Abstract
Background
Previous neuroimaging studies of recently detoxified alcohol-dependent patients (RDA) have found significant loss of white matter integrity associated with the shrinkage of the frontal lobes and thinning of the corpus callosum, especially the genu. The current study hypothesized that, in addition to exhibiting the most microstructural white matter disruption in RDA, the genu will also evidence the most recovery after abstinence. This microstructural recovery will be associated with improvements in executive functioning measures.
Methods
Fifteen RDA were examined approximately 2 weeks after abstinence and again after 1 year of abstinence and compared to 15 age- and education-matched nonalcoholic controls using diffusion tensor imaging (DTI). The effects of group, time, and their interactions on fractional anisotropy, radial diffusivity, and axial diffusivity were evaluated with repeated measures MANOVA; in addition, 2 × 2 ANOVA was used to test changes in measures of executive functioning in the 2 groups.
Results
At 2 weeks of abstinence, DTI of RDA showed significantly lower fractional anisotropy and greater radial diffusivity compared to controls in the genu and body of the corpus callosum. Reexamination after 1 year showed significant time by group interaction with fractional anisotropy increasing and radial diffusivity decreasing in RDA but not controls in these 2 regions. A smaller relapsed group did not show improvements between the 2 time points. Abstinent RDA also showed improvement on Digit Span Backward, a measure of working memory, but did not benefit from practice effects on the Halstead Category Test compared to controls.
Conclusions
The results suggest susceptibility of the genu and body of the corpus callosum to the effects of alcohol, and the potential for recovery of both these regions after abstinence, perhaps via mechanisms involving myelin reconstitution.
Keywords: Diffusion Tensor Imaging, White Matter, Alcohol Dependence, Abstinence, Tract-Based Spatial Statistics
White matter damage from chronic alcohol abuse is a consistent finding in neuropathological and structural magnetic resonance imaging (MRI) studies (Estruch et al., 1997; Harper, 1998; Harper and Kril, 1991; Harper et al., 1985; Hommer et al., 1996; Pfefferbaum et al., 1996). According to postmortem analyses, white matter anomalies associated with alcohol abuse include overall volume reduction, demyelination, microtubule disturbance, and axonal subtraction (Alling and Bostrom, 1980; Harper and Kril, 1989; Harper et al., 1987; Kril et al., 1997; Paula-Barbosa and Tavares, 1985). Pathological evidence also indicates that these white matter anomalies are especially prominent in the frontal lobes (Harper and Kril, 1989; Kril et al., 1997; Torvik, 1987).
Consistent with neuropathological research, structural MRI studies report loss in white matter integrity that is associated with shrinkage to the frontal lobes (Dirksen et al., 2006; Oscar-Berman and Marinkovic, 2007; Rosenbloom et al., 2003; Schlaepfer et al., 2006). For example, referring to statistically significant regional brain volume differences measured with MRI, Pfefferbaum and colleagues (1997) described greater white matter deficits in the prefrontal and other frontal regions compared to anterior temporal, anterior parietal, and posterior parietal lobes in chronic alcoholics. Diffusion tensor imaging (DTI) studies have identified decreases in fractional anisotropy values in frontal white matter and limbic pathways (Yeh et al., 2009) as well as in the 5 main subregions of the corpus callosum–the rostrum, genu, body, isthmus, and splenium–with the greatest loss to the genu subregion and less shrinkage to the body and splenium (Pfefferbaum et al., 2000, 2006a).
Neuropsychological changes from alcohol exposure parallel modifications in brain structure specified in the neuropathology and neuroimaging studies, especially cognitive deficits associated with frontal lobe dysfunction (Dirksen et al., 2006). Broadly speaking, functional deficiencies in alcohol dependence include deficits in abstract thinking, problem-solving, spatial and verbal learning, working memory, attention, and perceptual motor skills (Grant, 1987; Grant et al., 1979, 1984; Harper and Matsumoto, 2005; Pfefferbaum et al., 2000, 2006a; Rourke and Grant, 2009). More specifically, general disruptions to the integrity of the corpus callosum correlate with impairments in interhemispheric processing speed (Davies et al., 2005; Schulte et al., 2005). Callosal micro-structure integrity also predicts, at least in part, visuospatial global–local integration ability in alcohol-dependent subjects, a function also requiring the integration of information from both hemispheres (Muller-Oehring et al., 2009).
DTI studies using fiber tractography as well as postmortem analyses indicate that the genu, the anterior section of the corpus callosum, connects left and right hemispheres of the pre-frontal cortex while the posterior segment, the splenium, connects temporal, parietal, and occipital cortices (Abe et al., 2004; Hofer and Frahm, 2006; Huang et al., 2005; Pandya and Seltzer, 1986; Sullivan et al., 2006). Previous research has found that changes in the genu are associated with alcohol-related neuropsychological impairments on frontal lobe-mediated tasks such as Trail Making and Digit Symbol Tests (Jokinen et al., 2007). Furthermore, deficiencies in the genu, specifically, contribute to deficits in attention and working memory (Jokinen et al., 2007; Pfefferbaum et al., 2000, 2006a).
Neuroimaging studies of detoxified alcoholics also report evidence of reversal of alcohol-related structural changes to the brain after the periods of abstinence (Sullivan and Pfefferbaum, 2005). As with the greater original harmful effects in white matter (Harper and Kril, 1991; Harper et al., 1985), recovery is also often observed in the white matter. In their MRI study, Shear and colleagues (1994) discovered that recently detoxified subjects examined 1 month after their last drink and after 3 months of abstinence exhibited white matter volume increases, while those who resumed drinking did not. Another study that used deformation-based morphometry (Cardenas et al., 2007) found that the frontal and temporal lobes are vulnerable to the effects of heavy alcohol consumption and that recovery is observed in these abstinent heavy drinkers compared to relapsers. Finally, a multimodal MRI and DTI study demonstrated that alcohol-dependent subjects showed white matter recovery–both in microstructural integrity and volume–depending on smoking status (Gazdzinski et al., 2010).
Additional studies confirm white matter recovery during periods of abstinence through changes in magnetic resonance spectroscopy (MRS) metabolites that have been observed to be correlated with white matter improvements. Ende and colleagues (2005) found a statistically significant increase in choline-containing compounds in their follow-up measurement in abstinent alcohol-dependent patients indicating an improved cerebral metabolism of lipids in membranes and myelin. Bartsch and colleagues (2007) also found that 1H-MRS levels of cerebellar choline and frontomesial N-acetylaspartate were significantly augmented after short-term sobriety, and that these increases were associated with global brain volume recovery.
The current study hypothesized that the significant declines in microstructural integrity as measured with DTI in the corpus callosumof recentlydetoxifiedalcohol-dependentpatients (RDA) will be partially ameliorated after a year of abstinence fromalcohol. As previously reported, the frontal lobesmay be especially susceptible tomicrostructural whitematter changes in alcoholic patients. The majority of fibers that connect the 2 sides of the frontal lobes course through the genu and body of the corpus callosum; while the splenium connects temporal and occipital regions. Therefore, the current study hypothesized that, paralleling previous findings of preferential frontal damage, the genu of the corpus callosum would exhibit the greatest white matter disruption in RDA, followed by the body and the splenium. Finally, the current study hypothesized that frontal/executive functions similar to those previously found to be susceptible to the effects of alcohol dependence (Jokinen et al., 2007; Pfefferbaum et al., 2000, 2006a) will show the significant improvements after a year of abstinent that parallels improvements inDTImeasures. 2 sides of the frontal lobes course through the genu and body of the corpus callosum; while the splenium connects temporal and occipital regions. Therefore, the current study hypothesized that, paralleling previous findings of preferential frontal damage, the genu of the corpus callosum would exhibit the greatest white matter disruption in RDA, followed by the body and the splenium. Finally, the current study hypothesized that frontal/executive functions similar to those previously found to be susceptible to the effects of alcohol dependence (Jokinen et al., 2007; Pfefferbaum et al., 2000, 2006a) will show the significant improvements after a year of abstinent that parallels improvements in DTI measures.
MATERIALS AND METHODS
This longitudinal study was approved by the Institutional Review Boards of the University of California, San Diego and the VA San Diego Healthcare System (VASDHS). Informed consents were obtained from all participants prior to enrollment in the study.
Participants and Procedures
Alcohol-dependent participants in this study were recruited from the VASDHS Alcohol and Drug Treatment Program, while the matched nonalcoholic controls were recruited from the community through local newspaper advertisement. To be included in the RDA group, participants had to meet the following criteria: At baseline, (Time 1) participants must have met Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994) criteria for alcohol dependence based on a Structured Clinical Interview for the DSM-IV administered by trained research psychiatrists at the VASDHS Alcohol Research Center. Furthermore, participants must have consumed the equivalent of 560 g of pure ethanol (EtOH) (approximately forty 5-ounce glasses of wine or forty 12-ounce cans of beer) each week for the most recent 5 years. To be classified as abstinent at follow-up (Time 2), participant must have had no drinks at all during the interim period between testing. Individuals who went back to regular drinking at pre-baseline levels for at least 9 months during the interim period were classified as relapsers. Finally, the control participants had never met DSM-IV criteria for substance dependence or other Axis-I disorders at either time points.
Participants were excluded if they had a history of neurological disease or other systemic illness that may interfere with cognitive functioning (e.g., liver disease, chronic obstructive pulmonary disease, cardiac disease, or kidney disease). Results of blood laboratories and physical examinations were used to exclude individuals with evidence of overall poor physical health, including malnutrition and liver disease. Additional exclusions were head injury with loss of consciousness greater than 15 minutes, Axis-I psychiatric diagnosis other than major depressive disorder, and history of substance dependence other than alcohol. Although we did not specifically exclude patients with remote abuse history of other drugs, we did exclude patients with DSM-IV diagnosis of (nonalcohol) substance abuse disorder in the preceding 5 years. Individual with intermittent use of marijuana (not diagnosable as abuse or dependence) or nicotine was not excluded from the study.
Fifteen participants met inclusion and exclusion criteria at both time points and were classified as abstinent RDA. Four participants met criteria for relapse and were classified as relapsed RDA; the rest did not meet criteria and were excluded. Finally, 15 controls were matched on a 1-to-1 basis to the abstinent RDA based on age and education. The 4 alcohol-dependent patients who relapsed to pre-baseline levels of drinking were used as a comparison group in a post hoc analysis, but were not included in the main analysis because of the small sample size.
All participants underwent a structured interview to gather information on participants’ drug use history, history of mild head trauma, family history of alcoholism, and estimated lifetime history of alcohol consumption using the timeline followback method (Adams et al., 1981; Sobell et al., 1988). Alcohol amounts were calculated based on the following: an alcoholic beverage was considered to have approximately 12.5 g of pure EtOH and was equivalent to: (i) one 12-ounce can of beer, (ii) 4 ounces of wine, or (iii) 1.5 ounces of distilled spirits.
Regular monitoring of blood and urine assured sobriety in RDA during their inpatient treatment. Controls had no blood-alcohol concentration at the time of the examinations as determined by breathalyzer (ALCO-Sensor III, St. Louis, MO). The RDA received MRI scans after a minimum of 2 weeks sobriety to minimize the effects of acute detoxification. Approximately a year later (Time 2), participants underwent the same MRI procedure. Sobriety was determined by self- and collateral report, in addition to blood-alcohol concentration at the time of the examinations as determined by breathalyzer.
Diffusion Tensor Imaging Protocol
DTI data were collected on a General Electric LX 1.5T scanner (GE, Milwaukee, WI) at the VASDHS using a single-shot, stimulated-echo sequence with spiral acquisition (TE = 100 ms, TR = 6,000 ms, slice thickness = 3.8 mm, FOV = 240 mm and in-plane resolution of 3.75 9 3.75 mm). A b-value of 1,745 s/mm2 was acquired in 42 directions, and 1 nondiffusion-weighted image (b = 0) was acquired. For each direction and for the nondiffusion-weighted image, 4 acquisitions were acquired and averaged to produce the images.
The functional MRI of the Brain Software Library (FSL 4.1.4, http://www.fmrib.ox.ac.uk/fsl; Smith et al., 2004) was used to process the images. FSL FLIRT was used to perform motion and eddy current correction for each scan by registering each diffusion direction to the nondiffusion-weighted image using a 12-parameter affine transformation. A brain mask was created and applied to perform skull stripping. This was performed using the 3dAutomask routine of Analysis of Functional NeuroImages (AFNI) program (Cox, 1996; Cox and Hyde, 1997). Further removal of eyeballs and nasal sinus artifacts was performed by hand. Diffusion tensor estimation was performed using AFNI's 3dDWItoDT program, using a nonlinear estimation of the diffusion tensor model. Eigenvectors and associ ated eigenvalues of the tensor describing the diffusion ellipsoid were computed, and fractional anisotropy, axial diffusivity, and radial diffusivity were derived using standard formulas (Song et al., 2003).
Tract-based Spatial Statistics version 1.2 (Smith et al., 2006) was chosen for data extraction for 2 reasons. First, this method mitigates partial volume effects by restricting the analysis to those voxels comprising the local tract center. Second, it reduces misregistration of multisubject DTI images using nonlinear registration methods combined with the projection of data onto tract representation that are insensitive to alignment discrepancies. A nonlinear registration algorithm (FSL FNIRT) was used to align all fractional anisotropy data to a template and then affine-transformed into MNI152 space by upsampling to 1 × 1 × 1 × 1 mm3. The transformed images were used to create mean fractional anisotropy images, which were then subjected to non-maximum-suppression perpendicular to local tracts (Smith et al., 2006) to produce the mean fractional anisotropy skeleton, which specifies the centers of fiber tracts common to all images. A fractional anisotropy threshold of 0.2 was applied to the mean skeleton to minimize nonwhite matter voxels. The thresholded skeleton was then overlayed on individual fractional anisotropy images and a spatial search algorithm mapped local maximum fractional anisotropy values on each skeleton voxel. This process was used to correct between-subject registration error. Spatial voxel mapping using fractional anisotropy images was also used to map axial diffusivity and radial diffusivity values to the skeleton. This method produced 68 aligned fractional anisotropy, axial diffusivity, and radial diffusivity skeletons with voxel values unique to each participant at each visit (see Figs 1 and 2).
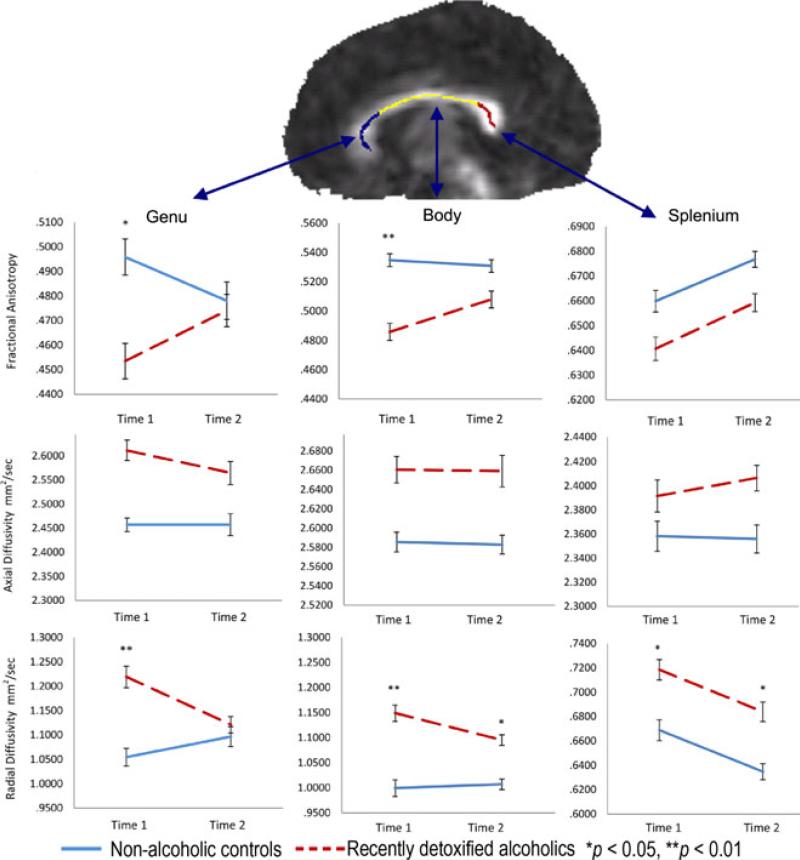
Fig. 1.
Fractional anisotropy, axial diffusivity, and radial diffusivity changes between baseline and follow-up in recently detoxified alcohol-dependent patients (RDA) and control participants in the genu, body, and splenium of the corpus callosum (error bars represent the standard error of the mean) and a sagittal fractional anisotropy exemplar image with the skeletonized map from an RDA.
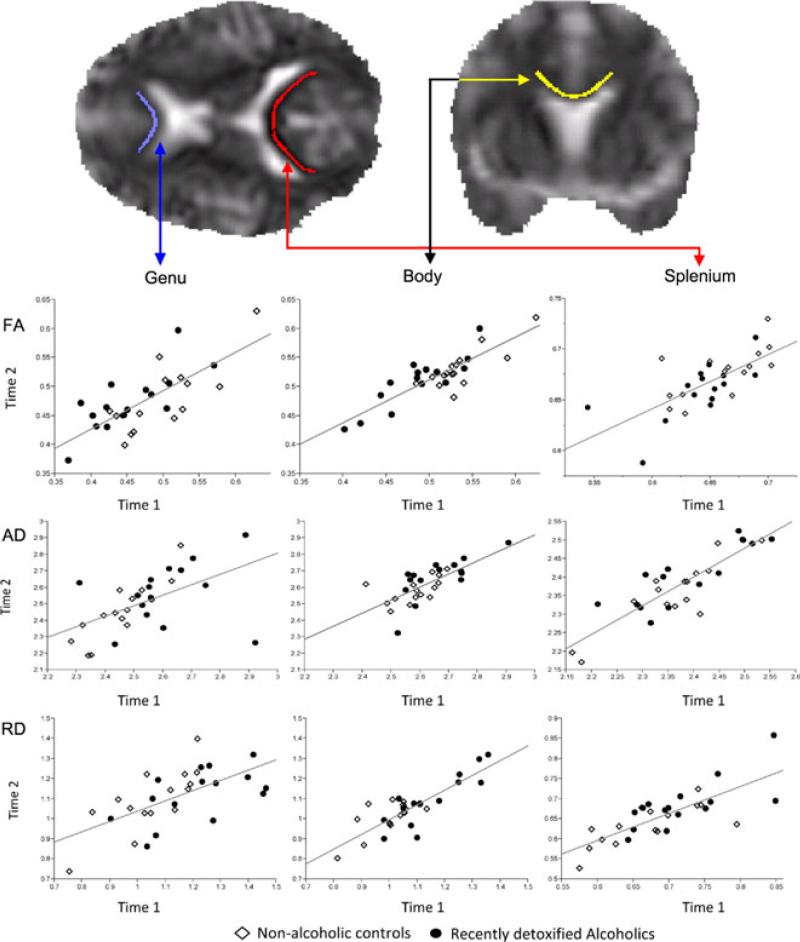
Fig. 2.
Relationship between baseline and follow-up fractional anisotropy, axial diffusivity, and radial diffusivity in recently detoxified alcohol-dependent patients (RDA) and control participants in the genu, body, and splenium of the corpus callosum (linear least squares regression line represents both groups) and transaxial and coronal radial diffusivity exemplar images with the skeletonized map from an RDA.
The genu, splenium, and body of the corpus callosum were defined based on the ICBM-DTI-81 white matter labels atlas (Mori et al., 2008) Average fractional anisotropy, axial diffusivity, and radial diffusivity from the 3 subregions of the corpus callosum were then extracted for each participants.
Neuropsychological Assessment
Participants were tested by a trained psychometrician on several components of the Expanded Halstead Reitan Battery (Heaton et al., 2004), which included 3 previously identified tests sensitive to the detrimental effect of alcohol on frontal/executive function. These were the Halstead Category Test, the Trail Making Test– Part B, and the Wechsler Adult Intelligence Scale-III (WAIS-III) Digit Span Backward. Using published normative data (Heaton et al., 2004), T scores adjusting for age, education, sex, and ethnicity were calculated for each of these tests and used in the analysis.
Statistical Analysis
To test our main hypothesis, a repeated measures multivariate analysis of variance (MANOVA) was performed to test the null hypothesis that groups did not change over time on their DTI measures in the 3 regions of the corpus callosum. Specifically, we tested the multivariate overall group by time interaction for statistical significance. This was followed up, when appropriate, with an examination of the interaction effect within each region and univariate tests of simple main effects. In addition, 2 (Time) × 2 (Group) ANOVAs were conducted to evaluate the 3 neuropsychological measures as dependent variables.
RESULTS
Demographics of the Samples
A detailed comparison of demographic and other characteristics of the 2 groups is presented in Table 1. The 2 groups were well matched on age, education, ethnicity, and time between baseline and follow-up scan. All participants were men. Moreover, a detailed comparison of demographic and alcohol-use histories was performed to examine potential differences between those alcohol-dependent patients who were followed up versus those who were not. The 2 groups did not differ on any of the demographic or alcohol consumption measures assessed at baseline (see Table 2). In addition, none of the patients in this study had past history of abuse or dependence on substances, other than alcohol.
Table 1.
Demographic, Alcohol Use, and Smoking Characteristics of Recently Detoxified Alcohol-Dependent Patients and Control Participants
| Characteristics | Recently detoxified alcoholics n = 15 | Controls n = 15 | Test value | p-Value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age | 51.4 ± 6.0 | 51.8 ± 7.4 | t = 0.16 | 0.87 |
| Education, years | 13.6 ± 2.4 | 13.9 ± 2.6 | t = 0.29 | 0.77 |
| Percentage non-white | 13% | 6% | χ2 = 3.0 | 0.23 |
| Alcohol use history | ||||
| Number of years of alcoholism | 28.2 ± 5.1 | — | ||
| Number of drinks lifetime | 121,996 ± 42,997 | — | ||
| Total drinks last 5 years | 28,116 ± 9,077 | — | ||
| Drinks per day last 30 days | 18.1 ± 6.1 | — | ||
| Smoking history | ||||
| Percent current smokers | 73.3% (11/15) | 20% (3/15) | χ2 = 8.57 | 0.003 |
| Cigarettes per day among smokers | 18.5 ± 6.2 | 11.3 ± 9.0 | t = 1.62 | 0.13 |
| Changed status between visit 1 and 2 | 1 | 0 | ||
| Number of days between scans | 372 ± 46 | 374 ± 24 | t = 0.19 | 0.85 |
Table 2.
Baseline Demographic and Alcohol-Use Characteristics of Recently Detoxified Alcohol-Dependent Patients Who Were Followed Up Versus Those Who Were Not
| Characteristics | Patients who completed follow up n = 26 | Patients who did notcomplete follow up n = 54 | Test value | p-Value |
|---|---|---|---|---|
| Sociodemographics | ||||
| Age | 48.7 ± 7.3 | 48.5 ± 10.2 | t = 0.523 | 0.60 |
| Education, years | 13.2 ± 2.2 | 12.9 ± 1.9 | t = 0.63 | 0.53 |
| Percent non-white | 19% | 20% | χ2 = 0.01 | 0.91 |
| Alcohol use history | ||||
| Number of years of alcoholism | 25.0 ± 9.4 | 21.3 ± 10.1 | t = 1.57 | 0.12 |
| Number of drinks lifetime | 117,714 ± 54,511 | 101,648 ± 52,440 | t = 1.27 | 0.21 |
| Total drinks last 5 years | 28,547 ± 12,216 | 26,501 ± 10,853 | t = 0.76 | 0.45 |
| Drinks per day last 30 days | 19.6 ± 9.5 | 19.0 ± 8.6 | t = 0.25 | 0.80 |
Fractional Anisotropy Analyses
The overall multivariate test confirmed a group by time interaction, Wilks’ λ = 0.705, F(3, 26) = 3.625, p = 0.026, η2 = 0.295. Follow-up analyses showed statistically significant interaction effects between time and group in both the genu, F(1, 28) = 7.534, p = 0.010, η2 = 0.212; and the body of the corpus callosum, F(1, 28) = 11.222, p = 0.002, η2 = 0.286 (see Fig. 1). On the other hand, changes in the splenium were observed in both groups over time, F(1, 28) = 13.726, p = 0.001, η2 = 0.329, with no between-subjects effect, F(1, 28) = 3.178, p = 0.085, η2 = 0.102.
Further analysis of simple main effects in the genu and body of the corpus callosum showed that the interactions found in the fractional anisotropy of the 2 regions were the result of an increase in fractional anisotropy between baseline and follow-up in the genu, t(14) = 2.103, p = 0.054, d = 0.54, and a similar increase in fractional anisotropy in the body, t(14) = 3.956, p < 0.001, d = 1.02, among RDA. On the other hand, changes in controls did not achieve statistical significance in either the genu, t(14) = 1.782, p = 0.096, d = 0.46, or the body of the corpus callosum, t(14) = 0.724, p = 0.481, d = 0.19. At baseline, the RDA, as compared to controls, exhibited significantly lower fractional anisotropy values in both the genu, t(28) = 2.051, p = 0.050, d = 0.749, and the body of the corpus callosum, t(28) = 3.348, p = 0.002, d = 1.222. On the other hand, at follow-up, the 2 groups did not statistically differ from each other on fractional anisotropy values in either the genu, t(28) = 0.195, p = 0.847, d = 0.071, or the body of the corpus callosum, t(28) = 1.586, p = 0.124, d = 0.579.
Figure 2 provides a detailed representation of the changes in fractional anisotropy for each RDA and control participant. As can be observed, more RDA show improvements (are above the regression line for both groups combined) than control subjects in the regions of the genu and body. On the other hand, the distribution of changes in fractional anisotropy in the splenium is approximately the same for the 2 groups.
Radial and Axial Diffusivity Analyses
In addition to fractional anisotropy, we examined changes in both radial diffusivity and axial diffusivity using the same statistical analysis methods. For axial diffusivity, the omnibus multivariate analysis revealed neither an interaction nor any significant main effects between the 2 groups. Specifically, there was no significant change across the 2 time points, Wilks’ λ = 0.951, F(3, 26) = 0.446, p = 0.722, η2 = 0.049, nor was there a significant difference between groups, Wilks’ η = 0.763, F(3, 26) = 2.686, p = 0.067, η2 = 0.237. In addition, the interaction was also not signifi-cant, Wilks’ η = 0.933, F(3, 26) = 0.621, p = 0.608, η2 = 0.067 (see Fig. 1).
The axial diffusivity scatter plots in Fig. 2 provide detailed representation of the changes in axial diffusivity for each RDA and control participant. The 2 groups were generally equally represented above and below the regression line indicating that the average change in axial diffusivity was similar between groups.
On the other hand, the overall multivariate test for radial diffusivity showed a group by time interaction, Wilks’ η = 0.716, F(3, 26) = 3.431, p = 0.032, η2 = 0.284. We therefore examined this interaction separately for each region. Statistically significant interaction effect between time and group was found both in the genu, F(1, 28) = 9.687, p = 0.004, η2 = 0.257, and the body of the corpus callosum, F(1, 28) = 6.732, p = 0.015, η2 = 0.194 (see Fig. 1). On the other hand, changes in the splenium were observed in both groups over time, F(1, 28) = 16.773, p < 0.001, η2 = 0.375, with a statistically significant between-subjects effect, F(1, 28) = 6.001, p = 0.021, η2 = 0.176.
The interactions found in the radial diffusivity of the 2 regions of the corpus callosum were the result of a decrease in radial diffusivity between baseline and follow-up in the genu, t(14) = 2.669, p = 0.018, d = 0.689, and a decrease in radial diffusivity in the body, t(14) = 3.148, p = 0.007, d = 0.813, among RDA. On the other hand, changes in controls did not achieve statistical significance in either the genu, t(14) = 1.616, p = 0.128, d = 0.42, or the body of the corpus callosum, t(14) = 0.469, p = 0.646, d = 0.12 (see Fig. 3). At baseline, the RDA exhibited significantly higher radial diffusivity values in the genu, t(28) = 2.890, p = 0.007, d = 1.06; body, t(28) = 3.840, p = 0.001, d = 1.40; and splenium, t(28) = 2.064, p = 0.048, d = 0.76. On the other hand, at follow-up, the 2 groups did not statistically differ from each other on radial diffusivity values in the genu, t(28) = 1.379, p = 0.179, d = 0.50, but did differ in the body, t(28) = 2.270, p = 0.031, d = 0.829, and the splenium, t(28) = 2.386, p = 0.024, d = 0.871.
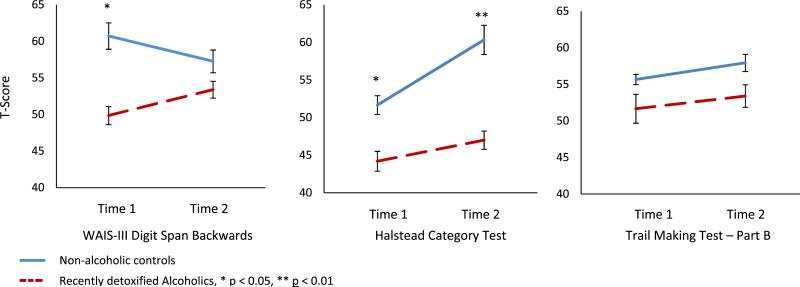
Fig. 3.
Digit Span Backward, Halstead Category Test, and Trail Making Test–Part B changes between baseline and follow-up in recently detoxified alcohol-dependent and control participants (error bars represent the standard error of the mean).
The radial diffusivity scatter plots in Fig. 2 provide detailed representation of the changes in radial diffusivity for each RDA and control participant. In general, more RDA showed a decrease (i.e., they were below the regression line) in both the genu and the body of the corpus callosum. It is also of note that the scatter plots overall show that the DTI values in the body of the corpus callosum are the most consistent (clustered close to the regression line) between baseline and follow-up scans indicating higher reliability in this measure and this is further shown in the short standard error bars on Fig. 1.
Neuropsychological Assessment Analyses
Digit Span Backward
A 2 (Time) × 2 (Group) ANOVA was performed on each of the neuropsychological measures chosen. For digit span, results showed a time by group interaction, F(1, 28) = 6.186, p = 0.019, in both the genu2 = 0.181. This significant interaction was the result of an improvement in the average T score of alcoholic patients, t(14) = 1.947, p = 0.036, d = 0.503, but not controls, t(14) = 1.612, p = 0.065, d = 0.416. The controls had significantly higher T scores at baseline (M = 60.7) compared to RDA (M = 49.9); t(28) = 2.482, p = 0.019, d = 0.906. At follow-up, the 2 groups did not differ significantly from each other; t(28) = 0.999, p = 0.326, d = 0.365 (see Fig. 3).
Halstead Category Test
The results of the 2 × 2 ANOVA revealed a statistically significant effect of time, F(1, 28) = 6.155, p = 0.019, η2 = 0.180. This result reflected the significant improvements in the Category Test T scores over time in the control group, t(14) = 2.035, p = 0.031, d = 0.525, but not in the patient group, t(14) = 1.588, p = 0.071, d = 0.410. The 2 groups were statistically significantly different from each other at both baseline, t(14) = 2.084, p = 0.046, d = 0.761, and follow-up, t(14) = 2.886, p = 0.007, d = 1.054.
Trail Making Test–Part B
The results of the ANOVA showed no statistically significant differences between the 2 groups, F(1, 28) = 1.305, p = 0.263, η2 = 0.045, or across the 2 time periods, F(1, 28) = 1.701, p = 0.203, η2 = 0.057.
Finally, Table 3 represents the correlation between change scores on DTI measure and change score on the neuropsychological measures in RDA. There were no statistically significant correlations between any of these measures.
Table 3.
Correlation Between Diffusion Tensor Imaging (DTI) and Neuropsychological Change Scores Between Baseline and Follow-Up of Recently Detoxified Alcohol-Dependent Patients Who Remained Abstinent
| Change in DTI measure | Change in Digit Span Backward | Change in Halstead Category Test | Change in Trail Making Test—Part B |
|---|---|---|---|
| Fractional anisotropy | |||
| Genu | 0.22 | –0.03 | 0.18 |
| Body | 0.10 | 0.10 | 0.14 |
| Splenium | 0.22 | 0.12 | 0.16 |
| Axial diffusivity | |||
| Genu | 0.28 | –0.09 | –0.46 |
| Body | –0.08 | 0.23 | –0.38 |
| Splenium | –0.35 | 0.43 | –0.42 |
| Radial diffusivity | |||
| Genu | 0.46 | –0.35 | –0.15 |
| Body | 0.01 | 0.15 | –0.09 |
| Splenium | –0.11 | –0.25 | –0.06 |
All p-values > 0.05.
Post Hoc Analysis of Relapsing Alcoholics
Table 4 presents post hoc analyses of fractional anisotropy, axial diffusivity, and radial diffusivity changes in RDA who relapsed to pre-baseline alcohol-use levels. As can been seen, there were no statistically significant changes in this group of relapsers between the 2 time points. However, we note that there is an increase in the axial and radial diffusivity in the genu of relapsers. The effect sizes of these 2 measures, d = 0.778 and d = 1.622, respectively, are substantial and indicate that these may be meaningful differences, but will require a larger follow-up sample to establish their statistical reliability.
Table 4.
Post Hoc Analysis of Fractional Anisotropy, Axial Diffusivity, and Radial Diffusivity Changes in Recently Detoxified Alcohol-Dependent Patients Who Relapsed to Pre-Baseline Alcohol-Use Levels
| DTI measure | Baseline n = 4 | Follow-up n = 4 | Test value | p-Value |
|---|---|---|---|---|
| Fractional anisotropy | ||||
| Genu | 0.27 ± 0.09 | 0.26 ± 0.09 | t = 0.31 | 0.78 |
| Body | 0.38 ± 0.05 | 0.37 ± 0.06 | t = 0.90 | 0.43 |
| Splenium | 0.42 ± 0.16 | 0.43 ± 0.15 | t = 0.96 | 0.41 |
| Axial diffusivity | ||||
| Genu | 1.02 ± 0.75 | 1.60 ± 0.74 | t = 2.33 | 0.10 |
| Body | 2.16 ± 0.27 | 2.20 ± 0.26 | t = 1.55 | 0.21 |
| Splenium | 2.18 ± 0.44 | 2.07 ± 0.37 | t = 1.07 | 0.36 |
| Radial diffusivity | ||||
| Genu | 0.62 ± 0.34 | 1.15 ± 0.31 | t = 2.84 | 0.07 |
| Body | 1.50 ± 0.12 | 1.56 ± 0.18 | t = 1.27 | 0.29 |
| Splenium | 1.58 ± 0.51 | 1.56 ± 0.37 | t = 0.29 | 0.79 |
DTI, diffusion tensor imaging.
DISCUSSION
The current study examined abstinence-related white matter recovery in alcoholics over a 1-year period and revealed significant microstructural white matter compromise as well as recovery in different subregions of the corpus callosum. Several studies have previously reported on the impact of alcohol dependence on the whole brain and corpus callosum in RDA (Gazdzinski et al., 2010; Pfefferbaum et al., 2000, 2006a,b; Rosenbloom et al., 2003; Sullivan and Pfefferbaum, 2005). More recent studies have reported regional variations in this type of white matter injury, with the genu showing some specific susceptibility to the effects of alcohol (Muller-Oehring et al., 2009; Rosenbloom et al., 2008).
This study extends these findings by examining the changes in the 3 main subregions between early treatment and after 1 year of abstinence. Significant increases in the fractional anisotropy were found in the genu and body of the corpus callosum in RDA but not controls. On the other hand, the 2 groups did not differ in the change in fractional anisotropy in the splenium over time. Given the vulnerability of the genu to the effects of alcohol (Kashem et al., 2007; Rosenbloom et al., 2008), the remarkable improvements in that region were not unexpected. However, the current study is the first to demonstrate such improvement using in vivo DTI measures in a longitudinal design with a 1-year abstinence period in RDA. This improvement is in contrast to the lack of change, and possible deterioration, in DTI measures among the small group of relapsers we examined. Finally, a new finding is that the body of the corpus callosum showed similar improvement as the genu.
It is noteworthy that the DTI data collected from the body of the corpus callosum appear to be the most reliable, both in terms of smaller standard errors of the mean, as well as a better fit to the linear least squares regression line, which represents both groups. This is probably due to the greater overall size of the body compared to the genu and splenium, which results in more reliable measures. This is one possible explanation of why the DTI data derived from the body of the corpus callosum shows that the controls remain relatively unchanged over the 1-year period of testing; while data from the genu and splenium show some unexpected, albeit not statistically significant, decreases in fractional anisotropy and increases in radial diffusivity. Despite the possibility of higher residual error in the data from the genu and splenium, our results are robust and can be interpreted with confidence because both a control and patient group were used and the 2 groups were well matched on the most significant variables that might affect the results, such as age and alcohol-use histories.
Although the results of the executive functioning assessments did not correlate with any of the DTI measures, the RDA group showed improvements on a measure of working memory after a year of abstinence (Digits Span Backward) while showing no improvement on a measure of abstraction and cognitive flexibility (Halstead Category Test). In contrast, the control participants remained relatively stable on the Digit Span Backward measure (or regressed slightly to the mean) while improving significantly on the Halstead Category Test. These findings illustrate a potential role that practice effects may play in re-testing and suggests that potential learning and memory deficits in the RDA may attenuate practice effects. Thus, it is possible that while the control group was able to learn and retain strategies used in performing the Halstead Category Test at baseline, the RDA group showed no such benefit of learning on this task. On the other hand, on the test that is less susceptible to practice effect (Digit Span Backward), the controls remained stable over the 2 testing periods while the RDA improved.
The results of the current study must be interpreted with a few limitations in mind. This study examined only men because of the preponderance of male patients seeking care at our facility. Our 2 groups were mismatched on smoking status. This mismatch could conceivably have impacted the baseline differences between the 2 group on fractional anisotropy (Gazdzinski et al., 2010). However, it is unlikely to have affected the longitudinal results because smoking status did not change significantly over time (only 1 alcohol-dependent patient quit smoking). Another limitation of this study is the difficulty interpreting the finding that both groups showed significant increases in fractional anisotropy and decreases in radial diffusivity in the splenium. This finding was not hypothesized a priori and requires replication to eliminate such potential explanations as scanner drift or a byproduct of an artifact in the imaging processing scheme.
Although the small size of our relapsing group made it impractical to statistically analyze it in combination with the larger data set, it did provide us interesting insights that would be useful for future research. The increases in both axial and radial diffusivity in the genu among relapsers might indicate that relapse could have an additive effect in terms of injury to regional white matter in the brain (in this case the genu). One of the difficulties of collecting data on relapsed alcoholic patients is the heterogeneity encountered in the length, severity, and timing of the relapse events. Therefore, future research will need to study large enough samples of relapsed patients in order to model the variety of ways relapse occurs (e.g., recency, length, or severity of relapse episodes) and its effect on the brain.
Despite these limitations, the current study contributes to an understanding of the mechanism of recovery in the corpus callosum of RDA. Based on an extra-cerebral white matter model, Song and colleagues (2003) have used axial and radial diffusivity to differentiate between axonal and myelin integrity, respectively. Although not uncontroversial (Wheeler-Kingshott and Cercignani, 2009), it is possible that by comparing patterns of changes in these 2 diffusivity measures, a better picture emerges about the role of myeli-nation in the recovery process. In both groups, axial diffusivity, a putative marker of axonal integrity, remained constant across the 2 time points. On the other hand, radial diffusivity, a putative marker of myelin integrity, decreased significantly in the genu and body of the corpus callosum in the RDA but not the controls. This is consistent with the possibility of a process involving reconstitution of myelin in these regions in the affected group. If our DTI findings are confirmed, they may provide a basis for understanding some of the mechanisms of neurocognitive recovery (Rourke and Grant, 2009) that has also been reported in alcoholics who achieve stable abstinence. Future research should attempt to replicate our finding and examine potentially interesting extra-callosal tracts, especially in the frontal lobe, which might lead to a better understanding of the relationship between white matter and functional recovery in abstinent alcoholics.
ACKNOWLEDGMENTS
This project was made possible by a grant from the Veterans Affairs Medical Research Service awarded to IG. We gratefully acknowledge June Allen for her invaluable assistance throughout the course of this project.
REFERENCES
- Abe O, Masutani Y, Aoki S, Yamasue H, Yamada H, Kasai K, Mori H, Hayashi N, Masumoto T, Ohtomo K. Topography of the human corpus callosum using diffusion tensor tractography. J Comput Assist Tomogr. 2004;28:533–539. doi: 10.1097/00004728-200407000-00016. [DOI] [PubMed] [Google Scholar]
- Adams KM, Grant I, Carlin AS, Reed R. Cross-study comparisons of self-reported alcohol consumption in four clinical groups. Am J Psychiatry. 1981;138:445–449. doi: 10.1176/ajp.138.4.445. [DOI] [PubMed] [Google Scholar]
- Alling C, Bostrom K. Demyelination of the mamillary bodies in alcoholism. A combined morphological and biochemical study. Acta Neuropathol. 1980;50:77–80. doi: 10.1007/BF00688539. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Author; Washington, DC.: 1994. [Google Scholar]
- Bartsch AJ, Homola G, Biller A, Smith SM, Weijers HG, Wiesbeck GA, Jenkinson M, De Stefano N, Solymosi L, Bendszus M. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain. 2007;130:36–47. doi: 10.1093/brain/awl303. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, Meyerhoff DJ. Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage. 2007;34:879–887. doi: 10.1016/j.neuroimage.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A. Is there cognitive impairment in clinically “healthy” abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Dirksen CL, Howard JA, Cronin-Golomb A, Oscar-Berman M. Patterns of prefrontal dysfunction in alcoholics with and without Korsakoff's syndrome, patients with Parkinson's disease, and patients with rupture and repair of the anterior communicating artery. Neuropsychiatr Dis Treat. 2006;2:327–339. doi: 10.2147/nedt.2006.2.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Welzel H, Walter S, Weber-Fahr W, Diehl A, Hermann D, Heinz A, Mann K. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry. 2005;58:974–980. doi: 10.1016/j.biopsych.2005.05.038. [DOI] [PubMed] [Google Scholar]
- Estruch R, Nicolas JM, Salamero M, Aragon C, Sacanella E, Fernandez-Sola J, Urbano-Marquez A. Atrophy of the corpus callosum in chronic alcoholism. J Neurol Sci. 1997;146:145–151. doi: 10.1016/s0022-510x(96)00298-5. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Mon A, Yeh PH, Meyerhoff DJ. Cerebral white matter recovery in abstinent alcoholics—a multimodality magnetic resonance study. Brain. 2010;133:1043–1053. doi: 10.1093/brain/awp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I. Alcohol and the brain: neuropsychological correlates. J Consult Clin Psychol. 1987;55:310–324. doi: 10.1037//0022-006x.55.3.310. [DOI] [PubMed] [Google Scholar]
- Grant I, Adams K, Reed R. Normal neuropsychological abilities of alcoholic men in their late thirties. Am J Psychiatry. 1979;136:1263–1269. doi: 10.1176/ajp.136.10.1263. [DOI] [PubMed] [Google Scholar]
- Grant I, Adams KM, Reed R. Aging, abstinence, and medical risk factors in the prediction of neuropsychologic deficit among long-term alcoholics. Arch Gen Psychiatry. 1984;41:710–718. doi: 10.1001/archpsyc.1984.01790180080010. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. Patterns of neuronal loss in the cerebral cortex in chronic alcoholic patients. J Neurol Sci. 1989;92:81–89. doi: 10.1016/0022-510x(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J. If you drink your brain will shrink. Neuropathological considerations. Alcohol Alcohol. 1991;1(Suppl):375–380. [PubMed] [Google Scholar]
- Harper C, Kril J, Daly J. Are we drinking our neurones away? Br Med J (Clin Res Ed) 1987;294:534–536. doi: 10.1136/bmj.294.6571.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5:73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: a pathological study. Br Med J (Clin Res Ed) 1985;290:501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Miller SW, Taylor MJ, Grant I. Psychological Assessment Resources. Odessa, FL.: 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Arch Neurol. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, Jiang H, Wakana S, Poetscher L, Miller MI, Van Zijl PC, Hillis AE, Wytik R, Mori S. DTI tractography based parcellation of white matter: application to the mid-sagittal morphology of corpus callosum. Neuroimage. 2005;26:195–205. doi: 10.1016/j.neuroimage.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Jokinen H, Ryberg C, Kalska H, Ylikoski R, Rostrup E, Stegmann MB, Waldemar G, Madureira S, Ferro JM, Van Straaten EC, Scheltens P, Barkhof F, Fazekas F, Schmidt R, Carlucci G, Pantoni L, Inzitari D, Erkinjuntti T. Corpus callosum atrophy is associated with mental slowing and executive deficits in subjects with age-related white matter hyperintensities: the LADIS Study. J Neurol Neurosurg Psychiatry. 2007;78:491–496. doi: 10.1136/jnnp.2006.096792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashem MA, James G, Harper C, Wilce P, Matsumoto I. Differential protein expression in the corpus callosum (splenium) of human alcoholics: a proteomics study. Neurochem Int. 2007;50:450–459. doi: 10.1016/j.neuint.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, Van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40:570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Schulte T, Fama R, Pfefferbaum A, Sullivan EV. Global-local interference is related to callosal compromise in alcoholism: a behavior-DTI association study. Alcohol Clin Exp Res. 2009;33:477–489. doi: 10.1111/j.1530-0277.2008.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya D, Seltzer B. The topography of commissural fibers. In: Lepore F, Petito M, Jasper H, editors. Two Hemispheres—One Brain: Functions of the Corpus Callosum. Alan R. Liss Inc.; New York: 1986. pp. 47–73. [Google Scholar]
- Paula-Barbosa MM, Tavares MA. Long term alcohol consumption induces microtubular changes in the adult rat cerebellar cortex. Brain Res. 1985;339:195–199. doi: 10.1016/0006-8993(85)90645-6. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: interaction of age and alcoholism. Neurobiol Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Supratentorial profile of white matter microstructural integrity in recovering alcoholic men and women. Biol Psychiatry. 2006b;59:364–372. doi: 10.1016/j.biopsych.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Adalsteinsson E, Lim KO, Moseley M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol Clin Exp Res. 2000;24:1214–1221. [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom M, Sullivan EV, Pfefferbaum A. Using magnetic resonance imaging and diffusion tensor imaging to assess brain damage in alcoholics. Alcohol Res Health. 2003;27:146–152. [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Sassoon SA, Fama R, Sullivan EV, Pfefferbaum A. Frontal callosal fiber integrity selectively predicts coordinated psychomotor performance in chronic alcoholism. Brain Imaging Behav. 2008;2:74–83. doi: 10.1007/s11682-007-9017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I. Neuropsychological correlates of alcoholism. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. Oxford University Press; New York: 2009. pp. 398–454. [Google Scholar]
- Schlaepfer TE, Lancaster E, Heidbreder R, Strain EC, Kosel M, Fisch HU, Pearlson GD. Decreased frontal white-matter volume in chronic substance abuse. Int J Neuropsychopharmacol. 2006;9:147–153. doi: 10.1017/S1461145705005705. [DOI] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, Pfefferbaum A. Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex. 2005;15:1384–1392. doi: 10.1093/cercor/bhi020. [DOI] [PubMed] [Google Scholar]
- Shear PK, Jernigan TL, Butters N. Volumetric magnetic resonance imaging quantification of longitudinal brain changes in abstinent alcoholics. Alcohol Clin Exp Res. 1994;18:172–176. doi: 10.1111/j.1530-0277.1994.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers’ reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–1039. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Torvik A. Brain lesions in alcoholics: neuropathological observations. Acta Med Scand Suppl. 1987;717:47–54. doi: 10.1111/j.0954-6820.1987.tb13041.x. [DOI] [PubMed] [Google Scholar]
- Wheeler-Kingshott CA, Cercignani M. About “axial” and “radial” diffusivities. Magn Reson Med. 2009;61:1255–1260. doi: 10.1002/mrm.21965. [DOI] [PubMed] [Google Scholar]
- Yeh PH, Simpson K, Durazzo TC, Gazdzinski S, Meyerhoff DJ. Tract-Based Spatial Statistics (TBSS) of diffusion tensor imaging data in alcohol dependence: abnormalities of the motivational neurocircuitry. Psychiatry Res. 2009;173:22–30. doi: 10.1016/j.pscychresns.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]