Abstract
Some studies have indicated that the consumption of chilli-containing foods can influence iron absorption and affect serum insulin and glucose concentrations, which may help to alleviate diabetes or prediabetes. The objective of this study was to explore the relationship between chilli food habits with iron status and insulin resistance in the Chinese population. Fasting blood samples, anthropometric data, and chilli food habit data collected from 8433 adults (aged 18 to 99), in 2009, as part of the China Health and Nutrition Survey, a large-scale longitudinal, household-based survey in China. Chilli food habits were assessed using chilli food eating frequencies (no eating, sometimes eating, often eating, and usually eating) and chilli food types (a little bit hot, moderately hot, and very hot). Fasting serum ferritin, insulin, and fasting plasma glucose were also measured. The homeostasis model assessment of insulin resistance (HOMA-IR) was used to estimate insulin resistance. Compared with the chilli-eating group, the no eating group had higher HOMA-IR levels for both men and women (P<.05). There were significant differences in HOMA-IR (P<.05) for both men and women and in ferritin (P<.001) for women according to different chilli food types. However, there was no significant difference in the ferritin level and HOMA-IR components for different chilli food eating frequencies in both sex groups. Chilli food habits, especially the different hotness levels of chilli food, were associated with iron status and insulin resistance in the Chinese population. Additional studies are needed to elucidate mechanisms of action and to establish causal inference.
Key Words: : diabetes, health functional food, iron, nutrition
Introduction
In the Chinese food culture, chillies are common and important spices, which contribute significantly to the taste and flavor of Chinese food. Research over the past three decades has indicated several potential beneficial health effects of spices (e.g., turmeric, cinnamon, garlic, and chilli), especially concerning lipid metabolism, diabetic control, digestive function, and antioxidative potential.1
Chillies, especially red chillies, contain large amounts of vitamin C and small amounts of carotene (provitamin A). They are also a good source of most B vitamins. In addition, they are very high in potassium, magnesium, and iron. Their very high vitamin C content can substantially increase the uptake of nonheme iron from other ingredients in food, such as beans and grains. However, vegetables and fruits, including chillies, also contain high amounts of polyphenols and phytate, which are the main inhibitors of iron absorption.2 Tuntipopipat et al.3 reported that chillies can inhibit iron absorption in young Thai women.
Iron is a transition metal that can convert poorly reactive free radicals into highly reactive moieties, which can cause oxidative damage to cells and tissues. Increased accumulation of iron in patients with hemochromatosis or hematological diseases affects the synthesis and secretion of insulin by the pancreas and compromises insulin action in target tissues.4 A recent study by Kim et al.5 reported that serum ferritin concentrations may be associated with insulin resistance and impaired glucose metabolism in the Korean population. Another study concluded that chilli consumption may attenuate postprandial hyperinsulinemia.1
Do chilli food habits affect iron status and insulin resistance in humans? We hope to explore the association between chilli food habits with iron status and insulin resistance among a free-living Chinese population by using a representative, randomly selected sample from nine provinces across China.
Materials and Methods
Study population
The data were extracted from the China Health and Nutrition Survey (CHNS), a large-scale longitudinal, household-based survey in China.6 The CHNS has followed individuals randomly selected from 228 communities since 1989, and was designed to represent a set of large provinces, with a range of economic and demographic variations, covering approximately 56% of China's population, including Heilongjiang, Liaoning, Shandong, Henan, Hubei, Hunan, Jiangsu, Guangxi, and Guizhou (from north to south).7–9 A multistage, random cluster process was used to draw the surveyed sample. Counties in the nine provinces were initially stratified by income (low, middle, and high) and a weighted sampling scheme was used to randomly select four counties in each province. A higher income city and a lower income city within each province were selected. In addition, the township capital and three villages within the counties were randomly selected. Finally, within each city, urban and suburban neighborhoods were randomly selected. In each community, 20 households were interviewed. The 2009 CHNS sample consists of 216 communities from 9 provinces, comprising 36 urban neighborhoods, 36 suburban neighborhoods, 36 towns, and 108 villages. Details about the study design and sampling strategies are reported elsewhere.6,10,11
In total, 8641 fasting blood samples were drawn from adult participants (aged 18–99 years). Two hundred eight participants who did not have serum ferritin due to a serious hemolytic state or did not have chilli food habit information were excluded in the analysis. Therefore, we analyzed the association between chilli food habits and iron status and insulin resistance among 8433 participants (aged 18–99 years).
Data collection methods
Trained interviewers (physicians and nutritionists) collected questionnaire-based demographic, anthropometric, and lifestyle data from each participant, including date of birth, sex, ethnicity, occupation, education level, health status, health behavior (smoking habit, alcohol use, and physical activity), food and beverage intake, tea intake, diseases under current or previous treatment, use of drugs and supplements, parental history of selected diseases, and blood collection control information (collection time, treatment time, transfer time, and storage time). Smoking status was grouped as nonsmokers, past smokers, and current smokers. Alcohol drinking was classified based on reported consumption frequencies (three or more times/week, one to two times/week, one to two times/month, less than one time/month, and nondrinker). The education level was categorized into three groups (primary school or below, middle and high school, and above high school).
Chilli food habits were classified based on chilli food eating frequencies (no eating, sometimes eating, often eating, and usually eating) and chilli food types (a little hot, moderately hot, and very hot).
Participants who signed the blood consent form in the study were required to fast overnight (at least 8 h) before the blood collection by trained phlebotomists under standard protocol.6 Blood was drawn from an antecubital vein in the morning and was transferred to the local hospital for further treatment within 2 h of blood collection. Blood specimens were collected in a 4-mL EDTA vacuum tube for routine examination and two 4-mL separation gel vacuum tubes for biochemical analysis, and were stored in an icebox in the field. When specimens were transferred to the local hospital, they were centrifuged at 3000 g for 10 min at room temperature as soon as possible and separated into nine aliquots. Aside from the samples for field tests, other samples were stored in −80°C freezers.
The fasting serum glucose (enzymatic method) was measured at local hospitals. The calibrator and control serums were provided by the Central Laboratory of the Clinical Laboratory Department of China-Japan Friendship Hospital (CJFH) using the same lot number. Other biochemical markers were analyzed at the Central Laboratory of CJFH. The concentration of fasting serum insulin and ferritin was determined by a commercial radioimmunoassay kit (Beijing North Institute of Biological Technology, Beijing, China). The interassay coefficient of variation (CV) was less than 15% and the intra-assay CV was less than 10%. The sensitivity was 2 μIU/mL. Insulin resistance was estimated using a homeostasis model assessment (HOMA-IR) equation: HOMA-IR=fasting serum insulin (μIU/mL)×fasting serum glucose (mM)/22.5.
Statistical analysis
The current analysis was restricted to 8433 participants who had completed fasting blood sampling and chilli food data collection. For baseline characteristics of participants, medians (interquartile range) were used for continuous variables, and counts and percentages for categorical variables. Unpaired t-tests were used to compare continuous variables, and chi-square tests to compare categorical variables between sex groups.
To examine whether the effects of chilli food eating habit differences on ferritin concentrations and HOMA-IR were independent of age, smoking use, alcohol consumption, nationality, and carbohydrate consumption, we performed linear regression analyses with ferritin levels and HOMA-IR as the dependent variables.
To examine whether differences of iron status and insulin resistance according to different chilli food habit populations were independent of age, smoking, alcohol consumption, nationality, and carbohydrate consumption, we used multivariable logistic regression analyses to examine the association between ferritin levels, HOMA-IR, and chilli food habits. The odds ratios (ORs) and 95% confidence intervals for the ferritin level and HOMA-IR were calculated for different chilli food habits, with the lowest chilli food type and chilli food frequency as the reference. Tests for trends across quartiles were computed by including a variable with the median value for each quartile as a continuous variable in the logistic regression models. All of the reported P values were two tailed, and those <0.05 were considered to be statistically significant. The ORs of all components were adjusted for age, nationality, smoking use, alcohol consumption, and carbohydrate consumption in different regression models. Statistical analyses were performed with SAS 9.2 (SAS Institute, Cary, NC, USA).
Results
In this survey, 8433 participants (3935 men and 4498 women) completed fasting blood samples and chilli food habit data collection. The baseline characteristics and the descriptive statistics of the study population are summarized in Table 1. The median age was 51.11 years (range, 18–99 years). Compared with women, men had higher body mass indices and systolic blood pressures. Men had a significantly higher rate of current smoking (54.89% versus 3.62%) and alcohol consumption (59.80% vs. 8.76%) than women.
Table 1.
Baseline Characteristics of Participants
| Total (n=8433) | Men (n=3935) | Women (n=4498) | Z for gender comparison | |
|---|---|---|---|---|
| Age, years | 51.11 (24.53–75.93) | 51.22 (24.23–75.95) | 50.99 (24.78–75.88) | 0.81 |
| BMIa, kg/m2 | 23.05 (18.23–29.62) | 23.09 (18.29–29.38) | 23.03 (18.20–29.74) | <0.05 |
| Waist circumferenceb, cm | 82.00 (66.50–100.10) | 84.00 (68.00–102.00) | 80.30 (65.20–99.10) | 0.80 |
| SBPc, mm Hg | 120.67 (100.00–160.00) | 122.00 (100.67–160.00) | 120.00 (97.67–161.33) | <0.001 |
| DBPc, mm Hg | 80.00 (62.00–100.00) | 80.67 (66.00–100.67) | 80.00 (60.67–100.00) | 0.08 |
| Smokers, n (%) | <0.001 | |||
| Never | 5836 (69.20) | 1516 (38.53) | 4320 (96.04) | |
| Former | 274 (3.25) | 259 (6.58) | 15 (0.33) | |
| Current | 2323 (27.55) | 2160 (54.89) | 163 (3.62) | |
| Alcohol use/last year, n (%) | <0.001 | |||
| No | 5686 (67.43) | 1582 (40.20) | 4104 (91.24) | |
| Yes | 2747 (32.57) | 2353 (59.80) | 394 (8.76) | |
| Education, n (%) | <0.001 | |||
| Low | 3656 (43.35) | 1336 (33.95) | 2320 (51.58) | |
| Medium | 2782 (32.99) | 1493 (37.94) | 1298 (28.66) | |
| High | 1995 (23.66) | 1106 (28.11) | 889 (19.76) | |
| History of diabetes | 890 (10.55) | 447 (11.36) | 443 (9.85) | <0.05 |
| History of cardiovascular disease | 82 (0.97) | 39 (0.99) | 43 (0.96) | 0.87 |
| History of stroke | 117 (1.39) | 79 (2.01) | 38 (0.84) | <0.001 |
| History of hypertension | 1114 (13.21) | 500 (12.71) | 614 (13.65) | 0.20 |
Data are presented as median (5th percentile–95th percentile) or as number (percent). Chi-square test for categorical variables, the unpaired t test or Mann–Whitney U test for continuous variables.
Information on BMI was available for 3904 men and 4478 women.
Information on waist circumference was available for 3894 men and 4457 women.
Information on SBP and DBP was available for 3933 men and 4495 women.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
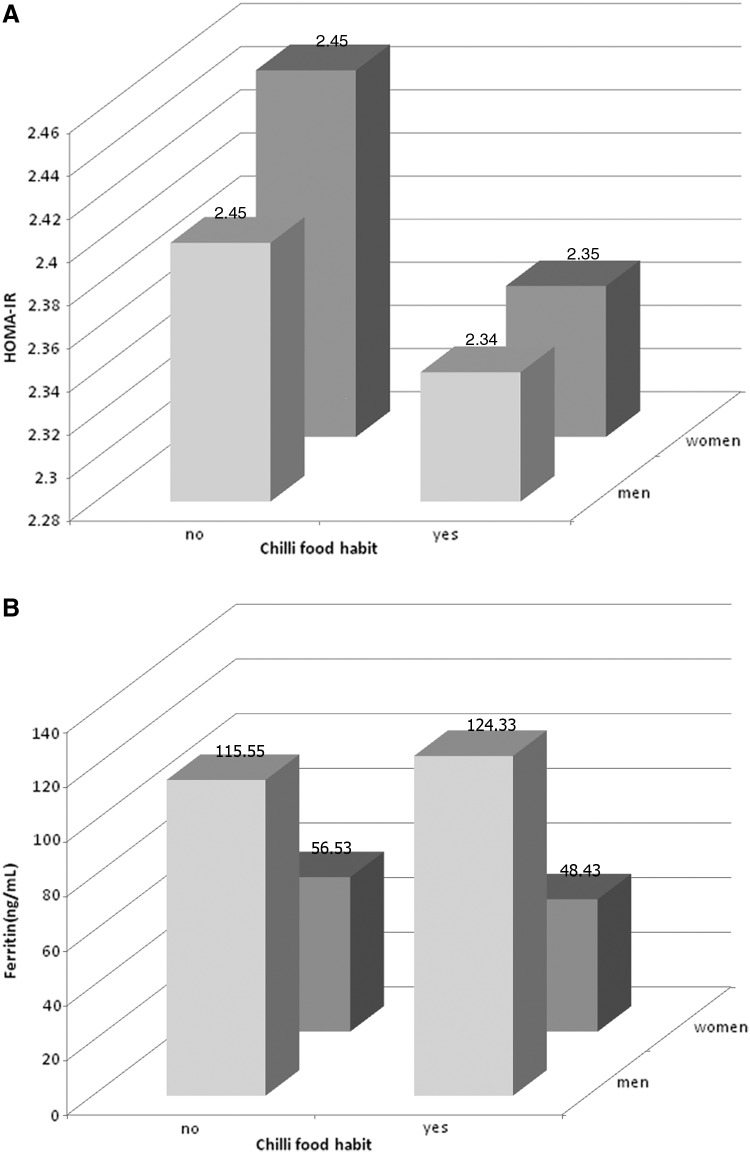
Based on the chilli food habit data, the study population can first be separated into two groups: the chilli food eating group (5881 participants) and the no chilli food eating group (2551 participants). There were significant differences in HOMA-IR for both groups in both men and women (P<.05); (Fig. 1A). The differences remained significant after further adjustment for age, nationality, smoking use, alcohol consumption, and carbohydrate consumption. Compared with the eating group, the no eating group had lower ferritin levels in men and higher ferritin levels in women (P<.05) (Fig. 1B). However, the difference became nonsignificant after adjusting for age in women (P=.35).
FIG. 1.
The HOMA-IR (A) and concentrations of ferritin (B) in different sex groups according to chilli food habits. HOMA-IR, homeostasis Model assessment of insulin resistance.
For the chilli food eating group, there was a significant difference in HOMA-IR (P<.05) for both men and women and in fasting serum Ferritin concentrations (P<.001) for women according to different chilli food types (Table 2).
Table 2.
Comparison of Ferritin Concentrations and Insulin Resistance in Different Chilli Food Types
| A little bit hot | Moderately hot | Very hot | P(trend) | |
|---|---|---|---|---|
| Men (n)a | 1330 | 1261 | 266 | |
| Ferritin (ng/mL) | 126.68 | 130.50 | 122.80 | 0.485 |
| HOMA-IR | 2.48 | 2.40 | 2.19 | <0.05 |
| Women (n)a | 1641 | 1190 | 194 | |
| Ferritin (ng/mL) | 60.52 | 58.75 | 46.46 | <0.001 |
| HOMA-IR | 2.40 | 2.47 | 2.12 | <0.05 |
One man and two women reported that information on chilli food types is unknown.
HOMA-IR, homeostasis model assessment insulin resistance.
The ORs for HOMA-IR decreased progressively across the chilli food levels (P<.05 for trend) in women and in men. After adjusting for age and nationality, the trend of the ORs of the men group remained significant. After adding smoking status, alcohol consumption, and carbohydrate consumption into the adjusted model, the trend significance disappeared (Table 3).
Table 3.
Comparison of Ferritin Concentrations and Insulin Resistance in Different Chilli Food Types
| A little bit hot | Moderately hot | Very hot | P (trend) | |
|---|---|---|---|---|
| Men | ||||
| Ferritin | 1 | 1.09 (0.95–1.25) | 1.00 (0.79–1.27) | 0.48 |
| Model 1a | 1 | 1.06 (0.92–1.21) | 0.98 (0.77–1.24) | 0.69 |
| Model 2b | 1 | 1.03 (0.90–1.19) | 0.94 (0.74–1.19) | 0.72 |
| HOMA-IR | 1 | 0.90 (0.78–1.03) | 0.76 (0.60–0.97) | <0.05 |
| Model 1 | 1 | 0.87 (0.76–1.00) | 0.74 (0.59–0.94) | <0.05 |
| Model 2 | 1 | 0.88 (0.76–1.01) | 0.80 (0.63–1.01) | 0.08 |
| Women | ||||
| Ferritin | 1 | 0.87 (0.76–1.00) | 0.64 (0.49–0.83) | <0.05 |
| Model 1 | 1 | 1.06 (0.93–1.22) | 0.84 (0.64–1.11) | 0.23 |
| Model 2 | 1 | 1.06 (0.92–1.21) | 0.83 (0.63–1.10) | 0.25 |
| HOMA-IR | 1 | 0.91 (0.80–1.04) | 0.73 (0.56–0.95) | <0.05 |
| Model 1 | 1 | 0.95 (0.83–1.08) | 0.77 (0.59–1.00) | 0.14 |
| Model 2 | 1 | 0.95 (0.83–1.08) | 0.75 (0.57–0.98) | 0.10 |
Model 1, odds ratio adjusted for age.
Model 2, odds ratio adjusted for model 1 plus nationality, smoking use, alcohol consumption, and carbohydrate consumption.
For chilli food eating frequencies, there was no significant difference in the fasting serum ferritin concentration and HOMA-IR for both sex groups (Tables 4 and 5).
Table 4.
Comparison of Ferritin Concentrations and Insulin Resistance in Different Chilli Food Eating Frequencies
| Sometimes | Often | Usually | P (trend) | |
|---|---|---|---|---|
| Men (n) | 1565 | 817 | 476 | |
| Ferritin (ng/mL) | 125.06 | 129.92 | 134.80 | 0.167 |
| HOMA-IR | 2.43 | 2.41 | 2.38 | 0.620 |
| Women (n) | 1715 | 812 | 500 | |
| Ferritin (ng/mL) | 58.70 | 55.30 | 61.75 | 0.167 |
| HOMA-IR | 2.39 | 2.38 | 2.48 | 0.467 |
Table 5.
Odds Ratios and Adjusted Odds Ratios of Ferritin Concentration and Insulin Resistance by Different Chilli Food Eating Frequencies
| Sometimes | Often | Usually | P (trend) | |
|---|---|---|---|---|
| Men (n)a | ||||
| Ferritin | 1 | 1.07 (0.92–1.24) | 1.13 (0.94–1.35) | 0.39 |
| Model 1a | 1 | 1.05 (0.90–1.23) | 1.12 (0.92–1.33) | 0.52 |
| Model 2b | 1 | 1.05 (0.90–1.23) | 1.09 (0.91–1.31) | 0.59 |
| HOMA-IR | 1 | 0.95 (0.82–1.11) | 0.91 (0.76–1.09) | 0.56 |
| Model 1 | 1 | 0.97 (0.83–1.13) | 0.94 (0.78–1.13) | 0.76 |
| Model 2 | 1 | 0.96 (0.83–1.12) | 0.94 (0.78–1.13) | 0.77 |
| Women (n)a | ||||
| Ferritin | 1 | 0.91 (0.78–1.05) | 1.08 (0.90–1.29) | 0.20 |
| Model 1 | 1 | 1.01 (0.86–1.17) | 1.12 (0.93–1.35) | 0.45 |
| Model 2 | 1 | 1.00 (0.86–1.17) | 1.15 (0.95–1.38) | 0.32 |
| HOMA-IR | 1 | 0.93 (0.80–1.08) | 1.02 (0.85–1.22) | 0.59 |
| Model 1 | 1 | 0.95 (0.82–1.11) | 1.02 (0.85–1.22) | 0.74 |
| Model 2 | 1 | 0.96 (0.82–1.11) | 1.03 (0.86–1.23) | 0.74 |
Data are presented as odds ratio (95% confidence interval).
Model 1, odds ratio adjusted for age.
Model 2, odds ratio adjusted for model 1 plus nationality, smoking use, alcohol consumption, and carbohydrate consumption.
CI, confidence interval.
Discussion
As common spices, chillies are a good source of vitamin C, carotene, and most B vitamins. They are also very high in potassium, magnesium, and iron. Their very high vitamin C content can substantially increase the uptake of nonheme iron from food such as beans and grains. However, as vegetables and fruits, they also contain significant amounts of polyphenols and phytates, which are dietary factors that can impair iron absorption. These factors have been shown repeatedly to influence iron absorption in single-food isotope studies, whereas in multifood studies with varied diet and multiple inhibitors and enhancers, the effect of single components has been, as expected, more modest. The influence of vitamin A, carotenoids, and nondigestible carbohydrates on iron absorption and the nature of the meat factor remain unresolved.2 Some researchers think that chillies' very high vitamin C content can substantially increase the uptake of nonheme iron from other ingredients in food, such as beans and grains. However, Tuntipopipat et al.3 indicated that being rich in phenolic compounds, chillies could be expected to bind iron (Fe)3+ in the intestine and inhibit iron absorption in humans. They concluded that both phenol quality and quantity determine the inhibitory effect of phenolic compounds on iron absorption.
On the other hand, some studies indicated that chilli consumption may attenuate postprandial hyperinsulinemia.1 Regular consumption of chilli may improve postprandial glucose, insulin, and energy metabolism, and attenuate postprandial hyperinsulinemia.12
Iron, a necessary trace element that participates in many biological oxidations and accumulates in tissue, can lead to pathological changes in the liver, heart, endocrine organs, and musculoskeletal system.13,14Some studies in patients with hemochromatosis or hematologic diseases indicated that increased accumulation of iron affects the synthesis and secretion of insulin by the pancreas15,16 and compromises insulin action in target tissues.17–19 Therefore, it might contribute to the development of insulin resistance. Ferritin is a ubiquitous intracellular protein that can store and release iron and act as a buffer against iron deficiency and iron overload. Ferritin is widely used as a clinical biomarker to evaluate iron status. Increased serum ferritin concentrations in nonpathologic conditions, reflecting subclinical iron overload, have been reported to be associated with insulin resistance and an increased risk of type 2 diabetes mellitus.5,20,21
Therefore, the influence of chilli food habits on iron status and insulin resistance needs more clarification and additional studies. In this analysis, we confirmed that the no chilli eating group had higher HOMA-IR levels than the chilli eating group among men and women (P<.05). The difference remained significant after further adjusting for age, nationality, smoking use, alcohol consumption, and carbohydrate consumption. Even after adjusting by different regression models, the trend of ORs for HOMA-IR decreased progressively across the chilli food levels (P<.05 for trend) in men and remained significant. However, there was no significant difference in fasting serum ferritin concentrations and HOMA-IR among different chilli food eating frequencies in both sex groups. These results indicated that chilli food habits in the Chinese diet may affect insulin resistance. Eating chilli food can decrease insulin resistance.
There was no significant difference in iron absorption and insulin resistance components according to different chilli food eating frequencies in both sex groups. This indicates that the type of chillies may influence insulin resistance more than the quantity of chillies, which is consistent with the results of the study by Tuntipopipat et al.3
All the results indicated no significant differences in fasting serum ferritin concentrations on chilli food eating, chilli food types, and chilli food frequencies. The relationship between chilli and iron absorption needs further investigation.
To our knowledge, this is the largest population-based study to describe the association between chilli food habits with iron status and insulin resistance in the Chinese population. One of the advantages of our study is that the data came from a large-scale longitudinal, household-based survey. Having a much larger sample size can provide a more representative sample of the Chinese population. The participants in this study were recruited from nine provinces, including urban, suburban, town, and village population, representing a wide variety of Chinese and their food cultures.
We have a few limitations as well. This cross-sectional analysis does not examine temporal changes in iron status and insulin resistance owing to the fact that biomarker data were only collected in the 2009 round of the CHNS. Also, due to the limitations of the survey design, some key variables are not available in this study.
Chilli food habits, including chilli food eating and chilli food type, were associated with insulin resistance in the Chinese population. Additional studies are needed to elucidate mechanisms of action and to establish causal inference. This may help to reduce diabetes or prediabetes.
Acknowledgments
The authors thank all the participants in China and the United States who were involved in the China Health and Nutrition Survey, in the blood sample test work, and in the related pilot studies, particularly Shengkai Yan, Shuang Li, Hongbing Jia, Hui Yang, and Bing Zhang. The authors also thank Shufa Du, Jim Terry, and Barry M. Popkin for their comments and technical support.
This study was supported by the NIH (R01-HD30880, DK056350, R24 HD050924, R01-HD38700, and R21DK089306) and Fogarty International Center, with added financial support from the Chinese Center for Disease Control and Prevention, National Institute of Nutrition and Food Safety, and the China-Japan Friendship Hospital, Ministry of Health of China (the National 12th Five-year Technology-based Plan Topic—2012BAH24F00).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ahuja KDK, Robertson IK, Geraghty DP, Ball MJ: Effects of chilli consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr 2006;84:63–69 [DOI] [PubMed] [Google Scholar]
- 2.Hurrell R, Egli I: Iron bioavailability and dietary reference values. Am J Clin Nutr 2010;91:1461S–1467S [DOI] [PubMed] [Google Scholar]
- 3.Tuntipopipat S, Judprasong K, Zeder C, et al. : Chilli, but not turmeric, inhibits iron absorption in young women from an iron-fortified composite meal. J Nutr 2006;136:2970–2974 [DOI] [PubMed] [Google Scholar]
- 4.Huang J, Jones D, Luo B, et al. : Iron overload and diabetes risk: a shift from glucose to fatty acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes 2011;60:80–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CH, Kim HK, Bae SJ, Park JY, Lee KU: Association of elevated serum ferritin concentration with insulin resistance and impaired glucose metabolism in Korean men and women. Metabolism 2011;60:414–420 [DOI] [PubMed] [Google Scholar]
- 6.China Health and Nutrition Survey. Carolina Population Center, University of North Carolina, Chapel Hill, NC, USA, and National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, Ministry of Health, Beijing, China: Available online at www.cpc.unc.edu/projects/china (accessed October31, 2013) [Google Scholar]
- 7.Ng SW, Norton EC, Popkin BM: Why have physical activity levels declined among Chinese adults? Findings from the 1991–2006 China Health and Nutrition Surveys. Soc Sci Med 2009;68:1305–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parvanta SA, Brown JD, Du S, Zimmer CR, Zhao X, Zhai F: Television use and snacking behaviors among children and adolescents in China. J Adolesc Health 2010;46:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tudor-Locke C, Ainsworth BE, Adair LS, Du S, Popkin BM: Physical activity and inactivity in Chinese school-aged youth: the China Health and Nutrition Survey. Int J Obes 2003;27:1093–1099 [DOI] [PubMed] [Google Scholar]
- 10.Popkin BM, Du S, Zhai F, Zhang B: Cohort Profile: The China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 2009;39:1439–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Popkin B, Keyou G, Zhai F, Guo X, Ma H, Zohoori N: The nutrition transition in China: a cross-sectional analysis. Eur J Clin Nutr 1993;47:333. [PubMed] [Google Scholar]
- 12.Green N, Ahuja K, Robertson I, Ball M: Effects of different amounts of chilli consumption on postprandial glucose and insulin. Am J Clin Nutr 2009;84:63–69 [DOI] [PubMed] [Google Scholar]
- 13.Wolff S: Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 1993;49:642. [DOI] [PubMed] [Google Scholar]
- 14.Powell L, George D, McDonnell S, Kowdley K: Diagnosis of hemochromatosis. Ann Internal Med 1998;129:925. [DOI] [PubMed] [Google Scholar]
- 15.Haan B, Scherrer J, Stauffacher W, Pometta D: Iron excess, early glucose intolerance and impaired insulin secretion in idiopathic haemochromatosis. Eur J Clin Invest 1973;3:179–187 [DOI] [PubMed] [Google Scholar]
- 16.Cario H, Holl R, Debatin K, Kohne E: Insulin sensitivity and beta-cell secretion in thalassaemia major with secondary haemochromatosis: assessment by oral glucose tolerance test. Eur J Pediatr 2003;162:139. [DOI] [PubMed] [Google Scholar]
- 17.Niederau C, Berger M, Stremmel W, et al. : Hyperinsulinaemia in non-cirrhotic haemochromatosis: impaired hepatic insulin degradation? Diabetologia 1984;26:441. [DOI] [PubMed] [Google Scholar]
- 18.Merkel P, Simonson D, Amiel S, et al. : Insulin resistance and hyperinsulinemia in patients with thalassemia major treated by hypertransfusion. New Engl J Med 1988;318:809. [DOI] [PubMed] [Google Scholar]
- 19.Dmochowski K, Finegood D, Francombe W, Tyler B, Zinman B: Factors determining glucose tolerance in patients with thalassemia major. J Clin Endocrinol Metab 1993;77:478. [DOI] [PubMed] [Google Scholar]
- 20.Fernández-Real JM, Ricart-Engel W, Arroyo E, et al. : Serum ferritin as a component of the insulin resistance syndrome. Diabetes Care 1998;21:62–68 [DOI] [PubMed] [Google Scholar]
- 21.Wrede C, Buettner R, Bollheimer L, Schölmerich J, Palitzsch K, Hellerbrand C: Association between serum ferritin and the insulin resistance syndrome in a representative population. Eur J Endocrinol 2006;154:333–340 [DOI] [PubMed] [Google Scholar]