of isotopic enrichment.
Mass spectrometry
The purified amino acids from plasma, as well as albumin hydrolysates, were derivatized with N-methyl-N-trimethylsilyltrifluoroacetamide plus 1% trimethylchlorosilane at 37°C, respectively, for 30 min. The isotopic enrichment of L-[2H5] phenylalanine from the plasma free amino acid pool and from albumin was measured by gas chromatography mass spectrometry analysis (model 5973; Hewlett-Packard, Palo Alto, CA) in the electron impact ionization mode. Mass-to-charge ratio (m/z) of 192 (m+0) and 197 (m+5) for phenylalanine were selectively monitored for unlabeled phenylalanine and [2H5] phenylalanine, respectively. The enrichment was expressed as tracer (labeled aminos acids) to tracee (unlabeled amino acids) ratio (TTR).
Other analytical procedures
White blood cell (WBC) count, plasma total protein content, albumin concentrations, plasma CRP concentrations and liver function tests were analyzed by routine clinical laboratory methods. Amino acid concentrations were performed on Agilent 1100 high performance liquid chromatograph (Santa Clara, CA). Plasma concentrations of cortisol and serum concentrations of GH, free triiodothyronine (FT3), free thyroxine, total triiodothyronine, total thyroxine, and thyroid-stimulating hormone were measured by chemiluminescent immunoassays on an Advia Centaur instrument (Bayer, Leverkusen, Germany).
Calculation
The FSR of albumin (i.e., the fraction of the total albumin-bound pool that is synthesized per day [%/d]), was calculated from the rate of incorporation of [ring-2H5]-phenylalanine into plasma albumin, using a standard precursor-product model as follows [17]:
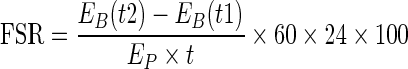 |
where EB (t1) and EB (t2) represent the isotopic enrichment (TTR) of albumin-bound phenylalanine at two consecutive time points after albumin enrichment becomes linearly increased, EP is the isotopic enrichment of precursor at plateau and t is the time interval in minutes between two blood samples. In this study, plasma [ring-2H5]-phenylalanine was used as surrogate for precursor enrichment according to previous studies [18].
Statistical analysis
Data are presented as means ± S.E.M. unless otherwise noted. Categorical variables were analyzed by Chi-square test or Fisher exact test, and continuous variables by Student t-test, one-way ANOVA, or Kruskal-Wallis H-test as appropriate. A repeated measures of analysis of variance with a post hoc LSD test was used when comparing more than two variables. Correlations between the protein synthesis rates and clinical parameters were tested by the Spearman test. All statistical analyses were performed with IBM SPSS Statistics 18 (SPSS Inc., Chicago, IL, USA) and p values < 0.05 were considered statistically significant. All statistical analyses were two-tailed.
Results
Subject characteristics
The subjects' anthropometric characteristics are presented in Table 1. The eight infectious patients consisted of six females and two males; eight sex-matched intestinal fistula patients were recruited. All of the patients with IAA were studied twice in a similar interval of seven days. The average age at study enrollment was 33.38 ± 3.48 yrs for patients with IAA and 34.0 ± 2.68 yrs for control patients. Body mass index and weight in participants with IAA was similar to that in control subjects and remained unchanged over the 7-d period of observation. All patients had increased body temperature, WBC counts and serum CRP concentrations and were diagnosed to have abdominal abscess by abdominal computed tomography (CT) scan. All patients had received antibiotic therapy, parenteral nutrition, sump drainage, and other supportive therapy.
Table 1.
Patient Characteristics
| Control patients | IAA patients | ||
|---|---|---|---|
| Period 0 | Period 1 | Period 2 | |
| Age (years) | 34.0±2.68 | 33.38±3.48 | 33.38±3.48 |
| Male/female | 6/2 | 6/2 | 6/2 |
| Weight (kg) | 64.26±3.38 | 58.28±2.94 | 58.9±2.25 |
| Height (cm) | 173.63±1.05 | 170.63±2.46 | |
| BMI (kg/m2) | 21.65±0.84 | 20.61±1.16 | 20.81±0.85 |
| Body temperature (°C) | 36.58±0.08 | 38.65±0.16* | 37.1±0.11 |
| WBC (109/L) | 5.9±0.26 | 12.38±1.79† | 8.3±0.35† |
| CRP (mg/L) | 9.73±1.74 | 116.07±29.1* | 35.48±6.59 |
| Albumin (g/L) | 38.46±1.66 | 31.71±1.14† | 35.21±1.05 |
| Cortisol (nmol/L) | 378.75±72.89 | 466.25±86.7 | 371.25±58.42 |
| GH (ug/L) | 1.57±0.38 | 1.49±0.30 | 1.59±0.30 |
| TSH (mU/L) | 2.37±0.42 | 1.77±0.33 | 2.07±0.33 |
| FT3 (pmol/L) | 4.56±0.32 | 3.41±0.41* | 4.49±0.29 |
| TT3 (nmol/L) | 1.47±0.24 | 1.26±0.21 | 1.27±0.24 |
| FT4 (pmol/L) | 11.1±0.79 | 11.39±0.78 | 10.76±0.85 |
| TT4 (nmol/L) | 100.2±5.87 | 84.86±6.39 | 95.63±5.81 |
p<0.05 compared with control patients (Period 0) and IAA patients following sump drainage (Period 2).
p<0.05 compared with control patients (Period 0).
IAA, intra-abdominal abscess; BMI, body mass index; WBC, white blood cell; CRP, C-reactive protein; GH, growth hormone; TSH, thyroid-stimulating hormone; FT3, free triiodothyronine; TT3, total triiodothyronine; FT4, free thyroxine; TT4, total thyroxine.
The median time from fistula development to study entry was 37.75 d in control patients and 21.63 d in IAA patients (Table 2). The site of the fistula in control patients was in duodenum in two cases, jejunum in three cases, ileum in two cases, and colon in one case. In IAA patients, four fistulas involved the duodenum, one involved jejunum, one involved ileum, and two involved colon. There were three high-output, three medium-output, and two low-output fistulas in control patients, whereas four fistulas of IAA patients were of high output, two were of medium output, and two were of low output. Six fistulas were believed to be secondary to original injuries from trauma in IAA patients, whereas there were four in control patients. Three control patients developed a fistula after previous abdominal surgery but only one in IAA patients. There was one patient in both groups, who developed spontaneous fistulation secondary to Crohn disease. Fistula closure was accomplished in all patients. Spontaneous closure occurred in five control patients and two patients with IAA, while surgical intervention achieved closure in three control patients and six patients with IAA.
Table 2.
Clinical Characteristics of Enteric Fistula in All Patients
| Control patients | IAA patients | p | |
|---|---|---|---|
| Duration of fistula (days) | 35.75±12.12 | 21.63±5.41 | 0.305 |
| Fistula origin | 0.506 | ||
| Duodenum | 2 | 4 | |
| Jejunum | 3 | 1 | |
| Ileum | 2 | 1 | |
| Colon | 1 | 2 | |
| Output (mL/day) | 0.842 | ||
| <200 | 2 | 2 | |
| 200–500 | 3 | 2 | |
| >500 | 3 | 4 | |
| Underlying surgery/disease | 0.497 | ||
| Trauma | 4 | 6 | |
| Operation | 3 | 1 | |
| Crohn disease | 1 | 1 | |
| Closure | 0.315 | ||
| Spontaneous | 5 | 2 | |
| Surgical | 3 | 6 |
IAA, intra-abdominal abscess.
Plasma albumin concentration and fractional synthesis rate
All participants' physiologic parameters were monitoring during the isotope infusion. Compared with control patients, though patients with IAA had increased body temperature, respiratory rates and heart rates, these parameters remained constant during the isotope infusion, indicating a physiological steady state. During a 6-h tracer infusion in both groups in the post-absorptive state, we observed a rapid, marked increase of the enrichments of plasma free phenylalanine in the first 2 h, and then the enrichments reached plateau values and remained unchanged (mean, 0.124884 in control and 0.128672 in IAA patients; p>0.05), indicating complicated intra-abdominal infections had no influence on plasma free phenylalanine enrichments. The enrichments of albumin-bound phenylalanine TTR increased linearly over the 6-h infusion of [2H5]-phenylalanine.
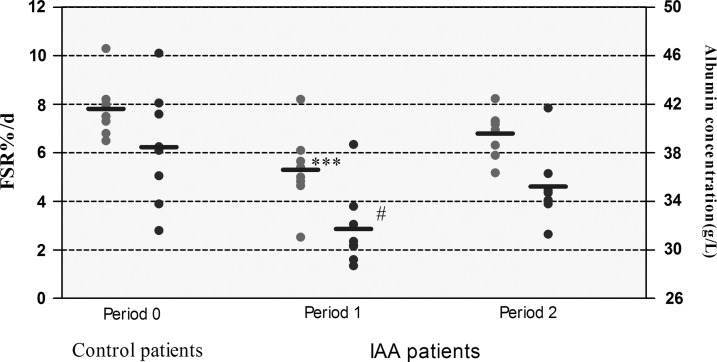
Patients suffering from IAA had reduced serum albumin concentrations (31.71±1.14 g/L vs. 38.46±1.66 g/L; p<0.05) and albumin fractional synthesis rates (5.3±0.56%/d and 7.8±0.41%/d, respectively; p<0.05), compared with control patients (Fig. 2).
FIG. 2.
Serum albumin concentrations and fractional synthesis rates in control patients (Period 0) and in intra-abdominal abscess patients immediately before (Period 1) and one week following sump drainage when clinical signs of intra-abdominal sepsis had been eradicated (Period 2). Circles represent individual values, horizontal lines represent means. ***p<0.05, compared with Period 0 and Period 2. #p<0.05, compared with Period 0.
When the source of infection was removed, fractional synthesis rate increase (5.3±0.56%/d and 6.8±0.33%/d; p<0.05, compared with phase of abscess formation, i.e., Period 1), whereas serum albumin concentrations did not differ from patient with IAA (Period 1). Both albumin synthesis rates and serum albumin concentrations were not significantly different between IAA patients during convalescence and control patients.
Plasma concentrations of free amino acids
Measurement of plasma free amino acids concentrations was performed once, immediately before the first isotope infusion. As shown in Table 3, the plasma concentration of phenylalanine and proline in patients with intra-abdominal abscess was higher than that in control subjects, whereas glutamic acid decreased, compared with controls. The plasma concentrations of other amino acids did not differ between both groups.
Table 3.
Comparison of Plasma Free Amino Acid Concentration between IAA and Control Patients
| IAA Patients | Control patients | p | |
|---|---|---|---|
| Essential amino acid | |||
| Leucine | 0.33±0.05 | 0.38±0.04 | 0.39 |
| Methionine | 0.038±0.01 | 0.041±0.01 | 0.76 |
| Valine | 0.73±0.09 | 0.88±0.04 | 0.15 |
| Threonine | 0.48±0.06 | 0.41±0.05 | 0.34 |
| Lysine | 0.45±0.04 | 0.49±0.04 | 0.52 |
| Isoleucine | 0.14±0.02 | 0.18±0.03 | 0.31 |
| Phenylalanine | 0.16±0.02 | 0.94±0.00* | 0.01 |
| Non-essential amino acid | |||
| Proline | 0.46±0.06 | 1.02±0.10* | <0.01 |
| Arginine | 0.28±0.02 | 0.31±0.02 | 0.27 |
| Serine | 0.20±0.04 | 0.27±0.02 | 0.15 |
| Glutamic acid | 0.66±0.12 | 0.26±0.02* | 0.017 |
| Alanine | 0.80±0.07 | 1.03±0.10 | 0.075 |
| Tyrosine | 0.25±0.02 | 0.28±0.01 | 0.133 |
| Histidine | 0.11±0.01 | 0.13±0.01 | 0.54 |
| Glycine | 0.74±0.10 | 0.86±0.05 | 0.3 |
| Cysteine | 0.08±0.00 | 0.08±0.00 | 0.52 |
| Aspartic acid | 0.18±0.00 | 0.16±0.00 | 0.59 |
p<0.05 compared with patients with IAA.
Data expressed as mg/mL.
IAA, intra-abdominal abscess.
Plasma endocrine hormone levels
The circulating concentration of FT3 in patients with IAA was significantly lower than that in both control patients and IAA patients after sump drainage. The concentration of all other endocrine hormones presented in Table 1 was not significantly different among the three groups.
Discussion
The primary goal of this study was to investigate the response of albumin synthesis to intra-abdominal abscess in patients with gastrointestinal fistula and the albumin synthesis kinetics during convalescence following sump drainage. We found that both serum albumin concentrations and albumin fractional synthesis rates were lower in patients with intra-abdominal abscess relative to control values. Furthermore, our results show that albumin fractional synthesis rates normalized during convalescence, whereas serum albumin concentrations did not differ significantly from the corresponding concentrations in control subjects and patients with intra-abdominal abscess.
The acute albumin synthetic response to stress has been examined in detail [19–21]. In 1987, reduced albumin synthesis was measured in four patients with inflammatory diseases, including periappendicular abscess, using the [14C]-carbonate technique [22]. In recent years, however, a decreased albumin synthesis rate has been challenged by several studies in human beings, indicating that the synthesis rate of albumin in patients with an acute-phase response is increased rather than decreased, as well as volunteers who received endotoxin [23–25].
These measurements were made at only one time point following stress, and it is confirmed that albumin synthesis responded differently depending on the stage of the inflammatory process. In particular, a dynamic response (no change, a decrease, and then an increase) of albumin synthesis has been demonstrated in a rat model of sepsis [8]. Also, several studies in humans have shown a biphasic change of albumin synthesis in different phases of cholecystitis, which corresponds to studies in small animals. An increased albumin synthesis is observed in acute cholecystitis patients (within 2–4 d after initiation of symptoms) [6], whereas in patients with cholecystolithiasis, a lower albumin synthesis (FSR, 5.9±1.2%/d) was found [26]. Taken together, these results suggest that timing of measurement should be taken into consideration to make comparisons.
The question remains: Why does IAA itself result in a decrease in albumin synthesis in patients with gastrointestinal fistula? First, unlike models based on intravascular administration of endotoxin or bacteria, an abscess will persist for several days until the diagnosis is made. Data presented in this study are only a glimpse of albumin synthesis at the time point when the diagnosis of intra-abdominal abscess was made and one week after sump drainage. On the contrary, many other studies in humans have been conducted at only one stage of the inflammatory process, mostly during the acute phase. In fact, when intra-abdominal infection was induced in rats, albumin synthesis was found to be decreased during the early acute phase [27] and the chronic phase [8], which was the case in present study. Thus, the degree or type of catabolic insult seem to be important.
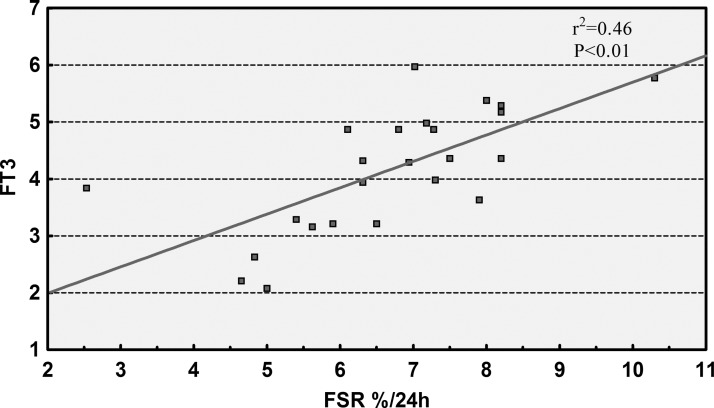
Second, the stress state of patients also appears to play an important role. It is accepted increasingly that stress hormones could be implicated in maintaining albumin synthesis in injury [28]. Although the two groups have similar growth hormone and cortisol concentrations (decreased compared with normal values), patients with IAA have lower levels of serum FT3, which have been dubbed a “nonthyroidal illness syndrome (NTIS)” or “low-T3 syndrome” [29]. In fact, we collected 226 intestinal fistula patients prospectively in our center, and found that NTIS occurred frequently, with a prevalence of 57.5% [30]. Once these patients complicated with sepsis, a significantly greater decline in plasma FT3 values was noted. Interestingly, we found positively correlations between the albumin synthesis rate and serum FT3 concentration (Fig. 3). Because thyroid hormones are known to have anabolic effects and FSRs of albumin correlated positively with plasma FT3 concentrations, the reduction of FT3 may be a pathogenic factor that contributes to the decreased synthesis rates of albumin in the present study. Further interventional investigation will make clear to what extent low FT3 plays a role in albumin synthesis.
FIG. 3.
The correlation between the fractional synthesis rates of albumin and plasma free triiodothyronine concentrations.
Albumin synthesis, which depends on the availability of intracellular amino acids, is well known to be influenced by the nutritional state [31]. Starvation and protein-deficient diets were associated with increased whole body protein kinetics and reduced albumin synthesis in vivo [32, 33], whereas the progressive restoration of food intake was associated with a progressive increase in albumin synthesis [8]. In the present study, to determine specifically the effect of infection, every patient received standard parenteral nutrition support and exercise program. Skeletal muscle wasting and weight loss was not observed in these patients. Moreover, liver functions were affected to a slight degree in few of the patients in this study and amino acid concentrations were consistent with values in control subjects (Table 3). Thus, the reduction of albumin synthesis in the present study was most likely not due to a decreased supply of amino acids to the liver or the result of a liver dysfunction.
It is worth mentioning that even with the increased albumin synthesis during convalescence, the synthesis rate of albumin remains relatively low, compared with its pool size. Further, depleted plasma albumin concentrations under pathological condition may result from reduced rate of synthesis, increased rate of degradation, or altered distribution between extra- and intravascular compartments. An increase in vascular permeability has the potential to change plasma albumin very rapidly and to a greater extent than changes in synthesis and degradation because its rate can be as much as several times higher than synthesis in patients with infections. Thus, it may take several days for changes in synthesis to be reflected in serum albumin concentrations, if other dynamic parameters remain unchanged. In other words, changes in albumin synthesis are more sensitive to the extent of inflammation, compared with serum albumin concentration. Further studies are needed to demonstrate whether albumin synthesis rate is a predictor of the degree of intra-abdominal infection and subsequent clinical outcome.
In summary, our study provides insight in the dynamics of albumin synthesis in patients with complicated intra-abdominal infections. Patients with IAA have decreased albumin synthesis and serum albumin concentrations. After removal of the sources of infection, that is, in convalescence, albumin synthesis rate was elevated, whereas serum albumin concentrations did not differ significantly from the corresponding concentrations in control subjects and patients with intra-abdominal abscess. These findings suggest that decreased serum albumin synthesis rates in patients with intra-abdominal abscess might partially contribute to the decline in serum albumin concentrations and albumin synthesis rate appears to be more sensitive to the degree of intra-abdominal infection.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant number 30872456) and the Climbing Program in Natural Science Foundation of Jiangsu Province for Distinguished Scholars (grants number BK2010017). We would like to thank the nursing staff for its excellent assistance with the isotope infusion and blood sampling.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect 2010;11:79–109 [DOI] [PubMed] [Google Scholar]
- 2.Wiedermann CJ, Wiedermann W, Joannidis M. Hypoalbuminemia and acute kidney injury: A meta-analysis of observational clinical studies. Intensive Care Med 2010;36:1657–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleck A, Raines G, Hawker F, et al. Increased vascular permeability: A major cause of hypoalbuminaemia in disease and injury. Lancet 1985;1:781–784 [DOI] [PubMed] [Google Scholar]
- 4.Ruot B, Papet I, Béchereau F, et al. Increased albumin plasma efflux contributes to hypoalbuminemia only during early phase of sepsis in rats. Am J Physiol Regul Integr Comp Physiol 2003;284:R707–R713 [DOI] [PubMed] [Google Scholar]
- 5.Ballmer PE, McNurlan MA, Grant I, et al. Down-regulation of albumin synthesis in the rat by human recombinant interleukin-1 beta or turpentine and the response to nutrients. JPEN J Parenter Enteral Nutr 1995;19:266–271 [DOI] [PubMed] [Google Scholar]
- 6.Barle H, Hammarqvist F, Westman B, et al. Synthesis rates of total liver protein and albumin are both increased in patients with an acute inflammatory response. Clin Sci (Lond) 2006;110:93–99 [DOI] [PubMed] [Google Scholar]
- 7.Sax HC, Talamini MA, Hasselgren PO, et al. Increased synthesis of secreted hepatic proteins during abdominal sepsis. J Surg Res 1988;44:109–116 [DOI] [PubMed] [Google Scholar]
- 8.Ruot B, Bechereau F, Bayle G, et al. The response of liver albumin synthesis to infection in rats varies with the phase of the inflammatory process. Clin Sci (Lond) 2002;102:107–114 [PubMed] [Google Scholar]
- 9.Gu G, Ren J, Yuan Y, et al. An innovative technique for intra-abdominal abscess drainage using a sump drain by trocar puncture. Am Surg 2011;77:E166-E167 [PubMed] [Google Scholar]
- 10.Schecter WP. Management of Enterocutaneous Fistulas. Surg Clin North Am 2011;91:481–491 [DOI] [PubMed] [Google Scholar]
- 11.Ballmer PE, McNurlan MA, Milne E, et al. Measurement of albumin synthesis in humans: a new approach employing stable isotopes. Am J Physiol 1990;259:E797-E803 [DOI] [PubMed] [Google Scholar]
- 12.Khan K, Blaak E, Elia M. Quantifying intermediary metabolites in whole blood after a simple deproteinization step with sulfosalicylic acid. Clin Chem 1991;37:728–733 [PubMed] [Google Scholar]
- 13.Spanik I, Horvathova G, Janacova A, et al. On the use of solid phase ion exchangers for isolation of amino acids from liquid samples and their enantioselective gas chromatographic analysis. J Chromatogr A 2007;1150:145–154 [DOI] [PubMed] [Google Scholar]
- 14.Debro JR, Korner A. Solubility of albumin in alcohol after precipitation by trichloroacetic acid: a simplified procedure for separation of albumin. Nature 1956;178:1067. [DOI] [PubMed] [Google Scholar]
- 15.Ballmer PE, Walshe D, McNurlan MA, et al. Albumin synthesis rates in cirrhosis: correlation with Child-Turcotte classification. Hepatology 1993;18:292–297 [PubMed] [Google Scholar]
- 16.Hunter KA, Garlick PJ, Broom I, et al. Effects of smoking and abstention from smoking on fibrinogen synthesis in humans. Clin Sci (Lond) 2001;100:459–465 [PubMed] [Google Scholar]
- 17.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. Hokoben (NJ): John Wiley & Sons, Inc.,2005: 326–335 [Google Scholar]
- 18.Ruot B, Breuille D, Rambourdin F, et al. Synthesis rate of plasma albumin is a good indicator of liver albumin synthesis in sepsis. Am J Physiol Endocrinol Metab 2000;279:E244-E251 [DOI] [PubMed] [Google Scholar]
- 19.Rittler P, Jacobs R, Demmelmair H, et al. Dynamics of albumin synthesis after major rectal operation. Surgery 2007;141:660–666 [DOI] [PubMed] [Google Scholar]
- 20.Martini WZ, Wolf SE, Chinkes DL, et al. Enhanced albumin synthesis in severely burned adults. Shock 2010;34:364–368 [DOI] [PubMed] [Google Scholar]
- 21.Verbruggen SC, Schierbeek H, Coss-Bu J, et al. Albumin synthesis rates in post-surgical infants and septic adolescents; influence of amino acids, energy, and insulin. Clin Nutr 2011;30:469–477 [DOI] [PubMed] [Google Scholar]
- 22.Moshage HJ, Janssen JA, Franssen JH, et al. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest 1987;79:1635–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansoor O, Cayol M, Gachon P, et al. Albumin and fibrinogen syntheses increase while muscle protein synthesis decreases in head-injured patients. Am J Physiol 1997;273:E898–E902 [DOI] [PubMed] [Google Scholar]
- 24.Fearon KC, Falconer JS, Slater C, et al. Albumin synthesis rates are not decreased in hypoalbuminemic cachectic cancer patients with an ongoing acute-phase protein response. Ann Surg 1998;227:249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barle H, Januszkiewicz A, Hallstrom L, et al. Albumin synthesis in humans increases immediately following the administration of endotoxin. Clin Sci (Lond) 2002;103:525–531 [DOI] [PubMed] [Google Scholar]
- 26.Barle H, Nyberg B, Essen P, et al. The synthesis rates of total liver protein and plasma albumin determined simultaneously in vivo in humans. Hepatology 1997;25:154–158 [DOI] [PubMed] [Google Scholar]
- 27.O'Leary MJ, Koll M, Ferguson CN, et al. Liver albumin synthesis in sepsis in the rat: influence of parenteral nutrition, glutamine and growth hormone. Clin Sci (Lond) 2003;105:691–698 [DOI] [PubMed] [Google Scholar]
- 28.McNurlan MA, Sandgren A, Hunter K, et al. Protein synthesis rates of skeletal muscle, lymphocytes, and albumin with stress hormone infusion in healthy man. Metabolism 1996;45:1388–1394 [DOI] [PubMed] [Google Scholar]
- 29.Plikat K, Langgartner J, Buettner R, et al. Frequency and outcome of patients with nonthyroidal illness syndrome in a medical intensive care unit. Metabolism 2007; 56: 239–244 [DOI] [PubMed] [Google Scholar]
- 30.Han G, Ren J, Liu S, et al. Nonthyroidal illness syndrome in enterocutaneous fistulas. Am J Surg 2013;206:386–392 [DOI] [PubMed] [Google Scholar]
- 31.Caso G, Feiner J, Mileva I, et al. Response of albumin synthesis to oral nutrients in young and elderly subjects. Am J Clin Nutr 2007;85:446–451 [DOI] [PubMed] [Google Scholar]
- 32.Afolabi PR, Jahoor F, Jackson AA, et al. The effect of total starvation and very low energy diet in lean men on kinetics of whole body protein and five hepatic secretory proteins. Am J Physiol Endocrinol Metab 2007;293:E1580-W1589 [DOI] [PubMed] [Google Scholar]
- 33.Pain VM, Clemens MJ, Garlick PJ. The effect of dietary protein deficiency on albumin synthesis and on the concentration of active albumin messenger ribonucleic acid in rat liver. Biochem J 1978;172:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]