Manfredi et al. [1] report in this issue of Helicobacter that, in their experience, in a large population, more than 95% of Helicobacter pylori infections were eradicated by the empiric strategy of administrating sequential therapy as the first-line therapy followed by a 10-day fluoroquinolone-containing triple therapy. The eradication rate was 92.6% with sequential therapy but only 75% with the second-line fluoroquinolone triple therapy yielding the cumulative result of 97.8% per-protocol eradication. Theirs is one of a few studies looking at overall community results rather than separately focusing on the results of first and second-line therapy [2,3]. They go on to suggest that fluoroquinolone triple therapy might be an excellent recue to eradicate H. pylori infection in only two rounds [1].
The Maastricht conferences have suggested use of treatment packages consisting of two different regimens designed such that failure of initial therapy would prompt treatment with a second-line therapy [4]. The ramifications and mathematics of this strategy are rarely discussed. Here, we consider the variables to take into account for devising such a strategy and recommend an approach to choosing the best option. All other things being equal, the first choice regimen should always be the one with the highest cure rates as that by definition produces the smallest proportion needing retreatment [5] (Fig. 1). Treatment failure results in risks and expenses of second-line treatment as well as lost to follow-up. For these reasons, it is both illogical and likely unethical the initiate therapy with the inferior of two regimens. Although the proportion of patients lost to follow-up was low in the study of Manfredi et al., [1] it is often much higher in routine clinical practice further compromising the real-life effectiveness of treatment strategies.
Figure 1.
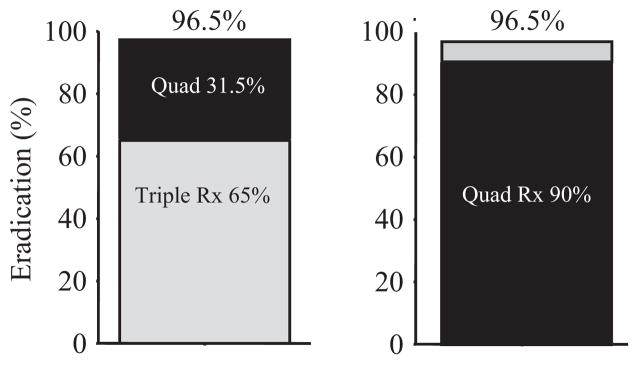
Theoretical model comparison of outcome with a “package” of two therapies such that treatment failure is followed by a different second-line regimen. We compare the sequence of therapy A (e.g. clarithromycin-containing triple therapy with 65% success) followed by treatment B (e.g. bismuth quadruple therapy with 90% success) and the opposite sequence (i.e. A + B, or B + A). While both sequences yield identical per-protocol overall results (e.g. 96.5%), 35% of those who received triple therapy first required retreatment and its attendant risks compared with only 10% in the B + A sequence. (modified from reference [5], with permission). In addition, a high rate of lost to follow-up may lead to a greatly reduced intention to treat cure rate when using less effective first-line treatments.
The goal of H. pylori therapy should be to cure all patients with therapies achieving at least 90% – and preferably 95% or more – cure rates. As such it seems logical to also choose the second-line therapy as the one with the greatest chance of reliably producing a high rate of treatment success. However, as shown in Table 1, if the success of first-line therapy is high, the goal of achieving per protocol 95% or greater overall strategy treatment results can be achieved with regimens that otherwise have individual unacceptably low cure rates (i.e. between 50% and 80%). The fact that it is possible to do so does not mean that it is to be recommended. As first-line sequential treatment was very effective in the Manfredi et al. [1] series, they could have achieved a 95% overall cure rate even with a poorly effective dual PPI plus amoxicillin therapy for 14 days (a 50% cure rate) and could then have recommended that dual therapy as an excellent choice to “eradicate Helicobacter pylori infection in only two rounds”.
Table 1.
Combination of first- and second-line therapies on cumulative outcome of Helicobacter pylori infections using either the minimally effective second-line regimen to achieve 95% success or the optimum therapy that reliably achieves 95% success
| Initial Rx (%) | Minimum 2nd line (%) | Cure rate (%) | Optimum 2nd line (%) | Cure rate |
|---|---|---|---|---|
| 90 | 50 | 95 | 95 | 99+% |
| 85 | 66 | 95 | 95 | 99+% |
| 80 | 75 | 95 | 95 | 99% |
| 75 | 80 | 95 | 95 | 98+% |
| 70 | 84 | 95 | 95 | 98+% |
| 60 | 88 | 95 | 95 | 98% |
When sequential therapy produced a cure rate of 92.6%, a regimen such as PPI and amoxicillin dual therapy for 2 weeks (50% eradication) would produce >95% cumulative cures (i.e. 100−92.6 = 7.4 and as 7.4 × 0.5 = 3.7 then 92.6 + 3.7 = 96.3 overall success) and even a “terrible” regimen with 40% success would still achieve 95.6% cumulative cures.
Here, we explore whether the choice of the second-line therapy should be based primarily on having reliably high treatment success as well as the roles of cost, safety, the consequences of developing resistance, or some other factor should be taken into account when making that choice. Table 1 shows that no matter how effective the initial treatment was, overall success will always be highest if one always chooses a reliably high success rate second-line regimen.
Manfredi et al. [1] had chosen a 10-day levofloxacin containing triple therapy that achieved a cure rate below 80%. Meta-analyses have shown that 7-day fluoroquinolone triple therapy typically provides unacceptably low treatment success, 10-day regimens yield borderline acceptable results (e.g. 84–89% treatment success), and neither provides reliable >90 or 95% cure rates [6,7]. Recently, a trial of 14-day fluoroquinolone triple therapy provided 95% success suggesting that it is possible to achieve high level success with this combination [8]. However, resistance to fluoroquinolones is rapidly increasing worldwide, and the presence of resistance is a likely explanation for the relatively low cure rates experienced by Manfredi et al. Increasingly common resistance suggests that fluoroquinolone-containing regimens should only be used in areas where resistance is known to still be low or pre-treatment susceptibility testing has been performed [9]. Furthermore, fluoroquinolones are expensive and have “black box” warnings. Thus, we can not concur with the Manfredi et al. [1] suggestion that a 10-day fluoroquinolone triple therapy would be an excellent choice to “eradicate Helicobacter pylori infection in only two rounds”.
Recommendations for second-line therapy
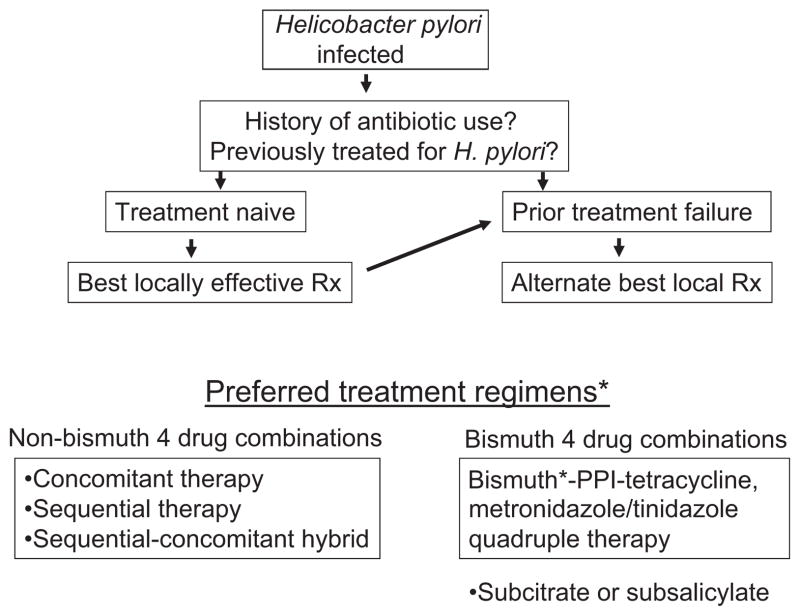
We recommend that the same considerations for choosing first-line empiric therapy be employed for choosing second-line therapy (i.e. that drugs used in previous H. pylori treatment schedules for which resistance has likely developed or those with predictable high primary resistance rates should be avoided). The second line should be the combination that is known to work best locally (Fig. 2) [5,9]. Where available, bismuth-containing quadruple therapy is often an excellent choice provided that one prescribe appropriate doses and for at least 10 or preferably 14 days. Seven-day bismuth-containing quadruple therapy is insufficient to overcome metronidazole resistance [10], which likely explains why a recent meta-analysis reported that 7-day bismuth quadruple was inferior to 10-day levofloxacin triple therapy as a second-line therapy [6].
Figure 2.
Suggested approach for empiric Helicobacter pylori therapy. *From reference [5] with permission.
In conclusion, the best locally available therapy should be used for both first-line and for second-line therapy. After the failure of a clarithromycin-containing four-drug first-line therapy (e.g. sequential or concomitant), current best alternatives are either a bismuth quadruple therapy where available or a fluoroquinolone-containing triple therapy. Our suggestion, however, is to give both for 14 days and avoid levofloxacin in areas where H. pylori fluoroquinolone resistance is known to have increased enough to jeopardize therapy results. The final goal should be achieving at least 90% treatment success also with second-line therapy.
Acknowledgments
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338, which funds the Texas Medical Center Digestive Diseases Center, DK067366 and CA116845.
Footnotes
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
Competing interests: the authors have no competing interests. Dr. Graham is a unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is also a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and for Otsuka Pharmaceuticals regarding diagnostic testing. Dr. Graham has received royalties from Baylor College of Medicine patents covering materials related to 13C-urea breath test. Xavier Calvet has participated in advisory boards for Astra-Zeneca, has served as a speaker for AstraZeneca and Almirall-Prodesfarma, and has received research support from AstraZeneca and Janssen-Cilag.
References
- 1.Manfredi M, Bazzarri B, de’Angelis GL. Helicobacter pylori infection: sequential therapy followed by levofloxacin-containing triple therapy provides a good cumulative eradication rate. Helicobacter. 2012 doi: 10.1111/j.1523-5378.2012.00945.x. (In press) [DOI] [PubMed] [Google Scholar]
- 2.Lee YC, Wu HM, Chen TH, et al. A community-based study of Helicobacter pylori therapy using the strategy of test, treat, retest, and re-treat initial treatment failures. Helicobacter. 2006;11:418–24. doi: 10.1111/j.1523-5378.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- 3.Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second-line regimens proposed by the Maastricht III consensus and a third-line empirical regimen. Am J Gastroenterol. 2009;104:21–5. doi: 10.1038/ajg.2008.87. [DOI] [PubMed] [Google Scholar]
- 4.Malfertheiner P, Megraud F, O’Morain C, Hungin AP, Jones R, Axon A, Graham DY, Tytgat G. Current concepts in the management of Helicobacter pylori infection– the Maastricht 2-2000 Consensus Report. Aliment Pharmacol Ther. 2002;16:167–80. doi: 10.1046/j.1365-2036.2002.01169.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Rimbara E. Helicobacter pylori therapy in the west. Japanese J Helicobacter Res. 2012;13:4–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Huang X, Yao L, Shi R, Zhang G. Advantages of moxifloxacin and levofloxacin-based triple therapy for second-line treatments of persistent Helicobacter pylori infection: a meta analysis. Wien Klin Wochenschr. 2010;122:413–22. doi: 10.1007/s00508-010-1404-3. [DOI] [PubMed] [Google Scholar]
- 7.Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. Am J Gastroenterol. 2006;101:488–96. doi: 10.1111/j.1572-0241.2006.00637.x. [DOI] [PubMed] [Google Scholar]
- 8.Miehlke S, Krasz S, Schneider-Brachert W, et al. Randomized trial on 14 versus 7 days of esomeprazole, moxifloxacin, and amoxicillin for second-line or rescue treatment of Helicobacter pylori infection. Helicobacter. 2011;16:420–6. doi: 10.1111/j.1523-5378.2011.00867.x. [DOI] [PubMed] [Google Scholar]
- 9.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol. 2011;8:79–88. doi: 10.1038/nrgastro.2010.210. [DOI] [PubMed] [Google Scholar]
- 10.Fischbach LA, van ZS, Dickason J. Meta-analysis: the efficacy, adverse events, and adherence related to first-line anti-Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther. 2004;20:1071–82. doi: 10.1111/j.1365-2036.2004.02248.x. [DOI] [PubMed] [Google Scholar]