Abstract
In regenerative medicine approaches involving cell therapy, selection of the appropriate cell type is important in that the cells must directly (differentiation) or indirectly (trophic effects) participate in the regenerative response. Regardless of the mode of action of the cells, angiogenesis underlies the success of these approaches. Stem cells derived from tooth tissues, specifically the periodontal ligament of teeth (periodontal ligament stem cells [PDLSCs]), have recently been identified as a good source of multipotent cells for cell therapies. PDLSCs have demonstrated properties similar to mesenchymal stem cells (MSCs), yet, unlike MSCs, their vascular potential has not been previously demonstrated. Thus, the aim of this study was to determine if PDLSCs could modulate angiogenesis. In comparison to MSCs and stem cells derived from tooth pulp tissues (SHEDs), we first determined if PDLSCs released soluble proangiogenic factors with the capacity to induce vessel formation by endothelial cells (ECs). Next, the ability of PDLSCs to modulate angiogenesis was examined through their cotransplantation with ECs in subcutaneous sites of immunocompromised mice. Finally, the stability of the PDLSC-mediated vasculature was determined through evaluation of the maturity and functionality of the vessels formed following PDLSC transplantation. It was determined that PDLSCs produced appreciable levels of vascular endothelial growth factor and basic fibroblast growth factor-2, and additionally, were able to initiate in vitro angiogenesis of ECs comparable to MSC- and SHED-mediated angiogenesis. In vivo cotransplantation of ECs with PDLSCs significantly (>50% increase) enhanced the number of blood vessels formed relative to transplantation of ECs alone. Finally, vessels formed following PDLSC cotransplantation were more mature and less permeable than those formed after transplantation of EC alone. These data demonstrate for the first time that PDLSCs have vascular potential, which could make them a very attractive cell population for utilization in regenerative cell therapies.
Introduction
It has been well established that the ability of a tissue to regenerate is dependent upon the nutrient support supplied by the local vasculature of the site.1,2 The developing vascular network of a regenerating site is typically dependent upon resident cells within the local microenvironment; however, because regeneration depends upon the new vasculature, strategies aimed to bolster the angiogenic process have become a focus of tissue engineering and regenerative therapies.2–4 One approach to enhancing angiogenesis involves cell transplantation, whereby delivery of different stem and vascular progenitor cells can serve to enhance angiogenesis directly (via differentiation into capillaries) or indirectly (through trophic effects on host vascular cells).5–7 Yet, a limiting factor in cell transplantation strategies is the identification of a readily available source of cells.
It has been demonstrated that mesenchymal stem cells (MSCs) derived from bone marrow have angiogenic properties and the ability to support angiogenesis when used clinically and in various preclinical model systems. However, because their procurement requires an invasive bone marrow aspiration procedure, the emergence of alternative sources of MSCs offers a less invasive and more user-friendly approach for the isolation of these cells. Recently, multipotent stem cells derived from internal (dental pulp stem cells [SHEDs]) and external (periodontal ligament stem cells [PDLSCs]) tooth tissues of extracted teeth have been shown to express phenotypes of osteoblasts, chondrocytes, adipocytes, fibroblasts, and neuronal cell types.8–10 Although there is evidence demonstrating that SHEDs, derived from within the highly vascular dental pulp of teeth, can differentiate into the functional endothelium,11,12 there is currently no evidence that PDLSCs, derived from the outer periodontal ligament of teeth, can support angiogenesis. Thus, it would be of great interest to determine whether or not PDLSCs have angiogenic potential, specifically, the ability to support the establishment of a functional vasculature.
Our group has developed and utilized different three-dimensional (3D) culture and in vivo systems for evaluating the ability of different interstitial cell types, such as MSCs, to establish and support angiogenesis. PDLSCs have been implicated as having phenotypic properties similar to those of MSCs, yet, the angiogenic potential of these cell populations has not been examined. Thus, the aim of this study was to address the hypothesis that PDLSCs have the ability to support angiogenesis. In this report, we investigated the effects of PDLSCs on endothelial cells' (EC) ability to initiate capillary tube formation in vitro and to establish a functionally stable vasculature in vivo.
Methods
Derivation of PDLSCs, SHEDs, and MSCs
PDLSCs were harvested as previously described.9 Briefly, PDLSCs were scraped from the root surface of a tooth into a p60 dish containing the alpha-minimum essential medium (αMEM; Gibco). After collection, the cells were centrifuged at 1600 rpm for 5 min at room temperature. The supernatant was aspirated and the cells were resuspended in a phosphate-buffered saline (PBS; Gibco #14190) solution with 4 mg/mL Dispase II (Roche #04942078001) and 2 mg/mL collagenase type II (Worthington # LS004196) and incubated at 37°C for 60 min. The enzyme solution was inactivated with 5 mL of αMEM, 15% fetal bovine serum (FBS), and 100 μM ascorbic acid 2 phosphate (ASAP; Sigma A-8960) and centrifuged at 1600 rpm for 5 min at room temperature. Cells were resuspeneded in 5 mL of αMEM, 15% FBS, and 0.1 mM ASAP and transferred to T-25 flasks. The medium was changed the next day and then every 2–3 days. SHEDs were isolated from extracted human teeth,13 as previously described, and human MSCs isolated from the iliac crest were commercially purchased (Lonza).
Flow cytometry and multipotency assay
Flow cytometry was performed to determine the expression of MSC markers according to the criteria proposed by the Mesenchymal and Tissue Stem Cell Committee of the International Society of Cellular Therapy (ISCT): CD45, CD90, CD73, CD105, CD34, CD19, and CD14.14 PDLSCs were harvested from T150 flasks, transferred into tubes, washed with PBS, and then incubated with the CD16/CD32 blocking solution, followed by incubation with specific antibodies conjugated with a fluorochrome (Biolegends). Cells were washed extensively at each step. Analysis was performed on either a Beckman Coulter MoFlo Astrios or CyAn flow cytometer and the following list of antibodies includes
PE anti-human CD105 antibody
FITC anti-human CD90 (Thy1) antibody
PerCP/Cy5.5 anti-human CD45 antibody
PE isotope antibody
FITC isotype antibody
PerCP/Cy5.5 isotype antibody
Multipotency of PDLSC cultures was determined by Alcian blue (chondrogenic), Oil Red O (adipogenic), and von Kossa (osteogenic) staining according to previously described methods.14 Briefly, following subculture of cells at >90% confluency, cells were induced toward osteogenic, adipogenic, and chondrogenic fates in the appropriate media according to previously described methods.15 Before staining, PDLSCs were cultured for 21 days with the respective induction media being changed every 2–3 days.
Angiogenic cytokine secretion
Overnight conditioned media from PDLSCs, MSCs, and SHEDs were collected and evaluated for the presence of vascular endothelial growth factor and basic fibroblast growth factor-2 (bFGF-2) using ELISAs for these two growth factors, according to the manufacturer's instructions (R&D Systems). Concentrations of growth factor were normalized to the number of cells.
Three-dimensional microcarrier angiogenesis assay
The angiogenic potential of various interstitial cell types was assessed using a microcarrier-based angiogenesis assay as described previously.16 Primary human umbilical vein ECs were cultured in the Endothelial Growth Medium-2 (Lonza), trypsinized at 90% confluence, and counted. Four million cells were combined with 10,000 Cytodex 3 microcarrier beads (Sigma-Aldrich) in 5 mL of EGM-2 and pipetted into an upright T-25 culture flask. The suspension was cultured for 4 h in a 37°C, 5% CO2 incubator and agitated gently every 30 min. After cell attachment, the suspension was removed and an additional 5 mL of EGM-2 was added. The suspension was then pipetted into a fresh T-25 and cultured in normal cell culture position overnight.
The following day, fibrin gels were prepared. Bovine fibrinogen (Sigma-Aldrich) was dissolved in the serum-free EGM-2 and sterile filtered. Gels were created by combining 2.5 mg/mL of fibrinogen solution containing approximately 100 HUVEC-coated microcarriers with bovine thrombin (Sigma-Aldrich), and pipetting the resulting solution into wells in a 24-well plate. The beads were allowed to settle for 5 min, and then the solution was incubated at 37°C for 25 min to finish gelation. During the incubation, PDLSCs, SHEDs, and MSCs were trypsinized and counted. After gelation was complete, 25,000 cells of the appropriate type were plated on top of each gel. Cocultures were incubated in a 37°C, 5% CO2 incubator. Media were changed every other day.
In vivo angiogenic potential of PDLSCs
Subcutaneous implantation of PDLSCs
Gelatin scaffolds (Gelfoam®; Pfizer) containing 1×106 ECs alone or in combination with 1×106 PDLSCs were implanted into subcutaneous pockets on the dorsal region of 7- to 9-week-old male severe combined immunodeficient (SCID) mice, as previously described17 (cb17/SCID) (Taconic Laboratories). Briefly, a small (about 1.5 cm) longitudinal incision was made into the skin at the center of a shaved area of the back. Blunt dissection of subcutaneous fascial tissue was performed to create pockets for implants to be placed and the incisions were closed with interrupted sutures. At 4 weeks, animals were sacrificed and implants were analyzed for blood vessel formation.
Cell transplantation for histological evaluation of angiogenesis
Implants were retrieved at various time points and fixed overnight in aqueous buffered zinc formalin (Z-Fix; Anatech). Implants were then transferred to 70% EtOH until they were processed and embedded in paraffin. Paraffin-embedded matrices were cut into serial sections (5 μm thick) and placed on glass slides for histological analysis. Tissue sections were stained with Gill's 3 hematoxylin (Sigma-Aldrich) and aqueous eosin Y solution (Sigma-Aldrich) (H&E) to visualize the overall tissue morphology. Immunostaining of human-derived blood vessels was performed using an anti-human CD31 antibody (DakoCytomation), respectively, as described previously.18 Tissue sections were visualized and photographed with the E-800 light microscope (Nikon). Five samples from each condition were analyzed manually and digitally. Blood vessels present in the implants were analyzed for total number. Blood vessels were identified in hematoxylin and eosin-stained tissues, at×200 magnification, by defined lumens and the presence of red blood cells within their boundaries. Mature vessels were defined as vessels whose diameter was >20 μm.
Cell transplantation for evaluation of vessel permeability with labeled tracer
For subcutaneous implants involving fluorescent labeling of dextran molecules, cell mixtures consisting of either ECs alone or in combination with PDLSCs or MSCs were spun down and resuspended in a previously prepared fibrinogen solution at a final concentration of 10 million cells/mL. Immediately before injection, 5% FBS and 6 μL of thrombin solution (50 U/mL; Sigma-Aldrich) were added to 300 μL of fibrinogen cell solution. Solutions were immediately injected subcutaneously on the dorsal flank of the mouse, with two implants per animal. Animals were kept stationary for 5 min to allow for implant polymerization, and were then placed in fresh cages for recovery. Five replicates were completed for each sample type (ECs only, EC-BMSCs, EC-PDLSCs).
Implants were retrieved after 7 days following systemic administration of a fluorescent tracer. Mature capillaries are impermeable to dextrans over a molecular weight of 65 kDa.19 Therefore, a 70-kDa Texas Red-conjugated dextran (λex/em of 595/615 nm; Invitrogen) was chosen as a functionally defining tracer as described previously.20,21 This dextran molecule contains free lysines, which are fixable in 4% paraformaldehyde (PFA). Before implant retrieval, mice were placed in a restraint device and 100 μL of a 5 mg/mL dextran solution in PBS was then injected through the tail vein. After injection, mice were placed into fresh cages, and the tracer was allowed to circulate systemically for 10 min. Animals were then euthanized and implants were surgically excised.
For immunofluorescence, explants were fixed in 4% PFA for 1 h and then moved to 0.4% PFA overnight. Implants were rinsed for three washes of 5 min each with PBS, pH 7.4, and incubated in 30% sucrose for 48 h at 4°C. Next, implants were transferred to a solution containing two parts 30% sucrose and one part optimal cutting temperature (OCT) embedding compound (Andwin Scientific) for 24 h. Each sample was embedded in 100% OCT in a disposable plastic mold (Fisher Scientific), and flash-frozen on the surface of liquid nitrogen. Frozen sections were generated from each sample by the histology core at the University of Michigan School of Dentistry. For staining, slides were prewarmed for 20 min at 25°C and then rinsed with three washes of PBS. Sections were blocked with 5% goat serum in PBS to eliminate nonspecific protein binding. The primary antibody (either anti-rabbit human CD31; Santa Cruz Biotechnologies or human anti-mouse alpha smooth muscle actin [αSMA]; Abcam) was diluted 1:50 in 5% goat serum and added to samples for an overnight incubation at 4°C. The unbound antibody was removed at the end of the incubation with three washes with PBS. The secondary antibody (Alexa Fluor-488 goat anti-rabbit; Invitrogen) was added to tissues at a 1:100 dilution and tissues were incubated 30 min at room temperature. The unbound antibody was removed by an additional three washes with PBS. Slides were then mounted with VectaShield (Vector Labs) and covered with a #1 glass coverslip before imaging. Imaging was performed on an Olympus IX81 microscope with a 100-W high pressure mercury lamp (Olympus) and a Hammamatsu camera (Bridgewater). Representative images were chosen for each condition.
Statistical analysis
Statistical analyses were performed with Instat software (Graphpad Software). Descriptive analyses were performed initially, followed by the use of Student's t-tests. Statistical significance was measured as p<0.05. All data are plotted as mean±SE. Statistically significant differences in histomorphometric analysis were determined by using two-tailed Student's t-tests, and statistical significance was defined by p<0.05.
Results
Multipotency of PDLCs
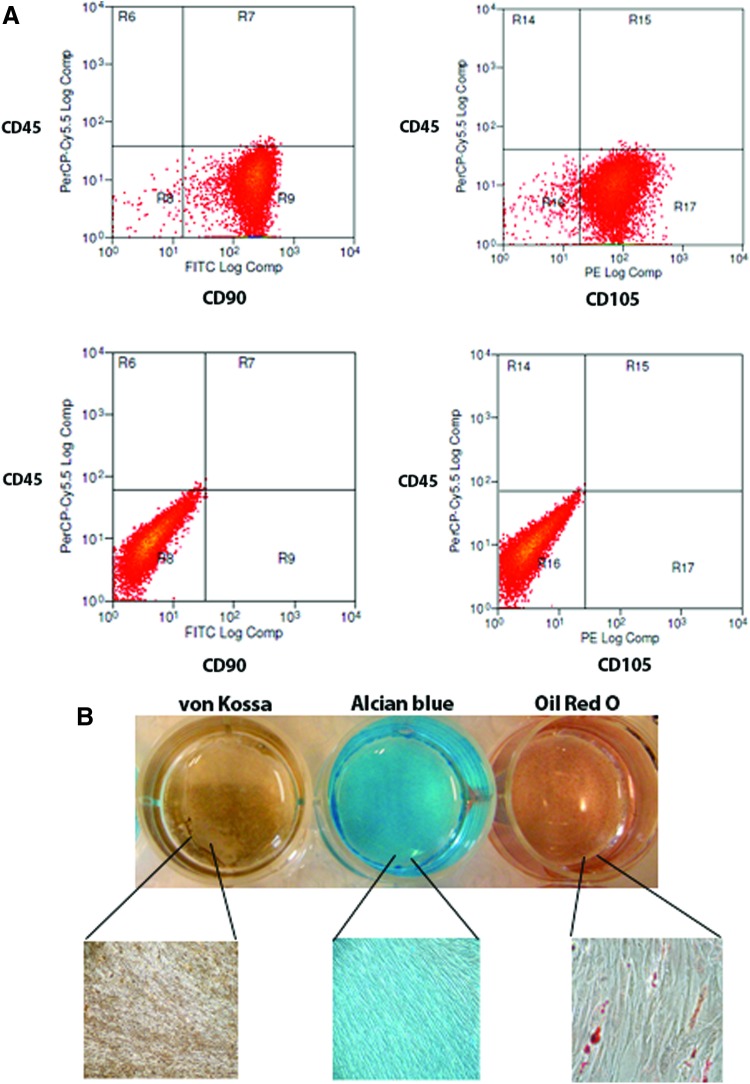
Cell surface marker expression of CD90 and CD105 was evaluated for PDLSCs in that these markers are all considered important determinants in defining stemness associated with MSCs.14 Following isolation and cell expansion, PDLSCs expressed high levels of CD90 (>98%), and CD105 (>85%) and did not express the hematopoietic stem cell marker CD45 (Fig. 1A). The stemness of PDLSCs was also confirmed through their capacity to differentiate toward different cellular lineages following culture under adipogenic, chondrogenic, and osteogenic conditions. After 3 weeks of culture, commitment toward different tissue lineages was evaluated through lineage-specific staining of induced cells. PDLSCs were stained with Oil Red O, Alcian blue, and von Kossa. In the uninduced control culture conditions, the Oil Red O, Alcian blue, and von Kossa stainings were all negative. In adipogenic conditions, Oil Red O staining was used to detect intracellular lipid-rich vacuoles and morphological changes in cell shape. The results confirmed that cells were differentiated toward an adipogenic lineage (Fig. 1B). Similarly, in cells grown under chondrogenic conditions, the presence of chondrogenic proteoglycans was indicated by positive Alcian blue staining (Fig. 1B), confirming chondrogenic differentiation. Under osteogenic culture conditions, deposition of mineralized matrix indicative of osteoblasts was evident through positive von Kossa staining (Fig. 1B).
FIG. 1.
Periodontal ligament stem cells' (PDLSCs) stemness. (A) PDLSCs express mesenchymal stem cell markers CD90 and CD105 and lack expression of the hematopoietic stem cell marker CD45 (bottom panels show negative controls). (B) Photograph and corresponding photomicrographs show multipotent mesenchymal differentiation of PDLSCs as measured by phenotypic expression of osteogenic mineralized matrix (von Kossa), chondrogenic proteoglycans (Alcian blue), and adipogenic lipid vacuoles (Oil Red O); low magnification images were taken at 40×; high magnification images are shown at 200×. Color images available online at www.liebertpub.com/tea
Angiogenic growth factor production
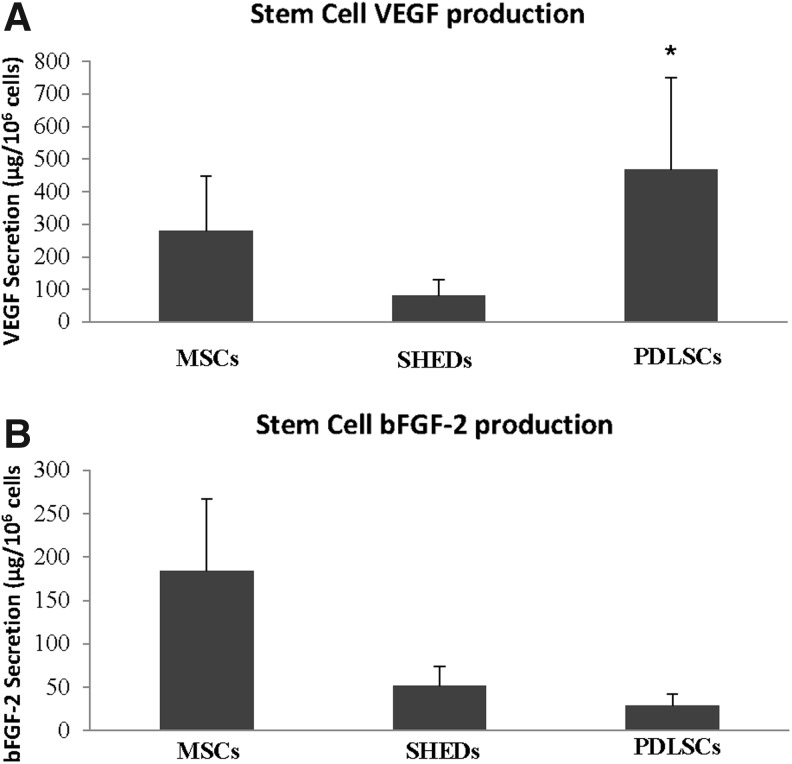
PDLSCs were evaluated in their ability to produce the highly angiogenic cytokine vascular endothelial growth factor (VEGF), and compared with production from MSCs and SHEDs, with both of these cell types being known to produce VEGF.22–25 PDLSCs showed equivalent levels of VEGF production to MSCs (Fig. 2A) and significantly (p<0.05) higher levels compared with SHEDs.
FIG. 2.
Angiogenic cytokine production. (A) Twenty-four-hour vascular endothelial growth factor (VEGF) secretion from mesenchymal stem cells (MSCs), dental pulp stem cells (SHEDs), and PDLSCs; (B) 24-h basic fibroblast growth factor-2 (bFGF-2) secretion from MSCs, SHEDs, and PDLSCs. *Indicates p<0.05 (compared to SHEDs).
PDLSCs were also evaluated for their ability to produce another proangiogenic cytokine, bFGF. Similar to VEGF production, bFGF secretion was detected from PDLSCs, MSCs, and SHEDs (Fig. 2B). All cell types produced significantly lower concentrations of bFGF than VEGF, yet differences between the cell types were not statistically significant.
PDLSCs support EC vessel formation
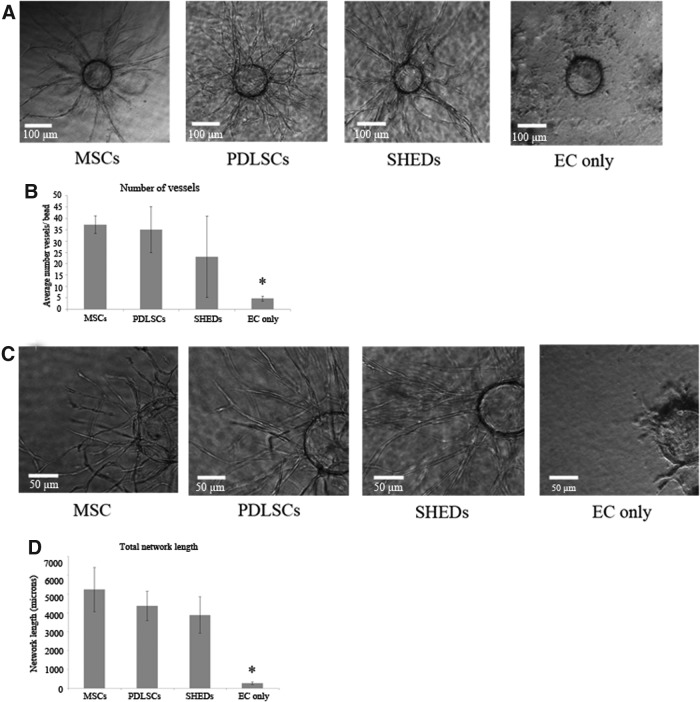
ECs were grown on microbeads placed in a 3D gel through which different angiogenic stimuli can induce EC differentiation and formation of capillary vessels. With this system, our group has previously demonstrated that interstitial cells such as MSCs have the ability to support EC differentiation into capillary tubes.26 Figure 3A shows vessel migration from microbeads under conditions where PDLSCs, MSCs, and SHEDs were cocultured in the 3D gel. Compared with when no supporting cells are present in the coculture, PDLSCs clearly supported EC vessel formation comparable to that seen with MSCs. SHEDs also supported angiogenesis to the same degree as MSCs. The total number of vessels formed in these conditions was quantified and demonstrated that PDLSCs were able to induce an equivalent number of vessels than those produced by MSCs and SHEDs (Fig. 3B). In addition to evaluating the number of vessels formed, an evaluation of the robustness of the angiogenic response was evaluated by determining the total network length of vessels formed in response to PDLSCs. In higher magnification, it was clear that MSCs, PDLSCs, and SHEDs were able to induce vessel structures that had branching and luminal morphologies indicative of EC capillary formation (Fig. 3C). Quantitatively, the total network length of vessels formed from ECs was the same between conditions containing cocultured MSCs, PDLSCs, and SHEDs (Fig. 3D). Again, EC cultured alone were not able to illicit this angiogenic response.
FIG. 3.
PDLSCs stimulate endothelial cell (EC) vessel formation. (A) Photomicrographs (10×magnification) of EC-coated microbeads placed within fibrin gels containing MSCs, PDLSCs, SHEDs, or no cells. Images show differentiating ECs forming vessels elongating from microbeads. (B) Quantification of the number of vessels formed by ECs when cultured in the presence of MSCs, PDLSCs, SHEDs, or no other interstitial cell type. (C) High magnification (20×) images of vessels highlighting branching of tubular networks forming from ECs on microbeads. (D) Quantification of the total vessel network length of tubular networks formed by ECs when cultured in the presence of MSCs, PDLSCs, SHEDs, or no other interstitial cell type. *Indicates p<0.05 (compared to MSCs, PDLSCs, and SHEDs).
In vivo angiogenic potential of PDLSCs
Histological analysis
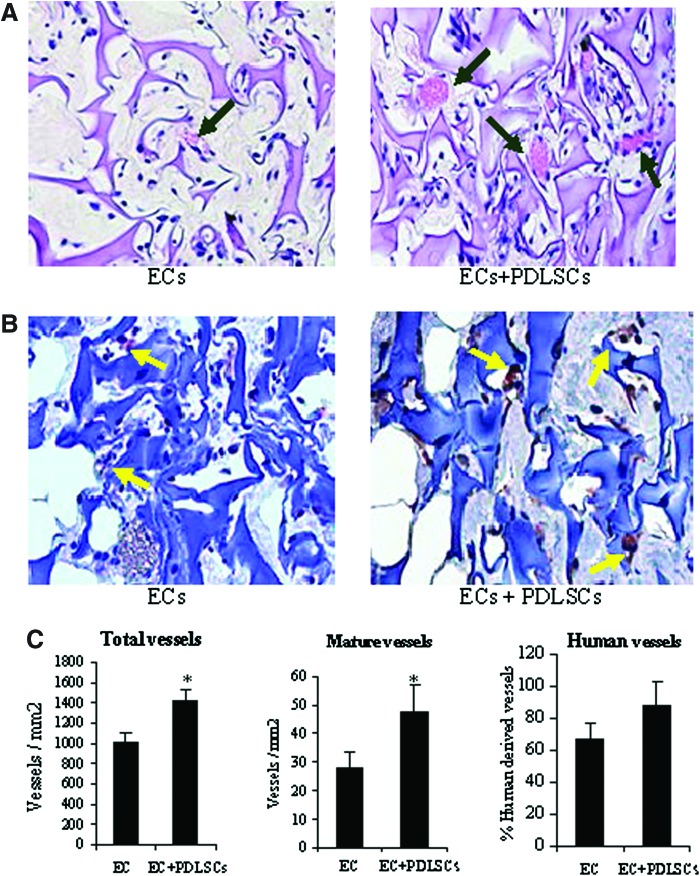
Four weeks after subcutaneous implantation of ECs, and ECs in combination with PDLSCs, implants were harvested and evaluated for neovascularization within the scaffolds. In evaluation of total blood vessel formation between ECs and ECs cotransplanted with PDLSCs, PDLSCs appeared to form more vessels throughout the implant (Fig. 4A). In evaluation of the maturity of the vessels between the two conditions, there appeared to be a greater number of larger, more mature vessels in the condition where the PDLSCs were cotransplanted. Of the vessels formed, we also wanted to determine the percentage of vessels that were of human origin (derived from transplanted cells) and thus sections were immunostained for detection of human CD31 antigen (Fig. 4B). Images clearly showed vessels in both groups derived from human cells and chimeric vessels derived from both human and host mouse cells. Quantitative analysis confirmed that there was a statistically significant increase in the number of total vessels formed and in the number of mature vessels formed when ECs were cotransplanted with PDLSCs (Fig. 4C). In the EC condition, 67% of the vessels were human derived or chimeric vessels from human and host origin. In the condition with PDLSCs, although 86% of the cells were human derived, this difference was not statistically significant.
FIG. 4.
PDLSC effect on in vivo angiogenesis. (A) Photomicrographs of histological sections (H&E stained) of subcutaneous implants containing ECs alone or ECs+PDLSCs (arrows point to blood vessels). (B) Photomicrographs of histological sections immunostained for human CD31 to identify blood vessels (arrows) formed from implanted human cells (ECs or PDLSCs). Quantification of (C) total vessels, human-derived vessels, and mature (diameter>20 μm) vessels between conditions. Values represent mean±SE (n=5). *p<0.05. Color images available online at www.liebertpub.com/tea
Vessel permeability
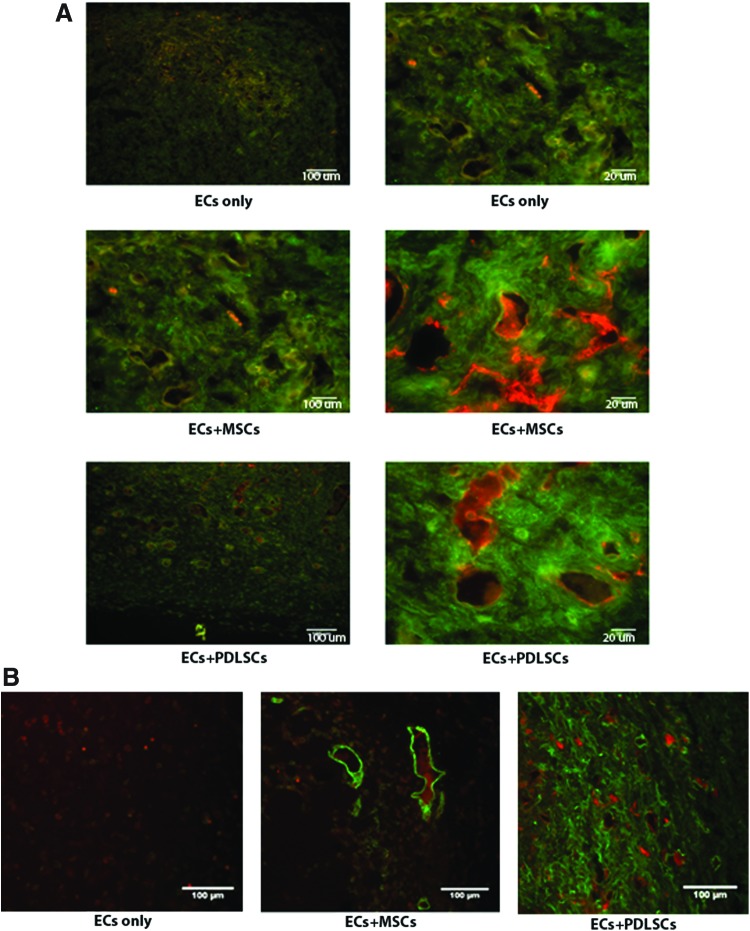
Evaluation of vessel maturation was further examined in another mouse model through the use of fluorescent labeling of vessels and their ability to maintain fluorescently labeled dextran molecules following their injection. In this model, we have previously demonstrated that MSC cotransplantation with ECs yields less permeable and more mature vessels than those formed from EC transplantation alone.20,21 In the present study, fluorescent staining showed more dextran contained within the vessels where either MSCs or PDLSCs were cotransplanted, relative to the condition where only ECs were tranplanted (Fig. 5A). Again, the appearance of vessels formed in these conditions, relative to transplantation of ECs alone, was that of larger, more mature vessels, characteristic of vessels that are associated with pericytes. Immunostaining for pericytes with αSMA demonstrated that transplanted PDLSCs were widely dispersed around vessels (Fig. 5B), confirming their pericytic role in the development of a de novo, functional vasculature.
FIG. 5.
PDLSC effect on functional vessel formation. (A) Red fluorescent 70 kDa dextran was administered systemically to mice before implant removal on day 7. Following fixation and cryosectioning, tissues were stained for human CD31 (green) and imaged. Stromal cell identity appeared to modulate the retention of dextran within the vessel lumen, which indirectly suggests variable maturity of the vessels across conditions. Implants contain ECs only, EC+MSCs, or ECs+PDLSCs. Images are 10×magnification; scale bar=100 μm and 40×magnification of the same region; scale bar=20 μm. (B) Cryosections from day 7 containing red dextran were stained for human alpha smooth muscle actin (αSMA, green) and imaged at 10×magnification. Implants contain ECs only, ECs+MSCs, or ECs+PDLSCs. Color images available online at www.liebertpub.com/tea
Discussion
Cell transplantation approaches in regenerative medicine rely heavily upon the establishment of a supporting vasculature to sustain tissue regeneration. In this report, we examined the angiogenic properties of PDLSCs in vitro and in vivo. PDLSCs secrete angiogenic cytokines and in a 3D culture system and demonstrated the ability to induce EC vessel formation through secretion of soluble factors. Additionally, when cotransplanted with ECs, PDLSCs significantly enhanced angiogenesis and even further, produced vessels that were larger and functionally more mature than those formed following transplantation of ECs alone.
It has been previously demonstrated that MSCs produce angiogenic factors, which can support the development of a functional vasculature and a number of studies have implicated VEGF as being a major mediator of this response.22,27,28 Oral-derived stem cells exhibit similar genotypic and phenotypic expression profiles to MSCs and the production of VEGF from PDLSCs validates yet another parallel between these cell types. Both SHEDs and PDLSCs are anatomically found in highly vascular regions, located in close proximity to blood vessels within the tooth (SHEDs) and surrounding the tooth (PDLSCs). It is thus not surprising that they have the capacity to produce factors that could initiate or sustain an angiogenic response, as this could be a physiological mechanism used when needed in response to changes in the local microenvironment (i.e., injury).
In demonstrating that PDLSCs produced proangiogenic cytokines VEGF and b-FGF, we wanted to determine whether or not soluble secretion of angiogenic factors played a role in the initiation of angiogenesis. Studies from our group and others have previously demonstrated that this model can be used to examine the effects of soluble signals on the EC's ability to form 3D vessel networks.29,30 Cells that have been implicated as pericytes or supporting cells show the capacity to induce EC vessel formation when cocultured in this system, without direct cell–cell contact. In this system, ECs will not spontaneously form elongated tubes and branches without coculture using an angiogenic supporting cell type. When we cultured both MSCs and PDLSCs, striking similarities were observed in the vessels and branching networks seen not only between these two cell types, but also in comparison to SHEDs. From a therapeutic standpoint, these findings are significant in that they provide evidence that PDLSCs have the capacity to elicit an angiogenic response, which could facilitate regeneration, regardless of the tissue of interest. There is evidence that ECs induce osteogenic and odontogenic induction of PDLSCs,31,32 yet, our study is the first report demonstrating a reciprocal relationship of the PDLSCs on ECs. The production of VEGF most likely contributes to this response, however, our study was not designed to address to what extent VEGF plays a role.
It was clear from the in vitro studies that PDLSCs had angiogenic properties, yet, we wanted to determine whether the vascular potential of these cells had an effect on a functional vasculature. Thus, PDLSCs were subcutaneously cotransplanted with ECs in an in vivo model of angiogenesis and although there was no difference in the number of human-derived vessels formed between implants with ECs and implants with ECs cotransplanted with PDLSCs, there was an overall net increase in the number of vessels formed. These data are consistent with other reports of enhanced wound healing models of MSC cotransplantation with ECs or ECs progenitors, yet, no other reports have investigated the effects of PDLSCs on angiogenesis when cotransplanted with ECs. In that there was no net increase in human-derived vessels between conditions, the data suggest that the transplanted PDLSCs did not contribute directly to the formation of vessels through their own differentiation into ECs, but instead may have been able to bolster the host angiogenic response. Again, lending to the similarity of their properties with MSCs, these data are in alignment with MSCs' ability to bolster angiogenesis in many different model systems of wound healing involving bone, cardiac, and cerebral tissue repair. Another possible function of transplanted oral-derived stem cells could be in their capacity to serve as EC supporting cells or pericytes. It has been recently reported that after isolation and expansion in culture, PDLSCs express cell surface markers associated with pericytes.33 In our in vivo model, αSMA staining confirmed the presence of pericytes in the condition where PDLSCs were cotransplanted with ECs. This role as a supporting pericyte is also supported by the observation that in the condition in which PDLSCs were transplanted, there were a greater number of larger, more mature vessels, relative to when only ECs were transplanted. Additionally, it appeared that these vessels were more functional in that they were able to maintain the dextran tracer within the lumens of the vessels to a greater extent than those in the implants where only ECs were transplanted.
Taken together, the findings of this investigation highlight the angiogenic potential of PDLSCs. This cell population demonstrated the capacity to stimulate EC differentiation into vessels through soluble signaling, and to support angiogenesis in vivo. Because these cells are derived from normally discarded tissues (i.e., extracted teeth), they could serve functional roles in cell therapy applications aimed not only to regenerate tissues, but also to enhance angiogenesis.
Acknowledgments
This study was funded by a Career Award for Medical Scientists from the Burroughs Wellcome Fund (D.K.) and by grant number R21 DE021537 from the National Institutes of Health (A.P./D.K.). The authors would also like to acknowledge the University of Michigan Flow Cytometry Core and the University of Michigan School of Dentistry Histology Core.
Disclosure Statement
No competing financial interests exist.
References
- 1.Santos M.I., and Reis R.L.Vascularization in bone tissue engineering: physiology, current strategies, major hurdles and future challenges. Macromol Biosci 10,12, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Ennett A.B., and Mooney D.J.Tissue engineering strategies for in vivo neovascularisation. Expert Opin Biol Ther 2,805, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Stosich M.S., Moioli E.K., Wu J.K., Lee C.H., Rohde C., Yoursef A.M., Ascherman J., Diraddo R., Marion N.W., and Mao J.J.Bioengineering strategies to generate vascularized soft tissue grafts with sustained shape. Methods 47,116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nolan K., Millet Y., Ricordi C., and Stabler C.L.Tissue engineering and biomaterials in regenerative medicine. Cell Transplant 17,241, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Yang F., Cho S.W., Son S.M., Bogatyrev S.R., Singh D., Green J.J., Mei Y., Park S., Bhang S.H., Kim B.S., Langer R., and Anderson D.G.Genetic engineering of human stem cells for enhanced angiogenesis using biodegradable polymeric nanoparticles. Proc Natl Acad Sci U S A 107,3317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szoke K., Beckstrom K.J., and Brinchmann J.E.Human adipose tissue as a source of cells with angiogenic potential. Cell Transplant 21,235, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Bhang S.H., Cho S.W., La W.G., Lee T.J., Yang H.S., Sun A.Y., Baek S.H., Rhie J.W., and Kim B.S.Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials 32,2734, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., and Shi S.SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100,5807, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., and Shi S.Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364,149, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S., Mankani M., Brahim J., Robey P.G., and Shi S.Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97,13625, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai V.T., Zhang Z., Dong Z., Neiva K.G., Machado M.A., Shi S., Santos C.F., and Nor J.E.SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89,791, 2010 [DOI] [PubMed] [Google Scholar]
- 12.d'Aquino R., Graziano A., Sampaolesi M., Laino G., Pirozzi G., De Rosa A., and Papaccio G.Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14,1162, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Batouli S., Miura M., Brahim J., Tsutsui T.W., Fisher L.W., Gronthos S., Robey P.G., and Shi S.Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res 82,976, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., and Horwitz E.Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8,315, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Ghajar C.M., Chen X., Harris J.W., Suresh V., Hughes C.C., Jeon N.L., Putnam A.J., and George S.C.The effect of matrix density on the regulation of 3-D capillary morphogenesis. Biophys J 94,1930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaigler D., Krebsbach P.H., West E.R., Horger K., Huang Y.C., and Mooney D.J.Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J 19,665, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Nor J.E., Peters M.C., Christensen J.B., Sutorik M.M., Linn S., Khan M.K., Addison C.L., Mooney D.J., and Polverini P.J.Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest 81,453, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Curry F.E., Huxley V.H., and Adamson R.H.Permeability of single capillaries to intermediate-sized colored solutes. Am J Physiol 245,H495, 1983 [DOI] [PubMed] [Google Scholar]
- 20.Grainger S.J., Carrion B., Ceccarelli J., and Putnam A.J.Stromal cell identity influences the in vivo functionality of engineered capillary networks formed by co-delivery of endothelial cells and stromal cells. Tissue Eng Part A 19,1209, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grainger S.J., and Putnam A.J.Assessing the permeability of engineered capillary networks in a 3D culture. PLoS One 6,e22086, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaigler D., Krebsbach P.H., Polverini P.J., and Mooney D.J.Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue Eng 9,95, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Duffy G.P., Ahsan T., O'Brien T., Barry F., and Nerem R.M.Bone marrow-derived mesenchymal stem cells promote angiogenic processes in a time- and dose-dependent manner in vitro. Tissue Eng Part A 15,2459, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Iohara K., Zheng L., Wake H., Ito M., Nabekura J., Wakita H., Nakamura H., Into T., Matsushita K., and Nakashima M.A novel stem cell source for vasculogenesis in ischemia: subfraction of side population cells from dental pulp. Stem Cells 26,2408, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Aranha A.M., Zhang Z., Neiva K.G., Costa C.A., Hebling J., and Nor J.E.Hypoxia enhances the angiogenic potential of human dental pulp cells. J Endod 36,1633, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Rao R.R., Peterson A.W., Ceccarelli J., Putnam A.J., and Stegemann J.P.Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis 15,253, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caplan A.I.Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213,341, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Nakamura S., Yamada Y., Baba S., Kato H., Kogami H., Takao M., Matsumoto N., and Ueda M.Culture medium study of human mesenchymal stem cells for practical use of tissue engineering and regenerative medicine. Biomed Mater Eng 18,129, 2008 [PubMed] [Google Scholar]
- 29.Ghajar C.M., Blevins K.S., Hughes C.C., George S.C., and Putnam A.J.Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng 12,2875, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Ghajar C.M., Kachgal S., Kniazeva E., Mori H., Costes S.V., George S.C., and Putnam A.J.Mesenchymal cells stimulate capillary morphogenesis via distinct proteolytic mechanisms. Exp Cell Res 316,813, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prgeeth Pandula P.K., Samaranayake L.P., Jin L.J., and Zhang C.F.Human umbilical vein endothelial cells synergize osteo/odontogenic differentiation of periodontal ligament stem cells in 3D cell sheets. J Periodontal Res 2013. [Epub ahead of print]; DOI: 10.1111/jre.12107 [DOI] [PubMed] [Google Scholar]
- 32.Zhao L., Wu Y., Tan L., Xu Z., Wang J., Zhao Z., Li X., Li Y., Yang P., and Tang T.Co-culture with endothelial cells enhances osteogenic differentiation of periodontal ligament stem cells via COX-2/PGE/VEGF signaling under hypoxia. J Periodontol 2013. [Epub ahead of print]; DOI: 10.1902/jop.2013.120548 [DOI] [PubMed] [Google Scholar]
- 33.Iwasaki K., Komaki M., Yokoyama N., Tanaka Y., Taki A., Kimura Y., Takeda M., Oda S., Izumi Y., and Morita I.Periodontal ligament stem cells possess the characteristics of pericytes. J Periodontol 84,1425, 2013 [DOI] [PubMed] [Google Scholar]